Abstract
Objective and design
We studied the involvement of calcium and calcium-activated NADPH oxidases in NLRP3 inflammasome activation and IL-1β release to better understand inflammasome signaling in macrophages.
Material or subjects
Human volunteer blood donors were recruited to isolate monocytes to differentiate them into macrophages. Wild-type or DUOX1-deficient C57/B6 mice were used to prepare bone marrow-derived macrophages.
Treatment
Murine or human macrophages were treated in vitro with NLRP3 inflammasome agonists (ATP, silica crystals) or calcium agonists (thapsigargin, ionomycin) in calcium-containing or calcium-free medium.
Methods
Intracellular calcium changes were followed by measuring FURA2-based fluorescence. Gene expression changes were measured by quantitative real-time PCR. Protein expression was assessed by western blotting. Enzymatic activity was measured by fluorescence caspase-1 activity assay. IL-1β release was determined by ELISA. ELISA data were analyzed by ANOVA and Tukey’s post-hoc test.
Results
Our data show that calcium is essential for IL-1β release in human macrophages. Increases in cytosolic calcium alone lead to IL-1β secretion. Calcium removal blocks caspase-1 activation. Human macrophages express Duox1, a calcium-regulated NADPH oxidase that produces reactive oxygen species. However, Duox1-deficient murine macrophages show normal IL-1β release.
Conclusions
Human macrophage inflammasome activation and IL-1β secretion requires calcium but does not involve NADPH oxidases.
Keywords: Calcium, Duox, macrophage, inflammasome, NADPH oxidase
1. Introduction
Interleukin-1β (IL-1β) is a major pro-inflammatory cytokine secreted by several cell types but its main sources are monocytes and macrophages.[1] IL-1β is produced as an inactive form, pro-IL-1β, (35 kDa molecular mass) that needs to be cleaved to form the bioactive 17 kDa form.[2] Proteolytic cleavage of IL-1β is carried out by caspase 1.[2, 3] Formation of active caspase 1 requires proteolysis of procaspase 1 by an intracellular multimeric protein complex called the inflammasome.[4, 2, 5]
Inflammasome activation and consequent IL-1β release can be triggered by diverse innate immune stimuli that are danger- or pathogen-associated molecular patterns including silica crystals and extracellular ATP.[2, 6, 7] Several different inflammasomes have been discovered but the best-described one is the NLPR3 inflammasome that mediates effects of both silica and ATP.[2]
Details of NLRP3 inflammasome regulation are largely unclear but ion fluxes and cell stress play a role in the process.[2] Earlier observations implicated a role for potassium efflux.[8, 9] Recent publications show that Ca2+ ions are essential in NLRP3 inflammasome activation.[1, 10, 11] The role of Ca2+ in NLRP3 inflammasome activation has been characterized in murine bone marrow-derived macrophages (BMDM) and human monocytes but this has not been described in human macrophages.
Reactive oxygen species (ROS) are also involved in NLRP3 inflammasome activation.[12, 13] The two main ROS sources in cells are mitochondria and NADPH oxidases.[14, 15] Mitochondrial-derived ROS have been already linked to the NLRP3 inflammasome.[16] The Nox/Duox family of NADPH oxidases consists of seven proteins (Nox1-5 and Duox1-2) that produce superoxide or hydrogen peroxide as their primary enzymatic products.[17, 18] Earlier observations suggested involvement of the phagocytic Nox2-based NADPH oxidase (also expressed in monocytes and macrophages) during inflammasome activation.[12] Later studies, however, disproved this theory since blood cells isolated from patients deficient in NADPH oxidase subunits (Nox2, p22phox) showed normal, or even enhanced, inflammasome activation.[10, 19, 20] Because p22phox is an essential subunit of not only the Nox2-based NADPH oxidase but also Nox1, Nox3 and Nox4, inflammasome activation does not require any of these oxidases (Nox1-4) either.[20] The remaining three NADPH oxidases (Nox5, Duox1 and Duox2) are all activated directly by increases in cytosolic Ca2+ levels [21–23]. It is unknown whether Nox5, Duox1 or Duox2 are expressed in monocytes or macrophages and could contribute to NLRP3 inflammasome activation.
In this article, we show that IL-1β secretion and NLRP3 inflammasome activation in human monocyte-derived macrophages (hMDMs) requires Ca2+. ATP- and silica-stimulated IL-1β secretion is abolished by chelating intra- and extracellular Ca2+. Cytosolic Ca2+-enhancing agonists induce IL-1β release. Among the three Ca2+-activated NADPH oxidases, only Duox1 expression could be detected in hMDMs. Duox1 is, however, not required for NLRP3 inflammasome regulation since Duox1-deficient murine BMDMs stimulated by silica or ATP had normal IL-1β release.
2. Materials and Methods
2.1. Purification of human monocytes and macrophage differentiation
Human subjects provided written informed consent for participation under the guidelines of NIH and UGA IRB-approved protocols.[24, 25] Whole blood was drawn at the Transfusion Medicine Branch of the National Institutes of Health or the University of Georgia Health Center. 40 mL blood was anticoagulated by ACD (anticoagulant citrate dextrose) or heparin. Red blood cells were removed by Dextran sedimentation. White blood cells were layered on 5-step Percoll (Sigma) gradient: 85%-80%-75%-70%-65% and centrifuged (800 xg, 30 min, 13° C). PBMCs containing monocytes (PBS/65% Percoll interface) were separated, washed, suspended in RPMI-1640 medium (Life Technologies, Grand Island, NY) and added to 6-well plates (BD Primeria, Swedesboro, NJ). After 30 min, adherent cells were washed four times with HBSS. Cells were differentiated into macrophages by incubating them in RPMI-1640 medium supplemented with 10% pooled human heat-inactivated serum and 50 ng/ml human macrophage colony stimulating factor (hM-CSF, R&D Systems, Minneapolis, MN) for 5–8 days. When mentioned, MDMs were primed with 1 μg/ml Salmonella typhimurium LPS (Sigma Aldrich, St. Louis, MO) for 30 min.
2.2. Collection of human serum
Blood (10 ml) was also collected from healthy volunteers to separate serum. After coagulation, sera were collected, centrifuged (10000g, 5min), filtered through 0.22 μm size sterile filter and stored at −80 °C for future use. Serum samples from at least 5 different donors were thawed, pooled and heat-inactivated (56 °C, 30 min). 10% heat-inactivated pooled human serum was used on MDM cultures.
2.3. Differentiation of murine BMDMs
Duox1-deficient mice (purchased from Lexicon Pharmaceuticals, Inc., The Woodlands, TX, USA) were previously characterized.[26] These mice were shown to contain a gene-trapping cassette between DUOX1 exons 20 and 21, resulting in the failure to produce detectable Duox1 protein in uroepithelial cells. Mice were maintained in a specific pathogen-free facility at the NIAID according to IUCAC animal protocols approved at the NIH. Bone marrow cell suspensions were isolated from tibias and femurs of 8- to 12-week-old C57Bl/6 mice by flushing bone marrows with RPMI1640 medium (+10% FCS, +1% Pen/Strep). Cell clumps were dislodged by pipetting, and debris was removed by passaging through a 70-μm cell strainer (Fisher Scientific, Pittsburg, PA, USA). Cells were washed twice with medium and seeded on 10 cm tissue culture dishes (for Ca2+ measurements) or on 24-well plates (for ELISA) (Corning Costar, Tewskbury, MA). Cells were grown in complete RPMI-1640 medium supplemented with 10 ng/mL recombinant murine M-CSF (R&D Systems, Minneapolis, MN) and cultured for 5–7 days in a humidified incubator at 37°C and 5% CO2.
2.4. Manipulations of intra- and extracellular calcium
To scavenge extracellular Ca2+, human and murine macrophages were incubated in HBSS supplemented with 1 mM MgCl2, 10 mM HEPES, 5 mM glucose and 100 μM EGTA, pH 7.4. Corresponding Ca2+-containing solutions contained 1 mM CaCl2 and no EGTA (pH 7.4). To chelate intracellular Ca2+, human MDMs were incubated with 50 μM BAPTA-AM for 15 min at 37°C in HBSS and washed twice in PBS afterwards to remove excess dye.
2.5. Calcium measurements
Murine macrophages were primed with 1μg/ml Salmonella typhimurium LPS (Sigma Aldrich, St. Louis, MO) for 1hr. After two washes, cells were incubated in HBSS containing 2 μM FURA2-AM (Life Technologies, Grand Island, NY) for 30 min in the dark. Cells were washed, suspended in HBSS and were added to black 96-well plates (Corning Costar, Tewksbury, MA). After 10-minute incubation, cells were stimulated by different doses of ATP (0–3 mM) (Sigma Aldrich, St. Louis, MO) and changes in 510nm fluorescence emission while exciting at two wavelengths (340nmex/510nmem, 390nmex/510nmem) were followed for 60 min with shaking. The ratio of 340 nm/510nm and 390 nm/510 nm values were calculated and presented to reflect kinetic changes in cytosolic Ca2+ levels.
2.6. RNA isolation and quantitative real-time RT-PCR
RNA was isolated by Trizol/chloroform extraction and isopropanol precipitation from differentiated hMDMs. cDNA synthesis was carried out (Thermoscript cDNA synthesis kit, Life Technologies, Grand Island, NY). Human DUOX1, DUOX2, NOX5, NLRP3 and P2RX7 gene expression was measured by reverse transcription/quantitative real-time PCR using SYBR Green (Invitrogen) and the following primers:
Human DUOX1 (F: 5′-CACCTCCTGGAGACCTTTTTC -3′, R: 5′ GGCCTGGTTGATGTCCAG -3′, 60 bp product), human DUOX2 (F: 5′-GATGGTGACCGCTACTGGTT -3′, R: 5′– GCCACCACTCCAGAGAGAAG -3′, 323 bp product), human NOX5 (F: 5′-CAAGAATGAAGCCGCAGAC -3′, R: 5′-CCTGCAATGGTCTTAAACTGC -3′, 95 bp product), human NLRP3 (F: 5′-CTTCTCTGATGAGGCCCAAG -3′, R: 5′-GCAGCAAACTGGAAAGGAAG -3′, 200 bp product), human P2RX7 (F: 5′-GGGAACCAGAAGACCTGTGA -3′, R: 5′-AGTTTTCGGCACTGTTCAAGA -3′, 94 bp product) and β-actin (F: 5′-CCAACCGCGAGAAGATGA-3′, R: 5′-CCAGAGGCGTACAGGGATAG-3′, 97 bp product). Changes in fluorescence were followed in 7500 Real-time PCR system (Applied Biosystems, Grand Island, NY). Reaction parameters were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The relative abundance of mRNAs was obtained using the comparative cycle threshold method and was normalized to beta-actin as the endogenous control.
2.7. Measurement of human and murine IL-1β release by ELISA
Human MDMs or mouse BMDMs cultured on 24-well plates were primed with Salmonella typhimurium LPS (1 μg/ml, Sigma Aldrich, St. Louis, MO) for 60 min. Cells were washed twice with Ca2+-free HBSS before addition of inflammasome stimuli (ATP, 0–4 mM or 100 μg/ml silica crystals (Sigma Aldrich, St. Louis, MO)). At indicated time points, supernatants were collected, centrifuged (500g, 10 min) to remove cell debris, crystals etc. Supernatants were either frozen or immediately used for further analysis. Human and murine IL-1β release in macrophage supernatants was determined by commercial ELISA kits (DuoSet, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Cytokine release was calibrated using standard curves.
2.8. Western Blotting
HEK-293 cells or human MDMs were washed three times with cold Ca2+- and magnesium-free PBS and lysed by NP-40 lysis buffer (Boston Biosciences, Ashland, MA) containing 150 μM PMSF (Fluka Biochemika-Sigma Aldrich, St. Louis, MO) and 1% protease inhibitor cocktail (dissolved in DMSO, Sigma Aldrich, St. Louis, MO). Lysates were centrifuged and protein concentrations in supernatants were determined using the BCA assay (Pierce, Rockford, IL). Equal amounts of protein were added to SDS sample buffer containing 2-mercaptoethanol (without boiling), and electrophoresed on SDS-polyacrylamide gels (8%; Tris-Glycine Gel, Life Technologies, Grand Island, NY). Gels were blotted on nitrocellulose membrane (Invitrogen) using the TE Series Transpher Electrophoresis Unit (Hoefer, Holliston, MA) wet blotting system. Blots were blocked overnight in TTBS (TBS-buffer containing 5% milk powder and 0.05% Tween-20). Blots were incubated with primary antibodies (RT, 1hr, TTBS), washed three times with TTBS and then probed with secondary HRP-linked antibodies (RT, 1hr, TTBS, GE Healthcare, Piscataway, NJ). After repeated washes, blots were developed by chemiluminescence using the Lumigen DS detection kit (GE Healthcare, Piscataway, NJ). The primary antibodies used were: anti-Duox1 (Clone T-14, 1:500, rabbit, Santa Cruz, Dallas, TX), anti-Hsp90 (1:1000, mouse, mouse, Santa Cruz, Dallas, TX), anti-caspase-1 p20 (rabbit, 1:500, Cell signaling, Danvers, MA), anti-actin (rabbit, 1:2000, Sigma Aldrich, St. Louis, MO), HA (mouse, 1:100, Covance, Princeton, NJ).
2.9. Caspase-1 enzymatic activity
Differentiated human MDMs were primed with 1 μg/ml Salmonella typhimurium LPS (Sigma Aldrich, St. Louis, MO, USA) for 30 min. To scavenge intracellular calcium, MDMs were treated with 50 μM BAPTA-AM (Dojindo laboratory, Rockville, Maryland USA) in a PBS with 5 mM glucose for 10 min. Equal volume of DMSO (Sigma Aldrich, St. Louis, MO,USA), solvent for BAPTA-AM, was used as a control. MDMs were then stimulated with 3mM ATP in HBSS containing 2mM glucose with or without 1mM Ca2+ for 10 min. Ca2+-free HBSS contained 1 mM MgCl2 (Sigma Aldrich, St. Louis, MO,USA) and 100 μM EGTA (Sigma Aldrich St. Louis, MO, USA) was used to chelate any remaining Ca2+. MDMs were then scraped off to measure caspase-1 enzymatic activity using caspase-1 fluorometric assay kit (Enzo Life Sciences, Farmingdale, NY, USA). Following the manufacturer’s instruction, cold lysis buffer was added to macrophages. Equal volume of the reaction buffer containing 10 mM DDT was then added to the lysis buffer. Finally, 50 μM of caspase-1 YFAD-AFC substrate was used to detect fluorescence at excitation and emission wavelengths of 450 nm and 505 nm, respectively. Results were reported as relative fluorescence units (RFU) and kinetics of the caspase-1 signal development was obtained using Varioskan™ Flash Multimode Reader (Thermo-scientific, USA) for 1.5 hours at 37 °C. Final endpoint fluorescent values were compared between 2 groups treated, one treated with media containing Ca2+ and once treated without Ca2+.
2.10. Statistical analysis
Data are represented as mean +/− S.E.M. or mean+/− S.D. Significance was calculated with ANOVA and Tukey’s pot-hoc test and was marked as * when p < 0.05, ** when p < 0.01 and *** when p < 0.001.
3. Results
3.1. Calcium is critical for IL-1β secretion of human macrophages stimulated by ATP or silica
Recently, a critical role of Ca2+ in NLRP3 inflammasome activation has been shown.[1] IL-1β release from murine BMDMs and human monocytes is inhibited by intra- or extracellular chelation of Ca2+.[1, 11] Since the role of Ca2+ in IL-1β secretion by human macrophages has not been demonstrated so far and differences exist between human monocytes and macrophages in terms of regulation of IL-1β expression and inflammasome activation, we differentiated monocytes isolated from peripheral human blood into macrophages by treatment with 50 ng/ml human M-CSF and measured IL-1β release by ELISA.[27, 2] We stimulated inflammasome activation by ATP, a well-described stimulus to induce IL-1β release in monocytes/macrophages.[28] Extracellular ATP enhanced IL-1β secretion in a concentration-dependent manner (Fig. 1A). Chelation of extracellular Ca2+ by ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) or intracellular Ca2+ by 1,2-bis(o-aminophenoxy)ethane- N,N,N′,N′-tetraacetic acid (BAPTA) decreased ATP- and silica-stimulated cytokine release (Fig. 1B). Combined effects of EGTA and BAPTA resulted in an 80.2+/−8.0% (mean+/−S.E.M., n=6) inhibition of silica- and 70.4+/−7.4% inhibition (mean+/−S.E.M., n=5) of ATP-activated IL-1β release (Fig. 2B). We observed similar robust inhibition of IL-1β secretion of human MDMs by using two additional known inflammasome activators, antimycin-A and calcium pyrophosphate dehydrate crystals (data not shown). Thus, Ca2+ plays an essential role in IL-1β release by human macrophages.
Figure 1. IL-1β secretion by human macrophages stimulated with ATP or silica requires calcium.
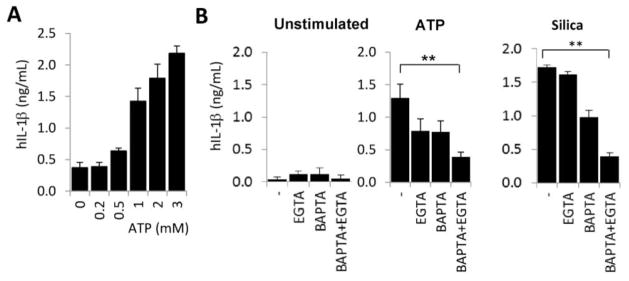
A) LPS-primed human MDMs were stimulated with different concentrations of ATP and release of human IL-1β was measured by ELISA (mean +/− S.E.M., n=3). B) LPS-primed human MDMs were treated (or not) with 50 μM BAPTA-AM and stimulated with 3 mM ATP or 100 μg/ml silica crystals in the presence (1 mM Ca2+) or absence (No Ca2+ and 100 μM EGTA) of extracellular Ca2+. Release of IL-1β was measured by ELISA. Mean+/−S.E.M. n=5. **, p<0.01.
Figure 2. Increased cytosolic calcium levels trigger IL-1β release by human macrophages.
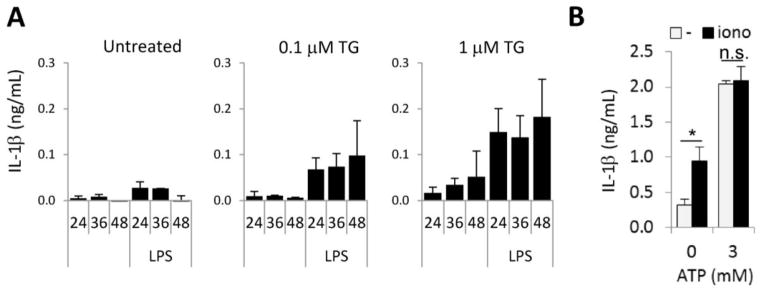
A) Human MDMs with or without LPS-priming (1 μg/ml, 1hr) were stimulated with thapsigargin (TG, 0.1 μM or 1 μM) and release of human IL-1β was measured at the indicated time points (24, 36 or 48 hrs post-treatment). Average +/−S.D. of two independent donors. B) LPS-primed human MDMs were stimulated with 1 μM ionomycin and/or 3 mM ATP and extracellular IL-1β release was measured by ELISA. Mean+/−S.E.M., n=4. *, p<0.05.
3.2. Calcium-inducing agonists trigger IL-1β release
To investigate whether the elevation in intracellular Ca2+ alone is sufficient to induce IL-1β secretion in human MDMs we used pharmacological manipulation to disrupt Ca2+ homeostasis. Thapsigargin (TG) is a widely used inhibitor of the sarco-/endoplasmic reticulum Ca2+ ATPase.[3] TG first empties intracellular calcium stores that activates the store-operated calcium entry pathway leading to subsequent calcium influx from the extracellular space.[3] TG-treatment of lipopolysaccharide (LPS)-primed human MDMs alone induced release of IL-1β (Fig. 2A). Ionomycin (iono), a calcium ionophore, enhances intracellular Ca2+ concentrations abruptly by releasing Ca2+ from both, extra- and intracellular stores.[6] Addition of ionomycin to human MDMs increased IL-1β secretion (Fig. 2B). ATP-induced IL-1β release did not differ in the presence or absence of ionomycin suggesting that ATP also releases Ca2+ from stores both inside and outside of the cell (Fig. 2B). Our data show that increases in cytosolic Ca2+ alone are enough to induce IL-1β secretion by human macrophages.
3.3. Macrophage inflammasome activation requires Ca2+
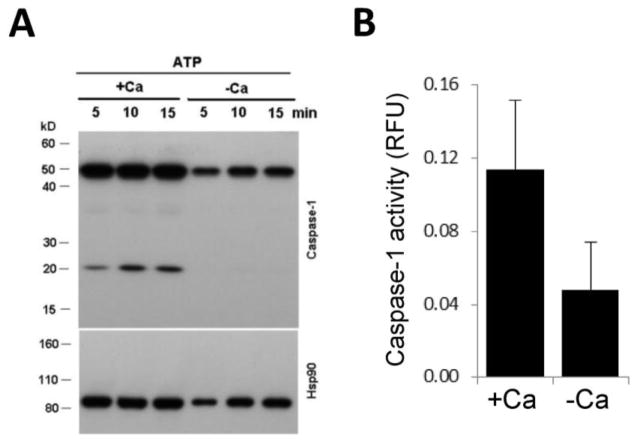
Next, we asked whether Ca2+ is required for IL-1β release either upstream or downstream of NLRP3 inflammasome activation. We stimulated human MDMs in the presence or absence of Ca2+ (BAPTA+EGTA) with 3 mM ATP, a well-characterized NLRP3 agonist, and measured the true indicator of inflammasome activation, procaspase-1 cleavage. Once assembled, the direct consequence of NLRP3 inflammasome activation is proteolytic activation of procaspase-1 into the bioactive, 20kD-size caspase-1.[20] While in presence of calcium the 20 kD caspase-1 cleavage product appeared upon stimulation by ATP, in absence of Ca2+, this band was missing (Fig. 3A). In addition, we measured caspase-1 enzymatic activity in human MDMs stimulated with 3mM ATP in the presence or absence of calcium (BAPTA+EGTA), and we experienced an inhibition in caspase-1 enzymatic activity upon calcium removal (Fig. 3B). These results show that Ca2+ is required for NLRP3 inflammasome activation in human macrophages (Fig. 3).
Figure 3. Human macrophage NLRP3 inflammasome activation requires calcium.
LPS-primed human MDMs were stimulated with 3 mM ATP in the absence (“−Ca”: treatment with 50 μM BAPTA-AM, absence of extracellular Ca2+, 100 μM EGTA) or presence (“+Ca”: no BAPTA-AM treatment, 1 mM extracellular Ca2+) of calcium. A) After 15 min, cell lysates were prepared and cleavage of 45 kD procaspase-1 into active 20kD capsase1 p20 was detected by western blotting. Hsp90 was used as loading control. Another donor gave similar data. B) After 10 min intracellular caspase-1 activity was measured (mean+/−S.E.M., n=5). RFU, relative fluorescence unit.
3.4. Duox1 is expressed in human macrophages
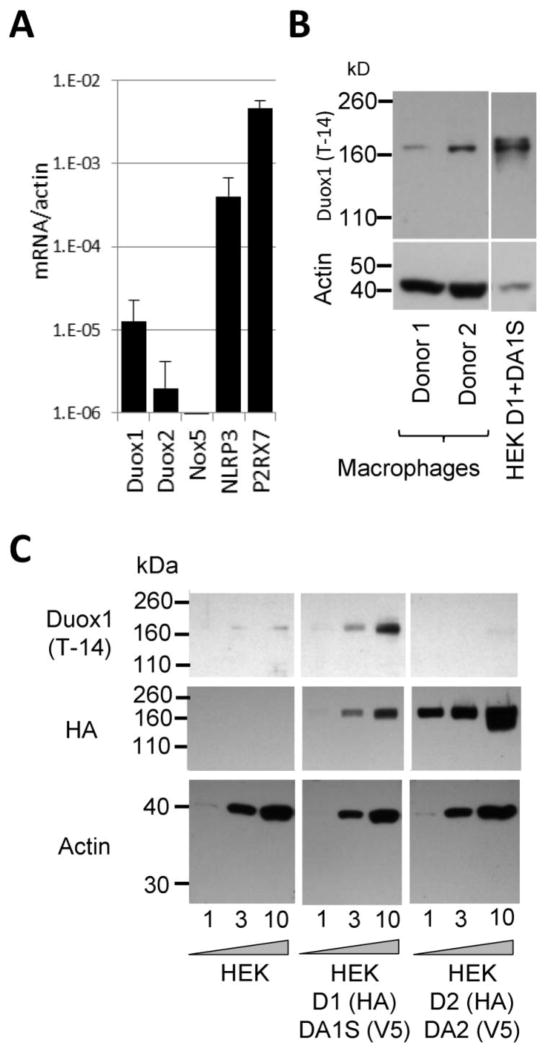
ROS were suggested to play a role in NLRP3 inflammasome regulation.[12] Mitochondria have been identified as the source of ROS but certain NADPH oxidases have been excluded.[16, 19, 20, 10] Studies on phagocytic NADPH oxidase-deficient monocytes showed that four members of the Nox/Duox family of NADPH oxidases (Nox1-4) that are dependent on p22phox are not involved in inflammasome activation.[10, 19, 20] There are, however, three other members of this family which could be ROS sources required for inflammasome activation in macrophages: Nox5, Duox1 and Duox2.[17] Whether these oxidases are required for inflammasome activation is unknown. Nox5, Duox1 and Duox2 are all directly activated by increases in intracellular Ca2+ levels through their intracellular Ca2+-binding EF-hand motifs.[22, 21, 23] Innate immune functions of Duox1 have already been previously proposed but it is unknown if these enzymes are expressed in human macrophages.[29, 17, 30] Therefore, we measured gene expression levels of NOX5, DUOX1 and DUOX2 in human MDMs by real-time PCR. Nox5 mRNA was not detected at all, Duox2 mRNA was only detected in one of the four donors whereas Duox1 mRNA was consistently detected (Fig. 4A). DUOX1 expression was significantly lower than that of NLPR3 or P2RX7 (Fig. 4A). Probing human MDM cell lysates of two donors with a commercial Duox1 antibody, we detected a clear band at about 180 kDa molecular mass in both donors (Fig. 4B). Thus, we identified a new cell type in humans expressing Duox1: macrophages.
Figure 4. Human macrophages express Duox1.
A) Gene expression levels of human DUOX1, DUOX2, NOX5, NLRP3 and P2RX7 were determined in LPS-primed human MDMs by real-time quantitative PCR using gene specific primers and SYBR Green. Mean +/− S.E.M. of gene expression levels relative to actin are shown (n=4). B) Immunoblots of LPS-primed human MDMs or Duox1+DuoxA1α-expressing HEK-293 cells were probed with anti-Duox1 (T-14) antibody. Macrophages from two different donors were tested side by side. C) Cell lysates were prepared from HEK-293 cell lines stably expressing bicistronic pcDNA5.1TM/FRT constructs of human Duox1+DuoxA1α (D1+DA1S) or human Duox2+DuoxA2 (D2+DA2) or from the parental line. D1 and D2 are HA-tagged, whereas DA1S and DA2 are tagged with V5. Different amounts of proteins (1, 3 and 10 μg) were loaded on western blots and probed with three different antibodies: Duox1 (Santa Cruz T-14 clone), HA-tag and actin. The anti-Duox1 and anti-HA antibodies were used on the same blot, the blots were stripped and reprobed. One representative gel, n=3.
To test the isoform specificity of the Duox antibody, we used cell lysates prepared from our previously characterized HEK-293 cell model lines stably expressing bicistronic pcDNA5.1™/FRT constructs of human Duox1+DuoxA1α or human Duox2+DuoxA2.[23] We used cell lines containing HA-tagged constructs to reliably determine the amounts of Duox by Western blotting.[10] The parental cell line (HEK-293) did not show detectable HA or Duox (Fig. 4C). The intensity of the HA-band was stronger in Duox2/DuoxA2-expressing cells compared to that of the Duox1/DuoxA1S sample, indicating higher protein/cell expression for Duox2 than Duox1 (Fig. 4C). The used Duox1 antibody detected Duox1 but not Duox2 (Fig. 4C), confirming that human MDMs express the Ca2+-activated NADPH oxidase Duox1.
3.5. Duox1 plays no role in NLRP3 inflammasome activation
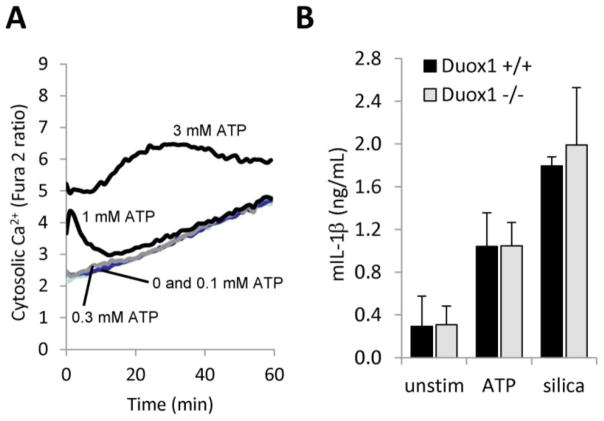
To address the question whether Duox1 is required for inflammasome activation and IL-1β release, first we tested whether murine BMDMs produce cytosolic Ca2+ responses to ATP. Cytosolic Ca2+ levels did not rise above baseline unless cells were stimulated with 1 mM or higher doses of ATP (Fig. 5A). 1mM ATP triggered a short, temporary Ca2+ transient whereas 3mM ATP, a concentration triggering maximal inflammasome activation resulted in a robust, long-lasting Ca2+ signal (Fig. 5A). We next exposed wild-type and Duox1-deficient murine BMDMs to ATP or silica and measured IL-1β release.[26] Duox1-deficiency does not impair the ability of murine BMDMs to secrete IL-1β (Fig. 5B). Thus, we conclude that Duox1 does not play a role in inflammasome activation in macrophages.
Figure 5. Macrophage IL-1β secretion is Duox1-independent.
A) LPS-primed murine BMDMs were loaded with Ca2+-sensitive dye FURA 2-AM and stimulated with different doses of extracellular ATP. Changes in cytosolic Ca2+ over 60 min time were monitored as a ratio of 510nm fluorescence emission based on excitation at two different wavelengths (340/510 nm and 390/510 nm) using a Fluoroskan microplate fluorimeter. One representative recording of three similar ones isolated from different mice. B) Duox1-deficient BMDMs produce normal amounts of IL-1β. Duox1-deficient and wild-type mouse BMDMs were primed with LPS and stimulated with 3 mM ATP (30 min) or 100 μg/ml silica crystals (6 hrs). Secretion of murine IL-1β in culture supernatants was determined by ELISA (mean+/−S.E.M., n=4).
Discussion
Several diverse stimuli (cytokines, crystals, danger signals, microbial products) converge on the NLRP3 inflammasome in monocytes/macrophages and activate production and secretion of bioactive IL-1β.[31–34] The links between extracellular stimuli and NLRP3 activation are still largely unknown. Decreased intracellular potassium concentrations have been implicated in inflammasome activation by ATP, crystals, toxins and bacteria.[35, 36] The proposed role of potassium was based on experiments showing that caspase 1 activation and IL-1β secretion is blocked in the presence of high extracellular potassium levels that inhibit potassium efflux from cells.[36, 35] More recently, another ion, Ca2+ has been shown to be essential for NLRP3 inflammasome activation in murine BMDMs and human monocytes.[1, 11] Our data (Fig. 1–3) show that Ca2+ has a pivotal role in IL-1β secretion and NLRP3 inflammasome activation in human MDMs as well. We experienced a difference in the magnitudes of the cytosolic Ca2+ increase between two Ca2+-releasing agonists, ionomycin and thapsigagrin (Fig. 3.). Ionomycin triggers an almost immediate, very large Ca2+ signal whereas thapsigargin requires time to deplete the intracellular Ca2+ stores to induce the store-operated Ca2+ influx.[37] We think these differences in their kinetics and modes of action are the reason for the different Ca2+ responses (Fig. 2.). Cytosolic Ca2+ has already been implicated in IL-1β secretion from macrophages in an earlier study but recent data link it to inflammasome activation.[38, 1] Several of the known NLRP3 inflammasome agonists induce cytosolic Ca2+ signals in monocytes/macrophages: ATP, MSU crystals, nigericin.[1, 38] Another inflammasome agonist, antimycin-A induces Ca2+ signal in osteoblasts.[39] Increase in extracellular potassium levels depolarizes the plasma membrane and blocks Ca2+ entry in phagocytes.[1, 40] This and the fact that potassium -unlike Ca2+- has no known direct role in cell signaling suggest that Ca2+ rather than potassium affects inflammasome activation directly. Most of the inflammasome agonists lead to plasma membrane damage or pore formation (crystals, toxins, ionophores, ATP) that always results in potassium efflux and Ca2+ influx at the same time according to their electrochemical gradients.[34] Thus, an increasing number of studies including ours show that Ca2+ is an essential element of NLRP3 inflammasome activation.
ROS were proposed to be the convergence point in inflammasome signaling.[12, 41] ATP and crystals stimulate ROS production in macrophages.[42–46] The phagocytic NADPH oxidase was first thought to be the ROS source promoting inflammasome activation, but studies on gp91phox- and p22phox-deficient human monocytes showed that inflammasome activation is NADPH oxidase-independent.[10, 19, 20] P22phox is also a partner of three other members of the NADPH oxidase enzyme family, Nox1, 3 and 4, so inflammasome activation does not require these proteins and has been declared to be NADPH oxidase-independent.[47, 29] There are, however, three other members of this family (Nox5, Duox1, Duox2) that are all directly activated by cytosolic Ca2+ and have never been studied before regarding their potential participation in IL-1β metabolism in human macrophages.[21–23] We could detect only DUOX1 gene expression and Duox1 protein in human MDMs (Fig. 4). Using Duox1-deficient murine BMDMs we show, however, that IL-1β secretion is not dependent on Duox1 (Fig. 5.). Our data indicate that Ca2+-activated NADPH oxidases are not ROS sources required for inflammasome activation and therefore none of the seven NADPH oxidases are involved in IL-1β processing or release. The contribution of ROS to NLRP3 activation is still controversial. Recent data suggest mitochondria to be the major source.[16] Another study suggests that ROS are only required for inflammasome priming but not activation.[48] Several studies proposed that primary monocytes, macrophages and their model cell lines have different overall redox states and this will ultimately determine the efficiency of inflammasome activation.[27, 2, 49]
The function of Duox1 in macrophages could be participation in ATP-induced inflammatory responses since Duox1 is known to be activated in airway epithelial cells in response to extracellular ATP and mediates wound inflammatory responses in Drosophila embryos.[50–52] Considering its low expression, Duox1 could have a role in different signaling pathways similar to its suggested roles in airway epithelial cells and lymphocytes.[53, 54] The detected low Duox1 expression makes it unlikely that Duox1-derived ROS have any role in macrophage bactericidal activities. The main NADPH oxidase in macrophages is the NOX2-based NADPH oxidase that has the potential to produce enough ROS to kill microbes. NOX2, however, has already been clearly shown to be dispensable for inflammasome activation.[19] Aside from these speculations, the function of Duox1 in macrophages remains an open question.
Abbreviations
- BMDM
bone marrow-derived macrophage
- Duox
dual oxidase
- DuoxA
Duox Activator
- MDM
monocyte-derived macrophage
- Nox5
NADPH oxidase 5
- ROS
reactive oxygen species
Contributor Information
Balázs Rada, Email: radab@uga.edu.
Jonathan J. Park, Email: jon.j.park@gmail.com.
Payel Sil, Email: payel@uga.edu.
Miklós Geiszt, Email: geiszt.miklos@med.semmelweis-univ.hu.
References
- 1.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11282–7. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. The Journal of biological chemistry. 2011;286(31):27069–80. doi: 10.1074/jbc.M110.203398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, et al. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents and actions. 1989;27(1–2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 4.Brayden DJ, Hanley MR, Thastrup O, Cuthbert AW. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. British journal of pharmacology. 1989;98(3):809–16. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10(2):417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. The Journal of biological chemistry. 1978;253(17):5892–4. [PubMed] [Google Scholar]
- 7.Joosten LA, Ea HK, Netea MG, Busso N. Interleukin-1beta activation during acute joint inflammation: a limited role for the NLRP3 inflammasome in vivo. Joint, bone, spine : revue du rhumatisme. 2011;78(2):107–10. doi: 10.1016/j.jbspin.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Oosting M, van de Veerdonk FL, Kanneganti TD, Sturm P, Verschueren I, Berende A, et al. Borrelia species induce inflammasome activation and IL-17 production through a caspase-1-dependent mechanism. European journal of immunology. 2011;41(1):172–81. doi: 10.1002/eji.201040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell metabolism. 2010;12(6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, et al. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3030–3. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–7. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews Immunology. 2010;10(3):210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 13.Jones JW, Broz P, Monack DM. Innate immune recognition of francisella tularensis: activation of type-I interferons and the inflammasome. Frontiers in microbiology. 2011;2:16. doi: 10.3389/fmicb.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conforti-Andreoni C, Beretta O, Licandro G, Qian HL, Urbano M, Vitulli F, et al. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. Journal of leukocyte biology. 2010;88(6):1207–16. doi: 10.1189/jlb.1009652. [DOI] [PubMed] [Google Scholar]
- 15.Mortellaro A, Wong SC, Fric J, Ricciardi-Castagnoli P. The need to identify myeloid dendritic cell progenitors in human blood. Trends in immunology. 2010;31(1):18–23. doi: 10.1016/j.it.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 17.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to microbiology. 2008;15:164–87. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orient A, Donko A, Szabo A, Leto TL, Geiszt M. Novel sources of reactive oxygen species in the human body. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(5):1281–8. doi: 10.1093/ndt/gfm077. [DOI] [PubMed] [Google Scholar]
- 19.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116(9):1570–3. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, et al. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115(26):5398–400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 21.Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, et al. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. The Journal of biological chemistry. 2001;276(40):37594–601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 22.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. The Journal of biological chemistry. 2005;280(34):30046–54. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 23.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23(4):1205–18. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo DG, Winn M, Pang L, Moskowitz SM, Malech HL, Leto TL, et al. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. Journal of immunology. 2014;192(10):4728–38. doi: 10.4049/jimmunol.1301589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PloS one. 2013;8(1):e54205. doi: 10.1371/journal.pone.0054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donko A, Ruisanchez E, Orient A, Enyedi B, Kapui R, Peterfi Z, et al. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free radical biology & medicine. 2010;49(12):2040–8. doi: 10.1016/j.freeradbiomed.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends in immunology. 2011;32(12):559–66. doi: 10.1016/j.it.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, et al. Inflammasomes: current understanding and open questions. Cellular and molecular life sciences : CMLS. 2011;68(5):765–83. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxidants & redox signaling. 2006;8(9–10):1549–61. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 30.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(11):1502–4. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 31.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunological reviews. 2011;243(1):174–90. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conforti-Andreoni C, Ricciardi-Castagnoli P, Mortellaro A. The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cellular & molecular immunology. 2011;8(2):135–45. doi: 10.1038/cmi.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. Journal of clinical immunology. 2010;30(5):628–31. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 34.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Arlehamn CS, Petrilli V, Gross O, Tschopp J, Evans TJ. The role of potassium in inflammasome activation by bacteria. The Journal of biological chemistry. 2010;285(14):10508–18. doi: 10.1074/jbc.M109.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation. 2007;14(9):1583–9. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 37.Gao YD, Hanley PJ, Rinne S, Zuzarte M, Daut J. Calcium-activated K(+) channel (K(Ca)3.1) activity during Ca(2+) store depletion and store-operated Ca(2+) entry in human macrophages. Cell calcium. 2010;48(1):19–27. doi: 10.1016/j.ceca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. Journal of immunology. 2003;170(6):3029–36. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 39.Choi EM. Regulation of intracellular Ca(2+) by reactive oxygen species in osteoblasts treated with antimycin A. Journal of applied toxicology : JAT. 2012;32(2):118–25. doi: 10.1002/jat.1642. [DOI] [PubMed] [Google Scholar]
- 40.Rada BK, Geiszt M, Van Bruggen R, Nemet K, Roos D, Ligeti E. Calcium signalling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clinical and experimental immunology. 2003;132(1):53–60. doi: 10.1046/j.1365-2249.2003.02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature immunology. 2010;11(2):136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 42.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. The Journal of biological chemistry. 2007;282(5):2871–9. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(26):9035–40. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic signalling. 2007;3(1–2):39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, et al. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free radical biology & medicine. 2007;42(10):1506–16. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geiszt M. NADPH oxidases: new kids on the block. Cardiovascular research. 2006;71(2):289–99. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. Journal of immunology. 2011;187(2):613–7. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tassi S, Carta S, Delfino L, Caorsi R, Martini A, Gattorno M, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9789–94. doi: 10.1073/pnas.1000779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. The Journal of biological chemistry. 2009;284(26):17858–67. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. Journal of immunology. 2008;181(7):4883–93. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Current biology : CB. 2013;23(5):424–9. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sham D, Wesley UV, Hristova M, van der Vliet A. ATP-mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PloS one. 2013;8(1):e54391. doi: 10.1371/journal.pone.0054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, et al. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Science signaling. 2010;3(133):ra59. doi: 10.1126/scisignal.2000976. [DOI] [PMC free article] [PubMed] [Google Scholar]