Summary
The NLRP3 inflammasome is an important component of the innate immune system. However, its mechanism of activation remains largely unknown. We show that NLRP3 activators including bacterial pore-forming toxins, nigericin, ATP and particulate matter caused mitochondrial perturbation or the opening of a large membrane pore; but this was not required for NLRP3 activation. Furthermore, reactive oxygen species generation or a change in cell volume was not necessary for NLRP3 activation. Instead, the only common activity induced by all NLRP3 agonists was the permeation of the cell membrane to K+ and Na+. Notably, reduction of the intracellular K+ concentration was sufficient to activate NLRP3 whereas an increase in intracellular Na+ modulated, but was not strictly required for inflammasome activation. These results provide a unifying model for the activation of the NLRP3 inflammasome in which a drop in cytosolic K+ is the common step that is necessary and sufficient for caspase-1 activation.
Introduction
A major signaling pathway of the innate immune system is the inflammasome, a multi-protein platform that activates caspase-1 (Schroder and Tschopp, 2010). Once activated, caspase-1 proteolytically processes several protein substrates including pro-interleukin-1β and pro-IL-18 into their biologically active forms. To date, four inflammasomes have been described of which three, the NLRP1, NLRP3 and NLRC4 inflammasomes, contain a PRR that belongs to the intracellular Nod-like receptor (NLR) family (Franchi et al., 2012). Among the NLR inflammasomes, NLRP3 has been under intense investigation given its link to inherited autoinflammatory syndromes (Hoffman et al., 2001) and to several acquired inflammatory disorders (Wen et al., 2012). Activation of the NLRP3 inflammasome is mediated by two signals. The first signal, referred as priming, is the NF-κβ-dependent transcription of NLRP3 and pro-IL-1β, through stimulation with Toll-like receptor (TLR) agonists or certain cytokines such as TNF-α or IL-1β (Bauernfeind et al., 2009; Franchi et al., 2009). The second signal activates NLRP3 and is induced by nigericin, ATP, bacterial pore-forming toxins (PFTs), or crystalline and particulate matter (Hornung et al., 2008; Mariathasan et al., 2006). However, how these structurally unrelated stimuli activate NLRP3 remains unclear.
Several events have been proposed to explain the activation of the NLRP3 inflammasome including the production of reactive oxygen species (ROS), mitochondrial damage, lysosomal damage, formation of large non-specific pore in the cell membrane, and cytosolic K+ efflux (Franchi et al., 2012). The identification of the cellular event responsible for NLRP3 activation is complicated by the fact that NLRP3 activators trigger multiple cellular signals. The paradigm to explain this complexity has been ATP, which causes all the aforementioned cellular events, that is, opens a large pore permeable to monovalent cations and molecules up to 900 Da (Steinberg et al., 1987), increases the production of ROS (Cruz et al., 2007) and damages several organelles including the mitochondria and lysosomes (Lopez-Castejon et al., 2010; Shimada et al., 2012). Furthermore, membrane permeation, lysosomal damage, mitochondrial damage and ROS production are interrelated cellular events that can mutually cause each other to occur (Guicciardi et al., 2004), complicating even further the distinction between bystander and causative events of NLRP3 activation. The objective of this study was to identify the cellular signal responsible for NLRP3 activation in response to diverse stimuli. For that purpose we analyzed and compared the cellular effects caused by NLRP3 activators including mitochondrial perturbation, ROS generation, change in cell volume, and membrane permeability to organic molecules and ions in order to define the minimal requirement(s) to trigger NLRP3. Our results suggest a unifying model for NLRP3 activation induced by various stimuli in which K+ efflux is the intracellular event that triggers NLRP3 activation.
Results
Mitochondrial perturbation is not required for NLRP3 activation
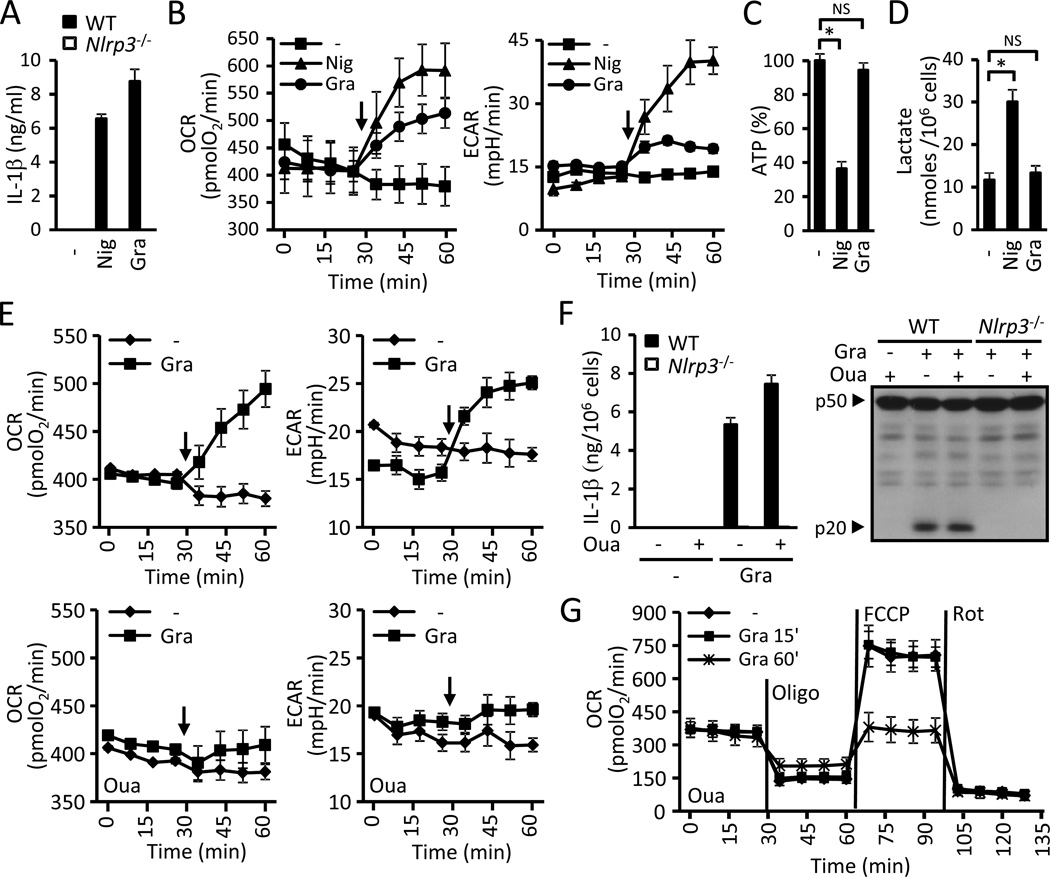
Mitochondrial damage has been implicated in NLRP3 activation; therefore we analyzed mitochondrial function in response to the NLRP3 agonists nigericin and gramicidin (Fig. 1A; Allam et al., 2011; Mariathasan et al., 2006). We monitored mitochondrial function in real-time during stimulation with the NLRP3 agonists by measuring the O2 consumption rate (OCR) and the extracellular acidification rate (ECAR). To ensure that the measured changes in mitochondrial function are upstream to NLRP3 and are not secondary to caspase-1 activation we performed all the bioenergetics studies in Nlrp3−/− macrophages unless otherwise specified. Both nigericin and gramicidin caused a rapid increase in OCR and ECAR in BMDMs (Fig. 1B). Nigericin, however, caused a much greater increase in ECAR than gramicidin (Fig. 1B). Furthermore, treatment with nigericin, but not with gramicidin, led to a significant decrease in the intracellular pool of ATP and an increase in lactate concentrations (Fig. 1C and D). These results suggest that while both nigericin and gramicidin are robust activators of NLRP3, gramicidin causes much less mitochondrial perturbation than nigericin. Thus, we sought to further understand the effects of gramicidin on the mitochondria to elucidate whether mitochondrial perturbation is required for the activation of the NLRP3 inflammasome.
Figure 1. Mitochondrial perturbation is not required to activate NLRP3.
(A) LPS-primed WT and Nlrp3−/−BMDMs were stimulated for 30 min with 10µM nigericin (Nig) or 0.5 µM gramicidin (Gra) and supernatants were analyzed for IL-βp by ELISA. (B) Effect of nigericin (10µM, Nig) and gramicidin (0.5 µM, Gra) on mitochondrial function. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured in BMDMs. Black arrows indicate the time of addition of the stimuli. (C and D) BMDMs were treated for 30 min with nigericin (10µM, Nig) or gramicidin (0.5 µuM, Gra) and the intracellular levels of ATP (C) and lactate (D) were determined. (E) Role of Na+/K+-ATPase in mitochondrial perturbation by gramicidin. OCR and ECAR triggered by the addition of 0.5 µM gramicidin (Gra) were measured in the absence (upper panels) and presence (lower panels) of 125 µM ouabain (Oua). Black arrows indicate the time of addition of gramicidin. (F) LPS-primed WT and Nlrp3−/−BMDMs were treated for 15 min with 0.5 µM gramicidin or vehicle in the presence or absence of 125 µM ouabain (Oua) and changed to their respective medium without gramicidin for an additional 15 min. IL-1β was measured in supernatants (left panel) and caspase-1 in cell extracts by immunoblotting (right panel). (G) BMDMs were stimulated for 15 min or 60 min with 0.5 µM gramicidin or left untreated in the presence of 125 µM ouabain (Oua) and the mitochondrial function was evaluated immediately after by performing a bioenergetic profile. Vertical lines indicate the injection of the specified mitochondrial inhibitors. Values represent mean ± standard deviation (n=3–4). Results are representative of at least three separate experiments. NS, not statistically significant (p ≥ 0.05). *, p < 0.05. See also Figure S1.
Gramicidin forms pores in lipid bilayers that are permeable to monovalent cations and H2O, collapsing the transmembrane gradient of Na+ and K+ in treated cells (Andersen et al., 2005). Therefore, we hypothesized that the rapid rise in the OCR triggered by gramicidin is secondary to an increase in energy consumption caused by the activation of the Na+- and K+-ATPase. Treatment of BMDMs with ouabain, an inhibitor of the Na+- and K+-ATPase, did not alter the basal amounts of the OCR and ECAR (Fig 1E). However, ouabain abolished the increase in the OCR and ECAR triggered by gramicidin (Fig 1E) without impairing its ability to activate NLRP3 (Fig. S1A). Similar to gramicidin, the NLRP3 agonist ATP, by acting on the P2x7 receptor (P2rx7), permeates the cell membrane and collapses cation gradients (Surprenant et al., 1996). Consistently, ATP also elicited a rapid increase in the OCR, which was inhibited by ouabain (Fig. S1B) and absent in P2rx7−/− BMDMs (Fig. S1C). To exclude the possibility that the observed inhibition in mitochondrial function by ouabain is due to off-target effects, we also inhibited the Na+- and K+-ATPase by culturing the cells in K+-free medium as the enzyme cannot function in the absence of extracellular K+ (Rose and Ransom, 1997). Like ouabain, medium lacking K+ did not change the basal OCR and ECAR, but completely blocked the increase in the OCR and ECAR triggered by gramicidin (Fig. S1D). Collectively, these results suggest that the effect of gramicidin on mitochondrial function as observed by increases in the OCR and ECAR is indirect and caused by the activation of the Na+- and K+-ATPase. Consistently, inhibition of the Na+- and K+-ATPase prevented gramicidin-induced changes in the OCR and ECAR but did not impair the ability of gramicidin to activate NLRP3.
Gramicidin can also perturb the mitochondria directly by permeating the mitochondrial membranes. To explore this possibility, we studied the effect of gramicidin on the coupling efficiency and the maximal respiratory capacity of BMDMs by performing bioenergetic profiles. For this purpose, macrophages were first stimulated with gramicidin, and the OCR was monitored while sequentially administering the mitochondrial inhibitors oligomycin, FCCP and rotenone. To demonstrate that the inhibitors were used at maximally effective doses, BMDMs were consecutively stimulated twice with the same dose of inhibitor (Fig. S1E). Importantly, stimulation of BMDMs with 0.5 µM gramicidin for 15 min resulted in NLRP3 activation (Fig. 1F) without affecting mitochondrial function (Fig. 1G). Longer stimulation with gramicidin (60 min), however, led to mitochondrial damage demonstrated by the uncoupling of the oxidative phosphorylation and a decrease in the maximal respiratory capacity (Fig. 1G). Collectively, these results demonstrate that perturbation of and damage to the mitochondria that can occur with NLRP3 activators are not necessary events to activate the NLRP3 inflammasome.
ROS production is not necessary for NLRP3 priming or activation
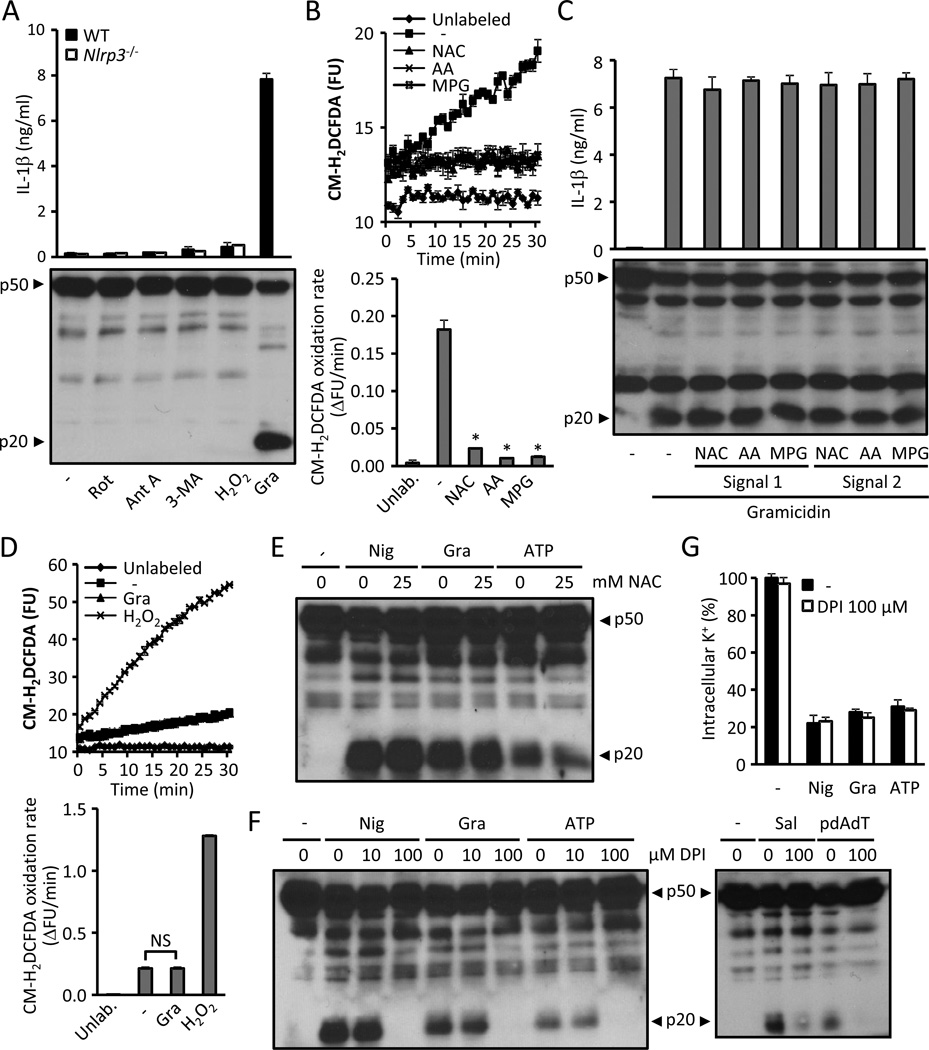
ROS generation resulting from mitochondrial damage has been implicated in NLRP3 activation (Zhou et al., 2011). Unlike this study, we could not detect NLRP3 activation following stimulation with the mitochondrial toxicants rotenone and antimycin A, the autophagy inhibitor 3-methyladenine, or H2O2 (Fig. 2A). To further clarify the role of ROS in NLRP3 activation, we studied the effect of the ROS scavengers N-acetyl-L-cysteine (NAC), ascorbic acid (AA) and N-(2-Mercaptopropionyl)glycine (MPG) on priming (signal 1) and NLRP3 activation (signal 2) in response to gramicidin. Using concentrations of ROS scavengers that had a profound inhibitory effect on the cellular redox state (Fig. 2B), we found no effect of these inhibitors on either the priming or activation of the NLRP3 inflammasome (Fig. 2C). In addition, treatment with gramicidin did not lead to an increase in ROS production (Fig. 2D). High concentrations of NAC have been reported to inhibit NLRP3 activation (Cruz et al., 2007). However, we did not observe this effect using NAC at neutral pH (Fig. 2E). Similarly, the NAPDH inhibitor DPI did not impair caspase-1 activation at 10 µM (Fig. 2F), a concentration that causes maximal NAPDH inhibition (Decleva et al., 2006). At a concentration 10 times higher, DPI prevented caspase-1 activation without altering the efflux of K+ (Fig. 2F–G). However, 100 µM DPI also impaired the activation of the NLRC4 and the AIM2 inflammasome (Fig. 2F). Thus, ROS do not play a role in NLRP3 activation.
Figure 2. ROS production is not required to activate NLRP3.
(A) LPS-primed WT and Nlrp3−/− BMDMs were stimulated for 6 hrs with 10 µM rotenone, 10 µg/ml antimycin A, 10 mM 3-methyladenine (3-MA) or 1 mM H2O2, or treated 30 min with 0.5 µM of gramidicin. NLRP3 activation was detected by measuring the secretion of IL-1β (upper panel) and caspase-1 activation (lower panel). (B) CM-H2DCFDA -labeled BMDMs were incubated in medium containing ROS scavengers N-acetyl-L-cysteine (NAC, 2.5 mM), N-(2-Mercaptopropionyl)glycine (MPG, 2.5 mM), ascorbic acid (AA,150 µM). The oxidation of CM-H2DCFDA (upper panel) and the oxidation rate (lower panel) were calculated as described in Experimental Procedures. (C) The effect of ROS scavengers on signal 1 and signal 2 of NLRP3 activation induced by gramicidin was evaluated. WT BMDMs were primed with LPS for 3 hrs in the presence of ROS scavengers and subsequently stimulated with 0.5 µM gramicidin (signal 1) or primed for 3 hrs with LPS and stimulated with gramicidin in the presence of ROS scavengers (signal 2). NLRP3 activation was assessed by measuring IL-1β release (upper panel) and caspase-1 activation (lower panel). (D) BMDMs labeled with the fluorescent ROS probe CM-H2DCFDA were stimulated with 0.5 µM gramicidin (Gra), 1 mM H2O2 or medium. The oxidation of CM-H2DCFDA was monitored (upper panel) as in (B) and the oxidation rate was calculated (lower panel). (E) LPS-primed WT BMDMs were stimulated with nigericin (10µM, Nig), gramicidin (0.5 µM, Gra) or 5 mM ATP 30 min with the indicated amount of NAC and caspase-1 activation was analyzed. (F and G) LPS-primed WT (F) and Nlrp3−/− (G) BMDMs were stimulated with nigericin (10µM, Nig), gramicidin (0.5 µM, Gra) or ATP (5 mM) for 30 min, with Salmonella (MOI 10, Sal) for 1 h or with pdAdT (5 µg/ml) for 4 hrs in the presence of the indicated amounts of DPI. Caspase-1 activation (F) and the intracellular content of K+ (G) were measured. Caspase-1 activation was analyzed by immunoblotting and IL-1β by ELISA. Cells were treated 30’ with the inhibitors or medium before adding the agonists. The intracellular levels of K+ were quantified by ICP-OES in Nlrp3−/− cells. Values represent mean ± standard deviation (n=3–4). Results are representative of at least three separate experiments. NS, not statistically significant (p ≥ 0.05). *, p < 0.05 (stimulated vs. unstimulated).
Phagocytosis of particulate matter triggers K+ efflux and NLRP3 activation
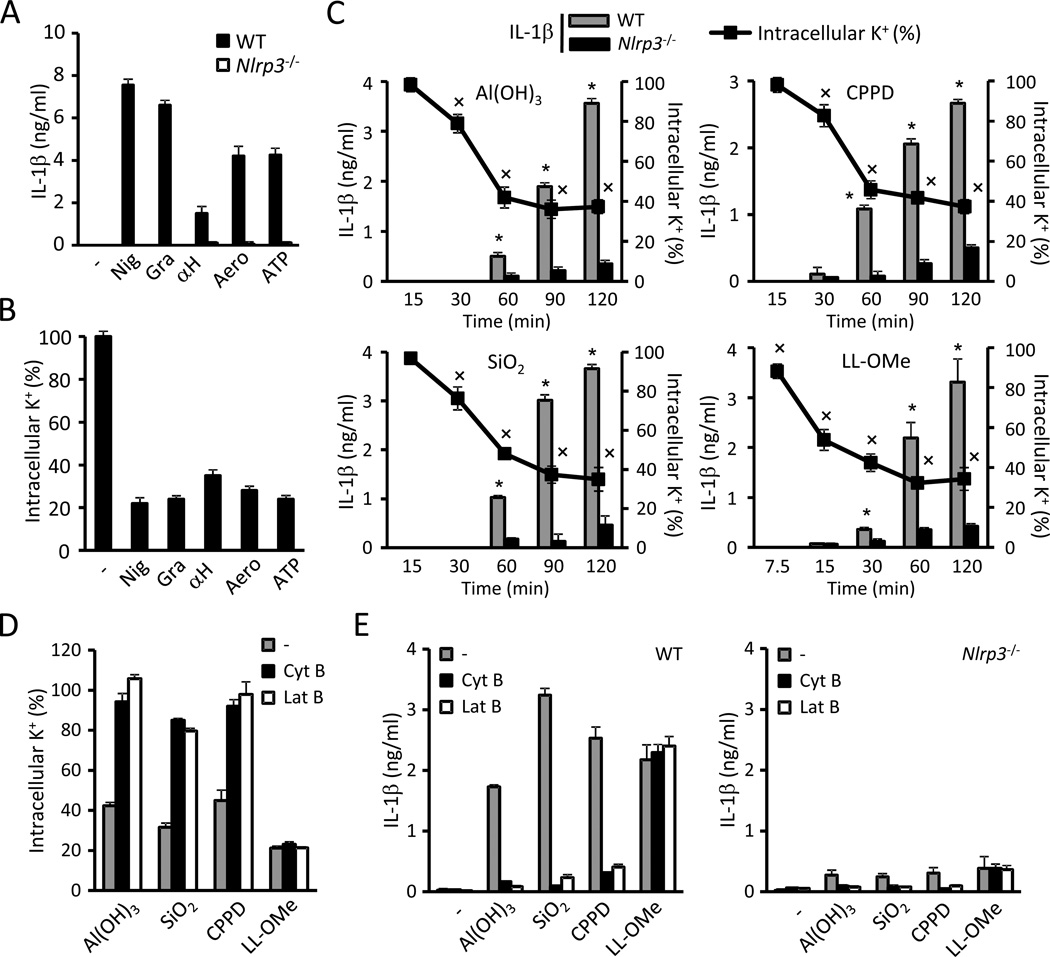
A role of K+ efflux in NLRP3 activation has been proposed because several NLRP3 activators can permeate the cell membrane to K+ (Fig. 3A and B; Perregaux and Gabel, 1994) and increasing the extracellular [K+] inhibits inflammasome activation by all tested NLRP3 activators (Pétrilli et al., 2007). However, particulate matter has not been reported to trigger the efflux of K+ and there is no evidence that reduction of cytosolic [K+] alone is sufficient to trigger NLRP3 activation. Therefore we studied whether NLRP3 activators that have been proposed to act via lysosomal damage, i.e. particulate matter and the lysosomal-damaging dipeptide LL-OMe (Hornung et al., 2008), also cause efflux of K+. To establish a reliable correlation between intracellular K+ concentrations and NLRP3 activation, we measured IL-1β release and K+ efflux in parallel. We determined K+ concentrations in Nlrp3−/− macrophages because caspase-1 activation can lead to pyroptosis and nonspecific membrane permeation (Fig. S2A). Time-course experiments revealed that a drop in the intracellular content of K+ preceded the release of IL-1β induced by Al(OH)3, silica , calcium pyrophosphate crystals (CPPD) and LL-OMe (Fig. 3C). Phagocytic uptake is also a requirement for NLRP3 activation induced by particulate matter (Hornung et al., 2008; Martinon et al., 2006). Therefore, we investigated a role for phagocytosis in K+ efflux elicited by particulate NLRP3 activators. Pretreatment of BMDMs with the phagocytosis inhibitors cytochalasin B and latrunculin B strongly impaired both the efflux of K+ (Fig. 3D) and NLRP3-dependent IL-1β secretion triggered by particulate matter (Fig. 3E) but not by LL-OMe (Fig. 3D and E). We did not observe any difference in K+ efflux caused by NLRP3 agonists among WT and Nlrp3−/− unprimed BMDMs (Figs. S2B). However, we observed a major effect of LPS priming on K+ efflux caused by particulate matter and LL-OMe. Specifically, LPS priming enhanced K+ efflux caused by SiO2, Al(OH)3, CPPD crystals and LL-OMe but not by nigericin, gramicidin and ATP (Figs. S2C, data not shown). These findings are consistent with a role of phagocytosis and pinocytosis in the uptake of these stimuli (Fig. 3D), as LPS treatment has been shown to enhance both processes (Chen et al., 2012; Peppelenbosch et al., 1999).
Figure 3. Phagocytosis of particulate matter triggers K+efflux and activates NLRP3.
(A,B) LPS-primed WT and Nlrp3−/− BMDMs were treated 30 min with 10 µM nigericin (Nig), 0.5 µM gramicidin (Gra), 10 µg/ml S. aureus α-hemolysin (αH), 10 ng/ml A. hydrophila aerolysin (Aero) or 5 mM ATP. Secreted IL-1β (A) and the intracellular content of K+ (B) were measured. (C) LPS-primed WT and Nlrp3−/− BMDMs were stimulated with 250 µg/ml of Al(OH)3, silica (SiO2) or calcium pyrophosphate crystals (CPPD) or with 1 mM L-leucyl-L-leucine methyl ester (LL-OMe) and secreted IL-1β and the intracellular content of K+ were determined at the specified time points. (D–F) Effect of inhibition of phagocytosis in K+ efflux and NLRP3 activation caused by particulate matter and LL-OMe. LPS-primed WT and Nlrp3−/− BMDMs were incubated 30 min with phagocytosis inhibitors cytochalasin B (Cyt B, 5 µM) or latrunculin B (Lat B, 200 nM) and subsequently treated with 250 µg/ml of Al(OH)3, silica (SiO2) or CPPD, or with 1 mM LL-OMe. The intracellular content of K+ (D) and IL-1β release in WT and Nlrp3−/−BMDMs (E) were measured. K+ determinations were performed by ICP-OES in Nlrp3−/− cells. Values represent mean ± standard deviation (n=3). Results are representative of at least three separate experiments. Asterisks (*) indicate NLRP3 activation (p < 0.05, WT vs. Nlrp3−/−). Crosses (×) indicate a drop in intracellular content of K+ (p < 0.05, stimulated vs. non-stimulated). See also Figure S2.
The lysosomal inhibitors Ca-074 Me and Bafilomycin A prevent NLRP3 activation induced by particulate matter, but not ATP (Hornung et al., 2008). In accord with these results, Ca-074 Me and Bafilomycin A prevented K+ efflux and NLRP3 activation triggered by particulate matter and LL-OMe (Fig. S2D and E), but not by nigericin or ATP (data not shown). However, IL-1β release was not impaired in cathepsin B-deficient BMDMs (Fig. S2F), suggesting that the inhibition by Ca-074 Me is due to off-target effects. Alternatively, Ca-074 Me could be targeting another cathepsin or multiple cathepsins which are responsible for membrane permeation following the uptake of particulate matter.
Extracellular Ca2+ activates NLRP3 through K+ efflux
Lee et al. have recently proposed that calcium signaling play a crucial role in NLRP3 activation (Lee et al., 2012). The authors showed that high extracellular Ca2+ activates NLRP3 in RPMI medium. We found that NLRP3 activation by Ca2+ was abolished by high extracellular K+ (Fig. S2G) and correlated with a decrease in the intracellular amounts of K+ (Fig. S2H). Furthermore, we found that high extracellular Ca2+ causes K+ efflux and NLRP3 activation in RPMI medium but not in IMDM, DMEM or HBSS (Fig. S2I). However, gramicidin causes K+ efflux and NLRP3 activation in all tested media (Fig. S2I). The concentration of PO43− in RPMI is significantly higher than that in IMDM, DMEM and HBSS, and calcium phosphate salts are insoluble in aqueous medium and can activate NLRP3 (Jin et al., 2011). We noticed that the addition of Ca2+ to RPMI leads to the formation of particulate matter (Fig. S2J). On the contrary, addition of either Ca2+ or PO43− to IMDM did not cause any particulate precipitation (Fig. S2J). However, when IMDM was supplemented with both Ca2+ and PO43− particulate matter was formed (Fig. S2J), which correlated with K+ efflux and NLRP3 activation that was inhibited by high extracellular K+ (Fig. S2K). These results suggest that high extracellular Ca2+ activates NLRP3 acting as particulate matter, i.e. triggering K+ efflux. The CASR agonist R-568 and the PLC activator m-3M3FBS have also been reported to activate NLRP3 (Lee et al., 2012). We could detect NLRP3 activation by m-3M3FBS, but not R-568 (Fig. S2L). Notably, caspase-1 activation by m-3M3FBS was inhibited by high extracellular K+ (Fig. S2L).
K+ efflux is a specific upstream requirement for the activation of the NLRP3 inflammasome
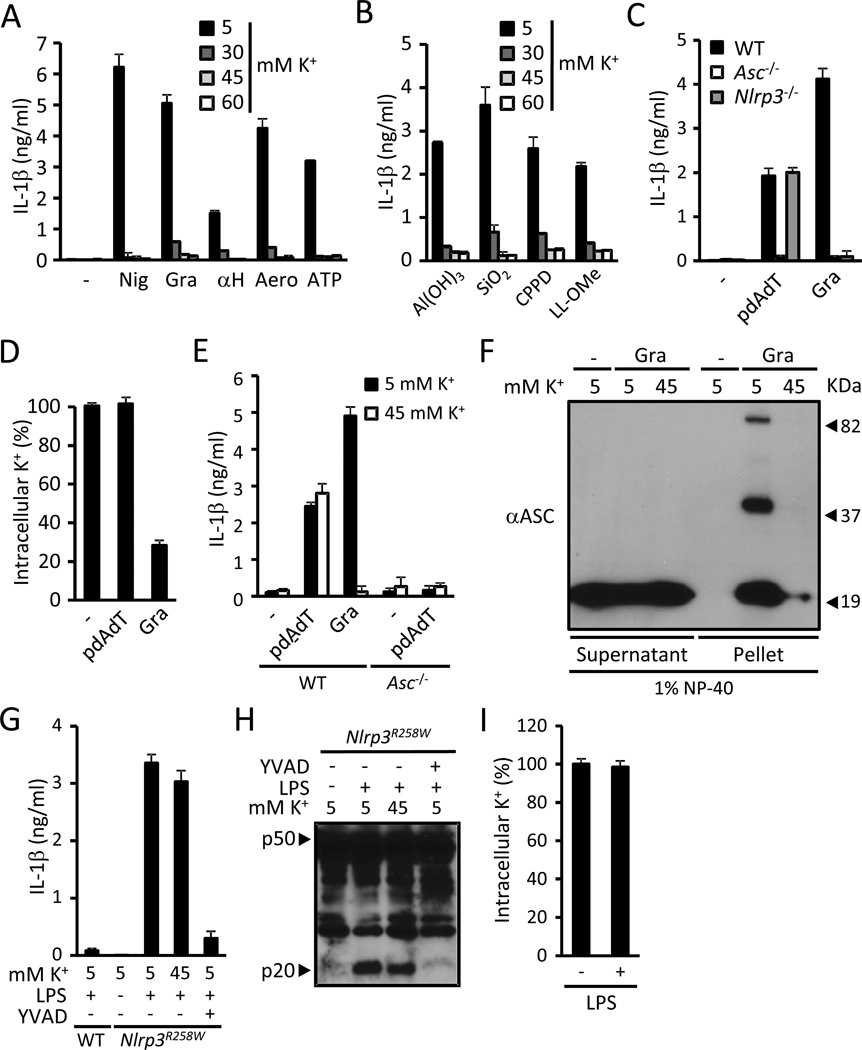
AIM2 recognizes cytosolic dsDNA and activates caspase-1, which was found to be inhibited by concentrations of extracellular K+ higher than 60 mM (Fernandes-Alnemri et al., 2010). Given the potential toxic effects of very high extracellular K+ concentrations, we determined the extracellular [K+] sufficient to prevent NLRP3 activation by nigericin, bacterial PFTs, ATP, particulate matter and LL-OMe. Increasing the extracellular [K+] from 5 to 30 mM had a major inhibitory effect on NLRP3 activation by all tested stimuli (Fig. 4A and B). Furthermore, increasing the extracellular [K+] from 30 to 45 mM had a minor or no additional inhibitory effect on NLRP3 activation, and raising it from 45 to 60 mM did not have any additional inhibitory effect for any of the stimuli (Fig. 4A and B). Thus, an extracellular [K+] of 45 mM provides maximal inhibition of NLRP3 activation by all activators tested.
Figure 4. Cytosolic K+is a specific upstream regulator of the NLRP3 inflammasome.
(A) LPS-primed WT BMDMs were stimulated for 30 min with 10 µM nigericin (Nig), 0.5 µM gramicidin (Gra), 10 µg/ml S. aureus α-hemolysin (αH), 10 ng/ml A. hydrophila aerolysin (Aero) or 5 mM ATP in medium containing the specified [K+] and secreted IL-1β was measured. (B) LPS-primed WT BMDMs were stimulated for 2 hrs with 250 µg/ml of Al(OH)3, silica (SiO2) or calcium pyrophosphate crystals (CPPD) or with 1 mM L-leucyl-L-leucine methyl ester (LL-OMe) in medium containing the specified [K+] and secreted IL-1β was measured. (C and D) LPS-primed WT, Asc/− and Nlrp3−/− BMDMs were stimulated with 5 µg/ml pdAdT for 4 hrs or 0.5 µM gramicidin (Gra) for 30 min and the release of IL-1β (C) and the intracellular content of K+ (D) were measured. K+ determinations were performed in Asc−/− macrophages. (E) LPS-primed WT and Asc−/− BMDMs were treated with 5 µg/ml pdAdT for 4 hrs or 0.5 µM gramicidin (Gra) for 30 min in medium containing 5 or 45 mM K+ and IL-1β was quantified in the supernatants. (F) LPS-primed WT BMDMs were stimulated 30 min with 0.5 µM gramicidin (Gra) in medium containing 5 or 45 mM K+. The cells were lysed with 1 % NP-40 and separated by centrifugation in supernatants and pellets. Proteins in cell pellets and supernatants were cross-linked with DSS and immunoblotted with anti-Asc antibody. (G–H) BMDM from WT and Nlrp3R258W mice were stimulated as indicated for 4 hrs (0.5 µg/ml LPS, 100 µM YVAD) and released IL-1β (G) and caspase-1 activation (H) were analyzed. (I) BMDMs from Nlrp3R258W mice were treated with 0.5 µg/ml LPS or vehicle for 4 hrs and the intracellular content of K+ was determined. IL-1β was measured by ELISA, caspase-1 activation by immunoblotting and intracellular K+ by ICP-OES. In experiments with high K+ medium, the osmolarity was maintained at 300 mOsm by isosmotic substitution of NaCl with KCl. Values represent mean ± standard deviation (n=3). Results are representative of at least three separate experiments.
Activation of AIM2 is associated with recruitment of the bipartite adaptor Asc leading to its oligomerization and the activation of caspase-1 (Fernandes-Alnemri et al., 2010). Consistent with this study, IL-1β secretion induced by the AIM2 activator polydAdT was totally dependent on Asc, but independent of NLRP3 (Fig. 4C). Unlike gramicidin, stimulation with polydAdT did not elicit a decrease in the intracellular content of K+ (Fig. 4D). Furthermore, IL-1β secretion triggered by polydAdT was not inhibited by an extracellular [K+] of 45 mM, which effectively blocked gramicidin-induced IL-1β secretion (Fig. 4E). Collectively, these results indicate that the efflux of K+ is specific to NLRP3 activation and does not play a role in the activation of the AIM2 inflammasome.
NLRP3 also requires the adaptor Asc to activate caspase-1 (Mariathasan et al., 2004). Similar to ATP (Juliana et al., 2010), gramicidin elicited Asc oligomerization and its migration to the detergent-insoluble protein fraction in cellular extracts (Fig. 4F). Asc oligomerization was inhibited by 45 mM extracellular K+ (Fig. 4F), suggesting that K+ regulates the NLRP3 inflammasome by acting upstream of caspase-1. To better define the step in the NLRP3 inflammasome signaling pathway regulated by K+, we analyzed the role of K+ in caspase-1 activation in BMDMs harboring the Nlrp3R258W mutation. This mutation corresponds to the R260W mutation in human NLRP3, which is associated with Muckle-Wells syndrome. In agreement with a previous study (Meng et al., 2009), Treatment of Nlrp3R258W BMDMs with LPS alone was sufficient to activate caspase-1 and was blocked by the caspase-1 inhibitor YVAD (Fig. 4G and H). However, caspase-1 activation elicited by LPS was not inhibited by medium containing 45 mM of K+ and did not correlate with an efflux of K+ (Fig. 4G–I). In sum, these results indicate that K+ regulates the activation of the NLRP3 inflammasome upstream of Asc, i.e. K+ efflux acts either on NLRP3 or upstream of NLRP3.
The formation of a large pore is not required to activate NLRP3
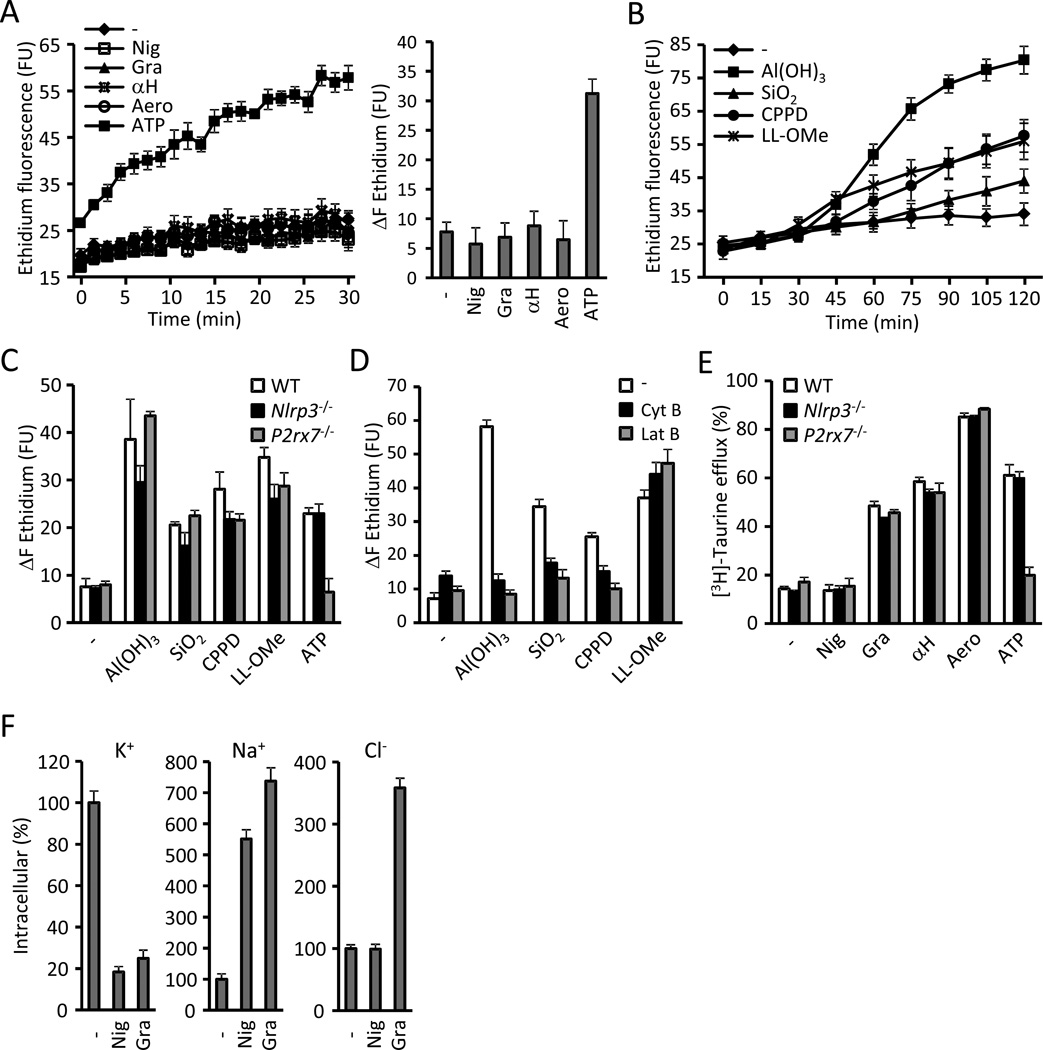
We showed above that particulate matter share with pore-forming toxins the ability to cause K+ efflux. However, it is unknown whether a drop in cytosolic [K+] is sufficient to activate NLRP3 because ATP also induces the formation of a large unspecific pore in the cell membrane that was suggested to be involved in caspase-1 activation (Pelegrin and Surprenant, 2006). Therefore, we determined whether permeation of the cell membrane to moieties larger than K+ is a common feature among NLRP3 activators. Stimulation of BMDMs with nigericin and the bacterial PFTs gramicidin, α-hemolysin and aerolysin did not permit the uptake of ethidium (molecular weight 314), as opposed to ATP (Fig. 5A). Strikingly, stimulation with particulate matter and LL-OMe increased ethidium uptake (Fig. 5B). In contrast to ATP, ethidium uptake triggered by particulate matter and LL-OMe was unimpaired in P2rx7−/− BMDM (Fig. 5C). Furthermore, ethidium uptake elicited by all stimuli was upstream to NLRP3 as it was undiminished in Nlrp3−/− BMDMs (Fig. 5C). Notably, inhibition of phagocytosis with cytochalasin B and latrunculin B strongly reduced ethidium uptake triggered by particulate matter, but not by LL-OMe (Fig. 5D).
Figure 5. NLRP3 activation correlates with K+ efflux and Na+ influx but not with the opening of a nonselective pore.
(A) Ethidium uptake kinetics in BMDMs treated with 10 µM nigericin (Nig), 0.5 µM gramicidin (Gra), 10 µg/ml S. aureus α-hemolysin (αH), 10 ng/ml A. hydrophila aerolysin (Aero) or 5 mM ATP. The total increase of ethidium fluorescence during the stimulation (ΔF ethidium) is shown in the right panel. (B) Ethidium uptake kinetics in BMDMs treated with 250 µg/ml of Al(OH)3, silica (SiO2) or calcium pyrophosphate crystals (CPPD) or with 1 mM L-leucyl-L-leucine methyl ester (LL-OMe) for 2 hrs. (C) WT, Nlrp3−/− and P2rx7−/− BMDMs were stimulated for 2 hrs with 250 µg/ml of Al(OH)3, silica (SiO2), CPPD crystals and 1 mM LL-OMe and the ethidium uptake was quantitated. ATP (5 mM, 30 min) was used as a control for P2rx7 signaling. (D) Ethidium uptake by BMDMs stimulated for 2 hrs with 250 µg/ml Al(OH)3, silica (SiO2), CPPD crystals and 1 mM LL-OMe in the presence of phagocytosis inhibitors cytochalasin B (5 µM, Cyt B) and latrunculin B (200 nM, Lat B). (E) [3H]-taurine efflux was determined in WT, Nlrp3−/− and P2rx7−/− BMDMs treated 30 min with 10 µM nigericin (Nig), 0.5 µM gramicidin (Gra), 10 µg/ml S. aureus α-hemolysin (αH), 10 ng/ml A. hydrophila aerolysin (Aero) or 5 mM ATP. (F) The intracellular content of K+, Na+ and Cl was determined in BMDMs treated 30 min with 10 µM nigericin (Nig) or 0.5 µM gramicidin (Gra). Na+ and K+ were measured by ICP-OES and Cl− by ICP-MS in Nlrp3−/− cells. Values represent mean ± standard deviation (n=3). Results are representative of at least three separate experiments. FU, fluorescence units. See also Figure S3.
Next, we analyzed whether NLRP3 activators can permeate the cell membrane to the organic osmolyte taurine (molecular mass of 125). Incubation of BMDMs in the presence of [3H]-taurine revealed that BMDMs avidly incorporate the organic osmolyte (Fig. S3A). Bacterial PFTs and ATP elicited the efflux of [3H]-taurine but, remarkably, nigericin did not (Fig. 5E). [3H]-taurine efflux occurred upstream of NLRP3 for all stimuli and was independent of the P2rx7 for bacterial PFTs, but not for ATP (Fig. 5E). Consistent with their ability to elicit the uptake of ethidium, particulate matter also triggered the efflux of the smaller molecule [3H]-taurine, which was detectable as early as 30 min after stimulation (Fig S3B) and strongly impaired by the phagocytosis inhibitors cytochalasin B and latrunculin B (Fig S3C). These results demonstrate that membrane permeation to chemical species larger than K+ is commonly caused by NLRP3 activators as eight out of the nine agonists tested permeated the cell membrane to [3H]-taurine and/or ethidium (Fig 5E and Fig. S3B). However, NLRP3 activation is unlikely to be triggered by the formation of a large nonselective pore as previously suggested (Pelegrin and Surprenant, 2006), because nigericin activates NLRP3 without permeating the cell to the small metabolite taurine (Fig 5E).
NLRP3 activation correlates with efflux of K+ and the influx of Na+ but not with the permeation to Cl−
To better define the minimal membrane permeation events required to activate NLRP3, we investigated whether NLRP3 activation requires increased cell membrane permeability to Na+ and Cl− ions. Treatment of BMDMs with gramicidin caused an increase in the intracellular levels of both Na+ and Cl− in addition to a decrease in the intracellular content of K+ and (Fig. 5F). Nigericin only caused a decrease in intracellular K+ and an increase in intracellular Na+, but did not produce a change in Cl− concentrations (Fig. 5F). Hence, NLRP3 activation correlates with the efflux of K+ and the influx of Na+, but not with Cl− fluxes.
K+-free medium alone activates the NLRP3 inflammasome
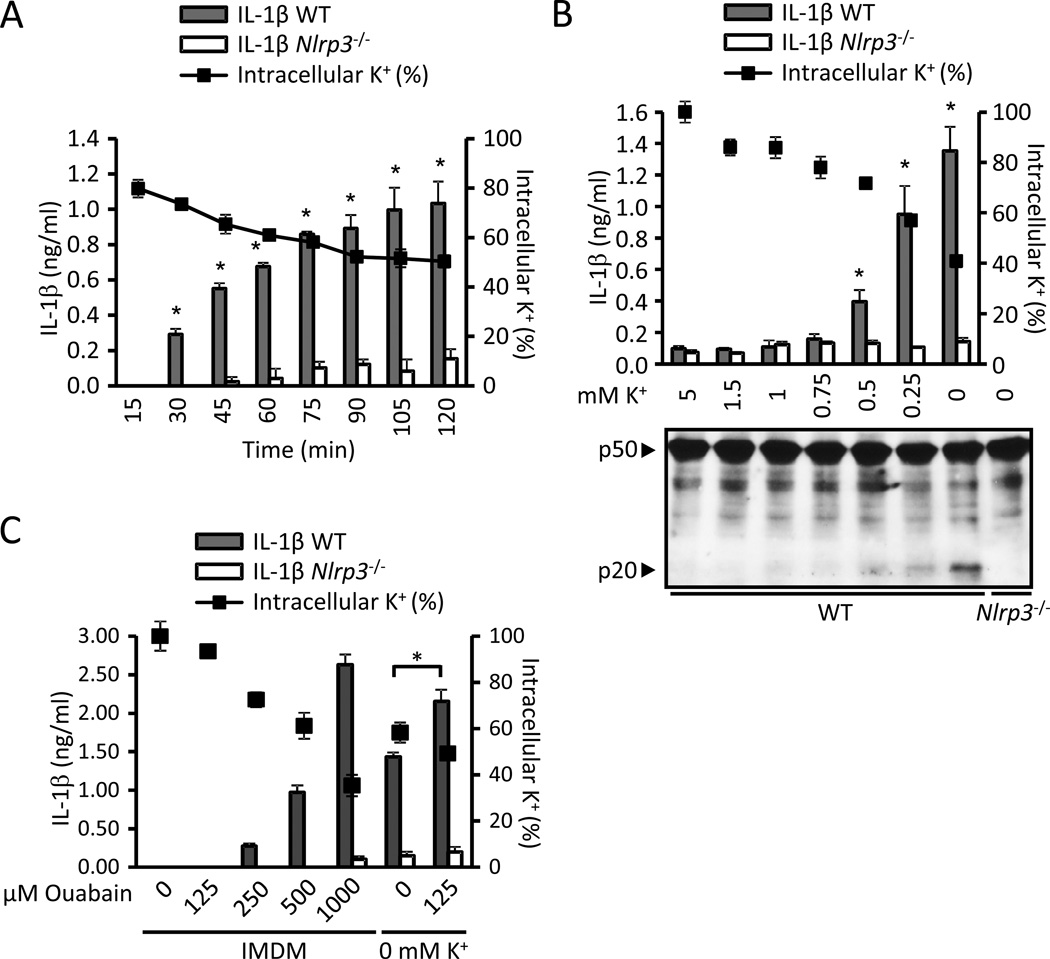
To further evaluate the role of K+ in NLRP3 activation, we tested whether decreasing the intracellular content of K+ by incubating the cells in low-K+ media is sufficient to activate the NLRP3 inflammasome. We examined this hypothesis using two different approaches. First, we incubated BMDMs from wild-type and Nlrp3−/− mice in medium containing 5 mM or 0 mM K+ and analyzed the secretion of IL-1β and the intracellular content of K+ at different time points. When macrophages were incubated in K+-free medium, IL-1β secretion was detected as early as 30 min in the absence of any stimuli and correlated with an intracellular content of K+ ≤ 74 % ± 1.34 % (average ± SEM of three independent experiments) (Fig. 6A). In a second approach, we incubated BMDMs for 2 hrs in media containing decreasing [K+] and measured the secretion of IL-1β, caspase-1 activation and the intracellular content of K+ (Fig. 6B). Under these conditions, IL-1β secretion in wild-type BMDMs was only detected when the extracellular [K+] was ≤ 0.5 mM and correlated with an intracellular content of K+ of ≤ 77 % ± 1.25 % (average ± SEM of three independent experiments) (Fig. 6B). These results demonstrate that incubating BMDMs in low-K+ medium is sufficient to activate NLRP3 in the absence of an NLRP3 agonist. Furthermore, our results indicate that the threshold of intracellular K+ to engage NLRP3 is in the range of 70–80 %. The Na+/K+-ATPase inhibitor ouabain enhanced NLRP3 activation induced by K+-free medium, and at higher doses it was sufficient to activate NLRP3 (Fig. 6C).
Figure 6. Incubation in low-K+ medium is sufficient to activate NLRP3.
(A) LPS-primed WT and Nlrp3−/− BMDMs were incubated in K+-free medium and the release of IL-1β (bars) and intracellular content of K+ (solid squares) were measured at the specified time points. (B) LPS-primed WT and Nlrp3−/− BMDMs were incubated for 2 hrs in medium containing the specified [K+] and the release of IL-1β (bars) and intracellular content of K+ (solid squares) were measured. (C) LPS-primed WT and Nlrp3−/− BMDMs were treated with ouabain for 2 hours in IMDM or K+-free medium and the release of IL-1β and the intracellular content of K+ were determined. IL-1β secretion was analyzed by ELISA. K+ determinations were performed in Nlrp3−/− cells. Values represent mean ± standard deviation (n=3). *, statistically significant (p < 0.05, WT vs. Nlrp3−/−). Results are representative of at least three separate experiments. See also Figure S4.
Compan et al. proposed that a regulatory volume decrease (RVD) following cell swelling is necessary in addition to K+ efflux to activate NLRP3 (Compan et al., 2012). However, the latter experiments were performed by diluting isotonic medium with distilled water and therefore did not consider the effect of reducing the extracellular [K+] in NLRP3 activation. Thus, we analyzed the individual contribution of lowering the osmolarity and the extracellular concentration of K+ to NLRP3 activation. Notably, only incubation of macrophages in highly hypotonic medium (90 mOsm) activated the NLRP3inflammasome (Fig. S4A), which correlated with nonspecific membrane permeation and significant cellular toxicity as evidenced by increased LDH release and a drop in ATP levels (Fig. S4B). 90 mOsm medium produced robust NLRP3 activation only when the extracellular [K+] was below 5 mM (Fig. S4A). Increasing the extracellular [K+] prevented NLRP3 activation, but did not decrease cytotoxicity, further suggesting that K+ efflux and not cytotoxicity resulted in NLRP3 activation (Fig. S4B). Furthermore, incubation of macrophages in 150–230 mOsm medium, induced significant cell swelling (Fig. S4D) and a regulatory volume decrease (RVD) response (Figs. S4C), but did not activate NLRP3 (Fig. S4A). Notably, K+-free medium activates NLRP3 (Fig. 6A–B) without causing cell swelling (Fig. S4E) and a RVD (Fig. S4C). Collectively, these results suggest that K+-free medium activates NLRP3 without swelling the cells. Furthermore, cell swelling or RVD does not activate NLRP3, and NLRP3 activation by severe hypo-osmolarity is largely due to the efflux of K+. In another study, Lee et al. reported that a decrease in cAMP leads to NLRP3 activation (Lee et al., 2012). However, we could not detect a decrease in the cAMP levels upon stimulation with K+-free medium (Fig. S4F).
Na+ influx is not an absolute requirement for NLRP3 activation
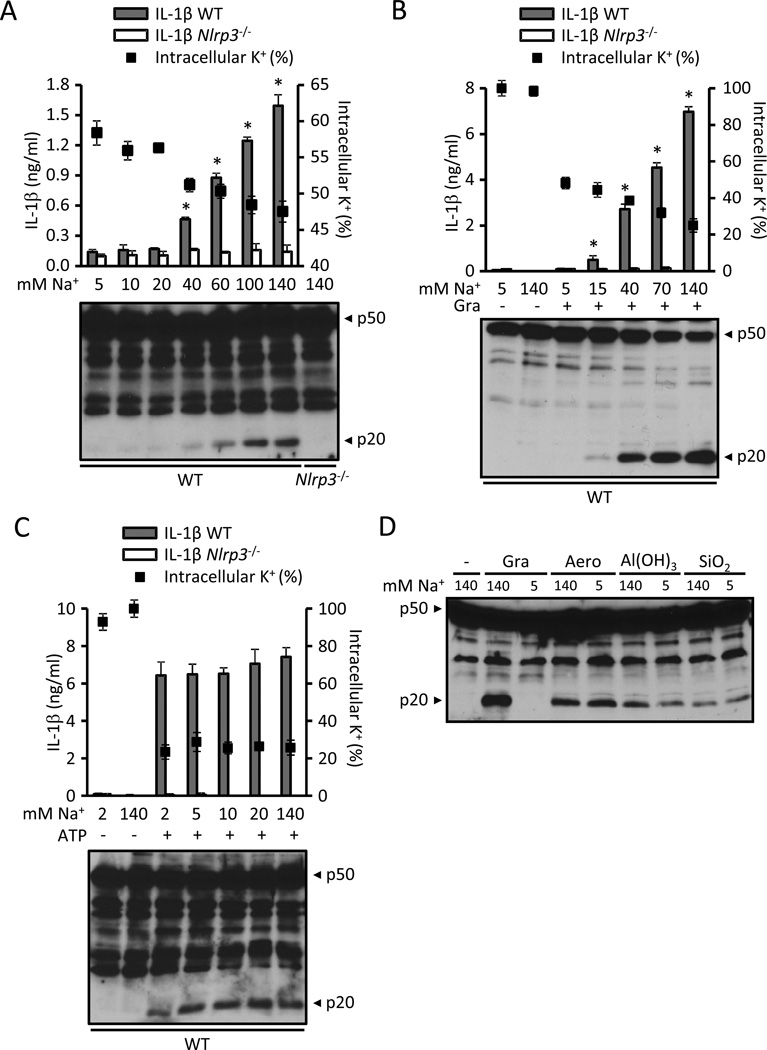
We next evaluated the role of Na+ influx in NLRP3 activation by iso-osmotically substituting extracellular Na+ by the cation choline. Remarkably, reducing the extracellular [Na+] had a strong dose-dependent inhibitory effect on NLRP3 activation induced by K+-free medium, gramicidin or nigericin (Fig. 7A and B and Fig. S5A). These stimuli required an extracellular [Na+] ≥ 40, 15 and 40 mM, respectively, to activate NLRP3 (Fig. 7A and B and Fig. S5A). Furthermore, lowering the extracellular [Na+] also reduced the drop in the intracellular K+ caused by all 3 stimuli (Fig. 7A and B and Fig. S5A). None of these stimuli activated NLRP3 in medium containing 5 mM Na+ despite the fact that all of them induced a reduction in the intracellular content of K+ below 60% (Fig. 7A and B and Fig. S5A). Indeed, lowering the extracellular [Na+] decreased the K+ threshold for NLRP3 activation induced by low-K+ medium from 70–80 % (Fig. 6A and B) to 50–60 % (Fig. 7A and B and Fig. S5A). These results suggest that Na+ influx can modulate NLRP3 activation independently of K+ efflux. However, substitution of extracellular Na+ with choline did not impair K+ efflux or NLRP3 activation elicited by ATP (Fig. 7 C), aerolysin, Al(OH)3 and silica (Fig. 7D and Fig. S5B and C). Thus, our results demonstrate that Na+ influx can modulate NLRP3 activation by certain agonists, but it is not a strict requirement for NLRP3 activation. To further assess a role for Na+ in NLRP3 activation, we tested whether the influx of Na+ is sufficient to activate NLRP3. Treatment of BMDMs with doses of the Na+ ionophore monensin previously shown to cause significant Na+ influx (Gurcel et al., 2006) did not cause NLRP3 activation (Fig. S5C). Next, we addressed a possible role of membrane depolarization in NLRP3 activation. Gramicidin forms pores in the cell membrane which allow K+ efflux and Na+ influx leading to membrane depolarization in high-Na+ medium (Chifflet et al., 2004). However, K+ efflux with decreased Na+ influx leads to hyperpolarization (Blaustein and Goldring, 1975; Langheinrich and Daut, 1997). To test whether membrane depolarization is required for NLRP3 activation, we treated the cells with gramicidin in K+-free medium in which Na+ was substituted by the membrane impermeable cation choline (Fig. S5D). Under these hyperpolarizing conditions (Blaustein and Goldring, 1975; Langheinrich and Daut, 1997), gramicidin caused robust caspase-1 activation (Fig. S5D). These results indicate that membrane depolarization is not required for NLRP3 activation and further support K+ efflux as the trigger of the NLRP3 inflammasome.
Figure 7. Na+ influx can modulate NLRP3 but is not a strict requirement for inflammasome activation.
(A) LPS-primed WT and Nlrp3−/− BMDMs were incubated for 3 hrs in K+-free medium containing the specified [Na+] and the release of IL-1β (bars) and the intracellular content of K+ (solid squares) were measured. (B and C) LPS-primed WT and Nlrp3−/− BMDMs were stimulated for 45 min with 0.5 µM gramicidin (Gra) (B) or 5 mM ATP (C) in media containing 5 mM K+ and the specified [Na+]. The release of IL-1β (bars), the intracellular content of K+ (solid squares) and caspase-1 activation were analyzed. (D) LPS-primed WT BMDMs were stimulated for 45 min with 0.5 µM gramicidin (Gra) or 10 ng/ml aerolysin (Aero) or for 3 hrs with 250 µg/ml Al(OH)3 or silica (SiO2) in medium containing 5 mM K+ and either 140 or 5 mM Na+ and caspase-1 activation was analyzed. In low Na+ medium, NaCl was isosmotically substituted with choline chloride to maintain a final osmolarity of 300 mOsm. IL-1β was measured by ELISA and caspase-1 activation by immunoblotting. K+ determinations were performed in Nlrp3−/− cells. Values represent mean ± standard deviation (n=3). Results are representative of at least three separate experiments. *, statistically significant (p < 0.05, WT vs. Nlrp3−/−). See also Figure S5.
Discussion
The fact that NLRP3 is activated by an array of chemically and structurally unrelated stimuli has led to the hypothesis that NLRP3 does not directly detect these stimuli, but instead senses a commonly induced intracellular signal. In line with this notion, several intracellular events have been proposed as the common signal upstream to NLRP3, including a change in the intracellular concentration of K+, the formation of a large pore in the cell membrane, lysosomal destabilization, mitochondrial damage, the production of ROS, changes in cell volume and Ca2+ signaling. The elucidation of the mechanism of NLRP3 activation is further complicated by the pleiotropic action of NLRP3 agonists. For example, ATP permeates the cell membrane to molecules up to 900 Da, damages lysosomes and the mitochondria, and increases the production of ROS. In this manuscript, we have analyzed the cellular events that have been proposed to serve as the common conduit to activate the NLRP3 inflammasome using a panel of stimuli. Our work indicates that intracellular K+ depletion alone acting on or upstream of NLRP3 is the minimal common cellular event that is necessary and sufficient to activate the NLRP3 inflammasome.
Mitochondrial damage has been suggested to be the upstream signal responsible for NLRP3 activation (Zhou et al., 2011), and cells treated with nigericin and ATP were found to have decreased OCR after 30–60 minutes of stimulation (Shimada et al., 2012). Unlike this study, we found that nigericin, gramicidin and ATP caused a rapid increase in the OCR. Robust NLRP3 activation by nigericin, gramicidin and ATP occurs within 30 minutes of stimulation. Therefore, it is possible that the mitochondrial damage in response to nigericin and ATP observed by Shimada et al. at later time points is due to cytotoxic effects of these stimuli and not involved in triggering NLRP3 activation. We found that the rapid increase in OCR and ECAR elicited by gramicidin was mediated by activation of the Na+/K+-ATPase and was unrelated to the activation of the NLRP3 inflammasome. Although gramicidin can damage the mitochondria after prolonged stimulation, we found conditions in which robust NLRP3 activation was observed in the absence of mitochondrial perturbation. Collectively, these results indicate that although gramicidin can alter the mitochondrial function through different mechanisms, these effects are not required for NLRP3 activation.
ROS generation secondary to mitochondrial damage has also been implicated in NLRP3 activation as manipulation of the respiratory chain with chemical inhibitors was reported to trigger NLRP3 activation (Zhou et al., 2011). However, unlike this study, we could not detect NLRP3 activation in cells treated with the mitochondrial toxicants rotenone and antimycin A at maximally effective doses or with the autophagy inhibitor 3-MA or H2O2. We do not have an explanation to account for the difference in results. High concentrations of ROS inhibitors block NF-κβ-mediated priming of the NLRP3 inflammasome, but not NLRP3 activation induced by nigericin and silica (Bauernfeind et al., 2011). Using lower concentrations of ROS scavengers which had a strong inhibitory effect on the cellular redox state, we could not see an inhibitory effect in either priming or NLRP3 activation. Thus, our results suggest that the effect of ROS generation does not play a crucial role in NLRP3 activation.
Pioneering studies proposed that the efflux of K+ is responsible for the maturation of proIL-1β as ATP and nigericin permeate the cell membrane to K+ and high extracellular K+ prevents proIL-1β processing (Perregaux and Gabel, 1994). Subsequent studies further supported the hypothesis that NLRP3 activation by all tested stimuli is inhibited by high extracellular [K+] (Pétrilli et al., 2007). However, the role of K+ in NLRP3 activation by particulate matter has been questioned as to date, there is no experimental evidence that K+ efflux occurs during stimulation with particulate matter (Tschopp and Schroder, 2010). Furthermore, a recent report suggested that particulate matter and soluble agonists activate NLRP3 through two distinct mechanisms (Shenoy et al., 2012). Notably, we found that K+ efflux precedes the activation of NLRP3 induced by particulate matter and the lysosomal damaging dipeptide LL-OMe. In addition, we found that NLRP3 activation by high extracellular Ca2+ is due to K+ efflux and could be explained by the formation of particulate matter. Therefore, our work supports a unifying model for NLRP3 activation by membrane permeating molecules and particulate matter in which a decrease in the cytosolic concentration of K+ engages the NLRP3 inflammasome. These results suggest that the internalization of particulate matter via phagocytosis induces lysosomal membrane damage, which triggers the opening of one or more membrane pores permeable to K+.
It has also been proposed that the opening of an unspecific pore formed by the hemichannel pannexin-1 after P2rx7 stimulation by ATP or a RVD response is necessary for NLRP3 activation in addition to K+ efflux (Compan et al., 2012; Pelegrin and Surprenant, 2006). However, recent studies using BMDMs deficient in pannexin-1 showed that this hemichannel is not the molecular substrate of the large pore opened by ATP (Qu et al., 2011). To clarify the role of a large pore in NLRP3 activation, we studied membrane permeation caused by NLRP3 activators to a set of molecular markers of decreasing size: ethidium, [3H]-taurine, Cl− and Na+. ATP, particulate matter, and LL-OMe caused membrane permeation to K+ and the larger chemical species [3H]-taurine and ethidium. Membrane permeation to [3H]-taurine and ethidium by particulate matter was not mediated by the P2rx7 and was secondary to phagocytosis, as it was strongly inhibited by cytochalasin B and latrunculin B. In contrast, the bacterial PFTs gramicidin, α-hemolysin and aerolysin did not permeabilize the cell membrane to ethidium, but caused the efflux of the smaller marker [3H]-taurine. However, the H+/K+-ionophore nigericin caused the efflux of K+ and the influx of Na+, but did not permeabilize the cell membrane to Cl−, [3H]-taurine or ethidium. Therefore, the minimal membrane permeabilization events associated with NLRP3 activation are the efflux of K+ and the influx of Na+. Depletion of intracellular K+ by incubating the cells in low K+ medium was sufficient to activate NLRP3, which occurred when the intracellular content of K+ dropped below 80 %. In addition, K+-free medium activated NLRP3 without causing a change in cell volume or a RVD response. It was suggested that K+ efflux might not be sufficient to activate NLRP3 as decreasing extracellular Na+ prevents NLRP3 activation (Perregaux and Gabel, 1998). Accordingly, substituting of extracellular Na+ by the cation choline had a strong inhibitory effect in NLRP3 activation by K+-free medium, nigericin and gramicidin. However, NLRP3 activation by ATP, aerolysin, Al(OH)3 and silica did not require extracellular Na+. Therefore, although all NLRP3 activators tested permeabilized the cell membrane to Na+, the influx of Na+ or membrane depolarization was not an absolute requirement for NLRP3 activation. Thus, our results demonstrate that a drop in the cytosolic content of K+ acts as the common signal triggered by bacterial PFTs and particulate matter which is sufficient to engage the NLRP3 inflammasome.
Experimental Procedures
Elemental analysis
Intracellular K+ and Na+ measurements were performed by inductively-coupled plasma optical emission spectrometry (ICP-OES) with a Perkin-Elmer Optima 2000 DV spectrometer using yttrium as internal standard. 35Cl was measured by inductively-coupled plasma mass spectrometry (ICP-MS) at the W. M. Keck Elemental Geochemistry laboratory (University of Michigan). The culture media were thoroughly aspirated and cells were extracted 30 min in 3% ultrapure HNO3. K+ determinations were done in 96-well plates. When K+, Na+ and Cl− were simultaneously analyzed 12-well plates were used. For accurate measurement of the intracellular ions, a control was performed in every experiment to determine the extracellular amount of the investigated ion remaining after aspiration and this value was subtracted from every measurement. Also for accurate elemental determinations, analyses were performed in infammasome deficient cells to avoid ion fluxes due to unspecific membrane permeation secondary to pyroptosis. In experiments using low extracellular K+ and/or Na+ cells were washed with medium low in the respective ion prior to stimulation to avoid ion carryover.
Metabolic analysis
Oxygen consumption rate and extracellular acidification rate were measured using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience). BMDMs were seeded in 24-well plates (1.25 × 105/well). The following day cells were changed to bicarbonate-free DMEM and incubated in a non-CO2 incubator for 1 h before the experiment. Mix, wait and measure times were 3, 2 and 3 min, respectively. Values were obtained from the average of 4 wells. Lactate was measured on the Siemen's Advia 2400 autoanalyzer using a lactate oxidase colorimetric method. Intracellular ATP was measured using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega).
[3H]-Taurine release
BMDMs were seeded in 96-well plates and six hours later the medium was replaced by 100 µl of medium containing 0.5 µCi/ml of [1,2-3H]-taurine. The following day, the cells were washed three times with PBS and stimulated. The stimulations were terminated by rapid aspiration of the medium and cells were lysed with 6 % trichloroacetic acid. Taurine efflux was calculated as a fractional release, i.e. the radioactivity released into the extracellular medium as a percentage of the total radioactivity present initially in the cells. The latter was calculated as the sum of radioactivity recovered in the supernatant and that remaining in the cells at the end of the assay. Cell volume measurements were performed with a Coulter Counter (Beckman).
Supplementary Material
Highlights.
NLRP3 activators can perturb the mitochondrial function.
Mitochondrial perturbation or ROS is not required for NLRP3 activation.
Phagocytosis of particulate matter leads to K+ efflux.
K+ efflux is sufficient to activate NLRP3.
Acknowledgements
The studies were supported by NIH grants AI063331 and AI064748 (to G.N.). Bioenergetics studies were done at the Molecular Phenotyping Core, supported by the Michigan Nutrition Obesity Research Center (NIH grant DK089503). G.M-C was supported by the post-baccalaureate Research Education Program grant 5 R25 GM086262 from the NIH. We thank Millenium Pharmaceuticals, George Dubyak, Eicke Latz and Warren Strober for generously supplying mutant mice; Yuumi Nakamura for maintaining the Nlrp3R258W knock-in colony; Stephen Fisher for help with [3H]-taurine experiments, Grace Chen and Luigi Franchi for critical review of this manuscript, Joel Whitfield for ELISA measurements; Sherry Koonse for animal husbandry; Sydney Bridges for performing Seahorse experiments; Ted Huston for ICP-MS measurements; Keith Matz for lactate measurements and James Windak for ICP-OES technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no financial conflict of interest.
References
- Allam R, Darisipudi MN, Rupanagudi KV, Lichtnekert J, Tschopp J, Anders HJ. Cutting edge: cyclic polypeptide and aminoglycoside antibiotics trigger IL-1beta secretion by activating the NLRP3 inflammasome. J Immunol. 2011;186:2714–2718. doi: 10.4049/jimmunol.1002657. [DOI] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE, Roux B. Gramicidin channels. IEEE Transactions on Nanobioscience. 2005;4:10–20. doi: 10.1109/tnb.2004.842470. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Goldring JM. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. The Journal of Physiology. 1975;247:589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Hsieh MY, Chang MY, Chen HC, Jan MS, Maa MC, Leu TH. Eps8 protein facilitates phagocytosis by increasing TLR4-MyD88 protein interaction in lipopolysaccharide-stimulated macrophages. The Journal of Biological Chemistry. 2012;287:18806–18819. doi: 10.1074/jbc.M112.340935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifflet S, Correa V, Nin V, Justet C, Hernandez JA. Effect of membrane potential depolarization on the organization of the actin cytoskeleton of eye epithelia. The role of adherens junctions. Exp Eye Res. 2004;79:769–777. doi: 10.1016/j.exer.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Compan V, Baroja-Mazo A, Lopez-Castejon G, Gomez AI, Martinez CM, Angosto D, Montero MT, Herranz AS, Bazan E, Reimers D, et al. Cell Volume Regulation Modulates NLRP3 Inflammasome Activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. The Journal of Biological Chemistry. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. Journal of Leukocyte Biology. 2006;79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunology. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nature Immunology. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature Genetics. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, Eisenbarth SC, Flavell RA. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. The Journal of Biological Chemistry. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheinrich U, Daut J. Hyperpolarization of isolated capillaries from guinea-pig heart induced by K+ channel openers and glucose deprivation. The Journal of Physiology. 1997;502(Pt 2):397–408. doi: 10.1111/j.1469-7793.1997.397bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185:2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. The EMBO Journal. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch MP, DeSmedt M, ten Hove T, van Deventer SJ, Grooten J. Lipopolysaccharide regulates macrophage fluid phase pinocytosis via CD14-dependent and CD14-independent pathways. Blood. 1999;93:4011–4018. [PubMed] [Google Scholar]
- Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. The Journal of Biological Chemistry. 1994;269:15195–15203. [PubMed] [Google Scholar]
- Perregaux DG, Gabel CA. Human monocyte stimulus-coupled IL-1beta posttranslational processing: modulation via monovalent cations. The American Journal of Physiology. 1998;275:C1538–1547. doi: 10.1152/ajpcell.1998.275.6.C1538. [DOI] [PubMed] [Google Scholar]
- Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death and Differentiation. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Regulation of intracellular sodium in cultured rat hippocampal neurones. The Journal of Physiology. 1997;499(Pt 3):573–587. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. The Journal of Biological Chemistry. 1987;262:8884–8888. [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature Reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nature Immunology. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.