Abstract
Receptor activator of NF-κB ligand (Rankl) is a TNF-like factor that induces the formation of osteoclasts responsible for bone resorption. Although T cell activation up-regulates this gene, the molecular mechanism of its transcriptional control remains unknown. We used ChIP-chip analysis in mouse primary T cells and a T cell hybridoma to define the regulatory enhancers responsible for this up-regulation and to characterize their properties. Elevated H3/H4 acetylation and increased RNA polymerase II density were evident at mRL-D5, a known enhancer located 76 kb upstream of the TSS, as well as at a cluster of regulatory sites located even further upstream between −123 to −156 kb, termed the T cell control region (TCCR). Based upon the ability of calcium signaling and MAPK inhibitors to block Rankl expression, we conducted further ChIP-chip analysis of the transcriptional mediators c-Fos, NF-κB, and Nfat. T cell activation induced c-Fos binding at the mRL-D5 enhancer and within the TCCR. The interaction of NF-κB was observed at the transcriptional start site and at mRL-D5. Both mRL-D5 and segments of the TCCR exhibited robust transcriptional activity in reporter assays, and site-specific mutagenesis of c-Fos and Nfat elements abrogated reporter activity, suggesting a role for both factors in the control of enhancer-mediated Rankl transcription. Finally, chromosome conformation capture analysis confirmed that mRL-D5 and segments of the TCCR were located in proximity to the Rankl gene promoter and thus potentially able to influence directly Rankl gene promoter activity. We conclude that both mRL-D5 and the TCCR represent control segments that play an integral role in Rankl expression in T cells.
Keywords: Fos, Gene Regulation, NF-κB, RNA Polymerase II, Transcription Factors, BMD, ChIP-chip, T Cell Activation, Distal Enhancers, Histone Acetylation
Introduction
Receptor activator of NF-κB (RANK)2 ligand (RANKL) is a membrane-bound TNF-like factor that interacts with RANK on the surface of target cells. RANKL-RANK signaling is important in the regulation of proliferation, differentiation, and/or survival of a variety of cell types (1–4). The absence of RANKL expression results in severe osteopetrosis caused by reduced bone resorption, defects in early T and B cell differentiation or in mammary gland differentiation, and increased production of auto-antibodies (3, 5, 6). Although RANKL is best known for its role in bone resorption where it controls the differentiation of precursors into functional osteoclasts (7, 8), this factor also plays multiple roles within the immune system to promote lymph node organogenesis (5), dendritic cell survival (1, 2, 9), tolerance to self-antigens (6), and T regulatory cell activation and proliferation (4, 10). RANKL is currently a key therapeutic target for the treatment of osteoporosis (11), rheumatoid arthritis (12), and the prevention and treatment of cancer metastases to the bone (13). Although inhibition of RANKL activity can prevent the loss of bone mass, concerns have been raised as to whether general inhibition of this factor may also impact the beneficial effects of RANKL-RANK signaling as indicated above. An understanding of the underlying mechanisms responsible for RANKL expression is therefore warranted and aimed at defining more cell-specific approaches to regulating RANKL activity, particularly at the level of the skeleton.
Rankl expression was first documented in T cells, where it was induced following T cell activation (1, 2). Subsequently, Rankl has also been found to be expressed in a variety of other cell types including osteoblasts (7, 8), keratinocytes (4), B lymphocytes (14, 15), mammary epithelial cells (3), and undefined cell types within the brain (16). Rankl expression in T cells is dramatically increased following activation of the immune system and during chronic inflammation, events that are associated with accelerated bone resorption (17). Moreover, a lack of T cells blocks the bone loss associated with ovariectomy in mice (18) and a lack of CD4+ T cells significantly decrease tumor metastasis (19) suggesting that T cell-mediated RANKL expression may be an important feature in the pathogenesis of several disease states. Although the rescue of Rankl ablation using a T cell-specific transgene showed a partial rescue of osteoclast development (20), its exact role in both disease-related bone loss as well as in normal bone remodeling remains unclear.
Transcriptional regulation of Rankl has been extensively studied in mouse osteoblasts where the expression of this gene is controlled through a series of regulatory domains that are located far upstream of the Rankl transcriptional start site (TSS) and that may cooperate with the Rankl promoter to modulate expression of the gene. Most notably, the mouse Rankl (mRL)-D5 distal enhancer located 76 kb upstream of the Rankl TSS is thought to mediate stromal cell production of Rankl via the inducers 1,25(OH)2D3 and PTH, whereas the mRL-D6 enhancer, located 88 kb upstream, mediates regulation by IL-6 and OSM (21–25). Activation of these enhancers leads to both local increases in the recruitment of RNA polymerase (pol) II and changes in chromatin structure, as evidenced by enhanced levels of histone H4 acetylation (H4ac) (21). A direct consequence of these actions is the production of not only Rankl mRNA, but also the synthesis of long RNA transcripts as well (26). Importantly, knock-out of the mRL-D5 enhancer in the mouse genome results in reduced basal expression of Rankl in both bone and spleen and desensitization of Rankl expression to treatment with 1,25(OH)2D3 and PTH in bone. These results highlight the importance of the mRL-D5 enhancer in bone in vivo and also suggest a possible role for mRL-D5 in the expression of Rankl in T cells (27).
In this report, we used ChIP-chip analysis to identify a series of potential regulatory regions in the Rankl gene in mouse T cells that are marked by increased histone H3/H4 acetylation and elevated RNA pol II density. These regions include the mRL-D5 enhancer that was previously characterized in osteoblasts as well as a novel series of putative regulatory enhancers located over 123 kb upstream of the Rankl TSS that we have termed the T cell control region (TCCR). We further characterized these enhancers for their role in T cell regulation of Rankl gene expression.
EXPERIMENTAL PROCEDURES
Reagents
General biochemicals were purchased from ThermoFisher Scientific (Waltham, MA), Sigma-Aldrich, or as previously described (21). Phorbol 12-myristate 13-acetate (P8139) and ionomycin (I0634) were purchased from Sigma-Aldrich. Anti-c-Fos (sc-7202), NF-κB p50 (sc-1190), and Nfat-pan (sc-7294) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-acetyl histone H4 (06-866) and anti-acetyl histone H3 Lys9 (06-942) antibodies were purchased from Millipore (Billerica, MA). Monomethyl histone H3 Lys-4 (ab8895–50) and trimethyl histone H3 Lys-4 (ab1012–100) antibodies were obtained from Abcam (Cambridge, MA). The mice were obtained from Harlan Laboratories (Madison, WI), and anti-RNA polymerase II 8WG16 (MMS-126R) was purchased from Covance (Princeton, NJ). U0126 (662005) was purchased from Calbiochem (San Diego, CA), cyclosporin A (BML-A195–0100) was obtained from Enzo Life Sciences (Farmingdale, NY), and EasySep mouse CD4+ T cell enrichment kits (19752) were purchased from Stem Cell Technologies (Vancouver, Canada). RNeasy Plus mini kits (74134) were purchased from Qiagen, and RP1640 (15-040-CV) was obtained from Mediatech (Manassas, VA). CD3e (553057) and CD28 (553294) antibodies were obtained from BD Biosciences (San Jose, CA). RPMI 1640 (11875) and RiboMinus eukaryote kit for RNA-Seq (A1083708) were purchased from Invitrogen. Small RNA sample prep kit v1.5 (FC-102-1009), mRNA-Seq prep kit (RS-100–1801), cBot Single Read Cluster Generation Kit (GD-300-1001), and the Illumina Sequencing Kit v4 (FC-104-4001) were purchased from Illumina (San Diego, CA).
Cell Culture and Isolation of Primary T Cells
2b4.11 cells were cultured in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (heat-inactivated), 50 μm β-mercaptoethanol, and 100 μg/ml gentamicin. Jurkat cells were cultured in RPMI 1640 (Mediatech) with 10% fetal bovine serum (heat-inactivated), 10 mm Hepes, 2.5 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Mouse ST2 osteoblastic cells were cultured in α-minimum essential medium supplemented with 10% fetal bovine serum (heat-inactivated), 100 units/ml penicillin, and 100 μg/ml streptomycin (21). Primary mouse CD4+ T cells were isolated from the spleen or whole blood of C57BL6/NHsd mice using the EasySep® mouse CD4+ T cell enrichment kit according to the manufacturer's protocol. The cells were cultured in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (heat-inactivated).
In Vitro and ex Vivo T Cell Activation
T cells were activated using 500 ng/ml ionomycin and 10 ng/ml phorbol 12-myristate 13-acetate (PMA) or through CD3/CD28 antibody activation. In the latter condition, the antibodies were first adsorbed overnight to ELISA plates using a solution of 10 μg/ml mouse CD3e antibody (clone 145–2C11) and 2.5 μg/ml CD28 antibody (clone 37.51). The wells were then blocked with 1 mg/ml BSA, and isolated cells were seeded at 0.2 million cells/well.
RNA Isolation and Analysis
RNA was isolated using two methods: 1) total RNA was isolated from cells using Tri-Reagent and DNaseI-treated, or 2) RNA was isolated using the RNeasy Plus mini kit according to the manufacturer's protocol. RNA was reverse-transcribed using the high capacity cDNA reverse transcription kit. The resulting cDNA was then subjected to quantitative PCR analysis.
Quantitative PCR Analysis
Real time PCR was performed on either an Eppendorf Realplex or ABI StepOnePlus using Power SYBR Master Mix with standard cycling conditions. The Mastercycler® ep Realplex software or StepOne Software was used for analysis. The primers used in the RNA analyses include: mouse Rankl mRNA, 5′-TGTACTTTCGAGCGCAGATG-3′ (forward) and 5′-AGGCTTGTTTCATCCTCCTG-3′ (reverse); mouse β-actin mRNA, 5′-TGTTTGAGACCTTCAACACCC-3′ (forward) and 5′-CGTTGCCAATAGTGATGACCT-3′ (reverse); mouse IL-2 mRNA, 5′-GCAGGATGGAGAATTACAGGA-3′ (forward) and 5′-TTGGCACTCAAATGTGTTGTC-3′ (reverse); mouse c-Fos mRNA, 5′-CGAAGGGAACGGAATAAGATG-3′ (forward) and 5′-GCTGCCAAAATAAACTCCAG-3′ (reverse).
RNA-Seq Analysis
Total RNA was isolated from cells using Tri-Reagent and then treated with DNaseI. Ribosomal RNA was depleted using RiboMinus eukaryote kit for RNA-Seq. rRNA depleted RNA (2 μg) was fragmented and a cDNA library prepared according to the Illumina Directional mRNA-Seq Library Prep protocol using the small RNA v1.5 and mRNA-Seq prep kits. Quality of cDNA library was assessed using the Agilent 2100 Bioanalyzer and high sensitivity DNA kit. DNA clusters were generated using a cBot Single Read Cluster Generation kit on an Illumina cBot and included the smRNA/GEX-DpnII sequencing primer in lieu of the typical genomic sequencing primer as described in the manufacturer's instructions, to obtain an average of 2.0 × 107 clusters for each flow cell lane. 75-bp reads of each library were performed on an Illumina Genome Analyzer IIX using the Illumina Sequencing kit (version 4). Fluorescent images were analyzed using the Illumina base-calling pipeline 1.6.0 to obtain FASTQ formatted sequence data. The sequences were mapped to the human genome (March 2006 assembly NCBI36/hg18) using Bowtie (Bowtie 0.12.5, mismatch = 3, solexa1.3-quals on) (28). Intron gaps and splice junctions were predicted and mapped by Tophat (Tophat 1.0.14, A spliced read mapper for RNA-Seq) (29). The data were further processed by ArrayStar 4.0 with Q-seq module (DNA-STAR Madison, WI) and entered into the UCSC Genome Browser.
Chromatin Immunoprecipitation-Microarray Analysis
ChIP analysis was preformed as previously described (23, 24, 30, 31). Immunoprecipitated DNA was blunt-ended by T4 DNA Polymerase, ligated to linkers with the sequence 5′-GAATTCAGATC-3′ and 5′-GCGGTGACCCGGGAGATCTGAATTC-3′ using T4 DNA ligase, and amplified by ligation-mediated PCR with Go Taq Flexi and linear PCR amplifications. PCR purification was performed with the QIAquick PCR purification kit. DNA samples were labeled with Cy3 or Cy5 9-mer wobble primers using Klenow fragment, and the reaction was then stopped with the addition of EDTA, precipitated with NaCl and isopropanol, washed with 80% EtOH, and then resuspended in water. Cy3- and Cy5-labeled DNA samples as indicated were co-hybridized to a custom oligonucleotide microarray using a NimbleGen hybridization kit and a MAUI hybridization system. Microarrays were washed using NimbleGen Wash Buffer and scanned using an Axon 4000B scanner with GenepixPro version 4.1 software at the appropriate wavelengths. Custom oligonucleotide microarrays were synthesized by Roche-NimbleGen Systems (Madison, WI). The microarray oligonucleotide probes were 50- to 70-mer in length with 65–70 bp resolution and synthesized using a mask-less array system tiled from 200 kb upstream of the mouse Rankl TSS to 200 kb downstream of the final 3′ coding exon. This custom array contained additional tiled genes not considered in this study. DNA samples were obtained by immunoprecipitation with antibodies specific for c-Fos, Nfat, p50, CBP, p300, H4K(5,8,12,16)ac, H3K9ac, H3K4me1, H3K4me3, or RNA polymerase II. A series of comparisons were used: 1) untreated and input control and 2) PI-treated and input control. The aforementioned co-hybridizations were analyzed and presented as previously described (24). NimbleScan version 1.9.0.05 was used for statistical analysis of data; a sliding window of 700 bp containing at least four of eight probes statistically above background was deemed significant at a false discovery rate (FDR) of <0.05.
Plasmids
The reporter constructs pTK mRL-D1, -D2, -D3, -D4, -D5a, -D5b, and -D6 were previously described (21, 24, 32). Similar methods were used to prepared pTK mRL-T1A (−122986 to −124293 nucleotides), mRL-T1B (−126338 to −127714 nucleotides), mRL-T1C (−129438 to −130831 nucleotides), mRL-T2 (−139923 to −141306 nucleotides), mRL-T3A (−150408 to −152161 nucleotides), and mRL-T3B (−152870 to −154315 nucleotides) using primers containing HindIII/BamHI/SalI restriction sites. Digested fragments were cloned into the corresponding sites within the pTK-luc vector. The QuikChange mutagenesis kit was used to introduce mutations into the pTK-mRL-D5b and mRL-T1A reporter constructs.
Reporter Transfections
ST2 cells were transfected using Lipofectamine as previous described (24). Jurkat cells (50,000 cells/well in 24-well plates) were transfected with 250 ng of reporter construct and 200 ng of RSV-βgal in serum-free medium using Lipofectamine and Plus reagent. After a 3-h incubation, the wells were supplemented with RPMI 1640 containing 20% FBS to a final concentration of 10% FBS and treated with 500 ng/ml ionomycin and 10 ng/ml PMA or Me2SO vehicle. The cells were harvested 20 h later for reporter assays using lysis buffer, and both luciferase and β-galactosidase activities were determined as previously described (23, 24). For inhibitor studies, the cells were treated with transfection reagent as above followed by a 30-min exposure to either U0126 or cyclosporin A (CSA) in medium containing 10% serum, and then treatment with 500 ng/ml ionomycin and 10 ng/ml PMA or Me2SO vehicle for an additional 20 h.
Chromosome Conformation Capture (3C) Assay
3C was performed as previously described (33–35). Briefly, 2b4.11 cells were grown to a density of 500,000 cells/ml and then treated with vehicle or 500 ng/ml ionomycin and 10 ng/ml PMA for 3 h. The cells were cross-linked for 10 min with 1% formaldehyde and then quenched with 0.125 m glycine. The nuclei were collected and disrupted using 20 strokes of a Dounce homogenizer. The nuclear suspension was divided into two tubes, washed, and resuspended in NEBuffer 3. SDS was added to 0.1% final concentration, the nuclei were incubated at 65 °C for 10 min, and Triton X-100 was added to 1% final concentration. Digestion was performed with 400 units of BtgI overnight at 37 °C. Enzyme was inactivated by addition of 2% final concentration SDS and further incubated at 65 °C for 30 min. The samples were diluted 10-fold (to 8 ml) and incubated with or without T4 DNA ligase for 4 h at 16 °C and an additional 30 min at room temperature. Proteinase K (100 μg) was added, and the samples were incubated overnight at 65 °C. The DNA was purified through two rounds of phenol:chloroform:isoamyl alcohol (25:24:1) extractions and a final ethanol precipitation. Final DNA was redissolved in TE buffer (10 mm Tris-HCl, pH 8.1, 1 mm EDTA) and treated with 4 μg of RNase A. As PCR product size control, DNA from BAC clones RP23-68C18 and RP23-52A3 spanning the entire Rankl gene locus were digested overnight with BtgI, religated, and then purified. This sample contained all possible Rankl PCR product combinations. PCR was performed using Go-Taq Flexi Green Master Mix for 35 cycles. The primers were designed on the forward genomic strand in proximity to the BtgI cut site. Analysis was performed from BtgI segments located between the distal elements and proximal promoter.
Statistical Analyses
All of the values were expressed as the means ± S.E. of the mean. All of the statistical calculations were performed using GraphPad PRISM version 4 statistical software package (GraphPad Software Inc., San Diego, CA) using either one-way nonparametric analysis of variance analysis with a Bonferroni post-test or nonparametric T tests as indicated. Activated samples were contrasted with vehicle-treated controls or other indicated samples.
RESULTS
Rankl mRNA Levels Increase with T Cell Activation
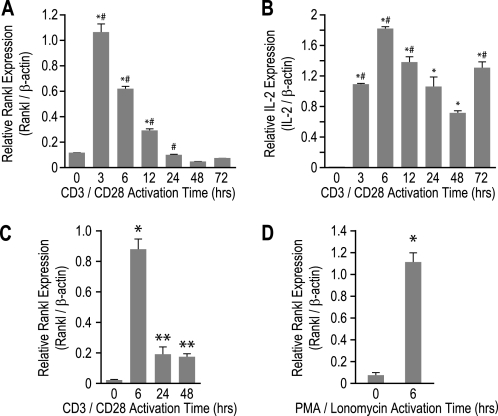
It is well established that Rankl gene expression is increased upon T cell activation both ex vivo and in vitro (1, 36, 37). To confirm these findings and to establish a model for further molecular exploration, we treated splenic CD4+ T cells with antibodies to CD3/CD28 from 0 to 72 h and then examined the levels of Rankl, IL-2, and β-actin mRNA by quantitative RT-PCR analysis. As shown in Fig. 1A, Rankl transcripts were rapidly and transiently induced with maximum expression occurring at 3 h followed by a return to base line at 24 h. This profile was similar to that for the IL-2 transcripts with respect to the time course of induction, but IL-2 expression remained highly elevated for the duration of the experiment (Fig. 1B).
FIGURE 1.
Rankl mRNA levels in mouse primary T cells and the mouse 2b4.11 T cell hybridoma are transiently induced upon activation. A and B, primary mouse CD4+ T cells were seeded into wells with or without plate-bound CD3e and CD28 antibodies for up to 72 h. Total RNA was analyzed using primers specific to Rankl (A) or IL-2 (B) and normalized to β-actin levels. Each value represents the average of three independent experiments ± S.E. *, p < 0.05 is statistically significant using a one-way nonparametric analysis of variance analysis with single variance followed by a Bonferroni multiple comparison post-test when compared with control sample. *#, p < 0.05 is statistically significant when compared with previous time point. C and D, 2b4.11 cells were seeded into wells for up to 48 h and analyzed as in A. C, 2b4.11 cells were seeded into wells and treated with plate-bound CD3e and CD28 antibodies for up to 48 h, and total RNA was analyzed using primers to Rankl and normalized to β-actin levels. D, 2b4.11 cells were treated for 6 h with either Me2SO vehicle or 500 ng/ml ionomycin and 10 ng/ml PMA (PI), and total RNA was analyzed using primers to Rankl and normalized to β-actin. Each value represents the average of three independent experiments ± S.E. *, p < 0.05 is statistically significant using a nonparametric t test when compared with control sample. **, p < 0.05 is statistically significant using a nonparametric t test when compared with both control samples and 6-h treatment samples.
We also analyzed the mouse 2b4.11 T cell hybridoma for its utility as an in vitro model to study T cell activation of Rankl expression (36). 2b4.11 cells were seeded into plates with or without plate-bound CD3/CD28 antibodies, and the concentrations of Rankl and β-actin mRNA were examined up to 48 h later. As in CD4+ T cells, Rankl transcripts were transiently induced as documented in Fig. 1C, with maximum expression at 6 h. Finally, we asked whether Rankl was induced with PMA/ionomycin, a classic inducer of T cell activation. 2b4.11 cells were treated with either Me2SO vehicle or both ionomycin (500 ng/ml) and PMA (10 ng/ml) (PI) for 6 h, and the isolated RNA was then evaluated. As seen in Fig. 1D, Rankl transcription was induced to a level comparable with that observed with CD3/CD28 activation, thereby providing an additional in vitro model system for studying Rankl gene expression.
ChIP-chip Analysis of Acetylated Histone H4/H3 and RNA pol II Is Used to Identify Putative T Cell Enhancer Regions in Primary Mouse CD4+ T Cells and the 2b4.11 Cell Line
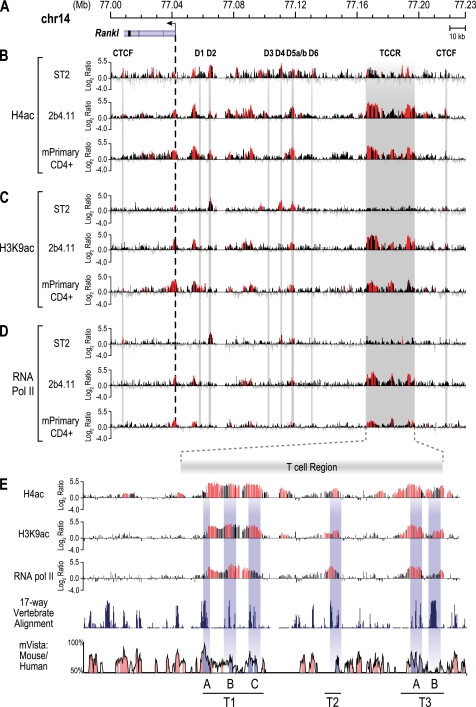
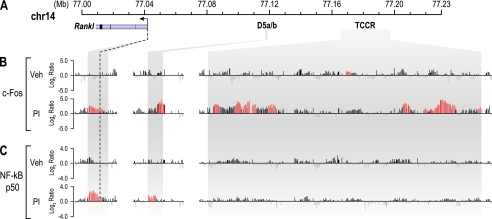
Osteoblasts control Rankl expression via a series of distal upstream enhancers (21, 23). Many of these enhancers are marked under basal conditions by elevated levels of H4ac and increased RNA pol II density; these activities were increased following induction by 1,25(OH)2D3, PTH, or IL-6 (21, 23, 24). These genetic features are now considered generally typical of functional enhancers (38). In an effort to identify enhancers responsible for Rankl expression in T cells, we conducted a ChIP-chip analysis of the levels of H4ac and histone H3 lysine 9 acetylation (H3K9ac) and of RNA pol II density across the Rankl gene locus in unstimulated 2b4.11 and primary CD4+ T cells and contrasted the results with those found in unstimulated osteoblastic ST2 cells. Chromatin was isolated and immunoprecipitated using antibodies to H4ac, H3K9ac, or RNA pol II, and the resulting DNA was co-hybridized with control input DNA overnight to custom microarrays. Fig. 2A depicts the Rankl gene locus on chromosome 14 (February 2006 assembly) with the nucleotide positions (megabases) indicated. Fig. 2B depicts the data tracks representing the log2 ratio of fluorescence obtained from a vehicle-treated sample precipitated with an antibody to tetra-acetylated histone H4 and co-hybridized with input DNA. Fig. 2 (C and D) depicts similar tracks from DNA precipitated with an antibody to either H3K9ac or RNA pol II. As shown in Fig. 2B, elevated levels of basal H4ac were found upstream of the Rankl TSS in ST2, 2b4.11, and mouse CD4+ T cells. Statistically significant elevation of this epigenetic mark was also found in unstimulated ST2 cells near the TSS and across many of the enhancer regions that were described previously (21), although these activities are relatively low in the unstimulated state. Elevated levels of H4ac were also found at significant levels in both untreated 2b4.11 and CD4+ T cell populations. Accordingly, although several of the enhancers discovered in the ST2 cells were also marked with H4ac in 2b4.11 cells, an extended region of particularly high H4ac was observed even further upstream in 2b4.11 cells between −123 and −155 kb that was largely absent in ST2 cells. Elevated levels of H3K9ac were also found similarly in both 2b4.11 and primary CD4+ cells (Fig. 2C); increased RNA pol II density was also observed in all three of the cell types (Fig. 2D). The activities of both H3K9ac and RNA pol II were most strikingly observed in the region upstream of −123 kb and restricted to the 2b4.11 and CD4+ T cells, suggesting strong similarity between the two cell types within this locus. Because of its unique nature in T cells, we tentatively designated this region the TCCR. These data also suggest that osteoblasts and T cells both may utilize a common enhancer such as mRL-D5 and cell type-specific enhancers such as the TCCR to regulate Rankl gene expression. Surprisingly, PI activation of 2b4.11 cells only modestly altered H4ac and H3K9ac levels and RNA pol II density profiles, suggesting that these cells show some degree of residual activation (data not shown).
FIGURE 2.
ChIP-chip analysis reveals the presence of elevated H4ac, H3K9ac levels, and RNA pol II density across the mouse Rankl gene locus. Mouse ST2 cells, 2b4.11 cells, and primary CD4+ T cells were subjected to ChIP analysis using antibodies to tetra-acetylated histone H4K5, K8, K12,K16 (H4ac), H3K9ac, and RNA pol II. Immunoprecipitated DNA was treated as described under “Experimental Procedures.” A, the Rankl gene locus on chromosome 14. The Rankl gene is indicated with the arrow representing the direction of transcription. The locations of established enhancers (D1–D6), the putative TCCRs, and CTCF sites are designated by descending bars. B, levels of H4ac across the Rankl gene locus in ST2, 2b4.11, and primary CD4+ T cells. The data tracks represent the log2 ratios of fluorescence obtained from samples precipitated with an antibody to H4ac and co-hybridized with a labeled ChIP input DNA. All of the peaks highlighted in red are statistically significant (FDR, p < 0.05). C, levels of H3K9ac across the Rankl gene locus in ST2, 2b4.11, and primary CD4+ T cells. The data tracks represent the log2 ratios of fluorescence obtained from samples precipitated with an antibody to H3K9ac and co-hybridized with a labeled ChIP input DNA. D, RNA pol II density across the Rankl gene locus in ST2, 2b4.11, and primary CD4+ T cells. The data tracks represent the log2 ratios of fluorescence obtained from samples precipitated with an antibody to RNA pol II and co-hybridized with a labeled ChIP input DNA. E, focused views of H4ac, HeK9ac, and RNA pol II activity across the TCCR interval in 2b4.11 cells. A 17-way vertebrate alignment and an mVista plot comparing sequence conservation in mouse and human are shown in the bottom two tracks. Highly conserved regions within the T region are indicated by gray dropdown bars and designed T1A, T1B, T1C, T2, T3A, and T3B.
Enhanced levels of H4ac and H3K9ac, as well as increased RNA pol II density across the TCCR in T cells, suggest the presence of at least three distinct regulatory regions, as depicted in Fig. 2E. To explore these regions in more detail, we conducted a 17-way vertebrate alignment and generated a mouse versus human Vista plot of the TCCR region using the UCSC Genome Browser to assess the degree of conservation located within this cluster of enhancers. As can be seen, each of the three regions within the TCCR contained components that were highly conserved both across species as well as between mouse and human genomes. These three regions within the TCCR were designated T1, T2, and T3.
Putative Rankl T Cell Enhancers Are Not Alternative Exons of the Rankl Gene
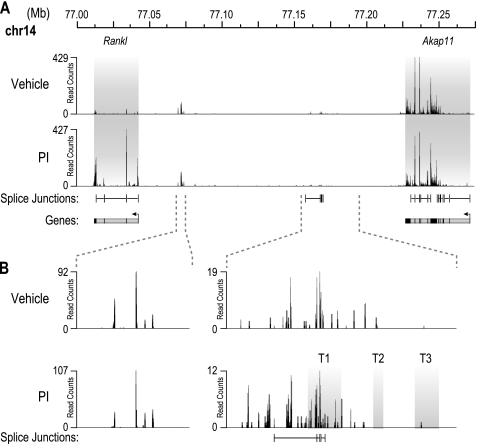
The mouse Rankl transcriptional unit is comprised of a single promoter that directs the synthesis in osteoblasts of five highly conserved and spliced exons; alternative or upstream promoters have not been identified in mouse osteoblasts. To confirm this in T cells, we employed high throughput RNA sequencing (RNA-Seq) analysis to characterize the Rankl RNA transcript and to assess whether components upstream could be identified that were spliced to the Rankl gene itself. 2b4.11 cells were treated with either vehicle or PI, and the isolated RNA was then fragmented, reversed transcribed, and analyzed using RNA-Seq analysis. Read counts for the interval 77.000–77.275 megabases that contain both the Rankl and adjacent Akap11 genes are shown in Fig. 3A. Spliced RNA was detected across each of the five exons of the Rankl gene under both basal and PI-induced conditions; induction was ∼11-fold, a level consistent with that observed via RT-PCR analysis (Fig. 1D). Interestingly, very low levels of RNA transcripts were also detected upstream of the Rankl promoter, including one spliced transcript that appeared to originate from the T1 enhancer within the TCCR; none of these, however, were found to be either spliced to the downstream Rankl transcript or regulated by PI. Inspection of the data more broadly also revealed that none of the genes located within 1 megabase of the Rankl transcriptional unit were similarly regulated by PI as well. These data suggest that the epigenetic and RNA pol II activities observed within the TCCR region do not represent alternative Rankl promoters, although the possibility that an unannotated and unregulated gene(s) may be present within the extended TCCR region cannot be ruled out.
FIGURE 3.
RNA-Seq analysis confirms upstream putative enhancer regions are not alternative Rankl exons. A, the Rankl transcript is composed of five spliced exons. 2b4.11 cells were treated with PI or vehicle for 6 h. Total RNA was isolated and depleted of ribosomal RNA, and a cDNA library was created according to the protocol described under “Experimental Procedures.” The cDNA library was then subjected to RNA-Seq analysis, and the reads were analyzed for the presence of transcript and splice junctions. Both Rankl and Akap11 genes are highlighted in gray, and the levels of transcript detected are displayed in read counts. Detected splice junctions and predicted genes are shown below the detected transcripts. B, a novel spliced intergenic transcript is detected upstream of the Rankl gene overlapping the T1 region of the TCCR. Two intergenic regions with transcript detected in A are enlarged.
MEK1/2 and Calcineurin Activation Are Necessary for Activation-induced Rankl Expression
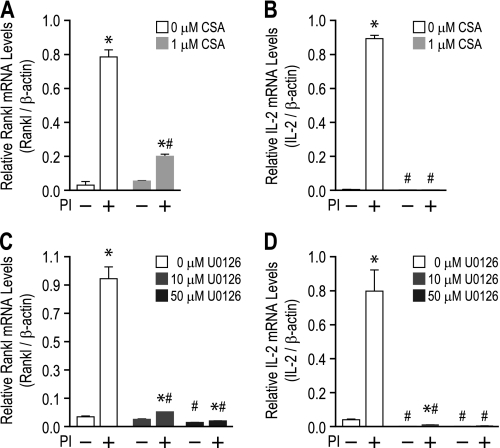
To gain further evidence of a role for the TCCR in Rankl expression, we next examined the effect of T cell activation on signaling pathways and transcription factor activity at the Rankl locus. T cell activation is known to stimulate calcium signaling, PKC, and MAPK pathways that ultimately activate specific regulatory factors that include AP-1, NF-κB, and Nfat. Previous studies suggest that Rankl expression in activated T cells and the 2b4.11 cell line is suppressed by inhibitors of the calcineurin/Nfat activation pathway such as FK506 and CSA (1, 39, 40). To confirm this finding and to explore the impact of the MAPK pathway on Rankl expression, 2b4.11 cells were pretreated with either Me2SO vehicle, CSA, or the MEK1/2 inhibitor U0126 and then treated with vehicle or PI for an additional 4 h. Total RNA was isolated, and Rankl and IL-2 mRNA levels were examined using quantitative RT-PCR. PI-induced Rankl mRNA levels were suppressed with both CSA (Fig. 4A) and U0126 (Fig. 4C) pretreatment. As was observed in osteoblasts, U0126 treatment also suppressed basal Rankl mRNA levels as well (41). T cell activation-induced IL-2 gene expression was also suppressed with both CSA and U0126 treatments (Fig. 4, B and D), a finding consistent with previous studies (42, 43).
FIGURE 4.
MEK1/2 and calcineurin signaling pathways are necessary for activation-induced Rankl gene expression. A and B, 2b4.11 cells were pretreated with either Me2SO vehicle or CSA for 30 min and then induced for an additional 4 h with either Me2SO vehicle or PI. Isolated total RNA was analyzed by quantitative PCR using primers specific to Rankl (A) or IL-2 (B) and normalized to β-actin. C and D, 2b4.11 cells were pretreated with Me2SO vehicle, 10 μm U0126, or 50 μm U0126 for 30 min and then induced for an additional 4 h with either Me2SO vehicle or PI. Isolated total RNA was analyzed by quantitative PCR using primers specific to Rankl (C) or IL-2 (D) and normalized to β-actin. Each value represents the average of three independent experiments ± S.E. *, p < 0.05 is statistically significant using a nonparametric t test when compared with vehicle-treated sample. #, p < 0.05 is statistically significant using a nonparametric t test when compared with vehicle- or inhibitor-treated cells.
IL-2 gene up-regulation in activated T cells is modulated by AP-1, NF-κB, and Nfat (42, 43). Given the similarities between both Rankl and IL-2 induction, we next conducted a ChIP-chip analysis of 2b4.11 cells following either vehicle or PI treatment using antibodies to c-Fos, Nfat, or the p50 subunit of NF-κB. Fig. 5 depicts data tracks representing the log2 ratios of fluorescence at the mRL-TSS, mRL-D5, and TCCR regions for c-Fos or NF-κB p50 binding activity. As can be seen, both c-Fos and NF-κB localized to the putative enhancer within the Rankl gene locus explored in this study as well as other upstream regions that are associated with increased H4ac levels and RNA pol II density (supplemental Fig. S1); however, c-Fos occupancy was particularly increased across the TCCR. Nfat occupancy was not detected, despite the appearance of this factor at several other gene loci present on the tiled array. The presence of c-Fos and NF-κB at these sites was validated using direct ChIP analysis (data not shown). The appearance of NF-κB near the Rankl promoter confirms a previous observation made using a transfected Rankl promoter-reporter gene constructs (36). These studies suggest that T cell activation prompts the appearance of both c-Fos and NF-κB at sites within the Rankl locus and with respect to c-Fos, across the TCCR that are likely responsible for inducing the Rankl gene. Similar studies revealed the presence of both CBP and p300 at these sites as well, but the recruitment of these acetyltransferases was not induced at the Rankl putative enhancers regions during T cell activation (supplemental Fig. S2). Selectively enhanced levels of H3K4me1 and H3K4me3 activity that mark enhancer and promoter regions, respectively, are also seen (supplemental Fig. S2).
FIGURE 5.
T cell activation induces c-Fos and NF-κB binding at the Rankl TSS and enhancer regions. 2b4.11 cells were treated with PI or vehicle (Veh) for 1 h and then subjected to ChIP analysis using antibodies to c-Fos and NF-κB (p50). A, the Rankl gene locus on mouse chromosome 14 with features as described in Fig. 2A. B and C, binding activity of c-Fos (B) and NF-κB (C) at the Rankl locus. The data tracks represent the log2 ratios of fluorescence obtained from either vehicle- or PI-treated samples precipitated with antibodies to c-Fos or NF-κB (p50). All of the peaks highlighted in red are statistically significant (FDR, p < 0.05).
The mRL-D5 and TCCR Enhancer Regions Show Strong Transcriptional Activity in Reporter Assays
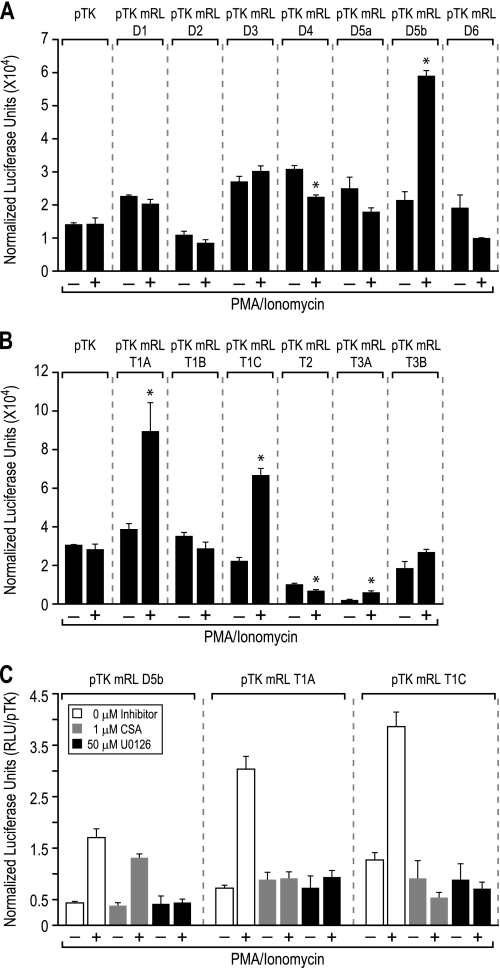
Enhancers that modulate transcription of the Rankl gene in osteoblasts are highly conserved across multiple species and capable of transcriptionally regulating reporter activity when cloned upstream of heterologous promoters (21–24). We therefore utilized this approach to assess whether the previously identified osteoblast enhancers or individual segments of the TCCR cluster (T1A-C,T2, T3A, and B) could mediate transcriptional regulation of reporter gene activity and whether these effects were precipitated by c-Fos or NF-κB following T cell activation. Accordingly, each of these individual constructs were transfected into human Jurkat T cells and reporter activity was assessed following T cell activation (these cells also express Rankl, and the locus is similarly responsive to PI activation; data not shown). Although different basal activities were evident, mRL-D5b, the mRL-T1A, mRL-T1C, and mRL-T3A reporter constructs all showed inducible reporter activity in response to PI treatment when compared with vehicle-treated samples, as documented in Fig. 6 (A and B). In contrast, none of the TCCR constructs themselves mediated transcriptional response to 1,25(OH)2D3, PTH, or IL-6 (data not shown), strong inducers of the Rankl enhancers mRL-D1 through mRL-D6 in ST2 cells. These data provide support for the idea that mRL-D5 together with enhancer elements of the TCCR may play a central role in Rankl induction in T cells but not in osteoblasts. Further exploration of the mRL-D5b, mRL-T1A, and mRL-T1C enhancers revealed that U0126 inhibited the PI inducible activity of all the constructs, whereas inhibition by CSA was restricted to the mRL-T1A and mRL-T1C constructs (Fig. 6C). This latter observation suggests that certain activities of the mRL-D5b enhancer may be unique relative to those seen at the mRL-T1A and mRL-T1C enhancers.
FIGURE 6.
PI induces the activity of the mRL-D5 enhancer and segments of the TCCR. Shown are the transcriptional activities of the Rankl enhancers in human Jurkat cells. A, Rankl enhancers mRL-D1–mRL-D6 were transfected into Jurkat cells and examined for basal and PI-inducible luciferase activity after 16 h as described under “Experimental Procedures.” B, Rankl enhancers T1A, T1B, T1C, T2, T3A, and T3B were transfected into Jurkat cells and examined as in A. C, Rankl enhancers D5b, T1A, and T1C were transfected into Jurkat cells and, following pretreatment with Me2SO vehicle, 1 μm CSA, or 50 μm U0126, were examined 16 h later for both vehicle and PI-inducible luciferase activity 16 h as described for A. Each value represents the average of three independent replicates ± S.E. *, p < 0.05 is statistically significant using a nonparametric t test when compared with vehicle-treated sample. #, p < 0.05 is statistically significant using a nonparametric t test when compared with the pTK control.
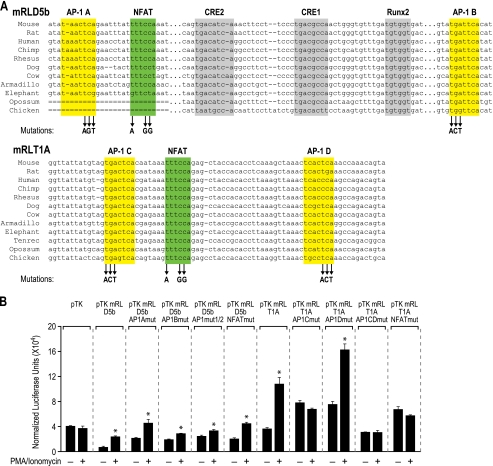
To assess whether c-Fos and NF-κB binding to the mRL-D5b region and to enhancers within the TCCR play a functional role in regulating transcription, we used an in silico approach to search for potential AP-1, NF-κB, and Nfat binding sites within the mRL-D5b and mRL-T1A regions (44, 45). Two highly conserved AP-1 and several Nfat consensus binding sites were identified in each region, as illustrated in Fig. 7; no NF-κB sites were uncovered. Interestingly, one of the sites within the T1A region was reminiscent of a composite AP-1 and Nfat site found within the IL-2 promoter (Fig. 7A) (46). To determine whether these sites were functional, we mutated each individually or in combination and then assessed the consequence of this activity on both basal and PI-inducible mRL-D5b or mRL-T1A enhancer activity following transfection into Jurkat cells. As documented in Fig. 7B, only mutation of the AP-1B site in mRL-D5b suppressed PI-inducible reporter activity. Interestingly, this AP-1 binding site was located immediately adjacent to two cAMP response elements and one Runx2 binding element that were shown previously to be important for PTH regulation of Rankl expression in osteoblasts (23). Mutation of AP-1A and the single Nfat sites in mRL-D5b was without effect and therefore consistent with the observation made in Fig. 6C that CSA did not prevent activation of mRL-D5b and was therefore unlikely to involve Nfat activation. Interestingly, mutation of either AP-1C or the adjacent Nfat site within the mRL-T1A enhancer reduced basal activity and prevented induction by PI. These results recapitulate Rankl mRNA data suggesting that AP-1 mediates the inducible reporter activity of both mRL-D5b and the mRL-T1A enhancers. They also indicate that despite our inability to detect its presence in the ChIP-chip analysis, Nfat or related transcription factor activation may be involved in transcriptional regulation through the putative mRL-T1A enhancer.
FIGURE 7.
Mutation reveals active AP-1 and Nfat sites in the mRL-D5b and the T1A enhancers. A, conserved sequence across portions of the mRL-D5b and mRL-T1A enhancers. Previously described transcription factor binding sites in the mRL-D5b enhancer for RUNX2 (Runx2, gray) and cAMP response element-binding proteins (CRE1 and CRE2; gray) are shown. Putative sites for c-Fos (AP-1A and AP-1B; yellow) and Nfat (Nfat1; green) in the mRL-D5b enhancer and for c-Fos (AP-1C and AP-1D; yellow) and Nfat (Nfat2; green) in the mRL-T1A enhancer are indicated. Site-directed mutagenesis was used to create triplet changes in each of the putative sites in mRL-D5b and mRL-T1A as indicated by the arrows. B, Jurkat cells were transfected with the pTK-luc control, native, and mutant pTK-D5b and native and mutant pTK-T1A constructs as indicated, treated with either vehicle or PI for 20 h, and then evaluated for luciferase and β-galactosidase activity. Luciferase activity was then normalized to β-galactosidase activity. Because of modest suppression of the activity of the pTK-luciferase control vector by cyclosporin A and U0126, the data for the additional constructs were normalized to the pTK-luciferase control vector activity. Each value represents the average of three independent experiments ± S.E. *, p < 0.05 is statistically significant using a nonparametric t test when compared with vehicle-treated sample. #, p < 0.05 is statistically significant using a nonparametric t test when compared with pTK-mRL-D5b or -T1A wild type vector.
Both mRL-D5 and the TCCR Enhancers Are Located in Close Proximity to the Rankl Promoter
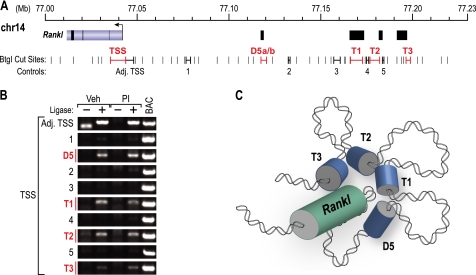
Long distance regulatory enhancers are thought to regulate their target genes via complex chromatin looping mechanisms. To determine whether looping might provide a mechanism for Rankl activation via the mRL-D5 and the TCCR regions, we treated 2b4.11 cells with either vehicle or PI and used 3C analysis to assess potential interactions between the promoter and enhancers as described under “Experimental Procedures.” The analysis was performed using the BtgI restriction enzyme as illustrated in Fig. 8A. As can be seen in Fig. 8B, BtgI-digested fragments located in or near the TSS, mRL-D5, mRL-T1, mRL-T2, and mRL-T3 regions region were all shown to undergo an enhanced frequency of ligation with a DNA fragment that spanned the Rankl promoter, suggesting that each of these segments had been linked via the initial cross-linking step. None of these interactions were sensitive to PI treatment. Control regions located between these enhancers did not show enhanced ligation. These data suggest that the mRL-D5 region and segments within the TCCR are located in close proximity to the Rankl promoter as depicted in the cartoon in Fig. 8C and are thus potentially able to regulate transcription at the TSS.
FIGURE 8.
3C analysis indicates that mRL-D5 and individual TCCR enhancers are located in proximity to the mouse Rankl TSS. 2b4.11 cells were treated for 3 h with either vehicle (Veh) or PI, and the isolated chromatin was subjected to 3C analysis as described under “Experimental Procedures.” A, the Rankl gene locus is depicted with the TSS and upstream enhancers designated in red. BtgI restriction sites are indicated in black. B, analysis of the ligation frequency of DNA fragments D5, T1, T2, and T3 as well as control segments 1–5 following treatment with either ligase or the PI inducer. C, model for upstream enhancer proximity to the Rankl TSS.
DISCUSSION
RANKL-RANK signaling has a profound effect on a variety of cell types including bone, immune, and cancer cells. Genome-wide association studies have identified Rankl as one of several primary genes associated with bone mineral density (47–50), likely for its role in promoting osteoclast differentiation, activation, and survival and enhancing bone resorption. The role of RANKL in the immune system is equally important because it modulates the activation and survival of dendritic cells and the expansion of regulatory T cells. As a result of its effects on the skeleton, RANKL is a major therapeutic target for the suppression of bone resorption, reduction in arthritic disease, and the prevention and treatment of cancer metastasis to bone (11, 13). Understanding the mechanism whereby the RANKL gene is regulated in a cell type-specific manner could therefore lead to more targeted approaches to the modulation of RANKL signaling, thus providing new therapeutic options that preserve the many beneficial effects of RANKL. The present work describes regulatory properties of the mouse Rankl gene locus using both in vitro and ex vivo T cell model systems. These studies revealed both striking similarities and distinct differences in the mechanisms and the components that regulate expression of this gene in osteoblasts and T cells.
Our previous ChIP-chip analyses of the mouse Rankl locus suggested that the long distance Rankl enhancers were routinely marked in osteoblasts by both basal and 1,25(OH)2D3-, PTH-, or cytokine-inducible levels of H4ac as well as increased RNA pol II density. We therefore utilized these markers to identify Rankl enhancers in T cells. Surprisingly, although we found strong intervals of basal H4ac and peaks of RNA pol II density across the Rankl locus, the regions that were most clearly marked in T cells were unique and located even further upstream (>123 kb) of those found in osteoblasts in a cluster that we designated the TCCR. These regions were strongly marked by focal H3K9ac as well. We also found that increased H3K9ac levels and RNA pol II recruitment were routinely associated with elevated levels of H3K4me1 (supplemental Fig. S2), an observation consistent with the emerging hypothesis that this epigenetic mark highlights enhancer domains (51–53). The linkage between both H3K9ac and H3K4me1 marks suggests that the former may be useful for identifying Rankl enhancers not only in different cell types, but in other genes as well. Nevertheless, our results support the idea that a regulatory region selective for T cells may play a dominant role in the control Rankl expression in these cells.
Both CSA and FK506 treatment block the induction of Rankl gene expression in T cells, indicating that the calcineurin activation pathway and its downstream transcription factor targets may participate in activation-induced Rankl expression (1, 39). We confirmed this finding and also observed that blockade of the MAPK/ERK1/2 pathway through inhibition of MEK1/2 by U0126 was also inhibitory to activation-induced Rankl expression. Our studies also demonstrate that c-Fos, a downstream effector of MEK1/2 activation, was recruited to several of the important Rankl enhancers including those identified through histone acetylation and RNA pol II recruitment profiling. Key AP-1 sites within both the mRL-D5 and the mRL-T1A regions were identified that represent functional binding sites for the c-Fos protein. We also confirmed by ChIP-chip analysis the presence of NF-κB binding at the Rankl promoter, as was previously described using more traditional methods (36). Interestingly, mutation of a putative Nfat binding site also abrogated mRL-T1A but not mRL-D5b reporter activity, suggesting that this regulatory protein, or perhaps an unidentified transcription factor, may also modulate Rankl expression.
The presence of a spliced transcript produced within the TCCR raises a question regarding the function of the TCCR and whether it is actually linked to activation-induced Rankl expression. Although it is possible that a unique promoter within the TCCR controls the expression of an unannotated gene in this region and that this promoter produces the uncharacterized transcript, it is clear that this RNA as well as RNAs from several genes within 1 megabase or more surrounding the Rankl gene are not regulated by T cell activation. This observation suggests that the increased epigenetic and transcription factor binding activities seen within the TCCR, coupled to our observation that the latter region lies in close proximity to the Rankl TSS, likely contribute to Rankl up-regulation during T cell activation.
Significant similarities were observed at the Rankl locus in both osteoblasts and T cells. Both cell types displayed elevated peaks of histone acetylation and RNA pol II density across the Rankl locus, likely marking the distal enhancers that are involved in controlling transcription. The relative levels of these epigenetic marks together with sites of increased RNA pol II activity are difficult to compare, however, because the state of activation and the levels of basal Rankl mRNA expression are largely different between the two cell types. Interestingly, the mRL-D5 enhancer is the only upstream region similarly marked in both cells types, suggesting that it may play an important role in controlling Rankl gene expression in both osteoblasts and T cells. This hypothesis is supported by the phenotype of the mRL-D5 knock-out mouse, which exhibits decreased levels of Rankl expression not only in bone cells, but in lymphoid tissues such as spleen and thymus as well. These findings suggest that mRL-D5 may participate in regulating the levels of basal Rankl transcription in both osteoblasts and T cells.
The approach we have taken also highlights striking differences, not only in the location of distal enhancer regions within the Rankl gene locus but also at the TSS of the gene. For example, although elevated H4ac levels were observed at the TSS in both cell types, increased RNA pol II density was apparent at this site only in T cells and never in osteoblasts. Additional activities were also restricted to the Rankl TSS in T cells, including elevated levels of both H4ac and H3K9ac. These findings suggest that the Rankl TSS may play a unique regulatory role in T cells as compared with that in the osteoblast. Perhaps the most striking difference was the restricted use of the upstream TCCR. These data, combined with similarities identified at mRL-D5 in both osteoblasts and T cells, suggests two categories of enhancers. The first is comprised of a common or “master” enhancer such as mRL-D5 that facilitates cell type-independent transcription. The second is comprised of cell type-specific or lineage-specific enhancers, including the Rankl proximal promoter, perhaps other proximal enhancers (mRL-D2) and the TCCR cluster that are involved in the cell-selective transcriptional regulation. Thus, in additional to cell type-specific transcription factor usage, combinatorial enhancer usage may also occur for genes as well. Combinatorial usage might be particularly useful in genes that express regulatory factors that are biologically important in a variety of related cells types or that are involved in temporal or site-specific activities such as those that occur at the skeleton.
The present studies define a novel mechanism whereby Rankl is expressed in T cells. Using ChIP-chip analysis of specific epigenetic marks, we identified a common osteoblast enhancer and a new set of enhancers unique to T cells that mediate gene activation via c-Fos and NF-κB. These studies suggest a potential cell type-specific mechanism through which the expression of Rankl is controlled in T cells that may provide new avenues for therapeutic modulation of Rankl gene expression in disease.
Supplementary Material
Acknowledgments
We thank members of the Gumperz and Hayes Lab for advice and expertise in developing T cell models systems and activation conditions, specifically Xiaohua Wang, Jenny Gumperz, Chris Mayne, Justin Spanier, and Faye Nashold. Discussions with Pike laboratory members are also gratefully acknowledged. We also thank Laura Vanderploeg for the contribution to the figures documented in this study.
This work was supported, in whole or in part, by National Institutes of Health Grant DK-74993 (to J. W. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- RANK
- receptor activator of NF-κB
- RANKL
- RANK ligand
- TSS
- transcriptional start site
- mRL
- mouse Rankl
- PTH
- parathyroid hormone
- H4ac
- histone H4 acetylation
- pol
- polymerase
- TCCR
- T cell control region
- PMA
- phorbol 12-myristate 13-acetate
- PI
- PMA/ionomycin
- H3K9ac
- histone H3 lysine 9 acetylation
- RNA-Seq
- RNA sequencing
- CSA
- cyclosporin A
- H3K4me1
- histone H3 lysine 4 monomethylation
- H3K4me3
- histone H3 lysine 4 trimethylation
- 3C
- chromosome conformation capture.
REFERENCES
- 1. Wong B. R., Rho J., Arron J., Robinson E., Orlinick J., Chao M., Kalachikov S., Cayani E., Bartlett F. S., 3rd, Frankel W. N., Lee S. Y., Choi Y. (1997) J. Biol. Chem. 272, 25190–25194 [DOI] [PubMed] [Google Scholar]
- 2. Anderson D. M., Maraskovsky E., Billingsley W. L., Dougall W. C., Tometsko M. E., Roux E. R., Teepe M. C., DuBose R. F., Cosman D., Galibert L. (1997) Nature 390, 175–179 [DOI] [PubMed] [Google Scholar]
- 3. Fata J. E., Kong Y. Y., Li J., Sasaki T., Irie-Sasaki J., Moorehead R. A., Elliott R., Scully S., Voura E. B., Lacey D. L., Boyle W. J., Khokha R., Penninger J. M. (2000) Cell 103, 41–50 [DOI] [PubMed] [Google Scholar]
- 4. Loser K., Mehling A., Loeser S., Apelt J., Kuhn A., Grabbe S., Schwarz T., Penninger J. M., Beissert S. (2006) Nat. Med. 12, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 5. Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 6. Rossi S. W., Kim M. Y., Leibbrandt A., Parnell S. M., Jenkinson W. E., Glanville S. H., McConnell F. M., Scott H. S., Penninger J. M., Jenkinson E. J., Lane P. J., Anderson G. (2007) J. Exp. Med. 204, 1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Cell 93, 165–176 [DOI] [PubMed] [Google Scholar]
- 8. Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong B. R., Josien R., Lee S. Y., Sauter B., Li H. L., Steinman R. M., Choi Y. (1997) J. Exp. Med. 186, 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghoreishi M., Bach P., Obst J., Komba M., Fleet J. C., Dutz J. P. (2009) J. Immunol. 182, 6071–6078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverman S. L. (2009) Curr. Osteoporos. Rep. 7, 91–95 [DOI] [PubMed] [Google Scholar]
- 12. Nakashima T., Wada T., Penninger J. M. (2003) Curr. Opin. Rheumatol. 15, 280–287 [DOI] [PubMed] [Google Scholar]
- 13. Burkiewicz J. S., Scarpace S. L., Bruce S. P. (2009) Ann. Pharmacother. 43, 1445–1455 [DOI] [PubMed] [Google Scholar]
- 14. Choi Y., Woo K. M., Ko S. H., Lee Y. J., Park S. J., Kim H. M., Kwon B. S. (2001) Eur. J. Immunol. 31, 2179–2188 [DOI] [PubMed] [Google Scholar]
- 15. Croucher P. I., Shipman C. M., Lippitt J., Perry M., Asosingh K., Hijzen A., Brabbs A. C., van Beek E. J., Holen I., Skerry T. M., Dunstan C. R., Russell G. R., Van Camp B., Vanderkerken K. (2001) Blood 98, 3534–3540 [DOI] [PubMed] [Google Scholar]
- 16. Hanada R., Leibbrandt A., Hanada T., Kitaoka S., Furuyashiki T., Fujihara H., Trichereau J., Paolino M., Qadri F., Plehm R., Klaere S., Komnenovic V., Mimata H., Yoshimatsu H., Takahashi N., von Haeseler A., Bader M., Kilic S. S., Ueta Y., Pifl C., Narumiya S., Penninger J. M. (2009) Nature 462, 505–509 [DOI] [PubMed] [Google Scholar]
- 17. Leibbrandt A., Penninger J. M. (2008) Ann. N.Y. Acad. Sci. 1143, 123–150 [DOI] [PubMed] [Google Scholar]
- 18. Cenci S., Weitzmann M. N., Roggia C., Namba N., Novack D., Woodring J., Pacifici R. (2000) J. Clin. Invest. 106, 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan W., Zhang W., Strasner A., Grivennikov S., Cheng J. Q., Hoffman R. M., Karin M. (2011) Nature 470, 548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim N., Odgren P. R., Kim D. K., Marks S. C., Jr., Choi Y. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 10905–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bishop K. A., Meyer M. B., Pike J. W. (2009) Mol. Endocrinol. 23, 2095–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu Q., Manolagas S. C., O'Brien C. A. (2006) Mol. Cell. Biol. 26, 6453–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim S., Yamazaki M., Shevde N. K., Pike J. W. (2007) Mol. Endocrinol. 21, 197–214 [DOI] [PubMed] [Google Scholar]
- 24. Kim S., Yamazaki M., Zella L. A., Shevde N. K., Pike J. W. (2006) Mol. Cell. Biol. 26, 6469–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Brien C. A. (2010) Bone 46, 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martowicz M. L., Meyer M. B., Pike J. W. (2011) J. Cell. Biochem., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galli C., Zella L. A., Fretz J. A., Fu Q., Pike J. W., Weinstein R. S., Manolagas S. C., O'Brien C. A. (2008) Endocrinology 149, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langmead B., Trapnell C., Pop M., Salzberg S. L. (2009) Genome. Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trapnell C., Pachter L., Salzberg S. L. (2009) Bioinformatics 25, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim S., Shevde N. K., Pike J. W. (2005) J. Bone Miner. Res. 20, 305–317 [DOI] [PubMed] [Google Scholar]
- 31. Yamamoto H., Shevde N. K., Warrier A., Plum L. A., DeLuca H. F., Pike J. W. (2003) J. Biol. Chem. 278, 31756–31765 [DOI] [PubMed] [Google Scholar]
- 32. Nerenz R. D., Martowicz M. L., Pike J. W. (2008) Mol. Endocrinol. 22, 1044–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kabotyanski E. B., Rijnkels M., Freeman-Zadrowski C., Buser A. C., Edwards D. P., Rosen J. M. (2009) J. Biol. Chem. 284, 22815–22824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vakoc C. R., Letting D. L., Gheldof N., Sawado T., Bender M. A., Groudine M., Weiss M. J., Dekker J., Blobel G. A. (2005) Mol. Cell. 17, 453–462 [DOI] [PubMed] [Google Scholar]
- 35. Meyer M. B., Goetsch P. D., Pike J. W. (2010) J. Biol. Chem. 285, 15599–15610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fionda C., Nappi F., Piccoli M., Frati L., Santoni A., Cippitelli M. (2007) J. Immunol. 178, 4039–4050 [DOI] [PubMed] [Google Scholar]
- 37. Josien R., Wong B. R., Li H. L., Steinman R. M., Choi Y. (1999) J. Immunol. 162, 2562–2568 [PubMed] [Google Scholar]
- 38. Kim T. K., Hemberg M., Gray J. M., Costa A. M., Bear D. M., Wu J., Harmin D. A., Laptewicz M., Barbara-Haley K., Kuersten S., Markenscoff-Papadimitriou E., Kuhl D., Bito H., Worley P. F., Kreiman G., Greenberg M. E. (2010) Nature 465, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang R., Zhang L., Zhang X., Moreno J., Celluzzi C., Tondravi M., Shi Y. (2002) Eur. J. Immunol. 32, 1090–1098 [DOI] [PubMed] [Google Scholar]
- 40. Liu J. O. (2009) Immunol. Rev. 228, 184–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsubaki M., Kato C., Manno M., Ogaki M., Satou T., Itoh T., Kusunoki T., Tanimori Y., Fujiwara K., Matsuoka H., Nishida S. (2007) Mol. Cell. Biochem. 304, 53–60 [DOI] [PubMed] [Google Scholar]
- 42. Crispín J. C., Tsokos G. C. (2009) Autoimmun. Rev. 8, 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim H. P., Imbert J., Leonard W. J. (2006) Cytokine Growth Factor Rev. 17, 349–366 [DOI] [PubMed] [Google Scholar]
- 44. Quandt K., Frech K., Karas H., Wingender E., Werner T. (1995) Nucleic Acids Res. 23, 4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. (2005) Bioinformatics 21, 2933–2942 [DOI] [PubMed] [Google Scholar]
- 46. Boise L. H., Petryniak B., Mao X., June C. H., Wang C. Y., Lindsten T., Bravo R., Kovary K., Leiden J. M., Thompson C. B. (1993) Mol. Cell. Biol. 13, 1911–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rivadeneira F., Styrkársdottir U., Estrada K., Halldórsson B. V., Hsu Y. H., Richards J. B., Zillikens M. C., Kavvoura F. K., Amin N., Aulchenko Y. S., Cupples L. A., Deloukas P., Demissie S., Grundberg E., Hofman A., Kong A., Karasik D., van Meurs J. B., Oostra B., Pastinen T., Pols H. A., Sigurdsson G., Soranzo N., Thorleifsson G., Thorsteinsdottir U., Williams F. M., Wilson S. G., Zhou Y., Ralston S. H., van Duijn C. M., Spector T., Kiel D. P., Stefansson K., Ioannidis J. P., Uitterlinden A. G. (2009) Nat. Genet. 41, 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roshandel D., Holliday K. L., Pye S. R., Boonen S., Borghs H., Vanderschueren D., Huhtaniemi I. T., Adams J. E., Ward K. A., Bartfai G., Casanueva F., Finn J. D., Forti G., Giwercman A., Han T. S., Kula K., Lean M. E., Pendleton N., Punab M., Silman A. J., Wu F. C., Thomson W., O'Neill T. W. (2010) J. Bone Miner. Res. 25, 1830–1838 [DOI] [PubMed] [Google Scholar]
- 49. Styrkarsdottir U., Halldorsson B. V., Gretarsdottir S., Gudbjartsson D. F., Walters G. B., Ingvarsson T., Jonsdottir T., Saemundsdottir J., Center J. R., Nguyen T. V., Bagger Y., Gulcher J. R., Eisman J. A., Christiansen C., Sigurdsson G., Kong A., Thorsteinsdottir U., Stefansson K. (2008) N. Engl. J. Med. 358, 2355–2365 [DOI] [PubMed] [Google Scholar]
- 50. Styrkarsdottir U., Halldorsson B. V., Gretarsdottir S., Gudbjartsson D. F., Walters G. B., Ingvarsson T., Jonsdottir T., Saemundsdottir J., Snorradóttir S., Center J. R., Nguyen T. V., Alexandersen P., Gulcher J. R., Eisman J. A., Christiansen C., Sigurdsson G., Kong A., Thorsteinsdottir U., Stefansson K. (2009) Nat. Genet. 41, 15–17 [DOI] [PubMed] [Google Scholar]
- 51. Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., Hawkins R. D., Barrera L. O., Van Calcar S., Qu C., Ching K. A., Wang W., Weng Z., Green R. D., Crawford G. E., Ren B. (2007) Nat. Genet. 39, 311–318 [DOI] [PubMed] [Google Scholar]
- 52. Wang Z., Zang C., Rosenfeld J. A., Schones D. E., Barski A., Cuddapah S., Cui K., Roh T. Y., Peng W., Zhang M. Q., Zhao K. (2008) Nat. Genet. 40, 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.