Abstract
Background
The role of poor diet quality in the rising incidence of colorectal cancer (CRC) diagnosed younger than age 50 years has not been explored. Based on molecular features of early-onset CRC, early-onset adenomas are emerging surrogate endpoints.
Methods
In a prospective cohort study (Nurses’ Health Study II), we evaluated 2 empirical dietary patterns (Western and prudent) and 3 recommendation-based indexes (Dietary Approaches to Stop Hypertension [DASH], Alternative Mediterranean Diet [AMED], and Alternative Healthy Eating Index [AHEI]-2010) with risk of early-onset adenoma overall and by malignant potential (high-risk: ≥1 cm, tubulovillous or villous histology, high-grade dysplasia, or ≥3 adenomas), among 29 474 women with 1 or more lower endoscopy before age 50 years (1991–2011). Multivariable logistic regressions were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs).
Results
We documented 1157 early-onset adenomas with 375 at high risk. Western diet was positively associated, whereas prudent diet, DASH, AMED, and AHEI-2010 were inversely associated with risk of early-onset adenoma. The associations were largely confined to high-risk adenomas (the highest vs lowest quintile: Western, OR = 1.67, 95% CI = 1.18 to 2.37; prudent, OR = 0.69, 95% CI = 0.48 to 0.98; DASH, OR = 0.65, 95% CI = 0.45 to 0.93; AMED, OR = 0.55, 95% CI = 0.38 to 0.79; AHEI-2010, OR = 0.71, 95% CI = 0.51 to 1.01; all Ptrend ≤ .03), driven by those identified in the distal colon and rectum (all Ptrend ≤ .04, except AMED: Ptrend = .14).
Conclusion
Poor diet quality was associated with an increased risk of early-onset distal and rectal adenomas of high malignant potential. These findings provide preliminary but strong support to the role of diet in early-onset CRC.
Despite falling sharply or leveling off in older adults (1), colorectal cancer (CRC) has increased among young adults aged younger than 50 years (early-onset) in 9 high-income countries over the past 2 decades, including the United States. Largely driven by the rise in distal colon and rectal tumors (1,2), early-onset CRCs are diagnosed at more advanced stages with more aggressive clinicopathological characteristics compared with CRC diagnosed at older ages (3). In contrast to the vast accumulated evidence on the etiopathogenesis of older-onset CRC (4), risk factors for early-onset CRC remain largely unknown.
Poor diet quality has been linked to elevated risk of older-onset CRC (5-8). Increasing evidence points to hyperinsulinemia (9), chronic inflammation (10), and gut dysbiosis (11-13) as the plausible mechanisms linking diet and CRC. Prior evidence on obesity and sedentary behaviors has indirectly implicated the role of lifestyle factors, likely tied to unhealthy diet, in the etiology of early-onset CRC (14,15). In the United States, diet quality declined steadily between 1985 and 2006 (16) and has remained stable thereafter. Further, diet quality among younger adults is consistently poorer compared with the older population (17). Based on data from the National Health and Nutrition Examination Survey 1999-2016, the estimated overall diet quality of younger individuals in the United States showed modest improvement, but more than half of youth still had poor-quality diets (18). Yet, the health impact of poorer diet among the younger population, including its role in early-onset colorectal neoplasia, has not been well examined, in part because of the lack of cohort studies that followed younger adults for an extensive period of time with validated dietary assessment.
The majority (approximately 80%) of early-onset CRCs exhibits microsatellite stable (MSS) phenotypes (19,20), leading to the postulation that adenomas but not serrated polyps are the precursors of early-onset CRC. In a recent analysis from the Genetics and Epidemiology of Colorectal Cancer Consortium (21), compared with CRC diagnosed after age 65 years, early-onset CRC was more likely to present with a molecular subtype (MSS or microsatellite instability low, non-CpG island methylator phenotype, BRAF and KRAS wild type) arising from the adenoma-carcinoma sequence (22). These findings lend additional support to the etiological relevance of adenomas, particularly those of high-malignant potential (23), in understanding the etiology of early-onset CRC.
We therefore conducted a comprehensive analysis in the Nurses’ Health Study II (NHSII) to elucidate the role of diet quality, as measured by empirical dietary patterns as well as recommendation-based indexes, in early-onset CRC using early-onset adenoma and that of high-malignant potential as surrogate endpoints. The NHSII, a well-established, large, ongoing prospective cohort of young women with detailed documentation of endoscopic history and indications and family history, as well as validated assessment of dietary intake and lifestyle factors, provides a unique opportunity to address these knowledge gaps.
Methods
Study Population
The NHSII is a prospective cohort study of 116 430 US female nurses aged 25 to 42 years at enrollment in 1989. Participants were followed biennially with self-administered questionnaires on demographics, lifestyle factors, and medical diagnoses. Dietary intake was assessed every 4 years through mailed food frequency questionnaires (FFQs). Return of the completed questionnaire implied informed consent to participate in the study. Overall, the active follow-up rate was approximately 90% (24).
In the current analysis, study baseline was set as 1991, the time of initial FFQ assessment. We excluded participants who had diagnoses of CRC, inflammatory bowel disease, or a previous history of colorectal polyps prior to baseline and each biennial follow-up cycle. After additional exclusions were made for those who had missing data on any of the exposures or reported implausible energy intake (<600 or >3500 kcal/d), 59 013 participants were identified to have undergone at least 1 lower endoscopy before 2011 (the end of follow-up). We further restricted to 29 474 women younger than age 50 years for our primary analyses (Supplementary Figure 1, available online). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating state cancer registries as required.
Ascertainment of Colorectal Adenoma
On each biennial questionnaire, participants reported whether they had undergone lower endoscopy and the corresponding reason(s). Investigators masked to exposure information reviewed all the retrieved medical records and extracted data on anatomical location, size, histological type, and number of polyps. If more than 1 adenoma was diagnosed, size and histology were categorized by the largest and most advanced polyp, respectively. Cases and noncases were defined every 2 years and updated through the 2011 questionnaire cycle: all confirmed newly diagnosed adenomas (tubular, villous, tubulovillous, or with high-grade dysplasia) were considered as cases and individuals who had a lower endoscopy but reported no adenomas as noncases.
We further categorized adenomas according to their malignant potential (25). High-risk adenomas were defined as adenomas with any of the following features: 1 cm or more in size, tubulovillous or villous histology, high-grade dysplasia, and the presence of 3 or more adenomas. Low-risk adenomas included all other adenomas. We defined advanced adenomas considering only size and histology (26). Adenomas in the cecum, ascending colon, hepatic flexure, and transverse colon were classified as proximal adenomas; those in splenic flexure, descending colon, and sigmoid colon as distal colonic adenomas; and those in the rectum or rectosigmoid junction as rectal adenomas, respectively (27).
Assessment of Diet Quality
Every 4 years since 1991, participants self-reported average food intake over the preceding year via validated semiquantitative FFQs (28). Briefly, to capture food consumption frequency, 9 response options were provided, ranging from “never or less than once per month (referred to never)” to “6 or more times per day”. Total nutrient intake was calculated as the sum of consumption frequency of each food item multiplied by the corresponding nutrient composition in the standard portion size.
Food items on the FFQ were categorized into 40 groups, and factor analysis was performed to derive 2 dominant dietary patterns: Western and prudent diet (29) for which the reproducibility and validity have been documented (30).
To capture the adherence to major dietary recommendations, we derived Dietary Approaches to Stop Hypertension (DASH) (31), Alternative Mediterranean Diet (AMED) (32), and Alternative Healthy Eating Index-2010 (AHEI-2010) (33). The DASH score consisted of 8 components and ranged from 8 to 40. The AMED score consisted of 9 components and ranged from 0 to 9. The AHEI-2010 score consisted of 11 items and ranged from 0 to 110. Scoring methods and dietary components are provided in Supplementary Table 1 (available online). For the 3 indexes, a higher score reflects higher diet quality.
Statistical Analysis
We calculated the cumulative average of all dietary scores available from 1991 to the questionnaire cycle (2 years) prior to the most recent endoscopy to represent long-term intake reflecting true changes and reduce random within-person variation by increasing the number of measurements (34). As primary analyses, we first investigated the associations between diet quality (Western and prudent patterns; DASH, AMED, and AHEI-2010 scores, all in period-specific quintiles) and risk of early-onset adenoma overall and according to high-risk vs low-risk adenoma. The associations between each of the dietary indexes and early-onset adenoma were evaluated in different models in the entire study population. As secondary analyses, we further examined the associations by anatomical location, size, and histology. We evaluated the associations according to malignant potential in 2 logistic regressions using the same reference group: 1 for high-risk vs no adenoma, and the other for low-risk vs no adenoma, and similarly for comparisons according to size and histology. Joint association of Western and prudent dietary patterns with risk of early-onset high-risk adenoma was further tested to take into account the combination of 2 distinct dietary patterns. Because some of these early-onset adenomas will be first captured through average-risk screening if they have not had an endoscopy at younger ages (35,36), we performed sensitivity analyses stratified by age of endoscopy (younger than 45 years vs 45 years and older). Also, we conducted a sensitivity analysis among only those who had a colonoscopy to address the likelihood of proximal adenoma not being detected if participants only had a sigmoidoscopy. To replace missing data for exposure in the subsequent cycles, we carried forward nonmissing dietary intake values from the prior questionnaire cycle. Missing data for covariates were treated similarly.
Similar to prior work (27,37-39), we identified the case-control sets every 2 years among participants with a lower endoscopy during the same period. Once a participant was diagnosed with an adenoma, she was censored in all subsequent follow-up cycles (27). To account for the possibility that an individual may have undergone multiple endoscopies over the study period and to handle time-varying exposure and covariates efficiently, we constructed a new record for each 2-year follow-up period during which a participant underwent a lower endoscopy, using Andersen-Gill data structure. Age-adjusted and multivariable logistic regressions for clustered data (PROC GENMOD) were used to account for repeated observations and estimate odds ratios (ORs) and 95% confidence intervals (CIs). Tests for trend were conducted using the median of each quintile of dietary patterns and scores as a continuous variable.
In age-adjusted models, we controlled for age, total caloric intake, time period of endoscopy, number of reported endoscopies, time in years since the most recent endoscopy, and reason for the current endoscopy. In multivariable models, we additionally adjusted for the following potential confounders: height (40), body mass index (41), history of CRC in a first-degree relative (42), menopausal status (43), menopausal hormone use (44), personal history of type 2 diabetes (45), pack-years of smoking (46), physical activity in metabolic equivalent of task-hours (47), current use of multivitamin (48), and regular use (≥2 times per week) of aspirin (49) or nonsteroidal anti-inflammatory drugs (50). For the DASH diet, we additionally adjusted for alcohol intake. All analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC). Two-sided P values less than .05 were considered statistically significant.
Results
Among 29 474 women who reported a lower endoscopy between 1991 and 2011 when they were younger than age 50 years, those with a higher Western dietary pattern score were more likely to have higher pack-years of smoking and less likely to exercise or use multivitamins (Table 1). In contrast, participants with greater adherence to the prudent dietary pattern and DASH, AMED, and AHEI-2010 indexes tended to engage in healthier behaviors.
Table 1.
Characteristics of participants who had undergone lower endoscopy before age 50 years according to period-specific quintiles of diet quality scores, Nurses’ Health Study II, 1991-2011a
| Characteristic | Western dietary pattern |
Prudent dietary pattern |
Dietary Approaches to Stop Hypertension |
Alternative Mediterranean Diet |
Alternative Healthy Eating Index-2010 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Age, yb | 45.1 (4.5) | 45.0 (4.4) | 44.9 (4.5) | 44.6 (4.6) | 45.1 (4.4) | 45.2 (4.3) | 44.8 (4.6) | 45.0 (4.4) | 45.2 (4.4) | 44.7 (4.6) | 45.0 (4.5) | 45.1 (4.5) | 44.7 (4.6) | 44.9 (4.5) | 45.3 (4.3) |
| Height, cm | 165 (6.7) | 165 (6.6) | 165 (6.7) | 165 (6.7) | 165 (6.6) | 165 (6.8) | 165 (6.8) | 165 (6.6) | 165 (6.7) | 165 (6.7) | 165 (6.8) | 165 (6.6) | 165 (6.7) | 165 (6.7) | 165 (6.6) |
| BMI, kg/m² | 24.0 (4.4) | 25.2 (5.2) | 27.1 (6.3) | 26.0 (6.1) | 25.2 (5.3) | 24.9 (5.0) | 26.3 (6.0) | 25.4 (5.4) | 24.3 (4.7) | 26.2 (5.8) | 25.5 (5.4) | 24.3 (4.7) | 26.3 (6.1) | 25.4 (5.3) | 24.1 (4.4) |
| Family history of colorectal cancer, % | 17 | 17 | 16 | 15 | 17 | 17 | 15 | 16 | 18 | 16 | 17 | 17 | 16 | 16 | 17 |
| History of diabetes, % | 1.4 | 2.7 | 4.3 | 3.0 | 2.7 | 2.5 | 3.3 | 2.9 | 1.9 | 3.0 | 2.9 | 2.1 | 3.4 | 2.9 | 1.7 |
| Time period of endoscopy after 2001, % | 49 | 52 | 55 | 59 | 50 | 47 | 56 | 51 | 48 | 56 | 51 | 47 | 57 | 52 | 46 |
| No. of previous lower endoscopies | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.5 (0.9) | 1.6 (1.0) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 1.6 (0.9) |
| Time since the most recent lower endoscopy, y | 3.4 (2.7) | 3.4 (2.8) | 3.5 (2.8) | 3.6 (3.0) | 3.5 (2.9) | 3.3 (2.6) | 3.6 (3.0) | 3.5 (2.9) | 3.4 (2.6) | 3.6 (3.0) | 3.4 (2.8) | 3.4 (2.7) | 3.6 (2.9) | 3.4 (2.8) | 3.3 (2.7) |
| Reasons for endoscopy | |||||||||||||||
| Screening, % | 55 | 51 | 46 | 46 | 51 | 55 | 46 | 52 | 55 | 47 | 50 | 54 | 45 | 51 | 55 |
| Symptoms, % | 43 | 47 | 52 | 52 | 47 | 43 | 52 | 46 | 44 | 50 | 48 | 44 | 53 | 47 | 43 |
| Missing, % | 2.0 | 2.3 | 2.0 | 2.1 | 2.1 | 2.0 | 2.2 | 2.1 | 1.9 | 2.3 | 1.9 | 1.9 | 2.0 | 2.2 | 1.8 |
| Current use of multivitamin, % | 62 | 54 | 46 | 48 | 56 | 59 | 44 | 56 | 62 | 47 | 55 | 61 | 49 | 55 | 59 |
| Regular use of aspirin, %c | 11 | 12 | 14 | 12 | 12 | 12 | 12 | 12 | 11 | 12 | 12 | 12 | 13 | 12 | 12 |
| Regular use of nonaspirin NSAIDs, %c | 27 | 33 | 36 | 35 | 33 | 30 | 34 | 34 | 29 | 34 | 32 | 30 | 35 | 33 | 29 |
| Postmenopausal, % | 16 | 17 | 19 | 19 | 17 | 17 | 19 | 17 | 16 | 19 | 17 | 16 | 18 | 17 | 16 |
| Current menopausal hormone therapy, % | 60 | 57 | 62 | 59 | 61 | 60 | 60 | 61 | 59 | 60 | 61 | 60 | 60 | 61 | 60 |
| Physical activity, MET-h/wk | 31.8 (30.6) | 20.4 (19.8) | 15.9 (17.5) | 15.5 (17.2) | 21.4 (22.1) | 32.0 (30.0) | 15.5 (17.5) | 21.0 (21.4) | 31.0 (28.5) | 16.1 (18.1) | 21.2 (21.6) | 29.8 (28.5) | 15.3 (16.2) | 21.1 (20.7) | 31.9 (30) |
| Ever smokers, % | 34 | 32 | 35 | 30 | 33 | 38 | 36 | 32 | 32 | 33 | 32 | 35 | 29 | 33 | 39 |
| Pack-years among ever smokers | 10.3 (7.8) | 11.8 (9.3) | 15.1 (11.3) | 14.5 (11.4) | 11.7 (9.2) | 11.4 (8.3) | 15.3 (11.5) | 11.5 (9.0) | 10.4 (8.0) | 14.8 (11.2) | 11.7 (9.1) | 10.6 (8.0) | 14.7 (11.4) | 11.7 (9.2) | 10.8 (8.1) |
| Dietary intake | |||||||||||||||
| Calories, kcal/d | 1937 (464) | 1730 (474) | 1951 (520) | 1938 (520) | 1737 (469) | 1929 (473) | 1619 (463) | 1835 (470) | 2070 (451) | 1578 (434) | 1826 (463) | 2124 (465) | 1970 (488) | 1798 (490) | 1743 (467) |
| Red and processed meat, svg/wk | 3.7 (2.8) | 5.8 (3.1) | 9.4 (4.4) | 8.6 (4.3) | 5.8 (3.4) | 4.4 (3.3) | 7.7 (4.0) | 6.4 (3.7) | 4.2 (3.2) | 7.1 (3.7) | 6.3 (3.9) | 5.1 (3.9) | 9.1 (4.1) | 6.1 (3.2) | 3.1 (2.4) |
| Fruit, svg/d | 2.0 (1.1) | 1.2 (0.7) | 0.8 (0.6) | 0.8 (0.6) | 1.2 (0.6) | 2.0 (1.1) | 0.6 (0.4) | 1.2 (0.6) | 2.1 (1.0) | 0.7 (0.5) | 1.2 (0.7) | 1.9 (1.0) | 0.8 (0.6) | 1.2 (0.7) | 1.9 (1.1) |
| Vegetable, svg/d | 4.7 (2.5) | 3.3 (1.6) | 2.9 (1.5) | 2.1 (1.0) | 3.2 (1.1) | 6.0 (2.4) | 2.2 (1.1) | 3.4 (1.5) | 5.2 (2.3) | 2.1 (1.0) | 3.5 (1.6) | 5.2 (2.2) | 2.5 (1.2) | 3.5 (1.7) | 4.8 (2.4) |
| Alcohol, g/d | 4.5 (6.6) | 3.5 (5.5) | 2.8 (5.3) | 2.5 (5.3) | 3.6 (5.5) | 4.6 (6.5) | 3.1 (5.8) | 3.7 (5.9) | 3.9 (5.7) | 2.5 (5.8) | 3.6 (5.9) | 5.0 (5.8) | 2.3 (6.2) | 3.7 (5.8) | 5.0 (5.4) |
| Total folate, μg/d | 630 (235) | 526 (222) | 428 (191) | 426 (195) | 534 (226) | 625 (231) | 426 (215) | 537 (227) | 621 (223) | 453 (230) | 534 (231) | 590 (217) | 441 (201) | 526 (222) | 611 (244) |
| Total calcium, mg/d | 1322 (422) | 1128 (398) | 911 (327) | 959 (359) | 1140 (410) | 1230 (423) | 920 (387) | 1134 (398) | 1293 (383) | 1048 (437) | 1121 (408) | 1157 (380) | 989 (365) | 1125 (413) | 1235 (438) |
| Total vitamin D, IU/d | 489 (223) | 404 (216) | 304 (181) | 325 (186) | 411 (219) | 451 (225) | 319 (214) | 411 (219) | 467 (207) | 362 (227) | 405 (225) | 427 (199) | 332 (185) | 401 (211) | 468 (238) |
| Dietary fiber, g/d | 23.7 (5.9) | 18.7 (4.0) | 15.9 (3.3) | 14.6 (2.9) | 19.1 (3.4) | 24.8 (5.3) | 14.8 (3.1) | 19.0 (3.8) | 24.2 (5.3) | 15.0 (3.5) | 19.2 (4.3) | 23.4 (5.2) | 14.9 (3.0) | 19.0 (3.6) | 24.6 (5.7) |
29 474 women with a total of 43 961 observations. BMI = body mass index; MET = metabolic equivalent of task; NSAID = nonsteroidal anti-inflammatory drug; Q1 = lowest quintile; Q3 = middle quintile; Q5 = highest quintile; svg = serving.
All values other than age have been directly standardized to age distribution (in 5-year age group) of all the participants. Mean (SD) was presented for continuous variables.
Regular use was defined as 2 or more times per week.
We documented 1157 cases of early-onset adenomas from 1991 to 2011. Compared with those in the lowest quintile of Western dietary pattern, individuals in the highest quintile had an increased risk of early-onset adenoma, after adjusting for a list of putative CRC risk factors (multivariable ORQ5 vs Q1 = 1.38, 95% CI = 1.13 to 1.68, Ptrend = .003; Table 2). In contrast, a higher prudent pattern score was associated with a lower risk of early-onset adenoma (ORQ5 vs Q1 = 0.81, 95% CI = 0.66 to 0.99, Ptrend = .03). For the same comparison, there were also suggestions of inverse associations between adherence to the DASH (ORQ5 vs Q1 = 0.84, 95% CI = 0.69 to 1.04, Ptrend = .04), AMED (ORQ5 vs Q1 = 0.80, 95% CI = 0.65 to 0.99, Ptrend = .07), and AHEI-2010 (ORQ5 vs Q1 = 0.85, 95% CI = 0.69 to 1.04, Ptrend = .11) and risk of early-onset adenoma. In a sensitivity analysis among only those who had a colonoscopy, effect estimates were slightly attenuated, but the overall direction of association was consistent (Supplementary Table 2, available online).
Table 2.
Diet quality and risk of early-onset (aged younger than 50 years) adenoma, NHSII, 1991-2011a
| Diet quality | Quintile |
P trend d | |||||
|---|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | |||
| Dietary pattern | |||||||
| Western dietary pattern | |||||||
| No. of cases | 183 | 213 | 223 | 238 | 300 | ||
| Unadjusted OR (95% CI) | 1 (Referent) | 1.14 (0.93 to 1.39) | 1.12 (0.92 to 1.37) | 1.18 (0.97 to 1.43) | 1.41 (1.17 to 1.70) | .001 | |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.13 (0.92 to 1.38) | 1.11 (0.91 to 1.36) | 1.18 (0.96 to 1.43) | 1.42 (1.17 to 1.71) | .001 | |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.11 (0.91 to 1.36) | 1.10 (0.89 to 1.35) | 1.16 (0.94 to 1.42) | 1.38 (1.13 to 1.68) | .003 | |
| Prudent dietary pattern | |||||||
| No. of cases | 297 | 263 | 214 | 201 | 182 | ||
| Unadjusted OR (95% CI) | 1 (Referent) | 0.99 (0.83 to 1.17) | 0.86 (0.72 to 1.03) | 0.85 (0.71 to 1.02) | 0.81 (0.67 to 0.97) | .01 | |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.97 (0.81 to 1.15) | 0.83 (0.69 to 1.00) | 0.82 (0.68 to 0.99) | 0.79 (0.65 to 0.95) | .007 | |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.97 (0.82 to 1.15) | 0.84 (0.70 to 1.02) | 0.84 (0.69 to 1.02) | 0.81 (0.66 to 0.99) | .03 | |
| Recommendation-based dietary index | |||||||
| Dietary Approaches to Stop Hypertension | |||||||
| No. of cases | 272 | 260 | 227 | 201 | 197 | ||
| Unadjusted OR (95% CI) | 1 (Referent) | 1.01 (0.85 to 1.20) | 0.88 (0.73 to 1.05) | 0.86 (0.71 to 1.03) | 0.85 (0.71 to 1.03) | .04 | |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.99 (0.83 to 1.18) | 0.84 (0.70 to 1.01) | 0.81 (0.67 to 0.99) | 0.81 (0.66 to 0.98) | .008 | |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.00 (0.84 to 1.20) | 0.86 (0.72 to 1.04) | 0.85 (0.69 to 1.03) | 0.84 (0.69 to 1.04) | .04 | |
| Alternative Mediterranean Diet | |||||||
| No. of cases | 293 | 236 | 221 | 210 | 197 | ||
| Unadjusted OR (95% CI) | 1 (Referent) | 0.88 (0.74 to 1.05) | 0.82 (0.69 to 0.98) | 0.88 (0.73 to 1.05) | 0.81 (0.67 to 0.97) | .11 | |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.86 (0.72 to 1.02) | 0.79 (0.66 to 0.95) | 0.83 (0.68 to 1.00) | 0.76 (0.62 to 0.92) | .01 | |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.88 (0.74 to 1.05) | 0.82 (0.68 to 0.98) | 0.86 (0.71 to 1.04) | 0.80 (0.65 to 0.99) | .07 | |
| Alternative Healthy Eating Index-2010 | |||||||
| No. of cases | 287 | 245 | 246 | 197 | 182 | ||
| Unadjusted OR (95% CI) | 1 (Referent) | 0.92 (0.77 to 1.09) | 1.00 (0.84 to 1.19) | 0.84 (0.70 to 1.01) | 0.84 (0.69 to 1.01) | .30 | |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.90 (0.76 to 1.07) | 0.98 (0.82 to 1.17) | 0.82 (0.68 to 0.99) | 0.82 (0.67 to 0.99) | .03 | |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.91 (0.76 to 1.08) | 0.99 (0.83 to 1.19) | 0.85 (0.70 to 1.03) | 0.85 (0.69 to 1.04) | .11 | |
CI = confidence interval; NHSII = Nurses’ Health Study II; OR = odds ratio.
Adjusted for age (continuous), total caloric intake (in quintiles), time period of endoscopy (in 2-year intervals), number of reported endoscopies (continuous), time in years since the most recent endoscopy (continuous), and reason for the current endoscopy (screening, symptoms, missing).
Additionally adjusted for height (continuous), body mass index (in quintiles), family history of colorectal cancer (yes, no), menopausal status (premenopausal, postmenopausal), menopausal hormone use (never, past, current use of menopausal hormones), personal history of type 2 diabetes (yes, no), pack-years of smoking (never, 1-4.9, 5-19.9, 20-39.9, ≥40 pack-years), physical activity (in metabolic equivalent of task-hours per week, quintiles), current use of multivitamin (yes, no), regular use of aspirin (yes, no), and regular use of nonsteroidal anti-inflammatory drugs (yes, no). For Dietary Approaches to Stop Hypertension, we additionally adjusted for alcohol intake (0, 0.1-14.9, ≥15 g/d).
Calculated using the median of each quintile as a continuous variable.
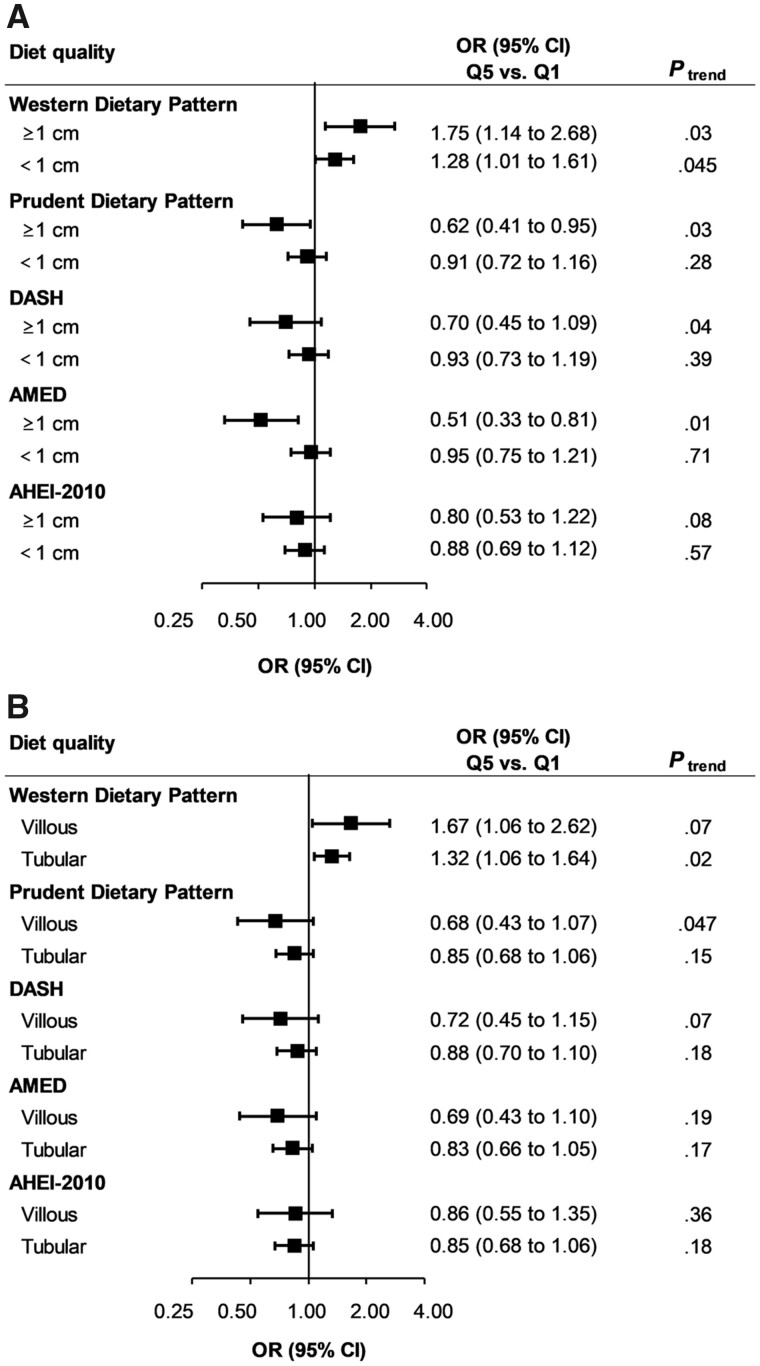
Notably, these associations appeared to be stronger for adenomas with higher malignant potential. We found a statistically significant positive association of the Western dietary pattern (multivariable ORQ5 vs Q1 = 1.67, 95% CI = 1.18 to 2.37, Ptrend = .01) and inverse associations of the prudent pattern (ORQ5 vs Q1 = 0.69, 95% CI = 0.48 to 0.98, Ptrend = .03), DASH (ORQ5 vs Q1 = 0.65, 95% CI = 0.45 to 0.93, Ptrend = .009), AMED (ORQ5 vs Q1 = 0.55, 95% CI = 0.38 to 0.79, Ptrend = .007), and AHEI-2010 scores (ORQ5 vs Q1 = 0.71, 95% CI = 0.51 to 1.01, Ptrend = .01; Table 3) with early-onset high-risk adenoma (n = 375 cases) but not with low-risk adenoma (n = 733 cases, all Ptrend ≥ .08). For early-onset adenoma overall and of high risk, we observed highly comparable results when stratified by age of endoscopy (younger than 45 years vs 45 years and older; data not shown). The stronger associations for high-risk adenoma were driven by large size (≥1 cm) and villous histology (Figure 1). Interestingly, the magnitude of these inverse associations was comparable between participants with or without symptoms (visible blood in stool specimen, positive result for fecal occult blood test, abdominal pain, and diarrhea or constipation) at the time of lower endoscopy (Supplementary Table 3, available online). In joint analyses, among women having the healthiest dietary pattern based on principal component analysis (ie, in the highest quintile of the prudent and the lowest quintile of the Western pattern), a statistically significantly lower risk was observed for early-onset high-risk adenomas, compared with those having the lowest score of the prudent and the highest score of the Western pattern (OR = 0.58, 95% CI = 0.36 to 0.92; Supplementary Table 4, available online).
Table 3.
Diet quality and risk of early-onset (aged younger than 50 years) adenoma according to malignant potential, NHSII, 1991-2011a
| Quintile |
P trend d | |||||
|---|---|---|---|---|---|---|
| Diet quality | 1 (lowest) | 2 | 3 | 4 | 5 (highest) | |
| Dietary pattern | ||||||
| Western dietary pattern | ||||||
| High-risk adenoma | ||||||
| No. of cases | 52 | 72 | 78 | 72 | 101 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.36 (0.95 to 1.94) | 1.38 (0.97 to 1.96) | 1.26 (0.88 to 1.80) | 1.67 (1.19 to 2.34) | .01 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.35 (0.95 to 1.94) | 1.38 (0.97 to 1.97) | 1.26 (0.88 to 1.80) | 1.66 (1.19 to 2.33) | .01 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.33 (0.93 to 1.91) | 1.37 (0.96 to 1.97) | 1.26 (0.88 to 1.82) | 1.67 (1.18 to 2.37) | .01 |
| Low-risk adenoma | ||||||
| No. of cases | 125 | 131 | 138 | 150 | 189 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.03 (0.80 to 1.32) | 1.01 (0.79 to 1.30) | 1.09 (0.86 to 1.38) | 1.30 (1.03 to 1.63) | .04 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.01 (0.78 to 1.29) | 1.00 (0.78 to 1.28) | 1.08 (0.85 to 1.38) | 1.31 (1.04 to 1.65) | .02 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.99 (0.77 to 1.28) | 0.98 (0.76 to 1.26) | 1.05 (0.81 to 1.35) | 1.25 (0.97 to 1.59) | .08 |
| Prudent dietary pattern | ||||||
| High-risk adenoma | ||||||
| No. of cases | 103 | 81 | 73 | 61 | 57 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.88 (0.65 to 1.18) | 0.85 (0.63 to 1.14) | 0.74 (0.54 to 1.02) | 0.73 (0.53 to 1.01) | .045 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.86 (0.64 to 1.16) | 0.82 (0.61 to 1.11) | 0.72 (0.52 to 0.99) | 0.70 (0.50 to 0.98) | .03 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.84 (0.62 to 1.13) | 0.80 (0.58 to 1.09) | 0.70 (0.50 to 0.98) | 0.69 (0.48 to 0.98) | .03 |
| Low-risk adenoma | ||||||
| No. of cases | 179 | 172 | 135 | 128 | 119 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.07 (0.87 to 1.32) | 0.90 (0.72 to 1.13) | 0.90 (0.71 to 1.13) | 0.87 (0.69 to 1.11) | .16 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.05 (0.85 to 1.30) | 0.87 (0.69 to 1.10) | 0.87 (0.69 to 1.10) | 0.86 (0.68 to 1.09) | .13 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.07 (0.86 to 1.32) | 0.90 (0.71 to 1.14) | 0.91 (0.72 to 1.16) | 0.91 (0.71 to 1.17) | .36 |
| Recommendation-based dietary index | ||||||
| Dietary Approaches to Stop Hypertension | ||||||
| High-risk adenoma | ||||||
| No. of cases | 97 | 77 | 83 | 59 | 59 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.84 (0.62 to 1.13) | 0.90 (0.67 to 1.21) | 0.70 (0.51 to 0.97) | 0.71 (0.52 to 0.99) | .02 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.81 (0.60 to 1.10) | 0.85 (0.62 to 1.15) | 0.65 (0.47 to 0.91) | 0.65 (0.46 to 0.91) | .006 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.81 (0.59 to 1.10) | 0.85 (0.62 to 1.16) | 0.65 (0.46 to 0.91) | 0.65 (0.45 to 0.93) | .009 |
| Low-risk adenoma | ||||||
| No. of cases | 160 | 177 | 133 | 132 | 131 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.17 (0.94 to 1.45) | 0.87 (0.69 to 1.10) | 0.96 (0.76 to 1.21) | 0.96 (0.76 to 1.22) | .44 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.15 (0.93 to 1.43) | 0.84 (0.66 to 1.06) | 0.92 (0.73 to 1.18) | 0.94 (0.73 to 1.20) | .29 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.18 (0.95 to 1.47) | 0.88 (0.69 to 1.11) | 0.98 (0.77 to 1.26) | 1.01 (0.78 to 1.31) | .69 |
| Alternative Mediterranean Diet | ||||||
| High-risk adenoma | ||||||
| No. of cases | 103 | 72 | 74 | 72 | 54 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.77 (0.57 to 1.04) | 0.79 (0.58 to 1.06) | 0.85 (0.63 to 1.16) | 0.63 (0.45 to 0.88) | .03 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.74 (0.54 to 1.00) | 0.74 (0.54 to 1.00) | 0.77 (0.56 to 1.06) | 0.55 (0.38 to 0.78) | .005 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.74 (0.54 to 1.01) | 0.73 (0.53 to 1.00) | 0.76 (0.55 to 1.06) | 0.55 (0.38 to 0.79) | .007 |
| Low-risk adenoma | ||||||
| No. of cases | 175 | 158 | 136 | 129 | 135 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.99 (0.80 to 1.23) | 0.85 (0.68 to 1.07) | 0.90 (0.72 to 1.13) | 0.93 (0.74 to 1.16) | .76 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.97 (0.78 to 1.20) | 0.83 (0.66 to 1.04) | 0.87 (0.68 to 1.10) | 0.91 (0.71 to 1.16) | .39 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.00 (0.81 to 1.25) | 0.87 (0.69 to 1.10) | 0.93 (0.73 to 1.19) | 1.00 (0.77 to 1.29) | .91 |
| Alternative Healthy Eating Index-2010 | ||||||
| High-risk adenoma | ||||||
| No. of cases | 103 | 86 | 73 | 54 | 59 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.90 (0.67 to 1.20) | 0.82 (0.61 to 1.12) | 0.64 (0.46 to 0.90) | 0.76 (0.55 to 1.04) | .02 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.89 (0.66 to 1.18) | 0.81 (0.60 to 1.10) | 0.63 (0.45 to 0.88) | 0.74 (0.53 to 1.03) | .01 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.87 (0.65 to 1.16) | 0.79 (0.58 to 1.07) | 0.61 (0.43 to 0.87) | 0.71 (0.51 to 1.01) | .01 |
| Low-risk adenoma | ||||||
| No. of cases | 172 | 147 | 163 | 135 | 116 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.92 (0.73 to 1.15) | 1.10 (0.89 to 1.37) | 0.96 (0.77 to 1.21) | 0.89 (0.70 to 1.13) | .52 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.90 (0.72 to 1.12) | 1.09 (0.87 to 1.35) | 0.94 (0.75 to 1.19) | 0.88 (0.69 to 1.12) | .51 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.92 (0.73 to 1.15) | 1.13 (0.90 to 1.41) | 1.01 (0.79 to 1.28) | 0.95 (0.74 to 1.23) | .90 |
High-risk adenoma includes adenoma at or greater than 1 cm, or with tubulovillous or villous histology or high-grade dysplasia, or 3 or more adenomas.CI = confidence interval; NHSII = Nurses’ Health Study II; OR = odds ratio.
Adjusted for age (continuous), total caloric intake (in quintiles), time period of endoscopy (in 2-year intervals), number of reported endoscopies (continuous), time in years since the most recent endoscopy (continuous), and reason for the current endoscopy (screening, symptoms, missing).
Additionally adjusted for height (continuous), body mass index (in quintiles), family history of colorectal cancer (yes, no), menopausal status (premenopausal, postmenopausal), menopausal hormone use (never, past, current use of menopausal hormones), personal history of type 2 diabetes (yes, no), pack-years of smoking (never, 1-4.9, 5-19.9, 20-39.9, ≥40 pack-years), physical activity (in metabolic equivalent of task-hours per week, quintiles), current use of multivitamin (yes, no), regular use of aspirin (yes, no), and regular use of nonsteroidal anti-inflammatory drugs (yes, no). For Dietary Approaches to Stop Hypertension, we additionally adjusted for alcohol intake (0, 0.1-14.9, ≥15 g/d).
Calculated using the median of each quintile as a continuous variable.
Figure 1.
Diet quality and risk of early-onset (aged younger than 50 years) adenoma according to size (A) and histology (B), Nurses’ Health Study II, 1991-2011. AHEI = Alternative Healthy Eating Index; AMED = Alternative Mediterranean Diet; CI = confidence interval; DASH = Dietary Approaches to Stop Hypertension; OR = odds ratio; Q1 = lowest quintile; Q5 = highest quintile. Odds ratio was adjusted for the covariates denoted in Table 2. Ptrend was calculated using the median of each quintile as a continuous variable.
By anatomical site, we observed stronger associations for the Western dietary pattern (multivariable ORQ4 vs Q1 = 1.65, 95% CI = 1.14 to 2.38, Ptrend = .01), prudent pattern (ORQ4 vs Q1 = 0.68, 95% CI = 0.47 to 0.99, Ptrend = .04), DASH (ORQ4 vs Q1 = 0.63, 95% CI = 0.42 to 0.94, Ptrend = .01), and AHEI-2010 scores (ORQ4 vs Q1 = 0.71, 95% CI = 0.49 to 1.03, Ptrend = .02), and risk of advanced adenomas in the distal colon and rectum (n = 271 cases; Table 4). However, we did not find any statistically significant associations for diet quality and risk of advanced adenoma in the proximal colon (n = 93 cases).
Table 4.
Diet quality and risk of early-onset (aged younger than 50 years) advanced adenoma according to anatomical locations, NHSII, 1991-2011a
| Quartile |
P trend d | ||||
|---|---|---|---|---|---|
| Diet quality | 1 (lowest) | 2 | 3 | 4 (highest) | |
| Dietary pattern | |||||
| Western dietary pattern | |||||
| Proximal | |||||
| No. of cases | 22 | 22 | 24 | 25 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.96 (0.53 to 1.73) | 1.00 (0.56 to 1.78) | 0.98 (0.55 to 1.75) | .90 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.93 (0.51 to 1.69) | 0.97 (0.55 to 1.73) | 0.97 (0.55 to 1.73) | .91 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.93 (0.50 to 1.71) | 0.96 (0.53 to 1.75) | 0.96 (0.52 to 1.78) | .88 |
| Distal and rectal | |||||
| No. of cases | 48 | 65 | 68 | 90 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.30 (0.89 to 1.88) | 1.30 (0.89 to 1.88) | 1.62 (1.14 to 2.31) | .01 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.31 (0.90 to 1.90) | 1.33 (0.91 to 1.93) | 1.61 (1.13 to 2.30) | .01 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.30 (0.89 to 1.91) | 1.34 (0.91 to 1.97) | 1.65 (1.14 to 2.38) | .01 |
| Prudent dietary pattern | |||||
| Proximal | |||||
| No. of cases | 25 | 25 | 21 | 22 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.14 (0.65 to 1.98) | 1.02 (0.57 to 1.82) | 1.13 (0.64 to 2.01) | .70 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.09 (0.62 to 1.91) | 0.97 (0.54 to 1.75) | 1.10 (0.62 to 1.95) | .79 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.10 (0.62 to 1.95) | 1.00 (0.55 to 1.82) | 1.14 (0.61 to 2.11) | .71 |
| Distal and rectal | |||||
| No. of cases | 91 | 73 | 56 | 51 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.91 (0.67 to 1.24) | 0.75 (0.53 to 1.04) | 0.72 (0.51 to 1.02) | .049 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.91 (0.67 to 1.23) | 0.73 (0.52 to 1.02) | 0.70 (0.49 to 0.99) | .03 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.89 (0.65 to 1.21) | 0.71 (0.50 to 1.01) | 0.68 (0.47 to 0.99) | .04 |
| Recommendation-based dietary index | |||||
| Dietary Approaches to Stop Hypertension | |||||
| Proximal | |||||
| No. of cases | 33 | 16 | 18 | 26 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.52 (0.29 to 0.95) | 0.58 (0.33 to 1.03) | 0.92 (0.55 to 1.54) | .83 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.51 (0.28 to 0.95) | 0.58 (0.32 to 1.05) | 0.92 (0.54 to 1.58) | .81 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.52 (0.28 to 0.95) | 0.59 (0.32 to 1.08) | 0.93 (0.53 to 1.65) | .85 |
| Distal and rectal | |||||
| No. of cases | 78 | 84 | 61 | 48 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 1.16 (0.85 to 1.58) | 0.83 (0.60 to 1.17) | 0.72 (0.50 to 1.03) | .04 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 1.10 (0.80 to 1.51) | 0.77 (0.54 to 1.09) | 0.63 (0.43 to 0.93) | .007 |
| Multivariable OR (95% CI)c | 1 (Referent) | 1.10 (0.80 to 1.52) | 0.77 (0.54 to 1.09) | 0.63 (0.42 to 0.94) | .01 |
| Alternative Mediterranean Diet | |||||
| Proximal | |||||
| No. of cases | 30 | 18 | 20 | 25 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.61 (0.34 to 1.10) | 0.73 (0.41 to 1.29) | 0.94 (0.55 to 1.60) | .84 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.62 (0.34 to 1.13) | 0.74 (0.41 to 1.34) | 0.94 (0.53 to 1.67) | .94 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.64 (0.34 to 1.17) | 0.76 (0.42 to 1.37) | 0.97 (0.54 to 1.76) | .86 |
| Distal and rectal | |||||
| No. of cases | 80 | 66 | 70 | 55 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.84 (0.61 to 1.17) | 0.96 (0.70 to 1.33) | 0.78 (0.55 to 1.10) | .34 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.80 (0.57 to 1.11) | 0.87 (0.62 to 1.22) | 0.67 (0.46 to 0.97) | .11 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.80 (0.57 to 1.13) | 0.86 (0.61 to 1.23) | 0.67 (0.45 to 0.99) | .14 |
| Alternative Healthy Eating Index-2010 | |||||
| Proximal | |||||
| No. of cases | 30 | 19 | 24 | 20 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.69 (0.39 to 1.23) | 0.95 (0.55 to 1.62) | 0.86 (0.49 to 1.52) | .93 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.67 (0.38 to 1.19) | 0.90 (0.52 to 1.58) | 0.82 (0.46 to 1.45) | .64 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.67 (0.37 to 1.20) | 0.91 (0.52 to 1.61) | 0.83 (0.47 to 1.49) | .69 |
| Distal and rectal | |||||
| No. of cases | 91 | 77 | 51 | 52 | |
| Unadjusted OR (95% CI) | 1 (Referent) | 0.93 (0.68 to 1.26) | 0.66 (0.47 to 0.94) | 0.74 (0.52 to 1.04) | .01 |
| Age-adjusted OR (95% CI)b | 1 (Referent) | 0.93 (0.69 to 1.26) | 0.66 (0.47 to 0.94) | 0.74 (0.52 to 1.05) | .02 |
| Multivariable OR (95% CI)c | 1 (Referent) | 0.91 (0.67 to 1.24) | 0.64 (0.44 to 0.92) | 0.71 (0.49 to 1.03) | .02 |
Advanced adenoma includes adenoma at or greater than 1 cm, or with tubulovillous or villous histology or high-grade dysplasia. CI = confidence interval; NHSII = Nurses’ Health Study II; OR = odds ratio.
Adjusted for age (continuous), total caloric intake (in quintiles), time period of endoscopy (in 2-year intervals), number of reported endoscopies (continuous), time in years since the most recent endoscopy (continuous), and reason for the current endoscopy (screening, symptoms, missing).
Additionally adjusted for height (continuous), body mass index (in quintiles), family history of colorectal cancer (yes, no), menopausal status (premenopausal, postmenopausal), pack-years of smoking (never, 1-20, ≥20 pack-years), physical activity (in metabolic equivalent of task-hours/week, quintiles), current use of multivitamin (yes, no), regular use of aspirin (yes, no), and regular use of nonsteroidal anti-inflammatory drugs (yes, no). For Dietary Approaches to Stop Hypertension, we additionally adjusted for alcohol intake (0, 0.1-14.9, ≥15 g/d).
Calculated using the median of each quartile as a continuous variable.
Discussion
In this large prospective cohort study of young women, Western diet was associated with an increased risk of early-onset adenoma, whereas intake more consistent with the prudent pattern and healthy DASH, AMED, and AHEI-2010 scores was associated with a lower risk. The associations were largely confined to high-risk adenomas, especially those in the distal colon and rectum. Coupled with secular trends toward low diet quality that is more prevalent among young adults in the United States (16), our findings suggest poor diet may partially contribute to the rapid increase in early-onset CRC.
Human nutrition has changed dramatically over the past century, represented by increased intake of meat, fats, oils, and added sugars and sweeteners, as well as reduced consumption of vegetables and whole grains (51). In addition to exploring the role of specific dietary elements in human health, researchers are increasingly focused on overall diet quality (52). Poor diet quality has been considered a putative CRC risk factor in older individuals. A meta-analysis of 40 studies showed that Western diet was associated with an increased risk of CRC, whereas a prudent pattern was associated with a lower risk (53). Findings have been mixed for recommendation-based indexes (52,54), together with no association observed in our parallel cohort of older women (52). Studies of early-onset CRC have been limited to case-control studies (55,56) from Pakistan and Italy. Our analyses are thus among the first prospective investigations of diet in early-onset colorectal neoplasia. The consistent findings across a posteriori dietary patterns and recommendation-based indexes, driven by high-risk adenomas, lend substantial support to the important and potentially stronger role of diet in early-onset than late-onset colorectal carcinogenesis.
Increases in early-onset CRC were primarily driven by distal colon and rectal tumors between 2004 and 2013, with incidence having increased by approximately 2% annually (57). Intriguingly, we observed stronger associations between diet quality and early-onset advanced adenomas in the distal colon and rectum, compared to those in the proximal colon. These results were in line with prior findings on diet quality demonstrating a prominent association for distal colon and rectal tumors in older adults (54,58). Proximal CRC is more likely to progress through the serrated neoplasia pathway (59), whereas the vast majority of distal cancers originate from conventional adenomas associated with molecular alterations such as APC and TP53 mutations (60). Indeed, prior analyses in 2 older cohorts showed that Western dietary pattern was more strongly associated with tumors that were MSS or microsatellite instability low, non-CpG island methylator phenotype, BRAF and KRAS wild type (58), a molecular subtype common in early-onset CRC (21). Taken together, our findings also indirectly support that diet may exert a stronger influence on neoplasia originating from the traditional adenoma-carcinoma sequence. As incidence for proximal tumors among younger individuals appears to be increasing with similar rates to distal colon and rectal tumors since 2012 (2), studies investigating the role of diet in recent years and other factors are warranted.
One of the potential mechanisms may relate to the summation of individual dietary constituents previously associated with CRC risk. For instance, a Western dietary pattern is high, whereas a prudent pattern and DASH, AMED, and AHEI-2010 scores are low in red and processed meats, which are known to be associated with increased risk of CRC (61). The DASH diet is rich in low-fat dairy products, a good source of dietary calcium. Higher calcium intake has been inversely associated with CRC risk, especially for distal colon cancer (62), which could be attributable to its known functions of reducing cellular proliferation and promoting cell differentiation and apoptosis (63). Secondly, diets may influence risk of adenoma by regulating levels of intestinal inflammation (64) and altering gut microbial composition and diversity (12,13). For advanced adenomas, microbial population shifts similar to CRC have been observed (11). For instance, gut commensals such as Bifidobacterium animalis and Streptococcus thermophilus that could inhibit potential pathogens in the colon were found to be relatively depleted in both advanced adenomas and CRC tissue (11). Last, unhealthy diets can lead to obesity (65), which is associated with increased risk of early-onset CRC (14), although our results did not change appreciably when body mass index was adjusted for.
Our study has several strengths. First, NHSII, with nearly 30 000 women younger than 50 years with at least 1 prior lower endoscopy and detailed data on indications, provided a unique opportunity to study early-onset adenomas, the most common precursors to early-onset CRC. Prior studies have reported comparable prevalence of adenomas in individuals aged 40-49 years vs 50-59 years who underwent employer-based screening colonoscopy (35,36,66,67). These studies also in part indicated the emerging lower endoscopy practice in the younger individuals. Our similar advanced adenoma detection rate for those aged 40-49 years with the prior report (36) lent additional support for the reliability and generalizability of our data. Second, our dietary data, assessed by FFQ, captured long-term intake and have been validated and collected in a prospective manner, limiting recall or ascertainment bias (68). Regular updates on dietary habits, accrued over 20 years of follow-up, also allowed us to evaluate long-term intake. Third, investigators masked to exposure information reviewed all the retrieved medical records and extracted data on histological subtype, which enabled us to perform subanalysis according to different adenoma subtypes as well as the malignant potential.
Several limitations need to be considered. First, as an observational study, the possibility of residual confounding could not be ruled out. Nevertheless, minimal changes after adjustment for a wide variety of putative risk factors of CRC indicated the robustness of our findings. Second, the dietary data were assessed using FFQs and subject to measurement errors. Using factor analysis to derive dietary patterns requires some decisions, such as the way to group individual food items into food groups, making it subjective to some extent for defining a posteriori dietary pattern. However, dietary measurement errors are expected to be nondifferential for CRC risk (52), and it has been well established that repeated FFQs can accurately capture long-term dietary intake (34). Finally, the generalizability of our findings to other populations, particularly men or other racial and ethnic groups, remains unknown.
In conclusion, higher scores for a Western diet were associated with an increased risk of early-onset adenoma overall, whereas intake of healthier dietary patterns (prudent diet and DASH, AMED, and AHEI-2010 scores) was associated with a lower risk, largely driven by associations for adenomas in the distal colon and rectum that were of high malignant potential. The slightly different associations based on dietary index classification system might inspire future work exploring the specific mechanisms involved. More detailed studies of differences in dietary index adherence and CRC risk by anatomic site in youth are also warranted.
Funding
This work was supported by the National Institutes of Health (NIH; NHSII cohort infrastructure grant of U01 CA176726, R00 CA215314 to MS; R03 CA197879 and R21 CA222940 to KW; R21 CA230873 to KW and SO; R01 CA151993 and R35 CA197735 to SO; K24 DK098311 to ATC; R37 CA246175 and K07 CA218377 to YC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. XZ was supported by International Program for PhD Candidates, Sun Yat-Sen University. LHN is supported by an NIH Loan Repayment Program Scholarship and a Crohn’s and Colitis Foundation Research Fellowship Award. MS is supported by a Mentored Research Scholar Grant in Applied and Clinical Research, MRSG-17–220-01-NEC, from the American Cancer Society. KW is supported by an Investigator Initiated Grant from the American Institute for Cancer Research. SO is supported by Nodal Award from the Dana-Farber Harvard Cancer Center and by grants from the Project P Fund for Colorectal Cancer Research, the Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance and American Association for Cancer Research (Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant). ATC is a Stuart and Suzanne Steele MGH Research Scholar.
Notes
Role of the funder: The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: Andrew T. Chan previously served as a consultant for Janssen Pharmaceuticals, Pfizer, Inc, and Bayer Pharma AG for work unrelated to the topic. The remaining authors disclose no conflicts of interest.
Author contributions: Drs. Zheng, Hur, and Cao had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: XZ, JH, ATC, EG, YC. Acquisition of data: MS, KW, WCW, ATC, EG, YC. Analysis and interpretation of data: all coauthors. Drafting of the manuscript: XZ, JH, YC. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: XZ, JH, YC. Obtained funding: KW, WCW, ATC, EG, YC. Administrative, technical, or material support: ATC, EG, YC. Study supervision: EG, YC.
Disclaimer: The authors assume full responsibility for analyses and interpretation of these data.
Acknowledgments: We thank the participants and staff of the Nurses’ Health Study II for their valuable contributions and the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8):djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. [DOI] [PubMed] [Google Scholar]
- 3. Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125(12):2002-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keum N, Giovannucci E.. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713-732. [DOI] [PubMed] [Google Scholar]
- 5. Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A.. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev. 2010;68(7):389-408. [DOI] [PubMed] [Google Scholar]
- 6. Fung T, Hu FB, Fuchs C, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163(3):309-314. [DOI] [PubMed] [Google Scholar]
- 7. Magalhaes B, Peleteiro B, Lunet N.. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev .2012;21(1):15-23. [DOI] [PubMed] [Google Scholar]
- 8. Wu K, Hu FB, Fuchs C, Rimm EB, Willett WC, Giovannucci E.. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Causes Control. 2004;15(9):853-862. [DOI] [PubMed] [Google Scholar]
- 9. Chan AT, Giovannucci EL.. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029-2043 e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song M, Garrett WS, Chan AT.. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244-1260.e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng Q, Liang S, Jia H, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6(1):6528. [DOI] [PubMed] [Google Scholar]
- 12. Louis P, Hold GL, Flint HJ.. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661-672. [DOI] [PubMed] [Google Scholar]
- 13. O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen LH, Liu PH, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectrum. 2018;2(4):pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sijtsma FP, Meyer KA, Steffen LM, et al. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2012;95(3):580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Rehm CD, Onopa J, Mozaffarian D.. Trends in diet quality among youth in the United States, 1999-2016. JAMA. 2020;323(12):1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cavestro GM, Mannucci A, Zuppardo RA, Di Leo M, Stoffel E, Tonon G.. Early onset sporadic colorectal cancer: worrisome trends and oncogenic features. Dig Liver Dis. 2018;50(6):521-532. [DOI] [PubMed] [Google Scholar]
- 20. Ballester V, Rashtak S, Boardman L.. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao Y, Harrison T, Liu J, et al. Integrative molecular marker analyses of early-onset colorectal cancer support the importance of the traditional adenoma-carcinoma sequence. Gastroenterology. 2020;158(6):S-202-203. [Google Scholar]
- 22. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113-130. [DOI] [PubMed] [Google Scholar]
- 23. Strum WB. Colorectal adenomas. N Engl J Med. 2016;374(11):1065-1075. [DOI] [PubMed] [Google Scholar]
- 24. Nimptsch K, Giovannucci E, Willett WC, Fuchs CS, Wei EK, Wu K.. Body fatness during childhood and adolescence, adult height, and risk of colorectal adenoma in women. Cancer Prev Res .2011;4(10):1710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR.. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. [DOI] [PubMed] [Google Scholar]
- 26. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. [DOI] [PubMed] [Google Scholar]
- 27. Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3-9. [DOI] [PubMed] [Google Scholar]
- 30. Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243-249. [DOI] [PubMed] [Google Scholar]
- 31. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB.. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713-720. [DOI] [PubMed] [Google Scholar]
- 32. Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD.. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466-472. [DOI] [PubMed] [Google Scholar]
- 33. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willett W. Nutritional Epidemiology. 3rd ed. Oxford; NY: Oxford University Press; 2013. [Google Scholar]
- 35. Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF.. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346(23):1781-1785. [DOI] [PubMed] [Google Scholar]
- 36. Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI.. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008;134(5):1311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M.. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. 2018;155(2):355-373.e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng X, Wu K, Song M, et al. Yogurt consumption and risk of conventional and serrated precursors of colorectal cancer. Gut. 2020;69(5):970.1-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hang D, Joshi AD, He X, et al. Colorectal cancer susceptibility variants and risk of conventional adenomas and serrated polyps: results from three cohort studies. Int J Epidemiol. 2020;49(1):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abar L, Vieira AR, Aune D, et al. Height and body fatness and colorectal cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Eur J Nutr. 2018;57(5):1701-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schlesinger S, Lieb W, Koch M, et al. Body weight gain and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Obes Rev. 2015;16(7):607-619. [DOI] [PubMed] [Google Scholar]
- 42. Roos VH, Mangas-Sanjuan C, Rodriguez-Girondo M, et al. Effects of family history on relative and absolute risks for colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(13):2657-2667.e2659. [DOI] [PubMed] [Google Scholar]
- 43. Freedman DM, Rajaraman P, Fuhrman B, Hoffbeck R, Alexander BH.. Sunlight, hormone replacement status and colorectal cancer risk in postmenopausal women. Int J Cancer. 2010;126(8):1997-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rennert G, Rennert HS, Pinchev M, Lavie O, Gruber SB.. Use of hormone replacement therapy and the risk of colorectal cancer. J Clin Oncol. 2009;27(27):4542-4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Larsson SC, Orsini N, Wolk A.. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(22):1679-1687. [DOI] [PubMed] [Google Scholar]
- 46. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P.. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778. [DOI] [PubMed] [Google Scholar]
- 47. Rezende LFM, Sá TH, Markozannes G, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52(13):826-833. [DOI] [PubMed] [Google Scholar]
- 48. Heine-Bröring RC, Winkels RM, Renkema JM, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. 2015;136(10):2388-2401. [DOI] [PubMed] [Google Scholar]
- 49. Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2(6):762-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nan H, Hutter CM, Lin Y, et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313(11):1133-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Das JK, Salam RA, Thornburg KL, et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann NY Acad Sci. 2017;1393(1):21-33. [DOI] [PubMed] [Google Scholar]
- 52. Petimar J, Smith-Warner SA, Fung TT, et al. Recommendation-based dietary indexes and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am J Clin Nutr. 2018;108(5):1092-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feng YL, Shu L, Zheng PF, et al. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev .2017;26(3):201-211. [DOI] [PubMed] [Google Scholar]
- 54. Park SY, Boushey CJ, Wilkens LR, Haiman CA, Le Marchand L.. High-quality diets associate with reduced risk of colorectal cancer: analyses of diet quality indexes in the multiethnic cohort. Gastroenterology. 2017;153(2):386-394.e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khan NA, Hussain M, Ur Rahman A, Farooqui WA, Rasheed A, Memon AS.. Dietary practices, addictive behavior and bowel habits and risk of early onset colorectal cancer: a case control study. Asian Pac J Cancer Prev. 2015;16(17):7967-7973. [DOI] [PubMed] [Google Scholar]
- 56. Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24(2):335-341. [DOI] [PubMed] [Google Scholar]
- 57. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177-193. [DOI] [PubMed] [Google Scholar]
- 58. Mehta RS, Song M, Nishihara R, et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology. 2017;152(8):1944-1953.e1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leggett B, Whitehall V.. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088-2100. [DOI] [PubMed] [Google Scholar]
- 60. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403-408. [DOI] [PubMed] [Google Scholar]
- 61. Alexander DD, Weed DL, Miller PE, Mohamed MA.. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang X, Keum N, Wu K, et al. Calcium intake and colorectal cancer risk: results from the Nurses’ Health Study and Health Professionals Follow-Up Study. Int J Cancer. 2016;139(10):2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lamprecht SA, Lipkin M.. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601-614. [DOI] [PubMed] [Google Scholar]
- 64. Tabung FK, Liu L, Wang W, et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4(3):366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR.. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Enwerem N, Cho MY, Demb J, et al. Systematic review of prevalence, risk factors, and risk for metachronous advanced neoplasia in patients with young-onset colorectal adenoma [published online ahead of print May 16, 2020]. Clin Gastroenterol Hepatol. 2020;S1542-3565(20)30679-0. doi: 10.1016/j.cgh.2020.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fedewa SA, Siegel RL, Goding Sauer A, Bandi P, Jemal A.. Colorectal cancer screening patterns after the American Cancer Society’s recommendation to initiate screening at age 45 years. Cancer. 2020;126(6):1351-1353. [DOI] [PubMed] [Google Scholar]
- 68. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC.. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126; discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.