Abstract
Background
The testes are suspected target organs of SARS‐CoV‐2. However, the results of studies on the effect of COVID‐19 on male reproduction are controversial.
Objective
To summarize current research on the effects of COVID‐19 on male reproduction.
Methods
A systematic review of English literature was performed using PubMed and Ovid Embase up to 18 August 2020. Research articles on the presence of SARS‐CoV‐2 in semen, the effects of the virus on semen parameters and any pathological changes in the testes were evaluated.
Results
Fourteen studies were included in this review. Six of 176 survivors (3.4%) and 1 of 13 decedents (7.7%) in 2 of 12 studies were positive for viral RNA in semen and testicular tissue, respectively. After stratification of patient groups, we found that the virus was detected in the relatively early stage of infection, 6–16 days after disease onset, in semen from survivors. Two of 3 studies reported that some participants had substandard semen quality after COVID‐19, and 1 study found that COVID‐19 may impair semen quality in a severity‐related manner. Pathological analyses showed that injuries to the seminiferous tubule occurred in all decedents (N = 11). Another study found that orchitic and testis fibrin microthrombi occurred in patients with fatal disease (100%, N = 2). Scrotal discomfort of orchiepididymitis or spermatic cord inflammation has also been reported in COVID‐19 patients.
Conclusion
Current studies suggest that semen is rarely considered a carrier of SARS‐CoV‐2 genetic material during the infection period but not in the semen of recovered patients. Fatal COVID‐19 may cause testicular structure damage without the presence of virus.
Keywords: COVID‐19, SARS‐CoV‐2, male reproduction, semen, testis
1. INTRODUCTION
Since the first case of coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was reported in Wuhan, China, it has rapidly spread and affected more than 21 million people worldwide as of 17 August 2020. 1 SARS‐CoV‐2 uses angiotensin‐converting enzyme II (ACE2) to enter host cells, similar to SARS‐CoV, which emerged 18 years ago. 2 COVID‐19 induces respiratory‐predominant multiorgan dysfunction, including myocardial, renal, enteric and hepatic dysfunction, which coincides with the tissue expression of ACE2. 3 Meanwhile, several studies have shown that ACE2 is expressed in human testes (eg spermatogonia, Leydig cells and Sertoli cells), 4 , 5 suggesting that the testes may be another organ affected by COVID‐19.
Numerous viruses have been detected in human semen. 6 Viruses may persist in semen and last longer in seminal fluid than in other body fluids due to the immune privilege of the testes and the contribution of the blood‐testes barrier to resistance to therapeutic agents. 7 , 8 Semen may also have higher loads of viruses, such as Zika virus, than blood. 9 , 10 Therefore, the testes may act as a reservoir of virus, which may cause imprecise evaluation of viral clearance in patients. Viruses, including Zika virus, Ebola virus, cytomegalovirus and human immunodeficiency virus (HIV), have been isolated from semen and can be sexually transmitted. 6 , 11 , 12 Furthermore, some viruses (eg HIV, Zika virus, herpes simplex virus (HSV) and human papillomavirus) can adhere to or be internalized by spermatozoa, 7 , 13 which may pose a risk for embryonic infection and cause adverse reproductive outcomes.
On the other hand, many viruses, such as mumps virus, HIV and HSV, 7 , 14 have been found to impair semen quality, and they may directly interact with spermatozoa or affect spermatogenesis by inducing local inflammation. 15 , 16 , 17 Previous studies found that SARS, 1 of the 3 epidemic coronaviruses to emerge in the past 20 years and that shows similar clinical presentations to COVID‐19, 18 could cause orchitis 19 and focal testicular atrophy. 20 Considering the tens of millions of COVID‐19 cases and that men are more vulnerable to COVID‐19 than women, 21 , 22 , 23 it is imperative to determine the effect of COVID‐19 on male reproduction. 24
Several studies have been performed on this topic. However, the results are controversial. For example, some researchers have reported that SARS‐CoV‐2 was not detected in the male reproductive tract, 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 while others reported that SARS‐CoV‐2 RNA was found in the semen or testes of COVID‐19 patients. 35 , 36 There are also unknown factors regarding COVID‐19 and male reproduction. Orchitis and broad destruction of the testes were found in deceased COVID‐19 patients, 35 , 37 while the pathological characteristics in survivors remain unknown. In this review, we summarize the current research focusing on the effects of COVID‐19 on male reproduction from the following 3 aspects: detection of SARS‐CoV‐2 in the male reproductive tract, determination of the impact of COVID‐19 on sperm quality and exploration of pathological changes in the testes of COVID‐19 patients. We further discuss the discrepancies and summarize the unknown topics, which we believe will be helpful for future research.
2. METHODS
A systematic search of published studies was conducted in the PubMed and Ovid Embase databases for studies published from December 2019 to 18 August 2020 in accordance with PRISMA. 38 All titles or abstracts of English‐language studies were reviewed for eligibility. Citations and references of the retrieved studies were used as additional sources. There was no limitation on sample size, and case reports were included. A full‐text review was performed by 2 independent reviewers (Y.Y. and X.Y.) on studies that reported the detection of SARS‐CoV‐2 in the male reproductive tract, determined the impact of COVID‐19 on sperm quality and explored pathological changes in the testes of COVID‐19 patients. Any disagreements between reviewers were discussed with a third reviewer (L.W.). The Cochrane RoB 2.0 tool was not applicable, and the Newcastle‐Ottawa Scale was not used due to the limited scope of the cohort studies among the included studies.
The literature search in PubMed used the following search terms: (“2019 new coronavirus” [All Fields] OR “2019 ncov” [All Fields] OR “severe acute respiratory syndrome coronavirus 2” [All Fields] OR “sars cov 2” [All Fields] OR “coronavirus disease 2019” [All Fields] OR “covid19” [All Fields] OR “covid 19” [All Fields]) AND (“semen” [All Fields] OR “sperm” [All Fields] OR “testis” [All Fields] OR “testes” [All Fields] OR “testicular” [All Fields] OR “epididymis” [All Fields] OR “spermatic fluid” [All Fields] OR “seminal fluid” [All Fields] OR “prostatic secretion” [All Fields] OR “prostatic fluid” [All Fields] OR “male reproductive tract” [All Fields] OR “male genital tract” [All Fields]). Searches in Ovid Embase used the following terms: (‘2019 ncov’ OR ‘sars cov 2’ OR ‘covid‐19’ OR covid19) AND (semen OR sperm OR testis OR testes OR testicular OR epididymis OR ‘spermatic fluid’ OR ‘seminal fluid’ OR ‘prostatic secretion’ OR ‘prostatic fluid’ OR ‘male reproductive tract’ OR ‘male genital tract’).
3. RESULTS AND DISCUSSION
After reviewing the studies retrieved from the database, citations and references were added based on a review of the title or abstract (Figure 1). Fourteen studies were eligible and were included in this study, with 12 studies detecting SARS‐CoV‐2 in the male reproductive tract, 3 determining the impact of COVID‐19 on sperm quality and 3 exploring pathological changes in the testes of COVID‐19 patients.
Figure 1.
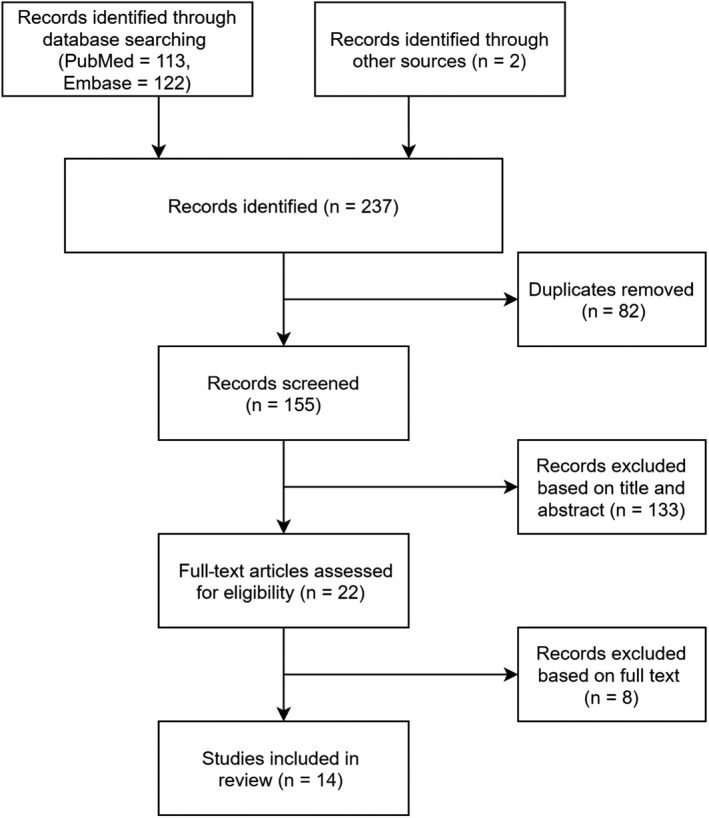
Flow chart of the study identification process.
3.1. Detection of COVID‐19 in the male reproductive tract
Twelve studies investigated the presence of SARS‐CoV‐2 in the male reproductive tract (eg semen, prostatic secretion or testicular tissue) and are shown in Table 1. Most studies were cross‐sectional in design and included mainly Chinese subjects. In brief, ten of 12 studies reported that none of the participants had SARS‐CoV‐2 RNA in semen, prostatic secretion or testicular tissue, 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 and 2 studies reported that some subjects were positive for viral RNA in semen or testicular tissue. 35 , 36 Among 12 studies, 170 of 176 survivors and 12 of 13 decedents did not have SARS‐CoV‐2 RNA in the reproductive tract (96.6% and 92.3%, respectively); however, 6 survivors (3.4%) and 1 decedent (7.7%) had viral RNA in semen and testicular tissue, respectively.
Table 1.
Summary of studies detecting SARS‐CoV‐2 in the male reproductive tract
| Reference | Region | Study design | Diagnostic criteria | Sample size | Clinical category | Age (year) | Specimen | Time since diagnosis (day) | Time since recovered a (day) | Detection method | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pan et al. 29 | China | Cross‐sectional | A | 34 in recovery phase | Mild‐moderate b | 18−57 | Semen | 8−75 | NP | RT‐PCR | Negative |
| Song et al. 26 | China | Cross‐sectional | B | 12 in recovered a phase |
11 mild and 1 asymptomatic |
22−38 | Semen | 14−42 | 0−21 c | RT‐PCR | Negative |
| 1 died | NA | 67 | Testicular tissue | 41 | NA | RT‐PCR | Negative | ||||
| Zhang et al. 44 | China | Cross‐sectional | B | 10 inpatients d | NP | 29−76 | Prostatic secretion | Median: 11 | NA | RT‐PCR | Negative |
| Paoli et al. 28 | Italy | Case report | A | 1 in recovery phase | Mild | 31 | Semen | 8 | NA | RT‐PCR | Negative |
| Nicastri et al. 32 | Italy | Case report | A | 1 found by screen | Asymptomatic | 20 | Semen | 1 | NA | RT‐PCR | Negative |
| Li et al. 36 | China | Cohort study | A | 38 from 50 e (23/15 in recovery/acute phase) | NP | ≥ 15 | Semen | 6–16 f | NA | RT‐PCR | Positive: 2/4 of recovery/acute cases |
| Ma et al. 30 | China | Cross‐sectional | B | 12 g | 1 mild and 11 moderate b | 25−46 | Semen | 56−109 h | NP | RT‐PCR | Negative |
| Guo et al.31 | China | Cross‐sectional | B | 23 in recovery phase i | 18 mild and 5 moderate b | 20−62 | Semen | 26−34 | NP | RT‐PCR | Negative |
| Holtmann et al. 27 | Germany | Cohort study | B | 18 in recovered phase | 14 mild j | 42.7 ± 10.4 k | Semen | 43.5 ± 6.2 k | 34.9 ± 11.7 l | RT‐PCR | Negative |
| 4 moderate j | 40.8 ± 8.7 k | Semen | 47.0 ± 5.3 k | 25.5 ± 8.3 l | RT‐PCR | Negative | |||||
| 2 in acute phase | NP | NP | Semen | NP | NA | RT‐PCR | Negative | ||||
| NA | 14 control cases | NA | 33.4 ± 13.1 k | Semen | NA | NA | RT‐PCR | Negative | |||
| Kayaaslan et al. 34 | Turkey | Cross‐sectional | A | 16 in acute stage | 11 mild and 5 moderate | 18−54 | Semen |
0−7 median: 1 |
NA | RT‐PCR | Negative |
| Pavone et al. 33 | Italy | Cross‐sectional | A | 9 in recovery phase | 8 mild j and 1 asymptomatic | 28−60 | Semen | 7−88 median:39 | NA | RT‐PCR | Negative |
| Yang et al. 35 | China | Cross‐sectional | C | 12 deceased patients | NA | 39−87 | Testicular tissue | 20−75 h | NA | RT‐PCR | Positive: one case |
| 3 of 12 deceased patients | NA | 61, 63, 73 | Testicular tissue | 36, 44, 49 h | NA | EM | Negative for viral particle |
Bold indicates that the study included specimens found positive for SARS‐CoV‐2 RNA. A: RT‐PCR for SARS‐CoV‐2 RNA from nasopharyngeal swab. B: RT‐PCR for SARS‐CoV‐2 RNA from nasopharyngeal swab or serum antibodies (IgM and IgG). C: RT‐PCR for SARS‐CoV‐2 RNA from oropharyngeal swabs or bronchoalveolar lavage fluid, or radiological features of viral pneumonia with clinical symptomatology.
EM: electron microscopy; RT‐PCR, reverse transcription polymerase chain reaction; NA, not applicable; NP, not provided.
Recovered was defined as viral clearance (two consecutive negative RT‐PCR tests) or a substantial resolution on chest CT scans with much lessened symptoms.
Clinical category was classified according to New Coronavirus Pneumonia Prevention and Control Program (7th edition)39.
One case persistently tested positive by pharyngeal swab.
Three patients had positive nasopharyngeal swabs for SARS‐CoV‐2 RNA within 3 days, and the other 7 were negative.
Twelve of the 50 initially identified patients were unable to provide a semen sample due to erectile dysfunction, being in a comatose state, or dying prior to recruitment, resulting in 38 patients available for semen testing. Recovery indicates clinical recovery.
Time since onset of symptoms of 6 cases who were positive for SARS‐CoV‐2 RNA in semen.
One case tested positive for SARS‐CoV‐2 RNA 5 days before trial, and 11 cases were negative on the day of trial.
Time since onset of disease.
Eleven cases tested negative for SARS‐CoV‐2 RNA in pharyngeal swabs, sputum or faecal specimens, and 12 were positive on the day of trial.
Mild COVID‐19 infection was defined as cases not requiring hospitalization. Moderate was defined for patients requiring hospitalization with up to 6 L oxygen supplied to achieve >92% peripheral oxygenation.
Data are presented as the means ±standard deviation.
Time since end of symptoms, presented as the means ± standard deviation.
Cases were stratified by infection status (recovered period defined as 2 consecutive negative reverse transcription polymerase chain reaction (RT‐PCR) tests or as defined in the article 26 ; otherwise, the participants were classified as being in the infection period) and clinical severity (ie mild, moderate, severe and critical 39 ). Patients with mild illness were negative for SARS‐CoV‐2 RNA in semen during the infection period 28 , 29 , 30 , 31 , 33 , 34 and recovered period. 26 , 27 Patients with moderate illness were negative for viral RNA in semen during the infection period 29 , 30 , 31 , 34 and recovered period. 27 Other findings include that asymptomatic subjects did not have viral RNA in semen, 26 , 32 , 33 that patients during the infection period who were not grouped by clinical severity did not have viral RNA in semen and prostatic secretions, 25 , 27 and that a deceased patient (with a positive RNA test from a pharyngeal swab) did not have viral RNA in his testicular tissue. 26 All 7 cases (from 2 studies) who were positive for SARS‐CoV‐2 RNA in semen or testicular tissue were in the infection period. 35 , 36 Four and 2 of the patients who were positive for viral RNA in semen were in the acute infection and clinical recovery periods, respectively, with interval times (from onset of symptoms to testing of semen) of 6–11 and 12–16 days, respectively. This was a shorter duration than most studies that reported negative viral RNA in semen 26 , 27 , 29 , 30 , 31 but a longer duration than 1 study that reported negative viral RNA within an interval time of 0–7 days (median: 1 day), 34 suggesting that SARS‐CoV‐2 may be shed into semen at a relatively early stage of COVID‐19 and then cleared. However, some details of the research were not available, and therefore, its accuracy cannot be fully verified. (1) The subjects may have included severe or critical cases. The study was conducted in the only COVID‐19‐designated hospital in a local city, and some patients were excluded because they were comatose or dying; however, the disease severity of the participants was not provided. (2) The detection of SARS‐CoV‐2 in semen is also an arguable point due to lacking information on RNA extraction, amplification, limit of detection (LoD), gene target and cycle threshold (Ct). 40 (3) In addition, the sample collection procedure was not described. In another study, 1 of 12 deceased COVID‐19 patients who died 30 days after the onset of disease had viral RNA in his testicular tissue and lung tissue. 35 However, considering that testicular tissue contains both fibrovascular tissue and seminiferous tubules, viral RNA loads from blood or semen are unknown.
Of 2 studies that reported the presence of SARS‐CoV‐2 RNA in the male reproductive tract, 1 provided the threshold cycle (Ct) value of the RT‐PCR test, with values of 31.68, 30.53 and 30.46 for the RdRP, E and N genes, respectively. 35 This patient had a high viral load, and his lung, kidney, spleen and testes were all positive for viral RNA, suggesting that the presence of virus in the testes was associated with whole‐body viral load. However, the relationship of viral load between the testes and other organs cannot be evaluated. On the other hand, almost all of the studies used RT‐PCR technology to detect SARS‐CoV‐2 RNA, which may have caused false‐negative results because the sensitivity of RT‐PCR is approximately 60–95%. 41 In addition, the detection of viral nucleic acids alone does not indicate the presence of infectious virus in testis or the infection of sperm cells. 42 , 43 Therefore, whether SARS‐CoV‐2 can be sexually transmitted is still unclear.
There are several limitations in the above studies. First, the collective sample size was relatively small, with viral RNA detected in a total of 189 samples from 12 studies, which may have led to imprecise estimates. For example, SARS‐CoV‐2 was detected and isolated in urine samples by some researchers, 21 , 44 while another study reported that none of their 72 urine samples were positive for viral RNA by RT‐PCR. 45 Second, the current research mainly consists of cross‐sectional studies with a single semen sample from each patient. However, a single sample may be insufficient for the determination of viral RNA. 46 Third, the studies were based on patients with asymptomatic, mild or moderate disease or deceased patients and lacked data on severe and critical cases. The degree of severity has been associated with viral load and illness duration 47 , 48 , 49 ; therefore, the reproductive tract may be more likely to be affected by COVID‐19 in patients who suffer more severe disease. Fourth, some details of the study process were not recorded or provided, such as sample collection, recovery criteria and viral detection. Despite these limitations, the above studies demonstrated that SARS‐CoV‐2 may be present in the male reproductive tract during a relatively early stage of COVID‐19, within approximately 2 weeks of infection. No evidence has shown that SARS‐CoV‐2 persists in the male reproductive tract of recovered patients. Therefore, the testes may not be a reservoir of SARS‐CoV‐2, and the risk for sexual transmission of SARS‐CoV‐2 is low, although it cannot currently be ruled out.
3.2. Association of COVID‐19 and semen quality
Three of the above studies further investigated the semen parameters (Table 2). Some patients who were enrolled during the infection period (prior to 2 consecutive negative RT‐PCR tests) showed decreased sperm quality parameters, such as altered sperm motility (progressive spermatozoa (PR) + non‐progressive (NP), 10.1–31.1%), sperm DNA fragmentation index (DFI, 16.2–23.7%), sperm morphology (normal spermatozoa, 3.4–3.5%), sperm volume (<0.5–1.2 ml per ejaculate) and sperm concentration (2 million/ml). 30 , 31 In 3 patients with moderate disease for whom semen parameter information from before they became ill with COVID‐19 was available, 1 patient showed a decrease in sperm motility (PR+NP, 26.7 + 35.0 = 61.7% before and 27.4 + 3.7 = 31.1% after), 1 patient showed a decrease in total mobile sperm number (111.67, 122.40 before and 97.93 after, million per ejaculate), and the remaining patient had been diagnosed with asthenospermia prior to becoming ill with COVID‐19 and showed little alteration after infection. 30
Table 2.
Summary of studies determining the impact of COVID‐19 on sperm quality
| Reference | Sample size | SARS‐CoV−2 RNA status | Abstinence duration (day) | Results |
|---|---|---|---|---|
| Ma et al. 30 | 12 | One mild case tested positive for viral RNA 5 days before trial, and 11 moderate cases tested negative on the day of trial. | 2−7 | Four of 11 moderate cases showed low sperm motility (PR +NPR, 10.1−31.1%) with higher sperm DFI (16.2−23.7%), and 2 of these 4 cases showed poor sperm morphology (normal spermatozoa, 3.4−3.5%). |
| Guo et al. 31 | 23 a | 12 cases tested positive for viral RNA, and 11 cases tested negative on the day of trial. | 3–6 | Seven cases showed low semen volume (<0.5−1.2 ml per ejaculate), 1 case showed low sperm concentration (2 million/ml), 4 cases showed PR <32% (10−30%), and 2 cases showed PR +NPR <40% (25−30%). |
| Holtmann et al. 27 | 4 recovered from moderate infection | NA | 2.5 ± 1.0 b | Lower sperm concentration, total no. of spermatozoa per ejaculate, and total no. of progressive motile, total no. of completely motile, and total no. of immotile spermatozoa than control or male who recovered from mild infection. |
| 14 recovered from mild infection | NA | 3.2 ± 1.1 b | No significant alteration. | |
| 2 in acute phase | NP | NP | NP | |
| 14 control cases | NA | 3.3 ± 1.9 b | NA |
DFI, DNA fragmentation index; PR, progressive motility; NP, not provided; NA, not applicable; NPR, non‐progressive motility.
Two cases were excluded due to sperm volume <0.5 ml and non‐liquefaction; the remaining 21 cases underwent semen parameter analysis.
Data are presented as the means ±standard deviation.
One study investigated the effect of COVID‐19 on semen quality among recovered males. 27 Subjects who had recovered from moderate disease (N = 4, defined as patients requiring hospitalization with up to 6 L of oxygen supplied to achieve >92% peripheral oxygenation) showed significantly lower sperm quality (ie sperm concentration, total number of spermatozoa per ejaculate, total number of spermatozoa with progressive motility and total number of completely motile spermatozoa) than those who recovered from a mild infection (N = 14, defined as cases that did not require hospitalization) or those in the control group (N = 14, healthy volunteers with no reported andrological pathology). These results suggested that COVID‐19 impairs semen quality in a disease severity‐related manner. When 2 patients with moderate illness were excluded due to cryptozoospermia, the sperm volume, sperm concentration, PR and spermatozoa with complete motility were lower in the moderately ill group (left N = 2) than in the mildly ill group or control group, but only the decrease in sperm concentration showed a significant difference between groups. Additionally, SARS‐CoV‐2 was not detected in the semen of any subject in these 3 studies.
Disease symptoms may affect semen quality. In Holtmann et al.’s study, when the recovered patients were stratified by whether fever occurred during infection, the fever‐positive group (N = 10) showed significantly lower sperm volume and total number of completely motile spermatozoa and a tendency for lower sperm concentration and total number of spermatozoa per ejaculate than the fever‐negative group (N = 8). 27 When 2 patients with cryptozoospermia were excluded, the fever‐positive group (N = 8) showed lower sperm volume, PR and complete motility than the fever‐negative group (N = 8). However, only sperm volume was significantly decreased. Moreover, this analysis may have been biased because the 2 cryptozoospermia patients were both in the moderately ill group, and the 4 moderately ill patients were all in the fever‐positive group. COVID‐19 may affect male reproduction by interfering with autophagy or inducing oxidative stress during spermatogenesis and endocrine processes. 50 , 51 Additionally, ACE2, as a component of the renin‐angiotensin‐aldosterone system (RAAS), plays an important role in male reproduction (eg steroidogenesis and spermatogenesis). 52 , 53 The entry of SARS‐CoV‐2 into cells via ACE2 led to marked downregulation of ACE2 receptors. 54 Therefore, virus‐induced ACE2 deficiency may impair male reproduction. More studies are needed to test these hypotheses.
In addition to sperm characteristics, other reproduction‐related symptoms of COVID‐19 have been reported. Ma et al. found an increase in serum luteinizing hormone (LH) and a decrease in the ratios of testosterone (T):LH and follicle‐stimulating hormone (FSH):LH among COVID‐19 patients. 30 This suggests that there is a relationship between hypogonadism and COVID‐19. 55 Similarly, Rastrelli et al. found that higher LH and lower testosterone were associated with adverse outcomes in male COVID‐19 patients. 56 Whether reduced testosterone in patients represents a pre‐existing condition or virus‐induced hypogonadism is unclear. It is also possible that testosterone might contribute to COVID‐19 by upregulating TMPRSS2 expression and modulating coagulation. 57 Conversely, testosterone may favour resistance to COVID‐19 by maintaining respiratory muscle activity and suppressing immune and inflammatory responses. 57 , 58 In summary, the relationship between testosterone and COVID‐19 is multifactorial and needs further research. 59 The research also reported that 3 of 12 enrolled patients experienced a loss of libido, and 1 experienced the loss of morning erections after having COVID‐19. 30 It was suspected that COVID‐19 might affect male sexual health. 60 However, a large‐sample cross‐sectional online survey found that sexual desire and sexual frequency were also decreased among the non‐COVID‐19 population during the pandemic, 61 suggesting that the COVID‐19 pandemic and related containment measures may generally affect sexual health.
Currently, the evidence is insufficient to link COVID‐19 and semen quality impairment. First, the results of the included studies were limited by small sample size or the lack of a control group. Second, most studies did not report semen parameters of participants before SARS‐CoV‐2 infection. Third, sperm parameters may also be affected by systemic disease, such as fever, which is a common symptom accompanying COVID‐19. 21 However, the semen parameters of patients with severe or critical disease in either the infection period or recovered period have not been investigated. In addition, since the duration of spermatogenesis in humans is approximately 74 days, 62 the long‐term effects of COVID‐19 on male fertility remain unclear, and it is important to clarify this for COVID‐19 patients. 55
3.3. Pathological characteristics of the testes of COVID‐19 patients
Three studies investigated the pathological changes in the testes of deceased COVID‐19 patients (Table 3). Yang et al. found that all the study cases (N = 11) showed seminiferous tubule injury, with 2 (18.2%), 5 (45.5%) and 4 (36.4%) cases showing <10%, 10–50% and >50% of seminiferous tubules affected, respectively. 35 The observed injuries included Sertoli cell damage (eg swelling, vacuolation, cytoplasmic rarefaction and detachment from the tubular basement membranes) and spermatogenesis alteration. Additionally, the number of Leydig cells in the testes of deceased COVID‐19 patients was lower than that in the testes of non‐COVID‐19 deceased patients. Interstitial oedema and lymphocytic inflammation (infiltrates of CD3+ T lymphocytes and CD68+ histiocytes) were also observed. In another study assessing the systemic pathological changes due to COVID‐19, Duarte‐Neto et al. found that orchitis and testes fibrin microthrombi occurred in all investigated testes samples from patients with fatal COVID‐19 (N = 2). 37 However, Barton et al. reported that a 77‐year‐old male who died 6 days after the onset of symptoms displayed pathologically normal testes. 63 Most of the above cases with damaged testes were sampled 20–75 days after the onset of disease 35 ; detailed interval times were not provided in 2 cases. 37 These results, combined with those of 1 patient who had pathologically normal testes 6 days after onset, 63 suggest that destruction of the testes may occur after the middle stage of infection.
Table 3.
Summary of studies exploring pathological changes in the testes in COVID‐19 patients
| Reference | Region | Sample size | Diagnostic criteria | Age (year) | Time since onset (day) | Detection methods | Results |
|---|---|---|---|---|---|---|---|
| Yang et al. 35 | China | 12 deceased COVID−19 patients a , 5 control cases b , Testicular tissue | C | 39−87 | 20−75 | HE, IHC | Sertoli cell swelling, vacuolation, cytoplasmic rarefaction, and detachment from tubular basement membranes; intratubular cell mass loss and sloughing into the lumens; 2, 5, and 4 of 11 cases showed mild, moderate, and severe seminiferous tubule injury, respectively c ; 8 of 11 cases showed spermatogenic alteration; reduced Leydig cells; interstitial oedema and inflammatory infiltrates. |
| Nunes Duarte‐Neto et al. 37 | Brazil | 2 fatal cases of COVID−19 d , Testicular tissue | D | 33−83 e | 3–16 e | HE, IHC | Orchitis (N = 2, 100%); fibrin microthrombi in testis (N = 2, 100%). |
| Barton et al. 63 | United States | Deceased patient | A | 77 | 6 | HE, IHC | Normal testes |
| Deceased patient f | A | 42 | NP | HE, IHC | Testicular atrophy |
A: RT‐PCR for SARS‐CoV‐2 RNA from nasopharyngeal swab. C: Positive for SARS‐CoV‐2 RNA from oropharyngeal swabs or bronchoalveolar lavage fluid, or radiological features of viral pneumonia with clinical symptomatology. D: Laboratory confirmation of SARS‐CoV‐2 infection or typical radiological and histological pulmonary changes.
HE, haematoxylin and eosin staining; IHC, immunohistochemistry; and NP, not provided.
Pathological examination was performed in 11 of the 12 cases. One case excluded due to containing predominantly fibrovascular tissue and very few seminiferous tubules.
Testicular tissue from 5 patients: death due to penile cancer (n = 1), castration to treat prostate cancer (n = 3) and perineal trauma (n = 1).
Seminiferous tubular injury was categorized as none, mild, moderate or severe if 0%, <10%, 10–50% or >50% of tubules were affected, respectively.
Ten cases (5 female and 5 male) of systemic autopsy; 2 of 10 cases underwent testis histological examination.
Age range or disease duration was provided for 10 cases.
Patient had progressive myotonic muscular dystrophy and died of acute bacterial bronchopneumonia, likely caused by aspiration.
In addition, several studies have reported scrotal discomfort in COVID‐19 patients during the infection period. In 2 of the studies referenced above, this symptom was reported in 1 of 4 patients with moderate illness 27 and 6 of 34 participants with mild‐moderate illness. 29 These findings were suggestive of viral orchitis, although a comprehensive genitourinary examination was not performed. The other 3 studies were case reports. Kim et al. reported a 42‐year‐old male COVID‐19 patient who had testicular pain, although no abnormalities were found by physical examination. 64 Özveri et al. reported a COVID‐19 patient with the first clinical sign of COVID‐19 being that of external genital pain. 65 A 49‐year‐old male was admitted to the clinic with initial symptoms of a swelling sensation and pain in his left groin and testicle, without other urinary symptoms. Scrotal ultrasound suggested spermatic cord inflammation with no signs of orchitis, while urinalysis, RT‐PCR and culture for potential sexually transmitted diseases were all negative. His pharyngeal swab for SARS‐CoV‐2 was positive, and he did not have any other systemic symptoms. Gagliardi et al. reported the first case of orchiepididymitis associated with COVID‐19 in a 14‐year‐old boy who presented at the hospital due to high fever, pain and swelling in his right testis, and the patient was diagnosed with orchiepididymitis after physical and ultrasonic examination. 66 The patient's nasopharyngeal swab was positive for SARS‐CoV‐2. The urine culture was sterile, and serologic studies for possible viruses and mycoplasma were negative, suggesting that the symptoms could be due to COVID‐19. Similar to the other case report, the boy had no respiratory or urinary tract symptoms. The simultaneous presence of male genital tract symptoms and COVID‐19 with the absence of other common aetiologies suggested that COVID‐19 may affect male genitalia.
Consistent with the pathological changes in the testes due to COVID‐19, a study of 6 patients who died of SARS found that orchitis occurred in all cases, with widespread germ cell destruction and decreased spermatozoa in the seminiferous tubule, a thickened basement membrane, and CD3+ T lymphocyte and CD68+ macrophage infiltration. Likewise, SARS‐CoV was not detected in the testes by in situ hybridization but was detected in the lungs. The study further found extensive IgG precipitation in the seminiferous epithelium, interstitium, some degenerated germ cells and Sertoli cells of the testes, 19 suggesting that immunological and inflammatory injury of the testes occurs during fatal SARS‐CoV infection. Immune and inflammatory reactions have been associated with virus‐related testis damage. For example, in mouse models, MuV and Zika virus could induce the innate immune response of Sertoli cells, Leydig cells or epididymal epithelial cells, resulting in the expression of proinflammatory cytokines and chemokines. 15 , 16 These findings indicated that, although SARS‐CoV‐2 was rarely detected in the reproductive tract of COVID‐19 patients, pathological damage to the testes may have occurred, and the impairment may be attributed to local inflammatory and immunological reactions, 67 , 68 hyperthermia, secondary infection, hypoxia, steroids 35 or vasculitis. 55 Similarly, pathological evidence of COVID‐19 destruction in the testes was derived from deceased patients, and data from survivors are lacking.
4. CONCLUSION AND FUTURE DIRECTIONS
Several conclusions can be drawn from these studies. First, SARS‐CoV‐2 was rarely present in the reproductive tract of men with mild‐moderate COVID‐19, and viral RNA was not present in the reproductive tract of recovered patients. Therefore, the testes may not be a reservoir of SARS‐CoV‐2, and the risk of sexual transmission of the virus is low. Second, COVID‐19 may cause testicular structure damage via immune or inflammatory reactions, which may further affect spermatogenesis. Based on the current preliminary understanding, we suppose that SARS‐CoV‐2 may shed into the male reproductive tract in the relatively early stage of COVID‐19 and then be cleared; the destruction of the testes may occur in the middle stage of infection, depending on the disease course and severity, and the immune and inflammatory reactions may be responsible for the impairment. More studies are needed to address this hypothesis.
With the efforts of numerous scientists, our understanding of the effects of COVID‐19 on male reproduction is expanding. However, there are still some unknown factors: (1) Does SARS‐CoV‐2 definitively present in the male reproductive tract? If so, (1.1) what is the prevalence and clearance time of SARS‐CoV‐2 in semen? (1.2) Is the virus present in semen infectious? In addition, (2) Does COVID‐19 impair semen quality? If so, (2.1) How? Most importantly, (2.2) Are impairments to the male reproductive system temporary or persistent? These questions require more research. Additionally, patients with different clinical severity of disease (ie mild, moderate, severe and critical) and infection statuses (infection period and recovery period) should also be considered in future research.
CONFLICT OF INTEREST
None.
AUTHOR'S CONTRIBUTIONS
YC Yao and YF Li conceived and designed the study. YC Yao, XQ Yuan and LJ Wu acquired the data. YC Yao and N Guo wrote the manuscript. YC Yao and L Yin revised the article. YF Li provided the final approval of the completed article.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China [grant numbers: 81771654 and 81701520].
Li Yin and Yufeng Li are jointly supervised this work.
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐2019) situation reports. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200817‐weekly‐epi‐update‐1.pdf?sfvrsn=b6d49a76_4 August 18, 2020.
- 2. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐Converting Enzyme 2: SARS‐CoV‐2 Receptor and Regulator of the Renin‐Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res. 2020;126(10):1456‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Douglas GC, O'Bryan MK, Hedger MP, et al. The novel angiotensin‐converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145(10):4703‐4711. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Xu X. scRNA‐seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS‐CoV‐2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells. 2020;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salam AP, Horby PW. The Breadth of Viruses in Human Semen. Emerg Infect Dis. 2017;23(11):1922‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50(11):e13140. [DOI] [PubMed] [Google Scholar]
- 8. Paz‐Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika Virus in Body Fluids ‐ Final Report. N Engl J Med. 2018;379(13):1234‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405. [DOI] [PubMed] [Google Scholar]
- 10. Medina FA, Torres G, Acevedo J, et al. Duration of the Presence of Infectious Zika Virus in Semen and Serum. J Infect Dis. 2019;219(1):31‐40. [DOI] [PubMed] [Google Scholar]
- 11. Mate SE, Kugelman JR, Nyenswah TG, et al. Molecular Evidence of Sexual Transmission of Ebola Virus. N Engl J Med. 2015;373(25):2448‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Counotte MJ, Kim CR, Wang J, et al. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Medicine. 2018;15(7):e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mansuy JM, Suberbielle E, Chapuy‐Regaud S, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis. 2016;16(10):1106‐1107. [DOI] [PubMed] [Google Scholar]
- 14. Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65(2):208‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu H, Shi L, Wang Q, et al. Mumps virus‐induced innate immune responses in mouse Sertoli and Leydig cells. Sci Reports. 2016;6:19507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma W, Li S, Ma S, et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell. 2016;167(6):1511‐1524. [DOI] [PubMed] [Google Scholar]
- 17. Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech. 2009;72(8):620‐628. [DOI] [PubMed] [Google Scholar]
- 18. Xie M, Chen Q. Insight into 2019 novel coronavirus ‐ An updated interim review and lessons from SARS‐CoV and MERS‐CoV. Int J Infect Dis. 2020;94:119‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu J, Qi L, Chi X, et al. Orchitis: a complication of severe acute respiratory syndrome (SARS). Biol Reprod. 2006;74(2):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papadopoulos V, Li L, Samplaski M. Why does COVID‐19 kill more elderly men than women? Is there a role for testosterone? Andrology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simoni M, Hofmann MC. The COVID‐19 pandemics: Shall we expect andrological consequences? A call for contributions to ANDROLOGY. Andrology. 2020;8(3):528‐529. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Wang X, Zhang H, et al. The absence of coronavirus in expressed prostatic secretion in COVID‐19 patients in Wuhan city. Reprod Toxicol. 2020;96:90‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song C, Wang Y, Li W, et al. Absence of 2019 novel coronavirus in semen and testes of COVID‐19 patients†. Biol Reprod. 2020;103(1):4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holtmann N, Edimiris P, Andree M, et al. Assessment of SARS‐CoV‐2 in human semen‐a cohort study. Fertil Steril. 2020;114(2):233‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paoli D, Pallotti F, Colangelo S, et al. Study of SARS‐CoV‐2 in semen and urine samples of a volunteer with positive naso‐pharyngeal swab. J Endocrinol Invest. 2020;43(12):1819‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome‐coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma L, Xie W, Li D, et al. Evaluation of sex‐related hormones and semen characteristics in reproductive‐aged male COVID‐19 patients. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo L, Zhao S, Li W, et al. Absence of SARS‐CoV‐2 in Semen of a COVID‐19 Patient Cohort. Andrology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicastri E, D'Abramo A, Faggioni G, et al. Coronavirus disease (COVID‐19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill. 2020;25:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pavone C, Giammanco GM, Baiamonte D, et al. Italian males recovering from mild COVID‐19 show no evidence of SARS‐CoV‐2 in semen despite prolonged nasopharyngeal swab positivity. Int J Impotence Res. 2020;32(5):560‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kayaaslan B, Korukluoglu G, Hasanoglu I, et al. Investigation of SARS‐CoV‐2 in Semen of Patients in the Acute Stage of COVID‐19 Infection. Urol Int. 2020;11:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang M, Chen S, Huang B, et al. Pathological Findings in the Testes of COVID‐19 Patients: Clinical Implications. Eur Urol Focus. 2020;6(5):1124‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duarte‐Neto AN, de Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement of COVID‐19 assessed by ultrasound‐guided minimally invasive autopsy. Histopathology. 2020;77(2):186‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. China. NHCotPsRo . New coronavirus pneumonia prevention and control program (7th edition). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml March 4, 2020.
- 40. Paoli D, Pallotti F, Turriziani O, et al. SARS‐CoV‐2 presence in seminal fluid: Myth or reality. Andrology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bahadur G, Acharya S, Muneer A, et al. SARS‐CoV‐2: diagnostic and design conundrums in the context of male factor infertility. Reprod Biomed Online. 2020;41(3):365‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atkinson B, Petersen E. SARS‐CoV‐2 shedding and infectivity. Lancet. 2020;395(10233):1339‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang Y, Chen S, Yang Z, et al. SARS‐CoV‐2 Viral Load in Clinical Samples from Critically Ill Patients. Am J Respir Crit Care Med. 2020;201(11):1435‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang N, Gong Y, Meng F, et al. Comparative study on virus shedding patterns in nasopharyngeal and fecal specimens of COVID‐19 patients. Sci China Life Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Team C‐I . Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID‐19) in the United States. Nat Med. 2020;26(6):861‐868. [DOI] [PubMed] [Google Scholar]
- 47. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu X, Sun S, Shi Y, Wang H, Zhao R, Sheng J. SARS‐CoV‐2 viral load in sputum correlates with risk of COVID‐19 progression. Crit Care. 2020;24(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen W, Lan Y, Yuan X, et al. Detectable 2019‐nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun J. The hypothesis that SARS‐CoV‐2 affects male reproductive ability by regulating autophagy. Med Hypotheses. 2020;143:110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sengupta P, Dutta S. Does SARS‐CoV‐2 infection cause sperm DNA fragmentation? Possible link with oxidative stress. Eur J Contracep Reprod Health Care. 2020:10;1‐2. [DOI] [PubMed] [Google Scholar]
- 52. Reis AB, Araujo FC, Pereira VM, Dos Reis AM, Santos RA, Reis FM. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: implications for male infertility. J Mol Histol. 2010;41(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 53. Younis JS, Abassi Z, Skorecki K. Is there an impact of the COVID‐19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 2020;318(6):E878‐e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med. 2020;76:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Corona G, Baldi E, Isidori AM, et al. SARS‐CoV‐2 infection, male fertility and sperm cryopreservation: a position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Societa Italiana di Andrologia e Medicina della Sessualita). J Endocrinol Invest. 2020;43(8):1153‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rastrelli G, Di Stasi V , Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cattrini C, Bersanelli M, Latocca MM, Conte B, Vallome G, Boccardo F. Sex Hormones and Hormone Therapy during COVID‐19 Pandemic: Implications for Patients with Cancer. Cancers (Basel). 2020;12(8):2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pozzilli P, Commentary LA. Testosterone, a key hormone in the context of COVID‐19 pandemic. Metabolism. 2020;108:154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ory J, Lima TFN, Towe M, et al. Understanding the Complex Relationship Between Androgens and SARS‐CoV2. Urology. 2020;144:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sansone A, Mollaioli D, Ciocca G, et al. Addressing male sexual and reproductive health in the wake of COVID‐19 outbreak. J Endocrinol Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li G, Tang D, Song B, et al. Impact of the COVID‐19 Pandemic on Partner Relationships and Sexual and Reproductive Health: Cross‐Sectional, Online Survey Study. J Med Internet Res. 2020;22(8):e20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Griswold MD. Spermatogenesis: The Commitment to Meiosis. Physiol Rev. 2016;96(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID‐19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: An atypical presentation of COVID‐19. Am J Emerg Med. 2020;38(7):1542.e1‐1542.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Özveri H, Eren MT, Kırışoğlu CE, Sarıgüzel N. Atypical presentation of SARS‐CoV‐2 infection in male genitalia. Urol Case Rep. 2020;33:101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gagliardi L, Bertacca C, Centenari C, et al. Orchiepididymitis in a Boy With COVID‐19. Pediatr Infect Dis J. 2020;39(8):e200‐e202. [DOI] [PubMed] [Google Scholar]
- 67. Dutta S, Sengupta P. SARS‐CoV‐2 and Male Infertility: Possible Multifaceted Pathology. Reprod Sci. 2020;28(1):23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Al NA. Histopathologic and Autopsy Findings in Patients Diagnosed With Coronavirus Disease 2019 (COVID 19): What we know So Far Based on Correlation With Clinical, Morphologic and Pathobiological Aspects. Adv Anat Pathol. 2020. [DOI] [PubMed] [Google Scholar]


