Abstract
Background
This is the first randomised controlled trial for assessment of the immunogenicity and safety of a candidate non-replicating adenovirus type-5 (Ad5)-vectored COVID-19 vaccine, aiming to determine an appropriate dose of the candidate vaccine for an efficacy study.
Methods
This randomised, double-blind, placebo-controlled, phase 2 trial of the Ad5-vectored COVID-19 vaccine was done in a single centre in Wuhan, China. Healthy adults aged 18 years or older, who were HIV-negative and previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-free, were eligible to participate and were randomly assigned to receive the vaccine at a dose of 1 × 1011 viral particles per mL or 5 × 1010 viral particles per mL, or placebo. Investigators allocated participants at a ratio of 2:1:1 to receive a single injection intramuscularly in the arm. The randomisation list (block size 4) was generated by an independent statistician. Participants, investigators, and staff undertaking laboratory analyses were masked to group allocation. The primary endpoints for immunogenicity were the geometric mean titres (GMTs) of specific ELISA antibody responses to the receptor binding domain (RBD) and neutralising antibody responses at day 28. The primary endpoint for safety evaluation was the incidence of adverse reactions within 14 days. All recruited participants who received at least one dose were included in the primary and safety analyses. This study is registered with ClinicalTrials.gov, NCT04341389.
Findings
603 volunteers were recruited and screened for eligibility between April 11 and 16, 2020. 508 eligible participants (50% male; mean age 39·7 years, SD 12·5) consented to participate in the trial and were randomly assigned to receive the vaccine (1 × 1011 viral particles n=253; 5 × 1010 viral particles n=129) or placebo (n=126). In the 1 × 1011 and 5 × 1010 viral particles dose groups, the RBD-specific ELISA antibodies peaked at 656·5 (95% CI 575·2–749·2) and 571·0 (467·6–697·3), with seroconversion rates at 96% (95% CI 93–98) and 97% (92–99), respectively, at day 28. Both doses of the vaccine induced significant neutralising antibody responses to live SARS-CoV-2, with GMTs of 19·5 (95% CI 16·8–22·7) and 18·3 (14·4–23·3) in participants receiving 1 × 1011 and 5 × 1010 viral particles, respectively. Specific interferon γ enzyme-linked immunospot assay responses post vaccination were observed in 227 (90%, 95% CI 85–93) of 253 and 113 (88%, 81–92) of 129 participants in the 1 × 1011 and 5 × 1010 viral particles dose groups, respectively. Solicited adverse reactions were reported by 183 (72%) of 253 and 96 (74%) of 129 participants in the 1 × 1011 and 5 × 1010 viral particles dose groups, respectively. Severe adverse reactions were reported by 24 (9%) participants in the 1 × 1011 viral particles dose group and one (1%) participant in the 5 × 1010 viral particles dose group. No serious adverse reactions were documented.
Interpretation
The Ad5-vectored COVID-19 vaccine at 5 × 1010 viral particles is safe, and induced significant immune responses in the majority of recipients after a single immunisation.
Funding
National Key R&D Programme of China, National Science and Technology Major Project, and CanSino Biologics.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 12·1 million cases of COVID-19 worldwide, resulting in 551 000 deaths and severe economic disruption.1, 2 After the initial outbreak, with more than 80 000 cases and 3000 deaths in China, COVID-19 has now spread to 216 countries and territories. Large numbers of cases and deaths are reported daily from Europe, the USA, Brazil, Russia, India, and many other countries.3, 4 The current pandemic has highlighted the need for effective preventive solutions to reduce burden and spread of the disease. As long as there is a COVID-19 epidemic in one area in the world, there is a risk of a pandemic.
Research in context.
Evidence before this study
We searched PubMed on July 16, 2020, for clinical trial reports with the terms “COVID-19” or “SARS-CoV-2”, “vaccine”, and “clinical trial”. Using the same terms, we also searched ClinicalTrials.gov for unpublished trials of COVID-19 vaccines. Except for the results of our earlier phase 1 study with the adenovirus type-5 (Ad5)-vectored vaccine and a phase 1 clinical trial with an mRNA vaccine (mRNA-1273) done in a small number of participants, no other COVID-19 vaccine data from clinical trials have been reported. We found registered trials with 11 candidate COVID-19 vaccines at ClinicalTrials.gov, including three recombinant protein-based vaccines, two viral vector-based vaccines, one DNA vaccine, two mRNA vaccines, two inactivated virus vaccines, and one autologous dendritic cell-based vaccine loaded with antigens from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The majority of the trials registered were in early phases; only ChAdOx1 nCoV-19 developed by the University of Oxford (Oxford, UK) is going to be evaluated in a phase 3 trial.
In the previously reported open-label, non-randomised, phase 1 trial, we found that the Ad5-vectored COVID-19 vaccine was tolerable and immunogenic in healthy adults. One dose of the vaccine induced rapid specific T-cell and humoral responses by 14 days.
Added value of this study
This study provides more evidence for the immunogenicity and safety of the Ad5-vectored COVID-19 vaccine in a larger population. To assess the vaccine in a more diverse population, we removed the age cap for the recruitment of participants for this phase 2 trial. Older individuals (ie, aged ≥55 years), many of whom often have chronic illness, have a high risk of serious illness and death associated with SARS-CoV-2 infection; thus, they are an important target population for a COVID-19 vaccine. Our results suggest a single-dose immunisation schedule of Ad5-vectored COVID-19 vaccine at 5 × 1010 viral particles is an appropriate regimen for healthy adults. Compared with the younger population, we found older people to have a significantly lower immune response, but higher tolerability, to the Ad5-vectored COVID-19 vaccine. Therefore, an additional dose might be needed to induce a better immune response in the older population, and this will be evaluated in a phase 2b trial.
Implications of all the available evidence
Evidence from this phase 2 study indicates the candidate Ad5-vectored COVID-19 vaccine has a good safety profile, with only mild, transient adverse events related to vaccination and no serious adverse events. Single-dose immunisation with the vaccine induced rapid onset of immune responses within 14 days and significant humoral and cellular immune responses within 28 days in the majority of the recipients. We are planning an international multicentre, randomised, double-blind, controlled phase 3 effectiveness trial to further evaluate the efficacy of the vaccine. We are in the midst of a global COVID-19 pandemic; thus, timely sharing of the results of clinical trials with candidate vaccines is critical.
Unlike typical vaccine development, which often takes decades, developing a vaccine to prevent COVID-19 has become a race between humans and the virus.5 Many countries have accelerated the process of clinical trials to determine an effective and safe vaccine to prevent COVID-19 and influence the course of the current pandemic.6, 7 Currently, about 250 candidate vaccines against SARS-CoV-2 are in development worldwide, including mRNA vaccines, replicating or non-replicating viral vectored vaccines, DNA vaccines, autologous dendritic cell-based vaccine, and inactive virus vaccines.8 To date, at least 17 of these vaccine candidates are under evaluation in clinical trials.
In March 2020, we did a single-centre, open-label, non-randomised, first-in-human phase 1 trial9 with CanSino Biologics' (Tianjin, China) adenovirus type-5 (Ad5)-vectored COVID-19 vaccine in a dose-escalating manner (5 × 1010, 1 × 1011, and 1·5 × 1011 viral particles). Generally, the candidate vaccines had acceptable safety and tolerability profiles and promising immunogenicity in healthy Chinese adults; however, the high-dose vaccine was associated with an increased risk of severe adverse reactions. We therefore carried forward the phase 2 trial with only the 5 × 1010 and 1 × 1011 viral particles doses of the candidate vaccine, aiming to further evaluate the immunogenicity and safety in a larger population, and to determine an appropriate dose for the efficacy study.
Methods
Study design and participants
This randomised, double-blind, placebo-controlled, phase 2 trial of the Ad5-vectored COVID-19 vaccine was done in a single centre in Wuhan (Hubei province, China). The study was done in accordance with the Declaration of Helsinki and Good Clinical Practice. An independent data safety monitoring board was established before the start of the trial to provide oversight of the safety data during the study. The trial protocol was reviewed and approved by the National Medical Products Administration, China, and the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention. The protocol is available online.
Eligible participants were healthy adults aged 18 years or older, who were HIV-negative and previous SARS-CoV-2 infection-free, confirmed by commercial human immunodeficiency virus antibody detection kit (InTec products, Xiamen, China) and SARS-CoV-2 rapid test kit (Jinwofu, Beijing, China) using fingertip blood at screening. To be included, participants needed to be able to understand the content of informed consent and willing to sign the informed consent; able and willing to complete all the scheduled study processes; have an axillary temperature of 37·0°C or less; have a body-mass index of between 18·5 and 30·0; and have general good health as established by medical history and physical examination. Pregnant or breastfeeding women were excluded. People with mental disease, history of allergies, or serious cardiovascular disease, and some other major chronic illnesses were also excluded. A complete list of the inclusion and exclusion criteria is provided in the protocol. Participants were recruited through online recruitment advertisements. Written informed consent was obtained from each participant before screening for eligibility.
Randomisation and masking
The Ad5-vectored COVID-19 vaccine was developed by the Beijing Institute of Biotechnology (Beijing, China) and CanSino Biologics, and contained replication-defective Ad5 vectors expressing the full-length spike gene based on Wuhan-Hu-1 (GenBank accession number YP_009724390). The placebo contained the vaccine excipients only, with no viral particles. The experimental vaccines and placebos had identical packaging with a randomisation number on each vial as the only identifiers. The vaccines of 1 × 1011 and 5 × 1010 viral particles and placebo were randomised at a 2:1:1 ratio. Eligible participants were sequentially assigned a randomisation number, according to a blocked randomisation list (block size 4) generated by an independent statistician using SAS software (version 9.4), and injected with an experimental vaccine or placebo labelled with the same number. Individuals involved in randomisation and masking had no involvement in the rest of the trial. Participants, investigators, and staff undertaking laboratory analyses were masked to group allocation.
Procedures
A single injection of the vaccines of 1 × 1011 or 5 × 1010 viral particles per mL, or placebo, were given to participants intramuscularly in the arm. Participants were monitored for 30 min post injection for immediate adverse reactions, and followed up for any injection site or systemic adverse reactions within 14 days and adverse events within 28 days post vaccination. Serious adverse events self-reported by participants were documented throughout the study.
The detailed methods of the assays have been reported previously.9 In brief, blood samples were taken from participants at day 0 immediately before vaccination, and at days 14 and 28 post vaccination for the measurement of specific antibody responses against the receptor binding domain (RBD) using ELISA kits (Beijing Wantai BioPharm, Beijing, China). The detection limit for the RBD-specific ELISA antibody test was 1:40. The neutralising antibody responses to live SARS-CoV-2 virus (strain SARS-CoV-2/human/CHN/Wuhan_IME-BJ01/2020, GenBank number MT291831.1) or a pseudovirus (a vesicular stomatitis virus pseudovirus system expressing the spike glycoprotein),10 and cellular immune responses before the vaccination and 28 days after the vaccination were also measured. The detection limits for the neutralising antibody tests to live SARS-CoV-2 virus and a pseudovirus were 1:8 and 1:10, respectively. Undetectable antibody titres in serum were assigned values of half the detection limits for calculation. The cellular immune responses of the expression of interferon (IFN) γ stimulated by the overlapping peptide pool of spike glycoprotein were detected by enzyme-linked immunospot (ELISpot) assay (Mabtech, Stockholm, Sweden). Positive IFNγ-ELISpot response was defined as at least five spot-forming cells per 1 × 105 peripheral blood mononuclear cells, and a minimum of a two-times increase from baseline. Neutralising antibody titres against the vaccine vector Ad5 were measured with the serum neutralisation assay.11 The follow-ups were scheduled at days 14 and 28, and month 6 post vaccination for safety and immunogenicity assessment.
Outcomes
The primary objectives were to evaluate immunogenicity and safety of the Ad5-vectored COVID-19 vaccine, and to determine a vaccine dose for a phase 3 efficacy study. The primary endpoint for safety evaluation was the incidence of adverse reactions within 14 days after the injection. The primary endpoints for immunogenicity were the geometric mean titres (GMTs) of RBD-specific ELISA antibody responses and neutralising antibody responses against live virus or pseudovirus at day 28 post vaccination. The secondary endpoints for immunogenicity were RBD-specific ELISA antibody responses at day 14 and month 6 (6 month data not yet available), and specific T-cell responses at day 28 post vaccination. Seroconversion of the humoral immune responses was also a secondary endpoint, and was defined as an increase in post -vaccination titre of at least four-times from baseline. The secondary safety outcomes included the occurrence of adverse events from days 0 to 28 after vaccination, and serious adverse events reported up to 6 months. Investigators did severity grading of the adverse events according to the standard guidelines issued by the China State Food and Drug Administration, and the causality with immunisation before unmasking. Stratified analysis of safety and immunogenicity of the participants was done based on the baseline Ad5 neutralising antibody titres with a cutoff at 1:200. Post-hoc analysis of the immune responses by age and sex, and the proportion of vaccine recipients with the composite endpoint of either positive cellular or seroconversion of humoral immune responses at day 28 post vaccination were presented.
Statistical analysis
This phase 2 trial was launched before the immunogenicity data from the phase 1 trial were obtained; therefore, the sample size was not calculated at the design stage. An overall sample size of 500 participants (n=250 in the 1 × 1011 viral particles dose group; n=125 in the 5 × 1010 viral particles dose group; and n=125 in the placebo group) was determined, based on expert opinion and the minimum sample size requirement in the technical guidelines for vaccine clinical trials issued by the National Medical Products Administration, China.12 We did an ex-post power calculation of this study after the immunogenicity data from the phase 1 study were available, using PASS software (version 11.0). For the RBD-specific antibody, 250 individuals in the 1 × 1011 viral particles dose group and 125 individuals in the 5 × 1010 viral particles dose group were able to provide at least a power of 90% to show a difference of the log-transferred titre of 0·176 with an SD of 0·4 between the dose groups, at a level of significance of 0·017, considering multiple comparisons.
Statistical tests were two-sided with an α value of 0·05, and analysed by an independent statistician using SAS (version 9.4). The primary immunogenicity analysis was done in the full-analysis cohort, including all participants who were injected and donated blood samples for antibody tests post vaccination, while the safety analysis was done in all enrolled participants who received the vaccination. Correlation analysis of the RBD-specific ELISA antibody and neutralising antibody was done, and the Pearson correlation coefficient was calculated. Antibody responses are reported as the GMT with 95% CI. ANOVA was used for log-transformed antibody titres, and the Wilcoxon rank-sum test for data that were not normally distributed. The χ2 test or Fisher's exact test was used for categorical data. Multiple comparisons were done if a significant difference across the treatment groups was noted, using Student Newman-Keuls test or Bonferroni-adjusted α value when relevant. This trial is registered with ClinicalTrials.gov, NCT04341389.
Role of the funding source
The funders of the study were involved in protocol design, but not in data collection, statistical analysis, data interpretation, or writing of the report. All the authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
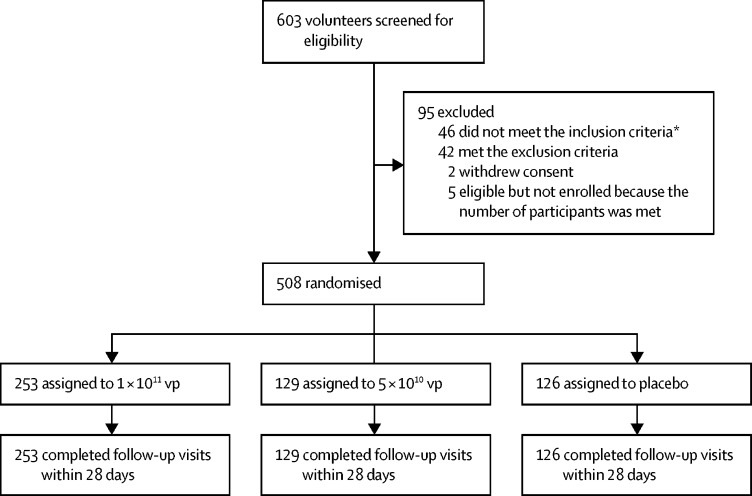
603 volunteers were recruited and screened for eligibility between April 11 and 16, 2020 (figure 1 ). 95 individuals were excluded, leaving 508 eligible participants who consented to participate in the trial and were randomly assigned to vaccine or placebo. 253 were randomly assigned to the 1 × 1011 viral particles dose group, 129 to the 5 × 1010 viral particles dose group, and 126 to the placebo group. The mean age of the participants was 39·7 years (SD 12·5; range 18–83), with 309 (61%) individuals aged 18–44 years, 134 (26%) aged 45–54 years, and 65 (13%) aged 55 years or older across the treatment groups (table 1 ). 254 (50%) of 508 participants were male. Baseline characteristics of the participants and the pre-existing Ad5 neutralising antibody titres were largely similar across the treatment groups. Among the 508 participants, 266 (52%) had high pre-existing immunity and 242 (48%) had low pre-existing immunity to the Ad5 vector. All the participants completed the scheduled safety visits within 28 days post vaccination and gave blood samples at days 0 and 28, and 506 (>99%) donated blood samples at day 14.
Figure 1.
Trial profile
vp=viral particles. *26 did not meet the inclusion criteria of negative IgM/IgG to severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline characteristics
| Vaccine at 1 × 1011 vp (n=253) | Vaccine at 5 × 1010 vp (n=129) | Placebo (n=126) | ||
|---|---|---|---|---|
| Age, years | ||||
| 18–44 | 152 (60%) | 80 (62%) | 77 (61%) | |
| 45–54 | 67 (26%) | 32 (25%) | 35 (28%) | |
| ≥55 | 34 (13%) | 17 (13%) | 14 (11%) | |
| Mean | 40·0 (12·8) | 39·7 (12·1) | 39·2 (12·5) | |
| Sex | ||||
| Male | 126 (50%) | 64 (50%) | 64 (51%) | |
| Female | 127 (50%) | 65 (50%) | 62 (49%) | |
| Body-mass index, kg/m2 | 24·2 (2·8) | 24·2 (2·7) | 23·3 (2·6) | |
| Underlying diseases | ||||
| Yes | 8 (3%) | 8 (6%) | 6 (5%) | |
| No | 245 (97%) | 121 (94%) | 120 (95%) | |
| Pre-existing adenovirus type-5 neutralising antibody | ||||
| ≤1:200, titre | 127 (50%) | 54 (42%) | 61 (48%) | |
| >1:200, titre | 126 (50%) | 75 (58%) | 65 (52%) | |
Data are number of participants (%) or mean (SD). vp=viral particles.
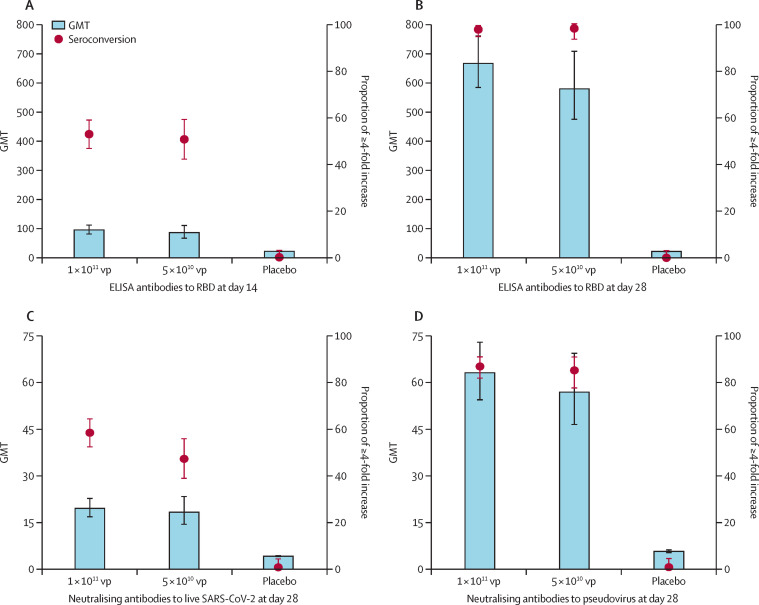
The baseline antibody titres of the participants are given in the appendix (p 1). RBD-specific ELISA antibody responses induced by the Ad5-vectored COVID-19 vaccine were detected from day 14 onwards, with GMTs of 94·5 (95% CI 80·5–110·8) and 85·1 (66·0–109·7) in the 1 × 1011 and 5 × 1010 viral particles dose groups, respectively (figure 2 ). At day 28, the RBD-specific ELISA antibodies peaked at 656·5 (575·2–749·2) in the 1 × 1011 viral particles dose group and 571·0 (467·6–697·3) in the 5 × 1010 viral particles dose group. 244 (96%, 95% CI 93–98) of 253 participants in the 1 × 1011 viral particles dose group and 125 (97%, 92–99) of 129 participants in the 5 × 1010 viral particles dose group showed seroconversion of RBD-specific ELISA antibodies at day 28, whereas the participants in the placebo group showed no antibody increase from baseline.
Figure 2.
Specific antibody responses to RBD, neutralising antibodies to live severe acute respiratory syndrome coronavirus 2 and pseudovirus post vaccination
Seroconversion was defined as an increase in post-vaccination titre of at least four-times baseline. The baseline antibody titres are shown in the appendix (p 1). All comparisons across the three treatment groups are p<0·0001. Multiple comparisons showed no significant difference between the 1 × 1011 vp and 5 × 1010 vp dose groups. GMT=geometric mean antibody titre. RBD=receptor binding domain. vp=viral particles.
Both vaccine doses induced significant neutralising antibody responses to live SARS-CoV-2, with GMTs of 19·5 (95% CI 16·8–22·7) and 18·3 (14·4–23·3) in participants in the 1 × 1011 and 5 × 1010 viral particles dose groups, respectively, at day 28 post vaccination (figure 2). Seroconversion of the neutralising antibody responses to live SARS-CoV-2 occurred in 148 (59%, 95% CI 52–65) of 253 participants receiving the 1 × 1011 viral particles dose, and in 61 (47%, 39–56) of 129 participants receiving the 5 × 1010 viral particles dose 28 days post vaccination. The GMTs of neutralising antibody responses to pseudovirus were 61·4 (95% CI 53·0–71·0) in the 1 × 1011 viral particles dose group and 55·3 (45·3–67·5) in the 5 × 1010 viral particles dose group, with seroconversion of 214 (85%, 95% CI 80–89) and 107 (83%, 76–88), respectively. No significant differences were observed between the two dose groups in the neutralising antibody responses to live virus and pseudovirus.
Before vaccination, 266 (52%) of 508 participants had high pre-existing anti-Ad5 neutralising antibodies (table 1). Participants with low pre-existing anti-Ad5 immunity had RBD-specific ELISA antibody and neutralising antibody levels that were approximately two-times higher than the participants with high pre-existing anti-Ad5 immunity (appendix pp 2–3). Increasing age was found to be another independent negative impact factor on the RBD-specific ELISA antibody (p=0·0018), and neutralising antibody responses to live virus (p<0·0001) or pseudovirus (p=0·046; appendix pp 4–6). The stratified analysis based on age found that participants aged 55 years or older were associated with relative low antibody responses in both dose groups post vaccination, particularly in terms of neutralising antibodies to live virus (appendix pp 7–8). Nevertheless, the ELISA antibodies to RBD and neutralising antibodies at day 28 in vaccine recipients were still significantly higher than in placebo recipients in this population. Male and female participants who received the vaccine showed similar RBD-specific ELISA antibody and neutralising antibody responses post vaccination (appendix pp 9–10). Both the ELISA antibody titres to RBD and neutralising antibody titres to pseudovirus were significantly correlated with the neutralising antibody titres to live virus, with a correlation coefficient of 0·75, and 0·72 (p<0·0001), respectively.
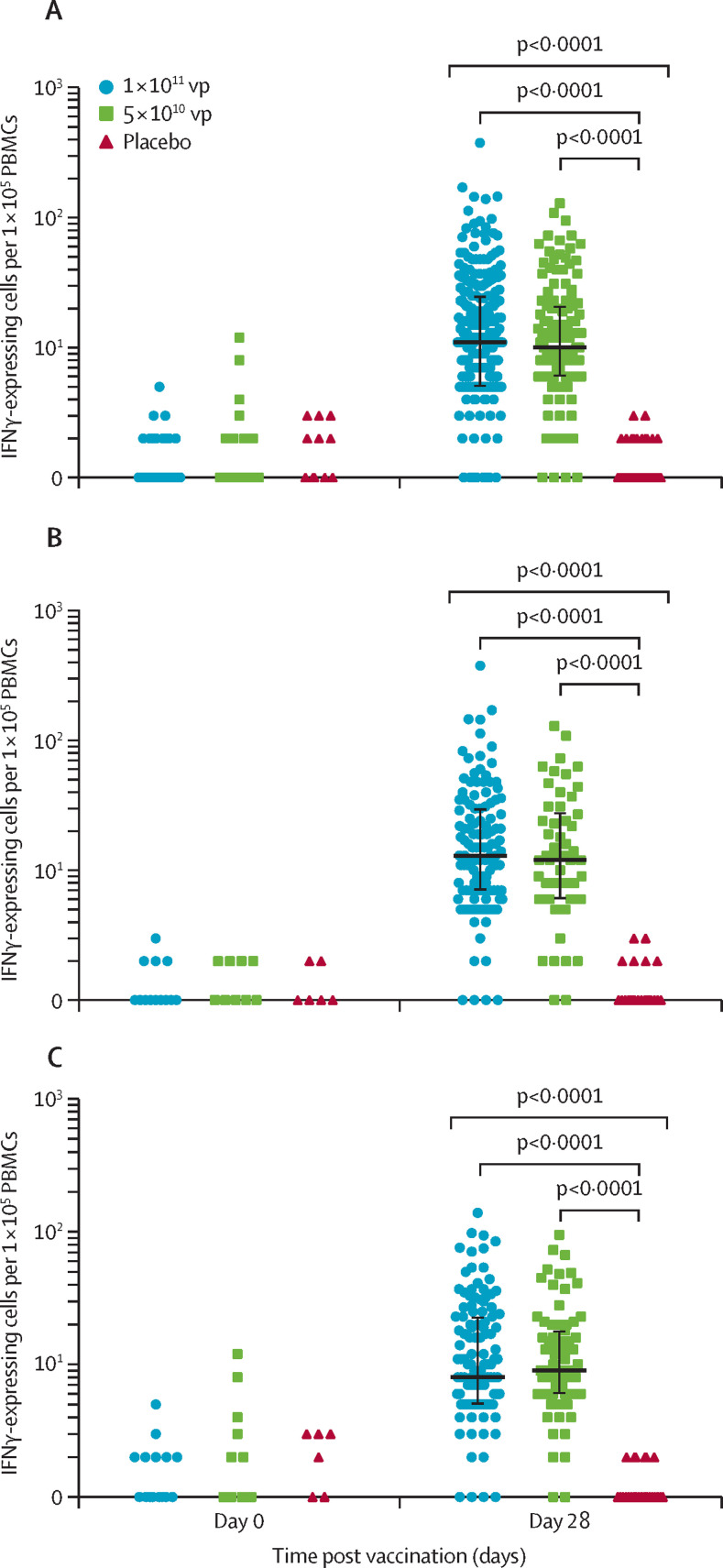
Baseline ELISpot T-cell responses were negative in 506 (>99%) of 508 participants. Ad5-vectored COVID-19 vaccine induced significant SARS-CoV-2 spike glycoprotein-specific IFNγ-ELISpot responses in 227 (90%, 95% CI 85–93) of 253 participants receiving the 1 × 1011 viral particles dose, and 113 (88%, 81–92) of 129 participants receiving the 5 × 1010 viral particles dose at day 28 (figure 3 ). A median of 11·0 spot-forming cells (IQR 5·0–25·0) and 10·0 spot-forming cells (6·0–21·0) per 1 × 105 peripheral blood mononuclear cells in participants in the 1 × 1011 viral particles and 5 × 1010 viral particles dose groups, respectively, were observed at day 28, with increases of more than ten-times in both dose groups. The IFNγ-ELISpot responses were not significantly different between the dose groups at day 28. No positive IFNγ-ELISpot T-cell responses were detected in the placebo group post vaccination. Significant increases of post-vaccination T-cell responses in terms of spot-forming cells were observed both in participants with high and low pre-existing neutralising antibody at day 28. In the participants with high pre-existing immunity against Ad5, 88% of participants across both groups (111 of 126 in the 1 × 1011 viral particles dose group, and 66 of 75 in the 5 × 1010 viral particles dose group) showed positive IFNγ-ELISpot T-cell responses post vaccination. Sex and age of the participants did not differ their IFNγ-ELISpot T-cell responses induced by vaccination (appendix p 11). In addition, 241 (95%, 95% CI 92–97) of 253 participants in the 1 × 1011 viral particles dose group, and 118 (91%, 85–95) of 129 participants in the 5 × 1010 viral particles dose group showed either positive IFNγ-ELISpot T-cell response or seroconversion of neutralising antibody to live SARS-CoV-2 at day 28 post vaccination (appendix p 12).
Figure 3.
Specific T-cell responses measured by ELISpot
The number of specific T cells with secretion of IFNγ at days 0 and 28 in all participants (A), and stratified by pre-existing adenovirus type-5 neutralising antibody titres of less than or equal to 1:200 (B) and more than 1:200 (C). vp=viral particles. IFN=interferon. PBMC=peripheral blood mononuclear cell.
Within 14 days after the vaccination, 183 (72%) of 253 participants in the 1 × 1011 viral particles dose group, and 96 (74%) of 129 participants in the 5 × 1010 viral particles dose group reported at least one solicited adverse reaction, both of which were significantly higher than the 46 (37%) of 126 participants in the placebo group (p<0·0001; table 2 ). The most common systemic solicited reactions in the 5 × 1010 and 1 × 1011 viral particles dose groups were fatigue, reported by 42% and 34%; fever, reported by 32% and 16%; and headache, reported by 29% and 28%, respectively. The most common injection site solicited reaction was pain, reported by 57% of the 1 × 1011 viral particles dose group and 56% of the 5 × 1010 viral particles dose group. Although most adverse reactions were reported as either mild or moderate, 24 (9%) of 253 participants receiving the vaccine at 1 × 1011 viral particles had severe (grade 3) adverse reactions, which was significantly higher than those receiving the vaccine at 5 × 1010 viral particles (p=0·0011) or placebo (p=0·0004). The most commonly reported grade 3 adverse reactions was fever, in 20 (8%) of 253 participants in the 1 × 1011 viral particles dose group, and one (1%) of 129 participants in the 5 × 1010 viral particles dose group. High pre-existing Ad5 immunity, increasing age, and male sex were associated with significantly lower occurrence of fever post vaccination (appendix p 13). The grade 3 reactions were self-limited and resolved within 72–96 h without medication (appendix pp 14–15). The unsolicited adverse reactions within 14 days post vaccination were reported by 19 (8%) participants in the 1 × 1011 viral particles dose group, seven (5%) in the 5 × 1010 viral particles dose group, and seven (6%) in the placebo group, showing no difference across the groups. Overall, 196 (77%) participants in the 1 × 1011 viral particles dose group, 98 (76%) in the 5 × 1010 viral particles dose group, and 61 (48%) in the placebo group experienced at least one or more adverse event within 28 days after the vaccination. No serious adverse events were documented within 28 days.
Table 2.
Adverse reactions within 14 days and overall adverse events within 28 days after vaccination
| Vaccine at 1 × 1011 vp (n=253) | Vaccine at 5 × 1010 vp (n=129) | Placebo (n=126) | p value | |||
|---|---|---|---|---|---|---|
| Solicited adverse reactions within 14 days | ||||||
| Any | 183 (72%) | 96 (74%) | 46 (37%) | <0·0001 | ||
| Grade 3 | 24 (9%) | 1 (1%) | 0 | <0·0001 | ||
| Injection site adverse reactions | ||||||
| Pain | 145 (57%) | 72 (56%) | 11 (9%) | <0·0001 | ||
| Induration | 12 (5%) | 2 (2%) | 0 | 0·014 | ||
| Grade 3 induration | 2 (1%) | 0 | 0 | 0·75 | ||
| Redness | 5 (2%) | 1 (1%) | 2 (2%) | 0·81 | ||
| Swelling | 10 (4%) | 5 (4%) | 0 | 0·049 | ||
| Grade 3 swelling | 1 (<1%) | 0 | 0 | 1·0 | ||
| Itch | 14 (6%) | 3 (2%) | 0 | 0·0075 | ||
| Systemic adverse reactions | ||||||
| Fever (all grades) | 82 (32%) | 21 (16%) | 12 (10%) | <0·0001* | ||
| Grade 3 fever | 20 (8%) | 1 (1%) | 0 | 0·0001† | ||
| Headache | 73 (29%) | 36 (28%) | 17 (13%) | 0·0031 | ||
| Grade 3 headache | 2 (1%) | 0 | 0 | 0·75 | ||
| Fatigue | 106 (42%) | 44 (34%) | 21 (17%) | <0·0001 | ||
| Grade 3 fatigue | 1 (<1%) | 0 | 0 | 1·0 | ||
| Vomiting | 4 (2%) | 1 (1%) | 1 (1%) | 0·88 | ||
| Diarrhoea | 19 (8%) | 10 (8%) | 4 (3%) | 0·22 | ||
| Muscle pain | 39 (15%) | 23 (18%) | 3 (2%) | 0·0002 | ||
| Grade 3 muscle pain | 1 (<1%) | 0 | 0 | 1·0 | ||
| Joint pain | 34 (13%) | 13 (10%) | 4 (3%) | 0·0074 | ||
| Grade 3 joint pain | 1 (<1%) | 0 | 0 | 1·0 | ||
| Oropharyngeal pain | 22 (9%) | 7 (5%) | 6 (5%) | 0·27 | ||
| Cough | 12 (5%) | 2 (2%) | 3 (2%) | 0·24 | ||
| Nausea | 20 (8%) | 6 (5%) | 4 (3%) | 0·14 | ||
| Hypersensitivity | 0 | 0 | 2 (2%) | 0·061 | ||
| Dyspnoea | 1 (<1%) | 0 | 0 | 1·0 | ||
| Grade 3 dyspnoea | 1 (<1%) | 0 | 0 | 1·0 | ||
| Appetite impaired | 27 (11%) | 7 (5%) | 3 (2%) | 0·0089 | ||
| Syncope | 1 (<1%) | 1 (1%) | 0 | 1·0 | ||
| Mucosal abnormality | 2 (1%) | 2 (2%) | 2 (2%) | 0·65 | ||
| Pruritus | 6 (2%) | 4 (3%) | 6 (5%) | 0·40 | ||
| Unsolicited adverse reactions within 14 days | ||||||
| Any | 19 (8%) | 7 (5%) | 7 (6%) | 0·65 | ||
| Grade 3 | 1 (<1%) | 0 | 0 | 1·0 | ||
| Overall adverse events within 28 days | ||||||
| Any | 196 (77%) | 98 (76%) | 61 (48%) | <0·0001 | ||
| Grade 3 | 24 (9%) | 1 (1%) | 2 (2%) | 0·0002 | ||
Data are number of participants (%). Any refers to all the participants with any grade adverse reactions or events. Adverse reactions and events were graded according to the scale issued by the China State Food and Drug Administration. Grade 3 is severe (ie, prevented activity). The p value was generated by comparisons across the three treatment groups. vp=viral particles.
Multiple comparisons of the dose groups 1 × 1011 vp versus 5 × 1010 vp; p=0·0008.
Multiple comparisons of the dose groups 1 × 1011 vp versus 5 × 1010 vp; p=0·0038.
All participants tested were seronegative for the antibodies to nucleocapsid protein of SARS-CoV-2 at day 28, by IgG and IgM rapid test kit (Vazyme Biotech, number CD101, Nanjing, China), suggesting none had exposure to SARS-CoV-2 during this study period.
Discussion
This study is the first randomised controlled trial for evaluation of the immunogenicity and safety of a candidate non-replicating Ad5-vectored COVID-19 vaccine. The phase 2 trial is a necessary and crucial step to turn an early-stage experimental vaccine into a promising vaccine candidate in an efficacy trial in a large population. In this study, a single injection of the Ad5-vectored COVID-19 vaccine at 1 × 1011 viral particles and 5 × 1010 viral particles induced comparable specific immune responses to the spike glycoprotein at day 28, with no significant differences noted between the two groups. The vaccine induced seroconversion of the neutralising antibodies in 59% and 47% of participants, and seroconversion of binding antibody in 96% and 97% of participants, in the 1 × 1011 and 5 × 1010 viral particles dose groups, respectively. Positive specific T-cell responses measured by IFNγ-ELISpot were found in 90% and 88% of participants receiving the vaccine at 1 × 1011 and 5 × 1010 viral particles, respectively. 95% of participants in the 1 × 1011 viral particles dose group and 91% of the recipients in the 5 × 1010 viral particles dose group showed either cellular or humoral immune responses at day 28 post vaccination (appendix p 12). Pre-existing immunity to the Ad5 vector and increasing age could partially hamper the specific immune responses to vaccination, particularly for the humoral immune responses.
In this study, most reactions reported post vaccination were mild or moderate. Although the proportions of participants who had adverse reactions such as fever, fatigue, and injection site pain were significantly higher in vaccine recipients than those in placebo recipients, adverse reactions within 28 days were generally not severe, and resolved within a short period of time (no more than 48 h). Among recipients of either dose of the Ad5-vectored COVID-19 vaccines, all grade 3 adverse reactions were reported from the dose group of 1 × 1011 viral particles, with the exception of one from the 5 × 1010 viral particles dose group. Although this study was powered only to capture common adverse events after immunisation, the results suggest that the experimental Ad5-vectored COVID-19 vaccine has a good safety profile, which was in line with the results of our phase 1 trial in healthy adults.9
We initiated this phase 2 trial before the full analysis of the data from the phase 1 study was available. The vaccine doses chosen were mainly based on the safety data from the dose-escalating phase 1 trial: a higher proportion of participants reported grade 3 adverse reactions in the high-dose group (1·5 × 1011 viral particles) compared with low-dose (5 × 1010 viral particles) or middle-dose (1 × 1011 viral particles) groups (17% vs 6% and 6%, respectively).9 Therefore, we assumed that the vaccine doses at 1 × 1011 and 5 × 1010 viral particles might have similar safety profiles. In addition, an increasing antigen dose is often associated with increasing immunogenicity; thus, we expected the vaccine dose at 1 × 1011 viral particles to be the better of the two doses. Therefore, we designed the randomisation of this study in a ratio of 2:1:1 for the dose groups of 1 × 1011 and 5 × 1010 viral particles, and placebo group, respectively, giving more weight to the first group. We found, in contrast to our expectations, that the vaccine at 5 × 1010 viral particles had a better safety profile than, and comparable immunogenicity to, the vaccine at 1 × 1011 viral particles.
Age and pre-existing anti-Ad5 immunity of the participants could have affected the candidate vaccine's safety and immunogenicity. We noted that the occurrence of fever was associated with decreasing age and low pre-existing immunity to the vaccine vector Ad5 virus. 19 (90%) of the 21 participants who experienced grade 3 fever had no pre-existing immunity to Ad5, with a neutralising antibody titre below detection. Increasing age and high pre-existing anti-Ad5 immunity were found to be able to significantly reduce the immune responses to the vaccine. In some participants with high pre-existing anti-Ad5 immunity, one injection of the vaccine might be inadequate to induce a high level of humoral immune responses, particularly for people aged 55 years or older. These results align with the finding that older people are more likely to have exposure history to Ad5, with higher baseline neutralising antibody to Ad5, which indicates that this population might be more tolerant of higher dose or a booster dose regimen of the Ad5-vectored COVID-19 vaccine than people who are young and naive to Ad5. Pre-existing anti-Ad5 immunity is considered to be the biggest obstacle for the candidate Ad5-vectored COVID-19 vaccine to overcome. A flexible additional dose (between months 3 and 6) might be a potential solution to provide enhancement of immune responses, according to our previous experience with an Ad5 vector-based Ebola vaccine in a homologous prime-boost immunisation study.13 More evidence about the immunogenicity and feasibility of additional dose immunisation in the older population will be evaluated in a phase 2b trial. However, the vaccine recipients in this study showed increased anti-Ad5 neutralising antibodies, by 5·0-times and 3·8-times in the 1 × 1011 viral particles and 5 × 1010 viral particles dose groups, respectively, at day 28 post vaccination (appendix p 17). The high level of anti-Ad5 immunity could affect the boosting effect of the vaccine; therefore, we planned to follow the dynamic change of the Ad5-specific antibodies in participants until month 6 to determine the timing of booster administration.
We determined the participants' serostatus before and after immunisation by ELISA, neutralising tests to live SARS-CoV-2 or pseudovirus, and ELISpot, providing evidence of humoral and cell-mediated immunity for the candidate vaccine. Because the live virus neutralising antibody test needs to be done in biosafety level three laboratories, a pseudovirus neutralising antibody test was developed to serve as an alternative.10 However, we found the magnitudes of neutralising antibody responses to pseudovirus were greater than those to the live virus, which might be associated with the different methodological principles of the two tests. In the pseudovirus neutralising test, when the specific antibody in serum binds to the pseudovirus, it inhibits the pseudovirus from entering the cells, reducing the expression of luciferase on the cell surface. Thus, we can calculate the amount of neutralising antibodies to pseudovirus by detecting the total fluorescence. The neutralising antibody is detected by measuring the cytopathic effect after the viral infection. Although, generally, the output values of the two methods are correlated, the two methods have different detection sensitivities and the detection values do not always have a one-to-one corresponding relationship.
Both neutralising antibody and T-cell responses were important in eliminating the virus and controlling disease development in patients with COVID-19 who were naturally infected by SARS-CoV-2.14, 15 Antibodies are very likely to be effective against SARS-CoV-2, considering that convalescent serum samples have been applied with apparently good clinical results in COVID-19.16 But for the vaccine-induced immune responses, whether neutralising antibody alone is capable of preventing infection remains undetermined. Specific T-cell responses are essential for directly attacking and killing virus-infected cells.15 In addition, the CD4 T-cell responses are critical for the cytotoxic T-cell response and the maturating of neutralising antibodies.17 Thus, the evaluation of the cell-mediated responses, in addition to the neutralising antibodies, is important for a successful candidate vaccine.
Our trial has some limitations. First, this phase 2 trial started before the full analysis of the data from the phase 1 study was available, so we did not calculate the sample size based on study power in advance, which might lead to a lack of power to show the difference between dose groups. Second, the participants included in this study are all from Wuhan, China. The baseline anti-Ad5 immunity of the participants seemed to be representative of Chinese adults, according to the previously reported studies;18 however, anti-Ad5 immunity in adults varies from place to place globally, with reported proportions of adults with neutralising antibodies titres for Ad5 of more than 1:200 of about 80% in India, 78% in Kenya, 67% in Thailand, 64% in Uganda, around 60% in South Africa, 45% in Sierra Leone, and less than 30% in the USA.19, 20, 21 We might expect the candidate Ad5-vectored vaccine to have a superior immunogenicity in the population with a lower pre-existing anti-Ad5 immunity, but an inferior immunogenicity in people with a higher pre-existing anti-Ad5 immunity than observed in this phase 2 trial. Third, this trial did not include children. Although COVID-19 appears to have a more benign course in children, with almost no fatalities reported,22, 23 an ideal candidate vaccine for the ongoing pandemic should cover susceptible populations in all ages. Fourth, we report only data within 28 days of vaccination, and do not include data about the durability of the vaccine-induced immunity, which was not available at the time of publication. A subset of patients infected with SARS-CoV-2 might not develop long-lasting antibodies to the virus, and S-antibodies were reported to rapidly decline for people infected with seasonal coronaviruses and who recovered from COVID-19, especially those with mild symptoms or asymptomatic infection.24 The ongoing phase 1 and 2 trials will enable the continued collection of safety data and assessment of antibody persistence over a 6-month period. Fifth, in this study, no participants had SARS-CoV-2 exposure after the vaccination, so we were unable to assess the efficacy of the candidate vaccine or any immunological risk associated with antibody induced by vaccination when exposed to the virus. However, the risk of COVID-19 and antibody-enhanced disease on exposure to the virus will be monitored long term. Finally, the clinical significance of these changes is difficult to assess because of the absence of an identified correlate of protective immunity, and reference standards for measuring neutralising antibodies against COVID-19. Future studies will need to establish an immune correlate of protection and a protective threshold to assess the feasibility of using the Ad5-vectored COVID-19 vaccine to provide protection for high-risk populations or for outbreak intervention.
WHO is facilitating collaboration and efforts to support vaccine development by defining the desired characteristics of promising candidate vaccines to combat COVID-19.25, 26 This situation emphasises the importance of being prepared to initiate an international multicentre, randomised, double-blind, controlled phase 3 effectiveness trial as soon as possible.25 One immunisation of the Ad5-vectored COVID-19 vaccine at 5 × 1010 viral particles has a good safety profile (limited to common adverse reactions following immunisation) and could elicit significant specific immune responses to SARS-CoV-2, making it a potential candidate for emergency vaccination of acute protective response.
In conclusion, the results of this trial have extended our knowledge of the immunogenicity and safety of the Ad5-vectored COVID-19 vaccines. The results support testing of the Ad5-vectored COVID-19 vaccine at 5 × 1010 viral particles in a phase 3 effectiveness trial in healthy adults.
Data sharing
We support sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after de-identification (text, tables, figures, and appendices) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents including study protocol, statistical analysis plan, and the informed consent form will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to jszfc@vip.sina.com or cw0226@foxmail.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.
Acknowledgments
Acknowledgments
We thank Yan-Song Sun, Sen Zhang, and Yu-Chang Li, from the Beijing Institute of Microbiology and Epidemiology (Beijing, China), for laboratory analysis. We thank Jian-Yuan Wu, Hu Wang, Chang Chen, and Han-Ning Hu, from Zhongnan Hospital of Wuhan University (Wuhan, China), and Hai-Ying Zhang, Li Liu, and Lei Wang, from the Hubei Provincial Center for Disease Control and Prevention (Wuhan, China), for on-site implementation. We thank Ke Zhang from the Academy of Military Medical Sciences (Beijing, China), Kun Liu from the General Hospital of Central Theater Command (Wuhan, China), and Chang-Long Fu and his team, from Wuhan Rest Center, Chinese People's Armed Police Force (Wuhan, China), for management of the clinical trial site.
Contributors
F-CZ was the principal investigator, and X-HG and J-YH were co-principal investigators. F-CZ, WC, X-HG, J-YH, L-HH, J-JX, Y-HL, J-XL, W-JW, ZW, J-BG, and S-YJ designed the trial and study protocol. J-XL drafted the Article. WC contributed to critical review and revision of the Article. F-CZ, W-JW, J-XL, H-XP, and L-HH contributed to the data interpretation and revision of the Article. X-WW was responsible for statistical analysis. J-JX, B-SW, and JZ contributed to study supervision. B-FY, W-JW, ZW, TJ, X-HWa, S-YJ, X-AQ, QL, PD, and H-DJ led and participated in the site work, including the recruitment, follow-up, and data collection. Y-HL, TJ, YH, LW, X-HWu, and J-JL were responsible for laboratory analyses. ZZ and S-YJ contributed to the literature search. J-BG and S-PW monitored the trial.
Declaration of interests
WC reports grants from the National Key R&D Program of China (2020YFC10841400), and grants from the National Science and Technology Major Project (2016ZX10004001, 2018ZX09201005). J-BG is an employee of CanSino Biologics. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200710-covid-19-sitrep-172.pdf?sfvrsn=70724b90_2
- 2.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 3.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2020. published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung K, Wu JT, Liu D, Leung GM. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395:1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lurie N, Saville M, Hatchett R, Halton J. Developing COVID-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 6.Caddy S. Developing a vaccine for covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m1790. [DOI] [PubMed] [Google Scholar]
- 7.WHO Draft landscape of COVID-19 candidate vaccines. 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 8.CEPI CEPI-funded COVID-19 vaccine candidates progress to clinical trials. 2020. https://cepi.net/news_cepi/cepi-funded-covid-19-vaccine-candidates-progress-to-clinical-trials/
- 9.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. 395: 1845–54. [DOI] [PMC free article] [PubMed]
- 10.Nie J, Li Q, Wu J. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprangers MC, Lakhai W, Koudstaal W. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Medical Products Administration, China Technical guidelines for clinical trials of vaccines. 2004. http://www.nmpa.gov.cn/WS04/CL2196/323471.html
- 13.Li JX, Hou LH, Meng FY. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Health. 2017;5:e324–e334. doi: 10.1016/S2214-109X(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 14.Grifoni A, Weiskopf D, Ramirez SI. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walls AC, Xiong X, Park YJ. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176:1026–1039. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu B, Zhou Y, Wu H. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J Med Virol. 2012;84:1408–1414. doi: 10.1002/jmv.23325. [DOI] [PubMed] [Google Scholar]
- 19.Thorner AR, Vogels R, Kaspers J. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J Clin Microbiol. 2006;44:3781–3783. doi: 10.1128/JCM.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilankatta R, Chawla T, Khanna N, Swaminathan S. The prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccines. J Med Virol. 2010;82:407–414. doi: 10.1002/jmv.21721. [DOI] [PubMed] [Google Scholar]
- 21.Barouch DH, Kik SV, Weverling GJ. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonker LM, Shen K, Kinane TB. Lessons unfolding from pediatric cases of COVID-19 disease caused by SARS-CoV-2 infection. Pediatr Pulmonol. 2020;55:1085–1086. doi: 10.1002/ppul.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long QX, Tang XJ, Shi QL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. published online June 18. [DOI] [PubMed] [Google Scholar]
- 25.Chi X, Yan R, Zhang J. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc6952. published online June 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Criteria for COVID-19 vaccine prioritization. 2020. https://www.who.int/publications/m/item/criteria-for-covid-19-vaccine-prioritization
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We support sharing of the individual participant data. The individual participant data that underlie the results reported in this Article, after de-identification (text, tables, figures, and appendices) will be shared. Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents including study protocol, statistical analysis plan, and the informed consent form will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to the individual participant data. Proposals should be directed to jszfc@vip.sina.com or cw0226@foxmail.com. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.