Abstract
Research question
What are the risks associated with cryopreserved semen collected during and after the coronavirus disease 2019 (COVID-19) pandemic wave in Wuhan, China?
Design
Retrospective cohort study involving young adult men who were qualified sperm donors at the Hunan Province Human Sperm Bank (China) during the pandemic wave (1 January 2020 to 30 January 2020) and after the wave and return to work (7 April 2020 to 30 May 30 2020). One hundred paired semen and blood specimens from 100 donors were included. One-step single-tube nested quantitative real-time polymerase chain reaction (OSN-qRT-PCR) was used to detect SARS-CoV-2. Moreover, to control the unacceptable risk of false-negative results, a second round of screening was performed with pooled RNA from negative semen samples using crystal digital PCR (cd-PCR).
Results
For individual blood and semen samples, the target genes, namely the nucleocapsid protein (N) and open reading frame (ORF-1ab) genes, tested negative in all of the 100 paired samples. Further, as per cd-PCR results, there were >20,000 droplets per well in the RNA for each combined sample and no positive droplets were present for either of the aforementioned target genes. A total of 100 paired semen and blood samples from these two groups tested negative for SARS-CoV-2.
Conclusions
Cryopreserved semen at the Hunan Province Human Sperm Bank during and after the COVID-19 pandemic wave was free of SARS-CoV-2 and was judged safe for external use in the future.
Keywords: COVID-19, Crystal digital PCR, Human sperm bank, OSN-qRT-PCR, SARS-CoV-2
Introduction
Several patients with pneumonia were admitted to hospitals in Wuhan (Hubei Province, China) from December 2019 onwards (Munster et al., 2020). On 11 February 2020, the World Health Organization (WHO) (Sun et al., 2020) officially named this condition as coronavirus disease 2019 (COVID-19). COVID-19 is the third known zoonotic coronavirus disease after severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and the causative agents belong to the beta-coronavirus cluster (Chen et al., 2020). COVID-19, caused by SARS coronavirus 2 (SARS-CoV-2), may manifest either as an asymptomatic infection or as mild to severe pneumonia. Considering the high infectivity of COVID-19, several countries implemented suppression and mitigation strategies, including mandated social distancing, restrictions on non-urgent medical care, and closure of non-essential businesses, to control the community spread of the virus. Despite these efforts, controlling COVID-19 is still a major challenge. As of December 2020, in excess of 64 million confirmed cases of SARS-CoV-2 infection have been reported globally (WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/).
Thus far, 27 viruses have been detected in semen and spermatozoa (Salam and Horby, 2017), and so many researchers have attempted to determine whether SARS-CoV-2 could be present in semen. Many published studies have showed that SARS-CoV-2 cannot be detected in the semen from patients with COVID-19 (Guo et al., 2020; Holtmann et al., 2020; Kayaaslan et al., 2020; Pan et al., 2020; Paoli et al., 2020; Pavone et al., 2020; Song et al., 2020). The available evidence indicates that the risk of significant virus shedding into the semen is low, but it cannot be assumed that this risk is acceptable if semen samples were to be cryopreserved during the pandemic. If SARS-CoV-2 with extremely low viral load is present in semen samples and liquid nitrogen in cryostores, the risk of transmission is bound to be amplified across the world.
Quantitative real-time polymerase chain reaction (qRT-PCR) may not be able to detect nucleic acids in clinical samples with a low viral load, such as the semen and blood. However, in a previous study (Wang et al., 2020), a novel one-step single-tube nested quantitative real-time PCR (OSN-qRT-PCR) assay was developed to detect SARS-CoV-2 in clinical samples, and this method showed 10-fold higher sensitivity and specificity than that of standard qRT-PCR. Thus, this study used OSN-qRT-PCR to assay cryopreserved semen and blood samples collected during and after the COVID-19 pandemic at the Hunan Province Human Sperm Bank. Moreover, to control the unacceptable risk of false-negative results, after screening, the highly sensitive crystal digital PCR (cd-PCR) was performed, using RNA collected from negative semen samples. The aim was to determine the risk associated with cryopreserved semen during and after the pandemic.
Materials and methods
Study population
This was a retrospective cohort study of young adult men who were qualified sperm donors at the Hunan Province Human Sperm Bank from 1 January 2020 to 30 May 2020. All donors signed informed consent forms during their first visit to the sperm bank, allowing the use of their semen samples or data for scientific research purposes. The Ethics Committee of the Hunan Provincial Center for Disease Control and Prevention approved this study (Hunan-CDC IRB2020005, date of approval: 4 March 2020).
Criteria for screening sperm donors in China
The guidelines are as follows (Huang et al., 2019): (i) donors must be between 22 and 45 years of age; (ii) donors must be in good health, based on the results of a physical examination and a psychological evaluation by a qualified doctor, and have no familial history of a genetic disease; (iii) fresh semen should have a liquefaction time of <60 min, sperm concentration ≥60 × 106/ml, progressive sperm motility ≥60%, and percentage of normal morphology >30%; (iv) post-thaw semen should have a motility of ≥40%, ≥12 × 106 motile spermatozoa per vial, and a frozen–thaw survival rate ≥60%; and (v) potential donors must undergo laboratory testing to exclude individuals at high risk for sexually transmitted infections and genetic diseases, including HIV-1 and HIV-2, hepatitis B and C, syphilis, gonorrhoea, mycoplasma, chlamydia, cytomegalovirus, Toxoplasma gondii, rubella virus, herpes simplex virus types 1 and 2, and undergo a karyotype analysis. If potential donors test negative for all the above tests and fulfil the Chinese Ministry of Health guidelines outlined above, the donation process is initiated and the semen samples are cryopreserved. The samples must be cryopreserved for a minimum 6-month quarantine period prior to rescreening for HIV.
Semen and blood samples
This study included 100 paired semen and blood samples from 100 donors; 50 paired semen and blood samples collected during the pandemic from 50 donors, and 50 semen and blood samples collected upon work resumption from another 50 donors. Semen specimens were collected by means of masturbation into a sterile container after 2–7 days of abstinence. All specimens were assessed according to WHO recommendations (WHO, 2020). After liquefaction within 1 h of ejaculation, the samples were analysed for volume and sperm concentration, morphology and motility, as per motility grades defined by the WHO (progressive, non-progressive or immotile). Whole blood samples (1.5 ml) were collected from each donor in blood tubes with EDTA and stored in 2 ml cryopreservation tubes at −80°C until required.
Nucleic acid extraction
The collection, transportation, storage and analysis of specimens were strictly performed according to the SARS-CoV-2 Laboratory Biosafety Guidelines (National Health Commission, 2020) and the Technical Guide for Laboratory Testing of COVID-19 (National Health Commission 2020) issued by the General Office of the State Health Commission. Total RNA was extracted from 200 µl of sample preservation solution using a commercial total nucleic acid extraction kit (Tian Long, China), as per the manufacturer's instructions. The RNA was eluted in 80 µl elution buffer and stored at −80°C until required.
OSN-qRT-PCR
The extracted RNA samples were subjected to OSN-qRT-PCR. The reaction mixture included 20 µl RNA template, 26 µl reaction buffer and 4 µl mixed enzyme (reverse transcriptase and Taq enzyme). After vortexing and centrifugation, the reaction mixture was analysed using the QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems, Beijing, China). OSN-qRT-PCR amplification involved the following steps: 50°C for 30 min; 95°C for 1 min; 10 cycles at 95°C for 30 s, 70°C for 40 s and 72°C for 40 s; and 40 cycles at 95°C for 15 s and 60°C for 30 s. The FAM (open reading frame [ORF-1ab] gene) and ROX (the SARS-CoV-2 nucleocapsid protein [N] gene) channels were selected to detect SARS-CoV-2, and the HEX channel was chosen to detect the reference gene (ribonuclease P, RNase P). Each run contained a positive control and negative control. The FAM, HEX and ROX channels showed a typical S-shaped amplification curve, and when the cycle threshold (Ct) values of both target genes (ORF-1ab and N genes) was ≤39, the result was considered to be positive.
cd-PCR
After screening, RNA was co-extracted from all negative semen samples in groups of 10, and these pooled RNA samples (n = 10 in total) were subjected to cd-PCR, which was performed on a Naica System™ for Crystal Digital PCR™ (Stilla Technologies, Villejuif, France) using a Novel Coronavirus (2019-nCoV) Digital PCR Detection Kit (Beijing Apex Biotechnology Co. Ltd). The reaction mixture included 12.5 µl of dPCR mix 1, 1 µl of dPCR mix 2, 1 µl of 2019-nCoV mix and 10.5 µl of 2019-nCoV RNA (total volume = 25 µl). After loading the sample on a Naica™ sapphire chip, the chip was placed into the Naica™ Geode equipment, and PCR was performed under the following conditions: partition 40°C for 12 min, cDNA synthesis 50°C for 10 min, initial denaturation 95°C for 60 s, 45 cycles of denaturation 95°C for 10 s and annealing/extension 55°C for 30 s, followed by pressure release for 33 min.
Data were captured by the Crystal Reader software. Image acquisition was performed using the Naica Prism3 reader using the following exposure times: blue channel 65 ms, green channel 250 ms and red channel 40 ms. The blue (FAM), green (VIC) and red (CY5) channels detected the N gene, ORF-1ab gene and the internal control, respectively. Each run contained a positive control and negative control. The total number of droplets per well was >20,000 to fit the test validity. The control of all three channels showed the number of droplets; a number of 3 or more was considered to be positive. The detection limit was 100 copies/ml.
Results
Semen sample analysis
The mean semen volume for sperm donors was 2.5 (1.5–4.5), and the mean sperm concentration (million/ml) and progressive motility (%) were 65 (62–70) and 52 (50–55), respectively (all expressed as median [5th–95th percentile]).
OSN-qRT-PCR
As per OSN-qRT-PCR results, the target ORF-1ab and N genes were not detected in any of the semen and blood samples (i.e. negative result, with Ct > 39). The reference gene was, however, detectable (mean Ct value of semen sample = 22.32 and of blood sample = 24.41; Table 1 ). The FAM, HEX and ROX channels of the positive control showed a typical S-shaped amplification curve, and the Ct value was >39 for the negative control.
Table 1.
OSN-qRT-PCR results
| Sample type | No. of samples | OSN-qRT-PCR | No. of positive samples (mean Ct value) | No. of negative samples |
|---|---|---|---|---|
| Semen | 100 | ORF-1ab | 0 (>39) | 100 |
| N | 0 (>39) | 100 | ||
| Ref-gene | 100 (22.32) | 0 | ||
| Blood | 100 | ORF-1ab | 0 (>39) | 100 |
| N | 0 (>39) | 100 | ||
| Ref-gene | 100 (24.41) | 0 |
Ct =cycle threshold; OSN-qRT-PCR = one-step single-tube nested quantitative real-time polymerase chain reaction.
cd-PCR
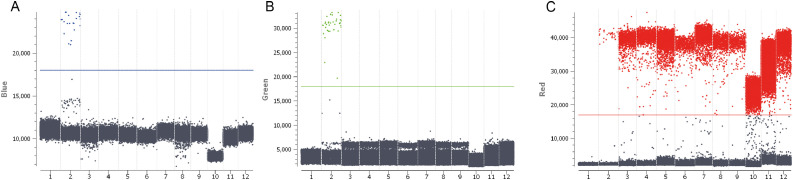
After screening by OSN-qRT-PCR, the 100 negative semen samples were divided into groups of 10, from group 1 (1–10) to 10 (90–100). RNA was extracted from these 10 groups, pooled and further analysed using cd-PCR. The total number of droplets per well for all the combined samples was >20,000, indicating good test validity. The threshold line for the blue (FAM), green (VIC) and red (Cy5) channel was 18,000, 18,000 and 17,500, respectively. As evident from Figure 1 , no positive droplets for the N (blue channel) and ORF-1ab (green channel) genes were detected in the 10 semen samples; all the (grey in Figure 1) droplets below the threshold line were negative. The human conserved region gene (internal control) showed (red in Figure 1) positive droplets, with the mean number of positive droplets being 2899. The number of positive droplets for the N gene was 21 (Blue-Pos) and that for the ORF-1ab gene was 34 (Green-Pos). No positive droplets were detected for the negative control.
Figure 1.
Absolute quantitative scatter plot for SARS coronavirus 2 genes detected using crystal digital PCR. Pooled semen samples were subjected to analyses. (A) N gene (grey: negative droplet, blue: positive droplet). (B) ORF-1ab gene (grey: negative droplet, green: positive droplet). (C) Human conserved region gene (grey: negative droplet, red: positive droplet). 1. Negative control; 2. Positive control; 3. Semen samples 1–10; 4. Semen samples 11–20; 5. Semen samples 21–30; 6. Semen samples 31–40; 7. Semen samples 41–50; 8. Semen samples 51–60; 9. Semen samples 61–70; 10. Semen samples 71–80; 11. Semen samples 81–90; 12. Semen samples 91–100.
Test results for qualified sperm donors
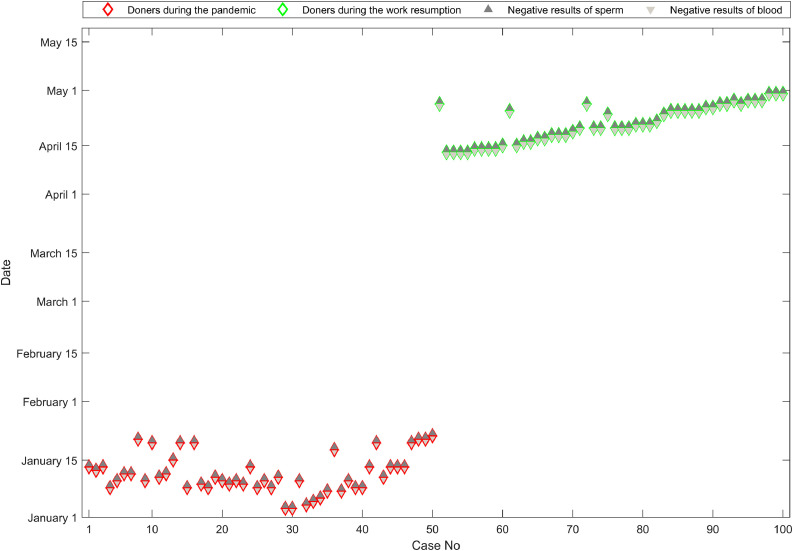
After screening with OSN-qRT-PCR and cd-PCR, all samples from qualified sperm donors during the pandemic wave (1 January 2020 to 30 January 2020) and after the wave and return to work (7 April 2020 to 30 May 2020), tested negative for SARS-CoV-2 (Figure 2 ; red diamonds represent samples collected during the pandemic from 50 donors, whereas green diamonds represent samples collected upon work resumption from another 50 donors). A total of 100 paired semen (dark grey triangles) and blood (light grey triangles) samples from these two groups tested negative for SARS-CoV-2.
Figure 2.
Test results for qualified sperm donors. Red diamonds: samples collected during the pandemic; green diamonds: samples collected upon work resumption; dark grey triangles: negative results for semen samples; and light grey triangles: negative results for blood samples.
Discussion
Several countries are currently dealing with SARS-CoV-2. The rapid emergence of the COVID-19 pandemic has presented a serious challenge to healthcare systems across the globe. Accordingly, many countries have implemented suppression and mitigation strategies to control the spread of SARS-CoV-2; social and economic activities are, however, gradually being resumed.
Hunan Province fully resumed work in April 2020, and the Hunan Human Sperm Bank resumed work on 17 April 2020. At present, the ‘lockdown’ has been completely lifted in China. However, asymptomatic people are a threat as they can infect healthy individuals, potentially leading to another outbreak (Zou et al., 2020). During the pandemic, several reproduction centres cancelled fertility treatments (La Marca et al., 2020; Rodriguez-Wallberg and Wikander, 2020). Although the association between SARS-CoV-2 and treatment involving assisted reproductive technologies remains uncertain, some infertility centres have provided guidance, including the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine (Ory et al., 2020; Veiga et al., 2020a, 2020b). They recommended suspending the initiation of new treatments and an alternative freeze-all protocol in cases where couples have already undergone human chorionic gonadotrophin triggering; further, cryopreservation of gametes was recommended for cases of urgent fertility preservation (Anifandis et al., 2020). It is noteworthy that most viruses remain viable at ultra-low temperatures if stored dried, in appropriate protein concentrations (Gould, 1999). SARS-CoV-2 is an enveloped RNA virus, so evaluation of the presence of SARS-CoV-2 in seminal samples is particularly important for semen cryopreservation, because viruses stored in liquid nitrogen retain their pathogenic properties (De Paoli, 2005). Angiotensin-converting enzyme 2 (ACE2) is highly tissue-specific, with significant levels being detected only in the heart, kidneys, testes and gastrointestinal tract (Harmer et al., 2002). In the testes, ACE2 is expressed in spermatogenic cells (Liu et al., 2020); a recent study reported that ACE2 is also expressed in human Leydig and Sertoli cells (Wang and Xu, 2020), implying a direct effect of the virus on the male reproductive system. Another study reported that SARS-CoV-2 infection can up-regulate ACE2 expression. Considering that SARS-CoV-2 acts through the ACE2 receptor (Li et al., 2003), a possible direct effect of SARS-CoV-2 on spermatozoa cannot be excluded. Only one paper has reported detection of the virus in semen of patients with COVID-19 (Li et al., 2020), but the paper lacks information on details of patient characteristics and does not report on the method used to detect the virus in semen, so this virus cannot be excluded from sources other than the male reproductive tract (Paoli et al., 2020).
A major challenge is the possibility of transmission by asymptomatic individuals. Not only is it crucial to detect SARS-CoV-2 in asymptomatic men who are going to freeze semen as donors, but also for cancer patients and in patients who are going to cryopreserve spermatozoa for use in ICSI (Jose et al., 2020). As yet, no studies have directly detected the presence of SARS-CoV-2 in semen obtained from sperm donors. These donors could be asymptomatic individuals or at the early stage of an infection, and asymptomatic individuals have a lower viral load than symptomatic individuals (Wiersinga et al., 2020); therefore, highly sensitive methods are needed to detect SARS-CoV-2, rather than simply using standard qRT-PCR. qRT-PCR is the gold standard method widely used in many diagnostic laboratories in China for the aetiological detection of SARS-CoV-2 (Udugama et al., 2020). However, depending on the sample type, sample quality, RNA extraction method and RNA quality, the viral load may be lower than the detection limit of qRT-PCR, leading to false-negative results (Winichakoon et al., 2020; Xie et al., 2020).
The sensitivity of OSN-qRT-PCR is reportedly one copy/reaction, which is 10-fold higher than that of qRT-PCR performed using a commercial kit. In addition, OSN-qRT-PCR is cost-effective and easy to perform, making it ideal for samples with a low viral load. However, in the case of semen and blood samples, their viral load is far lower than that of stool samples, sputum samples, and throat and nasal swabs; despite the fact that all blood and semen samples tested negative by OSN-qRT-PCR in this study, OSN-qRT-PCR remains a robust method to screen a large quantity of samples with a low viral load.
In this study a second round of screening was performed using cd-PCR, which is also suitable for screening samples with a low viral load (Li et al., 2018). This method involves using a single chip to partition samples into 2D droplet arrays, which are then subjected to thermal cycling and finally read using a three-colour fluorescence scanning device. This technology allows three-colour multiplexing, which entails a different approach to data analysis, making the results more reliable (Madic et al., 2016). As the use of cd-PCR involves specific reagents and instruments and professional operators, it is expensive with moderate throughput, and is thus unsuitable for screening a large number of samples. In this study, cd-PCR using a mixture of negative semen samples that were by first screened with OSN-qRT-PCR was performed; this substantially reduced the cost. The cd-PCR results confirmed the OSN-qRT-PCR results, markedly increasing the robustness of the data reported in this study.
This study has a few limitations. First, although SARS-CoV-2 was not detected in cryopreserved semen and blood samples, the possibility that the virus could be detected in samples collected in the acute phase cannot be excluded. Second, only 100 paired semen and blood specimens from 100 donors were studied; further studies involving a larger cohort are thus warranted. Finally, the study population represents only one geographical region of China, and the results may therefore not be applicable to China as a whole.
To conclude, this is thought to be the first study to use highly sensitive assays to determine the absence of SARS-CoV-2 in cryopreserved semen during and after the COVID-19 pandemic. The findings clearly illustrate that cryopreserved semen at the Hunan Province Human Sperm Bank during and after the COVID-19 pandemic was free of SARS-CoV-2. Nevertheless, it is advised that utmost care be exercised by all human sperm banks at such a time, and the use of highly secure devices and segregated cryovessels is encouraged.
Acknowledgements
We are grateful to the Chinese Center for Disease Control and Prevention and the National Institute for Viral Disease Control and Prevention. This study was supported by grants from the Key Technologies R and D Program of the National Ministry of Science (2018ZX10713002), the Research and Development Plan of Key Areas in Hunan Province (2020SK3012 and 2020SK3018), and China Mega-Projects for Infectious Disease (2017ZX10104001, 2018ZX10711001, 2018ZX10713-002); Chinese patent (201810054853.1) pending.
Biography

Chuan Huang obtained PhD degree in genetics in 2017 at the Central South University, China. Since 2017 she has been a member of the human sperm bank at the Reproductive and Genetic Hospital of CITIC-Xiangya. Her current research interest is male fertility preservation.
Key message.
This retrospective cohort study found that a sample cohort of cryopreserved semen at the Hunan Province Human Sperm Bank during and after the coronavirus disease 2019 (COVID-19) pandemic was free of SARS-CoV-2, and so safe for future external use. Utmost care should be exercised by all human sperm banks, and the use of highly secure devices and segregated cryo-vessels is encouraged.
Alt-text: Unlabelled box
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Anifandis G., Messini C.I., Daponte A., Messinis I.E. COVID-19 and fertility: a virtual reality. Reprod. Biomed. Online. 2020;41:157–159. doi: 10.1016/j.rbmo.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paoli P. Bio-banking in microbiology: from sample collection to epidemiology, diagnosis and research. FEMS Microbiol. Rev. 2005;29:897–910. doi: 10.1016/j.femsre.2005.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E.A. Methods for long-term virus preservation. Mol. Biotechnol. 1999;13:57–66. doi: 10.1385/MB:13:1:57. [DOI] [PubMed] [Google Scholar]
- Guo L., Zhao S., Li W., Wang Y., Li L., Jiang S., Ren W., Yuan Q., Zhang F., Kong F., Lei J., Yuan M. Absence of SARS-CoV-2 in Semen of a COVID-19 Patient Cohort. Andrology. 2020 doi: 10.1111/andr.12848. https://onlinelibrary.wiley.com/doi/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Holtmann N., Edimiris P., Andree M., Doehmen C., Baston-Buest D., Adams O., Kruessel J.S., Bielfeld A.P. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil. Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Lei L., Wu H.L., Gan R.X., Yuan X.B., Fan L.Q., Zhu W.B. Long-term cryostorage of semen in a human sperm bank does not affect clinical outcomes. Fertil. Steril. 2019;112 doi: 10.1016/j.fertnstert.2019.06.008. 663–669 e1. [DOI] [PubMed] [Google Scholar]
- Jose F.G., Gonzalez J.G.A., Molina J.M.C., Arnau L.B., Iribarren I.M., Jabaloyas J.M.M., Rico F.M., Garcia-Baquero R., Gaya M.R., Garcia E.L., Lopez C.L., Castro R.P., Salamanca J.I.M. [SARS-CoV-2 infection: implications for sexual and reproductive health. A position statement of the Asociacion Espanola de Andrologia, Medicina Sexual y Reproductiva (ASESA)] Revista internacional de andrologia. 2020;18:117–123. doi: 10.1016/j.androl.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaaslan B., Korukluoglu G., Hasanoglu I., Kalem A.K., Eser F., Akinci E., Guner R. Investigation of SARS-CoV-2 in Semen of Patients in the Acute Stage of COVID-19 Infection. Urol. Int. 2020;104:678–683. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A., Niederberger C., Pellicer A., Nelson S.M. COVID-19: lessons from the Italian reproductive medical experience. Fertil. Steril. 2020;113:920–922. doi: 10.1016/j.fertnstert.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA network open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Bai R., Zhao Z., Tao L., Ma M., Ji Z., Jian M., Ding Z., Dai X., Bao F., Liu A. Application of droplet digital PCR to detect the pathogens of infectious diseases. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20181170. 21 December 2018doi: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen Y., Tang W., Zhang L., Chen W., Yan Z., Yuan P., Yang M., Kong S., Yan L., Qiao J. Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci. China Life Sci. 2020;63:1006–1015. doi: 10.1007/s11427-020-1705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madic J., Zocevic A., Senlis V., Fradet E., Andre B., Muller S., Dangla R., Droniou M.E. Three-color crystal digital PCR. Biomolecular detection and quantification. 2016;10:34–46. doi: 10.1016/j.bdq.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A Novel Coronavirus Emerging in China–Key Questions for Impact Assessment. N. Engl. J. Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- National Health Commission. Technical Guide for Laboratory Detection of Pneumonia Infected by Novel Coronavirus (fourth edition)/Pneumonia Prevention and Control Program for Novel Coronavirus Infection (fourth edition) [EB/OL]. 2020http://www.nhc.gov.cn/jkj/s3577/ 202002/573340613ab243b3a7f61df260551dd4/ files/c791e5a7ea5149f680fdcb34dac0f54e.pdf (accessed 2020.2.8).
- National Health Commission. Novel Coronavirus Laboratory Biosafety Guide (second edition) [OB/OL] 2020.http://www.nhc.gov.cn/qjjys/s7948/202001/ 0909555408d842a58828611dde2e6a26.shtml (accessed 2020.2.6).
- Ory S.J., Miller K.A., Horton M., Giudice L. The global impact of COVID-19 on infertility services. Wolters Kluwer Public Health Emergency Collection. Glob. Reprod. Health. 2020;5 doi: 10.1097/GRH.0000000000000043. 2020 Aug 13PMCID: PMC7434007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P., Spivak A.M., Alukal J.P., Zhang X., Xiong C., Li P.S., Hotaling J.M. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil. Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D., Pallotti F., Turriziani O., Mazzuti L., Antonelli G., Lenzi A., Lombardo F. Andrology; 2020. SARS-CoV-2 presence in seminal fluid: Myth or reality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O., Antonelli G., Lenzi A., Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Invest. 2020 doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone C., Giammanco G.M., Baiamonte D., Pinelli M., Bonura C., Montalbano M., Profeta G., Curcuru L., Bonura F. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int. J. Impot. Res. 2020;32:560–562. doi: 10.1038/s41443-020-00344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Wallberg K.A., Wikander I. A global recommendation for restrictive provision of fertility treatments during the COVID-19 pandemic. Acta Obstet. Gynecol. Scand. 2020;99:569–570. doi: 10.1111/aogs.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam A.P., Horby P.W. The Breadth of Viruses in Human Semen. Emerg. Infect. Dis. 2017;23:1922–1924. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Wang Y., Li W., Hu B., Chen G., Xia P., Wang W., Li C., Diao F., Hu Z., Yang X., Yao B., Liu Y. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol. Reprod. 2020;103:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Veiga A., Gianaroli L., Ory S., Horton M., Feinberg E., Penzias A. Assisted reproduction and COVID-19: A joint statement of ASRM, ESHRE and IFFS. Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga A., Gianaroli L., Ory S., Horton M., Feinberg E., Penzias A. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Human reproduction open. 2020;2020:hoaa033. doi: 10.1093/hropen/hoaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cai K., Zhang R., He X., Shen X., Liu J., Xu J., Qiu F., Lei W., Wang J., Li X., Gao Y., Jiang Y., Xu W., Ma X. Novel One-Step Single-Tube Nested Quantitative Real-Time PCR Assay for Highly Sensitive Detection of SARS-CoV-2. Anal. Chem. 2020;92:9399–9404. doi: 10.1021/acs.analchem.0c01884. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia. Leydig and Sertoli Cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO: WHO Laboratory Manual for the Examination and Processing of Human Semen.5th ed, 2010
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Winichakoon P., Chaiwarith R., Liwsrisakun C., Salee P., Goonna A., Limsukon A., Kaewpoowat Q. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00297-20. e00297–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020;296:E41–E45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]