Abstract
Background
The current study aimed to determine the impact of SARS-CoV-2 infection on male fertility.
Methods
This is a single-center, hospital-based observational study that included autopsied testicular and epididymal specimens of deceased COVID-19 male patients (n=6) and recruited recovering COVID-19 inpatients (n=23) with an equal number of age-matched controls, respectively. We performed histopathological examinations on testicular and epididymal specimens, and also performed TUNEL assay and immunohistochemistry. Whereas, we investigated the semen specimen for sperm parameters and immune factors.
Findings
Autopsied testicular and epididymal specimens of COVID-19 showed the presence of interstitial edema, congestion, red blood cell exudation in testes, and epididymides. Thinning of seminiferous tubules was observed. The number of apoptotic cells within seminiferous tubules was significantly higher in COVID-19 compared to control cases. It also showed an increased concentration of CD3+ and CD68+ in the interstitial cells of testicular tissue and the presence of IgG within seminiferous tubules. Semen from COVID-19 inpatients showed that 39.1% (n=9) of them have oligozoospermia, and 60.9% (n=14) showed a significant increase in leucocytes in semen. Decreased sperm concentration, and increased seminal levels of IL-6, TNF-α, and MCP-1 compared to control males were observed.
Interpretation
Impairment of spermatogenesis was observed in COVID-19 patients, which could be partially explained as a result of an elevated immune response in testis. Additionally, autoimmune orchitis occurred in some COVID-19 patients. Further research on the reversibility of impairment and developing treatment are warranted.
Funding
This study was supported by Ministry of Science and Technology of China Plan, Hubei Science and Technology Plan, National Key Research and Development Program of China, HUST COVID-19 Rapid Response Call, China and National Natural Science Foundation of China; these funding bodies are public institutions, and they had no role in study conception, design, interpretation of results, and manuscript preparation.
Keywords: Autoimmune orchitis, Covid-19, Male infertility, Oligospermia, Spermatogenesis
Research in context.
Evidence before this study
The male reproductive system is vulnerable to viral infections, and previous viral epidemics had documented varying impacts on male reproductive functions. The preliminary studies on SARS-CoV-2 infection had indicated the possibility of SARS-CoV-2 outreach to male gonads. To date, the studies investigating patients with COVID-19 caused by SARS-CoV-2 had primarily focused on the detection of the virus in the male reproductive tract (MRT), which has yielded inconsistent results. The clarity of the pertinent information is still lacking.
Added value of this study
The current study is first to perform a thorough investigation of male reproductive health and bridge the existing gap in the field. The study identified significant impacts of SARS-CoV-2 on the male reproductive system, as demonstrated by impaired spermatogenesis with lower sperm count, inflammatory response in testis and epididymis, and altered seminal immune markers signifying immune impairment by COVID-19 illness. The impaired human spermatogenesis and retarding sperm maturation in COVID-19 patients could be a result of the immune response in testis and epididymis in COVID-19 patients, these factors are vital and determinant of healthy male fertility, which requires further care and support in male patients infected with SARS-CoV-2.
Implications of all the available evidence
SARS-CoV-2 detection in the MRT simply cannot be treated as a criterion for measuring the impact on the reproductive system. The discrepancy in the virus detection in MRT shall not dilute the known vulnerability of the male reproductive system. The current study observed a significant impact on male fertility and pathological changes in male gonads that might impact healthy reproductive well-being in male COVID-19 patients in the long-term. The current study recommends the clinician in the field to attach special attention and provide additional care to help these patients in restoring healthy reproductive life and encourage long-term screening of these patients. Also, the current study urges clinicians to determine whether these impacts are reversible or not. Furthermore, define particular strategies, including therapeutics approaches, to address these impacts in the SARS-CoV-2 infected male population.
Alt-text: Unlabelled box
1. Introduction
The first time that human coronaviruses were recognized as pathogens, that could cause severe respiratory tract infection was in 2002 with the outbreak of SARS. This disease was caused by SARS-CoV, a previously unrecognized coronavirus. Now, the world is again facing an imminent health threat from COVID-19, caused by another novel coronavirus (SARS-CoV-2), which is most closely related to SARS-CoV. However, the infection with SARS-CoV-2 is much more contagious and has already infected a much higher proportion of people worldwide. So far, the mortality recorded with COVID-19 is lesser compared to SARS (2.1% Vs. 9.6%, respectively) [1]. Also, COVID-19 is affecting more males than females, which contrasts with the SARS (male to female ratio of 2.7:1 Vs. 1:1.25, respectively) [1]. Furthermore, SARS-CoV-2 may persist in humans for a much longer duration than SARS-CoV. Kissler et al. projected that a recurrence of SARS-CoV-2 is possible even after its elimination [2], making it a pathogen of significant economic and medical concern.
The clinical characteristics of COVID-19 are quite similar to SARS [1], and noticeable impacts of COVID-19 on cardiovascular and gastrointestinal systems were observed [3,4]. However, does this pandemic spare the male reproductive system? To date, our knowledge of the male reproductive system indicates its fragility, and there is substantial recorded evidence that the male reproductive system is vulnerable to viral infections. Viruses like MUMPS, ZIKA, Hepatitis B virus (HBV), Hepatitis C Virus (HCV), Human Immunodeficiency Virus (HIV), Human papillomavirus (HPV), Herpes, EBOLA, and several others have been shown to exert varying impacts on male reproductive health [[5], [6]–7]. A few prominent examples of the adverse impact of these viruses include; disarrayed spermatogenesis, reduction in sperm counts, impacting sperm motility, altered hormonal levels, others [5–8]. Such consequences certainly raise pertinent questions: How do viruses create these impacts, and how do they gain access to the male reproductive tract (MRT)? In fact, during active viremia, the possibility of viral access to the male reproductive tract is possible [9]. Persistent high body temperatures, as such during viral infections, may tamper with the blood-testis barrier (BTB) [10], the experimental evidence also suggests that even mild scrotal heat stress could lead to the BTB leakages and allowing the passage for macromolecular substances to testis [11]. However, in several instances, it has been observed that direct virus access is not required to damage the male reproductive system [12]. Recent reports suggesting the role of Angiotensin-Converting Enzyme 2 (ACE2) as the cellular receptor for SARS-CoV-2 may be the mechanism for access to the male reproductive organs. ACE2 is abundantly expressed in testes, including spermatogonia, Leydig, and Sertoli cells [13]. Further, it is also hypothesized that the attachment of SARS-CoV-2 to the ACE2 receptor might in turn increases the expression of ACE2 and initiate an inflammatory response that could interfere with the normal functions of Sertoli and Leydig cells [14]. The potential for infection of the MRT by SARS-CoV-2 cannot be entirely ruled out, particularly if we take previous viral infections such as ZIKA, EBOLA, HIV, and others into account [15].
The majority of existing reports on SARS-CoV and SARS-CoV-2 have failed to identify the virus in MRT [[16], [17], [18], [19]–20], except a recent report on the presence of SARS-CoV-2 in human semen [21]. Also, there is a lack of substantial histopathological evidence to suggest the impact of COVID-19 illness on male reproductive organs. However, the absence of RNA virus detection in MRT, that is, in testes, epididymis, or seminal fluid does not infer that the male reproductive tract is therefore unaffected. Xu et al. identified orchitis as one of the prominent consequences of SARS virulence owing to an autoimmune response [16]. Whether SARS-CoV-2 has a similar impact requires investigation. However, SARS-CoV-2 was found to alter hormonal factors in COVID-19 patients [22] that are responsible for the healthy maintenance of male reproductive functions. Through this study, we aim to elucidate the impacts of SARS-CoV-2 on male fertility and determine the extent of damage incurred due to COVID-19 illness by investigating seminal parameters and histopathological changes in the male gonads, respectively.
2. Methods
2.1. Study design
The current observational study has a pair of two case-control investigations. The first part of the study is a case series in which autopsy specimens of testis and epididymis were obtained from patients who died of COVID-19 (n=6), and an equal number of surgical specimens from male patients other than of COVID-19 were taken as controls (n=6), the specimens included in the investigation for the control group were of the patients with prostate cancer and had no other history of medical illness (as per the documented medical history records). These specimens were obtained from the Department of Forensic Medicine, Tongji Medical College, Huazhong University of Science and Technology.
In the second part of the study (cross-sectional cohort), we recruited male COVID-19 inpatients and age-matching male healthy controls. A total of 31 eligible male COVID-19 inpatients showed interest in participating in this research between 26th February 2020 and 12th March 2020, of these, 23 patients provided their semen samples; the remaining eight patients declined to provide samples on the day of collection (primarily because of weakness and some did not explain the reasons) and thus were excluded from the study. The patients provided the semen samples were classified as mild cases (n=9) or ordinary cases (n=14) according to the “Guidance for COVID-19” (the sixth edition) from the National Health Commission of the PRC [23]. We also enrolled in age-matched healthy male controls (n=22), who visited the Center of Reproductive Medicine, Tongji Medical College, for counseling for fathering the second child or infertility due to his partner (wife) during August to October 2019 (before the COVID-19 pandemic). For the control group, a search was conducted in the hospitals’ research database using age range and reproductive health status. An independent investigator screened out the list of all healthy male volunteers and performed matching with COVID-19 group participants based on the age range of these participants. All controls provided written consent, allowing us to store their semen samples in liquid nitrogen for further research purposes. The COVID-19 inpatients were provided with a sterile 50-ml tube and asked to produce a semen sample by masturbation. The semen was sealed in the tube with a cap and allowed to liquefy 30 min at room temperature before transferring it to the laboratory for SARS-CoV-2 RNA detection.
The eligibility criteria for enrollment of male inpatients for COVID-19 group includes; (a) age above 18 years, (b) positive for SARS-CoV-2 RNA in throat swab tests within the recent seven days, (c) received no steroidal drug treatments while receiving anti-COVID-19 treatment, (d) have offspring through natural pregnancy or without offspring due to identified problem of his wife, (e) never received treatment for infertility, including drugs treatment or assisted reproductive techniques, (f) no history of diseases may influence spermatogenesis (obesity, diabetes, cryptorchidism, varicocele, testicular torsion, mumps, genital tract infection, exposure to environmental chemicals, etc.). While the eligibility criteria for the control group includes: (a) age above 18 years, (b) not receiving any steroidal drug treatments for any disease, (c) have offspring through natural pregnancy or without offspring due to identified problem of his wife, (d) never received treatment for infertility, including drugs treatment or assisted reproductive techniques, (e) no history of diseases may influence spermatogenesis. The participants were excluded from the study if they do not comprehend the defined criteria.
Both parts of the current study were reviewed and approved by the Ethics Committee of Center of Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology (No. 2020001). The participants provided written, informed consent for their participation in this study. The Study complies with STROBE guidelines for reporting study.
2.2. Histopathology
Tissues were fixed in 10% neutral-buffered formalin and then embedded in paraffin. Continuous 4-µm sections of the testis and epididymis were cut on a microtome (KD-3390, Huanxi), and used for hematoxylin and eosin staining, TUNEL assay, and immunohistochemistry. TUNEL assay was performed with an in-situ cell death detection kit (fluorescein, Roche) according to the manufacturer's instruction. Apoptotic cells were marked by green fluorescence, and DAPI was used to mark the nuclei. Photographs were taken under a fluorescence microscope at 200 × total magnification for cell counting. At least 5000 cells were counted for each sample. When the cell number reached 5000, other cells in this photograph were counted.
2.3. Immunohistochemistry
Immunohistochemistry was used to determine numbers of T lymphocytes (CD3+) and macrophages (CD68+) in specimens, and IgG distribution in testicular tissues, as well as the expression of the receptor for SARS-CoV-2, ACE2 in testicular and epididymal cells. The primary antibodies used included monoclonal mouse anti-human CD3 (clone F7.2.38, Dako), monoclonal mouse anti-human CD68 (clone KP1, Dako), monoclonal mouse anti-human ACE2 (MM0073-11A31, Abcam). Monoclonal rabbit anti-human DDX4 (EPR21789, Abcam) was used to marker germ cells in the testis. Antigen retrieval was performed in EDTA antigen retrieval solution (G1206, Servicer Bio.) by microwave oven heating, and then sections were incubated with a blocking solution. After blocking, sections were incubated overnight with the primary antibody at 4 °C. Biotinylated secondary antibodies and DAB (AR1022, Boster) were used for CD3, CD68. Alexa Flour 594 donkey anti-mouse IgG (Ref21203, Life) and Alexa Flour 488 donkey anti-rabbit IgG (Ref21206, Life) was used for the detection of ACE2 and DDX4, respectively. For IgG detection in the testis, sections were incubated with biotinylated rabbit anti-human IgG (SA1024, Boster) and stained with AEC (SA1020, Boster).
In order to count the percentage of T lymphocytes and macrophages in the interstitium of testicular tissue, at least 200 cells in the interstitium with round nucleus were counted for each specimen. Cells were counted under a light microscope at 200 × total magnification. When the cell number reached 200, other cells in this vision were counted.
2.4. Detection SARS-CoV-2 RNA in semen
There is no commercial kit specific for viral RNA isolation from semen. The Trizol LS reagent was recommended for reliable RNA isolation for viral RNA detection in semen by different groups [24,25]. Seminal plasma contains some agents that inhibit PCR. RNA from seminal plasma was isolated by Trizol LS (Invitrogen) as previously described [26] and subjected to SARS-CoV-2 viral RNA detection with RT-qPCR. For each sample, a total of 200 μl seminal plasma was used. The RNA pellet was dissolved in 30 µl RNase-free water. Moreover, 20 μl RNA was added to 20 µl RT-qPCR mix (Maccura, Chengdu, China), which determine ORF1ab, E gene, and N gene of SARS-CoV-2 within in a multiplex PCR reaction (primers are provided in Supplemental Table 1). RT-qPCR was performed on a PCR cycler (7500, ABI) with conditions: a cycle of reverse transcription at 55 °C for 15 min; a cycle for initial denaturation at 95 °C for 2 min, followed by 40 amplification cycles with denaturation at 95 °C for 15 s, annealing, extension, and signal detection at 58 °C for 35 s. SARS-CoV-2 positive culture medium from the throat swabs was spiked in the semen as the positive control.
2.5. Sperm concentration and leucocytes
The concentrations of sperm and leucocytes in semen were determined under a microscope according to the manual of WHO (Fifth Edition) [27]. Sperm motility was not determined because semen was transferred and stored in cold temperatures.
2.6. IL-6, TNF-α, MCP-1 determination in semen
Immune factors were determined in semen with ELISA kits following the manufacturer's protocol (Cusabio, Wuhan, China) to determine levels interleukin-6 (IL-6, CSB-E04638h), tumor necrosis factor-α (TNF-α, CSB-E04740h), monocyte chemoattractant protein-1 (MCP-1, CSB-E04655h) in semen.
2.7. Statistics
The statistical analysis was performed using SPSS (version: 20.0). Since the percentages of cells, levels of seminal immune factors, and sperm concentrations were positively skewed, these data were transformed to natural logarithms before analysis and expressed as geometric mean with its 95% CI. The difference between groups was analyzed using t-test, and mean ratios with their 95%CIs were calculated. The level of significance was determined as a p-value of less than 0.05.
2.8. Role of funding source
These funding bodies are public institutions, and they had no role in study conception, design, interpretation of results, and manuscript preparation.
3. Results
3.1. Study characteristics
Autopsied testicular and epididymal specimens of six deceased COVID-19 male cases (case no: A1, A4, A6, A7, A8, A10) were studied. The control specimen included in the study were of the patients with prostate cancers (PC1769, PC3894, PC2947, PC1074, PC9819, PC8664). The age of the COVID-19 and control patients ranges from 51 to 83 (69.3±11.5) and 56 to 85 years (69.0±9.4), respectively. The clinical characteristics of cases of both groups are shown in Supplemental Table 2.
In the second part of this study, a total of 23 Male COVID-19 inpatients and 22 age-matched healthy participants as controls were eligible for enrollment in this study according to our eligibility criteria described in the Methods and provided semen samples. The age of COVID-19 patients and controls ranges from 27 to 55 years old (40.8±8.5) and 30 to 54 years (40.1±5.8), respectively. All study participants of both groups had no history of infertility or ever had received any fertility treatment. Among these participants, a total of 21 COVID-19 inpatients and 16 control males have offspring through natural pregnancy, while remaining two COVID-19 inpatients and six control males yet have not attained fatherhood due to known infertile state of their partners (wife). That is, they were fertile or supposed to be fertile. No steroid treatment was applied in these COVID-19 inpatients. The clinical characteristics of both cohort groups are shown in Supplemental Table 3.
3.2. Histopathology
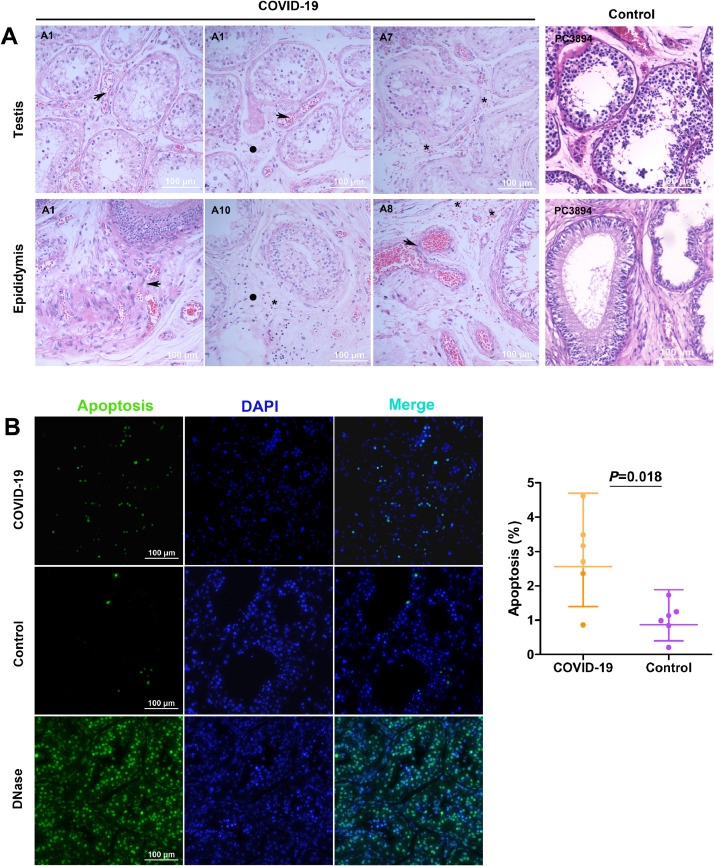
Histopathological assessment of testes and epididymides from deceased COVID-19 patient compared to age-matched control showed noticeable changes; it included the presence of interstitial edema and congestion both in testes and epididymides in all of the cases. Obvious red cell exudation was observed both in testes and epididymides of cases A7, A8, and in epididymides of case A10. Thinning of seminiferous epithelium (decreased cell layers) was observed (Fig. 1A). Also, the seminiferous tubules were seen to have higher spermatogenic epithelial shedding (Fig. 1A). This finding demonstrated impaired spermatogenesis in these deceased COVID-19 patients.
Fig. 1.
Histopathology of testes and epididymides and apoptosis of testicular cells. (A) Congestion (denoted by a solid circle) and interstitial edema (denoted by a star) were observed in all autopsy COVID-19 specimens of testes and epididymides. Red blood cell exudation (denoted by arrow) was observed in testes and epididymides of some (3 of 6) autopsy cases. Thinning of the seminiferous epithelium was observed. Case number is denoted on the up left of each photograph. Hematoxylin and eosin staining. (B) Increased apoptotic cells were seen in COVID-19 testes when compared with age-matched males. Compared to the control group, the mean ratio was 2.95 folds (95%CI: 1.26–6.90). TUNEL assay. DNase treatment was taken as a positive control. Data were presented as geometric mean with 95%CI. The graph was prepared using GraphPad Prism (Version 5.01).
The proportion of apoptotic testicular cells was determined using the TUNEL assay. Most apoptotic cells were located within seminiferous tubules. The proportion of apoptotic cells in the testes of COVID-19 deceased patients was significantly higher with 2.95 folds (95%CI 1.26–6.90, p=0.018) compared to the average of apoptotic cells in the testes of control patients (Fig. 1B).
3.3. Immunological mediators
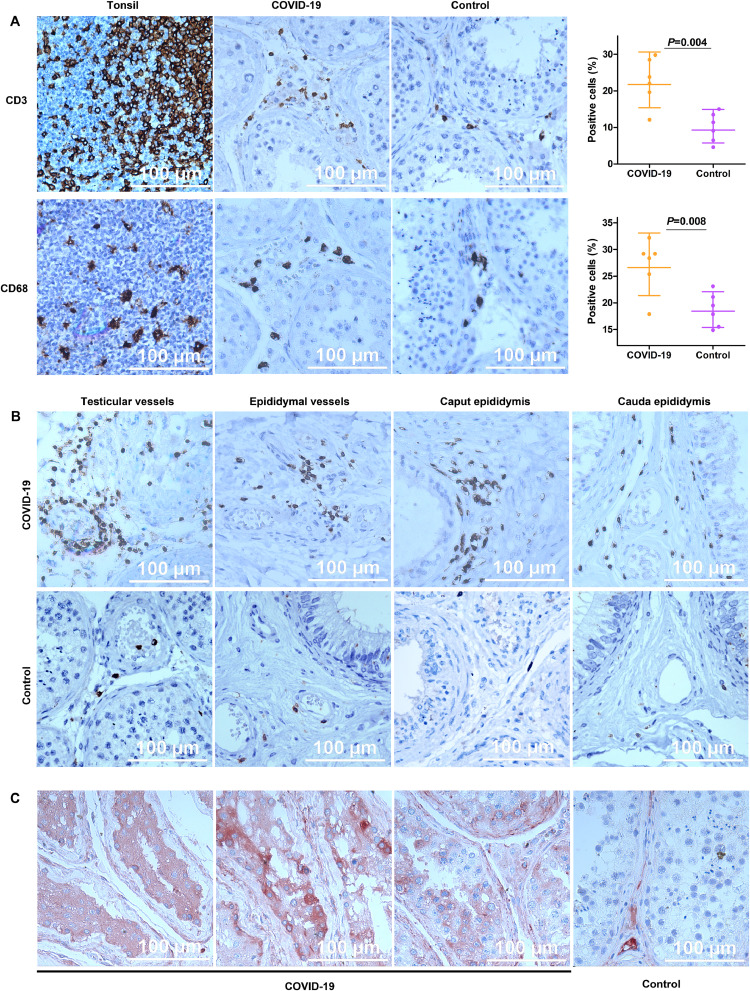
Observation of T-lymphocyte (CD3+) and macrophage (CD68+) infiltration in testicular tissues showed an increased concentration of T-lymphocyte and macrophage in the interstitium of testicular tissue of the COVID-19 patients compared to control patients testes, with mean ratios of 2.35 (95%CI 1.41–3.91, p=0.004) and 1.44 (95% CI 1.13–1.85, p=0.008), respectively (Fig. 2A). Additionally, we also found the obvious infiltration of T-lymphocytes around the blood vessels of the testes and epididymis tissue and noticed occasional T-lymphocytes presence around the epididymal duct of the COVID-19 patients (Fig. 2B).
Fig. 2.
Immune cell infiltration and the presence of IgG. (A) T-lymphocytes (CD3+) and macrophages (CD68) increased in the interstitium of COVID-19 testicular specimens when compared with age-matched males, with mean ratios of 2.35 folds (95%CI: 1.41–3.91), and 1.44 folds (95%CI: 1.13–1.85), respectively. Data were presented as geometric mean and 95%CI. (B) Apparent T-lymphocytes infiltration around blood vessels was observed in both testes and epididymides. And obvious T-lymphocytes infiltration was observed sparsely in the epididymis of autopsy cases. (C) The presence of IgG within seminiferous tubules was observed in some (4 of 6) autopsy cases but none in control. DAB was used for CD3 and CD68, and AEC was used for the IgG detection in the testis. Graphs were prepared using GraphPad Prism (Version 5.01).
We further investigated the distribution of IgG in COVID-19 patients' testis, of the six patients, we identified the IgG precipitation in seminiferous tubules of four patients (cases A1, A6, A8, A10) (Fig. 2C), while, no IgG precipitation within seminiferous tubules was observed in control cases.
3.4. ACE2 protein expression profiling in testicular cells
Immunochemistry with fluorescence was performed to detect the ACE2 protein expression in testicular cells using marker DDX4 expression by the spermatogenic cells. Consistent with the previous transcript expression results [13,19], we observed that the expression of ACE2 was highly expressed in Leydig cells (Supplemental Fig. 1). However, ACE2 is not strong in the spermatogonia, in which the ACE2 mRNA is abundant [13,19]. No obvious difference in the ACE2 expression between COVID-19 patients and the control was observed.
3.5. SARS-CoV-2 RNA detection in semen
Totally 23 COVID-19 inpatients provided semen samples. The days from the diagnosis to providing semen samples ranges from 4 to 42 days (averaged 25.8 days). All patients have positive SARS-CoV-2 RNA results in throat swab tests within seven days before the semen collection. All semen samples of inpatients showed negative results of SARS-CoV-2 RNA in semen, while the control semen sample with spiked SARS-CoV-2 positive culture medium showed a positive result.
3.6. Sperm concentrations and immune factors in semen
Upon examining semen specimens, nine out of the 23 COVID-19 inpatients (39.1%) showed sperm concentration less than 15 × 106/ml, which relates to the recommended criteria of WHO for diagnosing oligozoospermia [27]. Furthermore, all these nine patients who showed oligozoospermia had offspring without any history of the use of assisted reproductive technology or treatment for infertility (Supplemental Table 3). Moreover, the averaged sperm concentration of COVID-19 inpatients was significantly decreased when compared with the age-matched control males, with a mean ratio of 0.29(95%CI 0.12–0.71, p=0.008, Table 1).
Table 1.
Sperm concentration and seminal leucocytes of COVID-19 in patients.
| Group | Individuals | Age (years) | Sperm concentration (106/ml) | Cases with leucocytes>1 × 106/ml (%) | |
|---|---|---|---|---|---|
| Control | 22 | 40.5±5.9 | 40.9 (27.2, 61.5) | 4 (18.2%) | |
| COVID-19 | 23 | 40.8±8.5 | 11.9 (5.3, 26.8)a | 14 (60.9%)a | |
| Mild | 9 | 40.8±7.2 | 13.8 (5.2, 36.8) | 6 (66.7%) | |
| Ordinary | 14 | 40.9±9.6 | 10.9 (3.0, 39.1) | 8 (57.1%) | |
| Fever≥39 °C | 8 | 43.8±7.3 | 10.7 (2.0, 58.6) | 5 (62.5%) | |
| Fever<39 °C or no fever | 15 | 39.3±9.0 | 12.6 (4.5, 35.3) | 9 (60.0%) |
Denotes p<0.05 when compared with control. Age presented as mean ± SD. Sperm concentration presented as geometric mean with 95% CI.
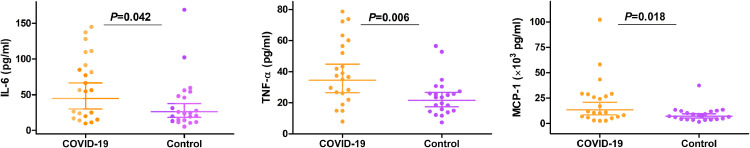
An increase in seminal leucocytes of more than 1 × 106/ml, which is the recommended criteria of WHO for leucocytospermia [27] was observed in 14 patients (60.9%). The percentage of cases with abnormal seminal leucocytes of COVID-19 inpatients was significantly higher than the control males (p=0.006, Table 1). Levels of proinflammatory cytokines and chemokines, including IL-6, TNF-α, and MCP-1, which play a significant role in the immunopathology of SARS-COV-2 [28] were determined in semen samples. Compared to controls, we observed a significant increase of seminal IL-6 (1.72 folds, 95%CI 1.02–2.89, p=0.042), TNF-α (1.60 folds, 95%CI 1.15–2.23, p=0.006), and MCP-1 (1.88 folds, 95%CI 1.12–3.16, p=0.018) levels were observed in COVID-19 patients (Fig. 3).
Fig. 3.
Seminal IL-6, TNF-α, and MCP-1 in COVID-19 inpatients measured by ELISA. The seminal levels of IL-6, TNF-α, and MCP-1 were increased in COVID-19 inpatients compared with age-matched males, the mean ratios observed were 1.72 folds (95%CI:1.02–2.89) for IL-6, 1.60 folds (95%CI:1.15–2.23) for TNF-α, and 1.88 folds (95%CI:1.12–3.16) for MCP-1. Graphs were prepared using GraphPad Prism (Version 5.01). Data were presented as geometric mean with 95%CI.
We further compared sperm concentration and seminal leucocytes among different subgroups of COVID-19 inpatients. However, no obvious difference of these parameters was observed between the mild cases and ordinary cases, or between the cases with or without a history of high fever (≥39 °C), maybe due to the limited size of patients (Table 1).
4. Discussion
Ever since the pandemic outbreak of COVID-19, several studies have been conducted primarily to understand the disease course, defining the therapeutic approach and strategies to contain the disease spread. It is of utmost importance to quantify the possible adverse effects extended due to COVID-19 illness on different human organ systems, including the male reproductive system, which remains of significant concern for clinicians. Several existing factors negatively impact male fertility, as revealed through a global decline in sperm quality [29] and infection of MRT, particularly caused by the virus, further deteriote sperm quality and negate fertility. To date, the potential impacts of SARS-CoV-2 infection on male fertility remains uncertain. Through this study, we aim to fill this existing gap through our obtained results, which demonstrate the vulnerability of the male reproductive system in COVID-19 inpatients. The findings of this study highlight the importance of delivering a detailed critical evaluation of the effect of SARS-CoV-2 on different male reproductive organs to provide a preliminary base for future studies.
Our first finding upon histopathological examination of testicular tissue samples of severely ill deceased COVID-19 revealed thinning of seminiferous epithelium (decreased cell layers), and higher spermatogenic epithelial shedding in the seminiferous tubules. In addition, we observed a significant increase in apoptotic cells within seminiferous tubules in deceased COVID-19 patients due to the extensive germ cell destruction, which is an indication of impaired spermatogenesis. Upon testing semen samples of mild and ordinary cases of COVID-19 inpatients, as we observed oligospermia (lower sperm count) in 9 out of 21 COVID-19 inpatients who have had offspring through natural pregnancy. And when compared with age-matched control males, the sperm concentration of COVID-19 inpatients was significantly decreased. A recent report also showed decreased sperm concentration in recovered males with a moderate COVID-19 infection [30]. However, the current study only enrolled patients who were fertile or supposed to be fertile, and these patients were detected for positive SARS-CoV-2 RNA in throat swab tests within seven days before the semen collection. Our results demonstrated the impaired spermatogenesis caused by COVID-19. Even for the patient who showed a sperm count above the lower reference limit, the impairment of spermatogenesis cannot be entirely excluded.
Examination of the testicular and epididymal tissues of deceased COVID-19 patients revealed the presence of interstitial edema, congestion, red blood cell exudation, and obvious T-lymphocytes (CD3+) infiltration around blood vessels. Additionally, it showed an increase in the concentration of both T-lymphocytes and macrophages (CD68+) in the interstitium of testicular tissue. These are all evidence indicated an inflammatory response in testes (orchitis) and epididymides (epididymitis). Orchitis is demonstrated by widespread germ cell damage, and destruction it causes with thinning in seminiferous epithelium and leukocyte infiltration; as a result, CD3+ T lymphocytes and CD68+ macrophages would display a significant increase in the interstitium of testicular tissue [16], which accounts for the difference observed in COVID-19 patients when compared to control group. Epididymitis can coexist with orchitis in viral infections [12]. This coexistence might lead to adverse effects like damaging of the spermatozoa, irregular sex-hormones secretion, and dysregulated release of inflammatory cytokines [22]. The deposition of IgG's in the seminiferous epithelium was observed in 4 out of 6 patients and could be a result of an autoimmune reaction [16]. It could be hypothesized that this observed IgG precipitation in the seminiferous tubules in these COVID-19 patients might be induced by a secondary autoimmune response to the viral infection similar to the autoimmune orchitis observed previously in SARS-CoV infected patients [16]. In addition, leukocyte infiltration could negatively impact the function of Leydig cells impairing the production of testosterone, damage the blood-testis barrier, and induce destruction upon seminiferous epithelium [16]. The total damage mentioned previously, combined with inflammatory cytokines, might activate an autoimmune response accompanied by IgG deposits inside the tubules [16]. Furthermore, previous study reported IgG deposition in the seminiferous epithelium, interstitium, and vascular endothelium in experimental autoimmune orchitis [31].
Increased seminal leucocyte concentration signifies the inflammation of MRT, in response to the inflammation of the MRT, the production of proinflammatory cytokines, and the recruitment of immune cells takes place [32]. Herein, the study observed increased seminal leucocyte concentration in most of the COVID-19 inpatients compared to control participants. Independently, immune factors, including IL6, TNF-α, MCP-1, were increased in semen. Leukocytes and cytokines could affect spermatogenesis and interfere with fertility. An increased concentration of seminal leukocytes may cause sperm abnormalities by activating reactive oxygen species. And it has been noted that cytokines could affect Sertoli cell functions and their altered level could impair spermatogenesis [33,34]. These higher concentrations of proinflammatory cytokines and chemokines further complement the findings of immune response observed in the autopsied specimens. The release of proinflammatory cytokines and chemokines, as a systemic immune response upon developing the SARS-CoV-2 infection could result in an uncontrolled inflammation, leading to a substantial reduction and dysfunction of lymphocytes, resulting in an inefficient initiation of the adaptive immune responses [28]. As the disseminated COVID-19 virus continues its attack, immune pathogenesis manifests through both systemic cytokine storm and microcirculation dysfunction [28].
Previous studies postulated that SARS-CoV-2 would impose a deleterious effect on the testes by gaining entry to testicular cells through ACE2 receptors, recent findings show that COVID-19 entry into target host testicular cells through ACE2 receptors is improbable based on studying ACE2 and TMPRSS2 expression in these cells [19]. SARS-CoV-2 entry into the cells requires the dual expression of both ACE and TMPRSS2 proteins [35]. The available data dictates that only 4 of 6490 testicular cells contain mRNA for both two genes [19]; hence the chances of SARS-CoV-2 are unlikely [35]. The cells contain mRNAs for both two genes should be most possibly spermatogonia [13]. However, our results showed that the expression of ACE2 protein is low in spermatogonia, implying low ACE2 protein translation in spermatogonia.
The current study does have some limitations, which include a relatively smaller sample size, and the study could not evaluate these COVID-19 patients prospectively. However, with these existing limitations, this study is first to do a comprehensive investigation to evaluate the virulence on the reproductive health in COVID-19 patients. Our results could be considered preliminary, and further studies are invited to verify our study findings.
In conclusion, altogether, these findings indicate that the male reproductive system could be vulnerable in COVID-19, as demonstrated by spermatogenic dysfunction with a significant decrease in sperm counts in COVID-19 patients, along with immune response in testis and epididymis. This implies to attach importance of applying further care in reproductive health in men infected with COVID-19. Screening of these patients is warranted to investigate whether these impacts are reversible or not and develop standard therapeutic strategies to aid these patients.
5. Contributors
Concept and design (C Xiong, H Li, Q Liu), specimen acquisition and preparation (X Xiao, J Zhang, F Pan), acquisition, analysis, or interpretation of data (all authors), manuscript drafting (H Li, MI Zafar), Critical revision of the manuscript for important intellectual content (C Xiong, Q Liu), Statistical analysis (MI Zafar, W Lu), Obtaining funding (L Liu, H Li, X Xiao), administrative, technical, or material support (C Xiong, L Liu, X Xiao, S Shen, W Lu, K Zhao, L Zhou), and Supervision (C Xiong).
Declaration of Interests
All authors declare no competing interests.
Acknowledgments
Acknowledgments
We would like to thank the study participants for their participation and kind cooperation in this study.
Data sharing
The study data will be made available on reasonable request to the corresponding author. Detailed description regarding the purpose of data request shall be send to the corresponding author. De-identified data of the participant will be provided after approval by principal authors and respective participating institutions.
Funding
This study was supported by Ministry of Science and Technology of China Plan-2020YFC0844700 (L Liu), Hubei Science and Technology Plan-2020FCA045 (L Liu), National Key Research and Development Program of China- 2017YFC1002001 (H Li), HUST COVID-19 Rapid Response Call-2020kfyXGYJ057 (H Li), China and National Natural Science Foundation of China-81874091 (X Xiao); these funding bodies are public institutions, and they had no role in study conception, design, interpretation of results, and manuscript preparation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100604.
Contributor Information
Qian Liu, Email: caixe_liu0222@tom.com.
Chengliang Xiong, Email: clxiong951@sina.com.
Appendix. Supplementary materials
References
- 1.Xu J, Zhao S, Teng T. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:E244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020 doi: 10.1126/science.abb5793. (published online: April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15047. (published online: March 25. [DOI] [PubMed] [Google Scholar]
- 5.Epelboin S, Dulioust E, Epelboin L, Benachi A, Merlet F, Patrat C. Zika virus and reproduction: facts, questions and current management. Hum Reprod Update. 2017;23:629–645. doi: 10.1093/humupd/dmx024. [DOI] [PubMed] [Google Scholar]
- 6.Le Tortorec A, Matusali G, Mahé D, Aubry F, Mazaud-Guittot S, Houzet L. From ancient to emerging infections: the Odyssey of viruses in the male genital tract. Physiol Rev. 2020 doi: 10.1152/physrev.00021.2019. (published online: February 07. [DOI] [PubMed] [Google Scholar]
- 7.Schindell BG, Webb AL, Kindrachuk J. Persistence and sexual transmission of filoviruses. Viruses. 2018;10:E683. doi: 10.3390/v10120683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50:e13140. doi: 10.1111/and.13140. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Jiang X, Gao Y. Mumps virus infection disrupts blood-testis barrier through the induction of TNF-α in Sertoli cells. FASEB. 2019;33:12528–12540. doi: 10.1096/fj.201901089R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Zafar MI, Wang X, Ding X, Li H. Heat stress and pulsed unfocused ultrasound: the viability of these physical approaches for drug delivery into testicular seminiferous tubules. Curr Drug Deliv. 2020;17(5):438–446. doi: 10.2174/1567201817666200514080811. [DOI] [PubMed] [Google Scholar]
- 12.Fijak M, Pilatz A, Hedger MP. Infectious, inflammatory and 'autoimmune' male factor infertility: how do rodent models inform clinical practice. Hum Reprod Update. 2018;24:416–441. doi: 10.1093/humupd/dmy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:E920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Illiano E, Trama F, Costantini E. Could COVID-19 have an impact on male fertility? Andrologia. 2020;52(6):e13654. doi: 10.1111/and.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23:1922–1924. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Qi L, Chi X. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y, He L, Zhang Q. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan F, Xiao X, Guo J. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil Steril. 2020 doi: 10.1016/j.fertnstert.2020.04.024. (E-Pub Ahead of Print). DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song C, Wang Y, Li W. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020 doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Jin M, Bao P. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Xie W, Li D, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. MedRxiv 2020.03.21.20037267. DOI: 10.1101/2020.03.21.20037267
- 23.National Health Commission of the PRC . People's Medical Publishing House; 2020. Guidance for corona virus disease 2019: prevention, control, diagnosis and management.http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf Available at. [Google Scholar]
- 24.Schulz C, van der Poel WH, Ponsart C. European interlaboratory comparison of Schmallenberg virus (SBV) real-time RT-PCR detection in experimental and field samples: the method of extraction is critical for SBV RNA detection in semen. J Vet Diagn Invest. 2015;27(4):422–430. doi: 10.1177/1040638715593798. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann B, Schulz C, Beer M. First detection of Schmallenberg virus RNA in bovine semen, Germany, 2012 [published correction appears in Vet Microbiol. 2014 May 14;170(1-2):179] Vet Microbiol. 2013;167(3-4):289–295. doi: 10.1016/j.vetmic.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Dong TT, Yu Q, Qing XR, Ma XL, Dong WW, Shi J, Li HG. Potential confounding factors in measurement of specific cell-free seminal mRNAs and microRNAs derived from human reproductive organs. Andrology. 2016;4(6):1010–1019. doi: 10.1111/andr.12238. [DOI] [PubMed] [Google Scholar]
- 27.WHO (World Health Organization) fifth ed. WHO Press; Switzerland: 2010. WHO laboratory manual for the examination and processing of human semen.https://apps.who.int/iris/bitstream/handle/10665/44261/9789241547789_eng.pdf Available at. (2010) [Google Scholar]
- 28.Li H, Liu L, Zhang D. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020 doi: 10.1016/S0140-6736(20)30920-X. (published online: April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen N, Andersen AG, Eustache F. Regional differences in semen quality in Europe. Hum Reprod. 2001;16(5):1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- 30.Holtman N, Edimiris P, Andree M. Assessment of SARS-CoV-2 in human semen – a cohort study. Fertil Steril. 2020 doi: 10.1016/j.fertnstert.2020.05.028. (E-Pub Ahead of Print). DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh M, Hiramine C, Tokunaga Y, Mukasa A, Hojo K. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. II. Immunohistochemical findings of fully-developed inflammatory lesion. Autoimmunity. 1991;10:89–97. doi: 10.3109/08916939109004812. [DOI] [PubMed] [Google Scholar]
- 32.Hedger MP, Meinhardt A. Cytokines and the immune-testicular axis. J Reprod Immunol. 2003;58:1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 33.Aziz N, Agarwal A, Lewis-Jones I, Sharma RK, Thomas AJ., Jr Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril. 2004;82(3):621–627. doi: 10.1016/j.fertnstert.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 34.Fraczek M, Kurpisz M. Cytokines in the male reproductive tract and their role in infertility disorders. J Reprod Immunol. 2015;108:98–104. doi: 10.1016/j.jri.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg ML. Coronavirus disease 2019 (COVID-19) and men's reproductive health. Fertil Steril. 2020 doi: 10.1016/j.fertnstert.2020.04.039. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.