Key Points
Question
Does remdesivir provide a benefit on clinical status for patients hospitalized with moderate coronavirus disease 2019 (COVID-19) pneumonia?
Findings
In this randomized, open-label, phase 3 trial that included 584 patients with moderate COVID-19, the day 11 clinical status distribution measured on a 7-point ordinal scale was significantly better for those randomized to a 5-day course of remdesivir (median length of treatment, 5 days) compared with those randomized to standard care. The difference for those randomized to a 10-day course (median length of treatment, 6 days) compared with standard care was not significantly different.
Meaning
Hospitalized patients with moderate COVID-19 randomized to a 5-day course of remdesivir had a statistically significantly better clinical status compared with those randomized to standard care at 11 days after initiation of treatment, but the difference was of uncertain clinical importance.
Abstract
Importance
Remdesivir demonstrated clinical benefit in a placebo-controlled trial in patients with severe coronavirus disease 2019 (COVID-19), but its effect in patients with moderate disease is unknown.
Objective
To determine the efficacy of 5 or 10 days of remdesivir treatment compared with standard care on clinical status on day 11 after initiation of treatment.
Design, Setting, and Participants
Randomized, open-label trial of hospitalized patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and moderate COVID-19 pneumonia (pulmonary infiltrates and room-air oxygen saturation >94%) enrolled from March 15 through April 18, 2020, at 105 hospitals in the United States, Europe, and Asia. The date of final follow-up was May 20, 2020.
Interventions
Patients were randomized in a 1:1:1 ratio to receive a 10-day course of remdesivir (n = 197), a 5-day course of remdesivir (n = 199), or standard care (n = 200). Remdesivir was dosed intravenously at 200 mg on day 1 followed by 100 mg/d.
Main Outcomes and Measures
The primary end point was clinical status on day 11 on a 7-point ordinal scale ranging from death (category 1) to discharged (category 7). Differences between remdesivir treatment groups and standard care were calculated using proportional odds models and expressed as odds ratios. An odds ratio greater than 1 indicates difference in clinical status distribution toward category 7 for the remdesivir group vs the standard care group.
Results
Among 596 patients who were randomized, 584 began the study and received remdesivir or continued standard care (median age, 57 [interquartile range, 46-66] years; 227 [39%] women; 56% had cardiovascular disease, 42% hypertension, and 40% diabetes), and 533 (91%) completed the trial. Median length of treatment was 5 days for patients in the 5-day remdesivir group and 6 days for patients in the 10-day remdesivir group. On day 11, patients in the 5-day remdesivir group had statistically significantly higher odds of a better clinical status distribution than those receiving standard care (odds ratio, 1.65; 95% CI, 1.09-2.48; P = .02). The clinical status distribution on day 11 between the 10-day remdesivir and standard care groups was not significantly different (P = .18 by Wilcoxon rank sum test). By day 28, 9 patients had died: 2 (1%) in the 5-day remdesivir group, 3 (2%) in the 10-day remdesivir group, and 4 (2%) in the standard care group. Nausea (10% vs 3%), hypokalemia (6% vs 2%), and headache (5% vs 3%) were more frequent among remdesivir-treated patients compared with standard care.
Conclusions and Relevance
Among patients with moderate COVID-19, those randomized to a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment. Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.
Trial Registration
ClinicalTrials.gov Identifier: NCT04292730
This open-label randomized trial compares the effect of remdesivir (5 or 10 days) vs standard care on clinical status 11 days after treatment initiation among patients with confirmed SARS-CoV-2 infection hospitalized with moderate pneumonia (room air oxygen saturation >94%).
Introduction
In the first 6 months of the pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide and has infected nearly 20 million people.1,2 As of August 10, 2020, coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2, resulted in more than 163 000 deaths in the United States and more than 730 000 worldwide.2 Many infected people are asymptomatic or experience mild symptoms and recover without medical intervention.3,4 However, older people and those with comorbid hypertension, diabetes, obesity, and heart disease are at higher risk of life-threatening illness.5,6
Remdesivir is a nucleotide prodrug whose active metabolite inhibits viral RNA-dependent RNA polymerases, structurally conserved enzymes that play a key role in the replication of a broad range of viruses, including Coronaviridae.7,8,9 A first randomized, placebo-controlled trial of remdesivir among patients with COVID-19 conducted in Wuhan, China, could not complete enrollment to meaningfully assess efficacy.10 However, in a larger randomized, double-blind clinical trial, patients with severe COVID-19 treated with a 10-day course of remdesivir had a significantly shorter time to recovery than those receiving placebo (11 days vs 15 days).11 Subsequently, a randomized, open-label trial showed that patients with severe COVID-19 with relative hypoxia or requiring oxygen support but not requiring ventilatory support had outcomes with 5- and 10-day courses of remdesivir that were not significantly different.12 These results prompted the US Food and Drug Administration to grant Emergency Use Authorization of remdesivir for patients with severe COVID-19 and the European Medicines Agency to grant conditional marketing authorization to remdesivir for treatment of COVID-19 in patients 12 years of age or older with pneumonia who require supplemental oxygen.13,14 Concurrent with these studies, this randomized, open-label, multicenter study was conducted to evaluate the efficacy and adverse events of remdesivir administered for 5 or 10 days vs standard care in hospitalized patients with moderate COVID-19.
Methods
The protocol was approved by the institutional review board or independent ethics committee from each site and conducted in compliance with the Declaration of Helsinki,15 Good Clinical Practice guidelines, and local regulatory requirements. The trial protocol and statistical analysis plan are included in Supplement 1 and Supplement 2. All patients or legally authorized representatives provided written informed consent.
Patients
Hospitalized patients with SARS-CoV-2 infection confirmed by polymerase chain reaction assay within 4 days of randomization and moderate COVID-19 pneumonia (defined as any radiographic evidence of pulmonary infiltrates and oxygen saturation >94% on room air) were enrolled. Patients with alanine aminotransferase or aspartate aminotransferase greater than 5 times the upper limit of normal or creatinine clearance of less than 50 mL/min were excluded. (Detailed eligibility criteria are provided in eAppendix 1 in Supplement 3.)
Study Design and Oversight
Patients were enrolled at 105 hospitals in the United States, Europe, and Asia between March 15, 2020, and April 18, 2020, and randomly assigned in a 1:1:1 ratio to receive up to a 5-day course of remdesivir, up to a 10-day course of remdesivir, or standard care. Randomization was not stratified. The randomization list was created and validated by the interactive web response system (IWRS) vendor. A dummy randomization list was provided in Microsoft Excel format to the biostatistician employed by the study sponsor for review. A separate list of sequential patient numbers within each treatment group was generated by the IWRS vendor. The randomization had a block size of 6. Based on the treatment from the randomization list, the IWRS provided the next sequential patient number to the site along with the treatment group assignment. The appropriate number of vials of open-label study drug were assigned to the patient. Sites did not have access to the randomization list and could not know the sequence of treatments. Treatment was open label because the sponsor had an insufficient number of placebo-containing vials to support this trial.
All patients randomized to a remdesivir group received 200 mg of remdesivir intravenously on day 1, followed by 100 mg of remdesivir once daily for the subsequent days, infused over 30 to 60 minutes. Remdesivir treatment was to be discontinued in any patient experiencing severe elevations in liver enzymes or decreases in estimated creatinine clearance to less than 30 mL/min (see eAppendix 1 in Supplement 3 for details). Patients who had sufficiently improved in the judgment of the investigator could be discharged from the hospital before finishing their assigned course of treatment.
The protocol was amended on March 15, 2020, on the basis of emerging understanding of the clinical presentation and assessment of COVID-19. The age limit for eligibility was lowered from 18 years to 12 years and the minimum temperature requirement was eliminated. The primary end point in the original protocol was the proportion of patients discharged by day 14 of the study. This was amended to assessment of clinical status on a 7-point ordinal scale by day 11 (described below).11,14,16 In addition, a nonrandomized extension phase was added in which up to 1000 additional patients could be enrolled to receive remdesivir; the results of the extension phase will be the subject of a subsequent report. The statistical analysis plan was approved on May 25, 2020, prior to the database lock for the day 11 analysis. The statistical analysis plan was amended on June 26, 2020, prior to the database lock for the day 28 analysis. A detailed account of changes to the protocol is provided in eAppendix 1 in Supplement 3. All protocol amendments were authorized and approved by the sponsor, the institutional review board or independent ethics committee, and the pertinent regulatory authorities (eg, Food and Drug Administration for the United States; European Medicines Agency for Europe). The original protocol allowed use of other agents with presumptive activity against SARS-CoV-2 if such use was local standard care. Although this exception was disallowed in a subsequent amendment, some patients had already received other concurrent therapies.
Clinical and Laboratory Monitoring
Patient assessments included physical examination, respiratory status (respiratory rate, type of oxygen supplementation, blood oxygen saturation, and radiographic findings), adverse events, and concomitant medications. On study days 1, 3, 5, 8, 10, and 14, blood samples were obtained for measurement of blood cell counts, serum creatinine, glucose, total bilirubin, and liver transaminases. Self-reported fixed race and ethnicity categories were collected as part of the demographic information to assess possible differences in disease severity or response to treatment.
Site investigators assessed clinical status daily from day 1 through day 14 or hospital discharge on a 7-point ordinal scale11,12 consisting of the following categories: 1, death; 2, hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation; 3, hospitalized, requiring noninvasive ventilation or use of high-flow oxygen devices; 4, hospitalized, requiring low-flow supplemental oxygen; 5, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (related or not to COVID-19); 6, hospitalized, not requiring supplemental oxygen or ongoing medical care; and 7, not hospitalized (eTable 1 in Supplement 3). If the clinical status of patients who remained hospitalized changed on a particular day, the worst score was documented. A final assessment was conducted on day 28 in person for hospitalized patients or by phone for those who had been discharged.
End Points
The primary efficacy end point was the distribution of clinical status assessed on the 7-point ordinal scale on study day 11. If remdesivir treatment improves outcomes, the distribution of scores among patients who received remdesivir should shift more toward the higher values of the scale than the distribution of scores among patients who received standard care.
The secondary end point was the proportion of patients with adverse events throughout the duration of the study. Prespecified exploratory end points were (1) time to recovery (improvement from a baseline score of 2-5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7); (2) time to modified recovery (improvement from a baseline score of 2-4 to a score of 5-7, improvement from a baseline score of 5 to a score of 6-7, or improvement from a baseline score of 6 to a score of 7); (3) time to clinical improvement (≥2-point improvement from baseline on the 7-point ordinal scale); (4) time to 1-point or larger improvement; and (5) time to discontinuation of any oxygen support. The proportion of patients with these end points was also assessed on days 5, 7, and 11. Other exploratory end points were duration of hospitalization, duration of different modes of respiratory support, and all-cause mortality. Due to logistical issues at the time of study implementation, virological and pharmacokinetic measurements were limited, including SARS-CoV-2 polymerase chain reaction tests on day 5 and day 10, and are not presented.
Statistical Analysis
We calculated that 600 patients (200 in each group) would provide greater than 85% power to detect an odds ratio of 1.8 for each remdesivir group vs the standard care group using a 2-sided significance level of .05. The odds ratio of 1.8 was calculated based on proposed group sizes at the time of study conception and was not intended as a minimum clinically meaningful treatment effect, as no prior data were available on the distribution of clinical status categories over time in patients with moderate COVID-19. An odds ratio greater than 1 indicates changes in clinical status across all categories toward category 7 for the remdesivir groups vs the standard care group. All patients who were randomized and received at least 1 dose of remdesivir, or for the standard care group, had the day 1 visit, were assessed for efficacy and adverse events. For clinical status, the ordinal score was recorded as 1 on the day of death and all subsequent days; if a patient was discharged, the ordinal score was recorded as 7 on the day of discharge alive and all subsequent days unless the patient was rehospitalized for COVID-19–related reasons; otherwise, the most recent assessment was used for missing values. We used SAS version 9.4 (SAS Institute Inc) for all analyses.
For the primary efficacy end point, each remdesivir group was compared with the standard care group at a 2-sided α = .025 (Bonferroni). Proportional odds models were used with treatment as the independent variable; odds ratios and 95% CIs are presented. The assumption of proportional odds was tested using the score test, and supporting P values from the Wilcoxon rank sum test are provided if the proportional odds assumption was not met. Analyses including baseline clinical status as a covariate were also performed.
For the secondary end point of proportion of patients with adverse events throughout the duration of the study, comparisons between each remdesivir group and the standard care group were performed using a Fisher exact test; point estimates of the group differences and corresponding 95% CIs were calculated. For the prespecified exploratory end points, death was considered the competing risk in these time-to-event analyses. Patients without the event of interest were censored on the day of the last nonmissing ordinal scale assessment.
All-cause mortality was estimated using the Kaplan-Meier product limit method with all available data. Each remdesivir group was compared with the standard care group using the log-rank test, and hazard ratios and 95% CIs were provided. Participants who did not die were censored on the last study day.
Durations of oxygen therapy and hospitalization were summarized and compared between groups using the Wilcoxon rank sum test.
Post Hoc Analyses
We conducted sensitivity analyses of the primary end point (1) adjusting for day 1 clinical score; (2) adjusting for duration of symptoms; (3) using day 28 visit data to confirm day 11 clinical status and imputing patients with missing status as dead; and (4) using all randomized patients whether they received treatment or not (intention-to-treat population). We calculated the proportions of patients with a 1-point or greater improvement at day 11 within subgroups based on symptom duration, body mass index, race, baseline oxygen support, region, sex, and age. To understand if the open-label design and assigned duration of treatment had an effect on hospital discharge, we calculated rates of discharge in each group over time.
Because of the potential for type I error due to multiple comparisons, findings for analyses of end points other than the primary end point should be interpreted as exploratory.
Results
Patient Disposition and Characteristics
Of 612 patients who consented and were assessed for eligibility, 596 underwent randomization and 584 began the study: 193 began a 10-day course of remdesivir, 191 patients began a 5-day course of remdesivir, and 200 continued standard care (Figure 1). Of the 16 patients who were not randomized, 13 did not meet eligibility criteria and 3 withdrew consent. Twelve randomized patients did not receive treatment: 8 withdrew consent, 3 had protocol violations, and 1 was withdrawn by investigator discretion.
Figure 1. Participant Flow in a Randomized Clinical Trial of Remdesivir vs Standard Care in Patients With Moderate Coronavirus Disease 2019.
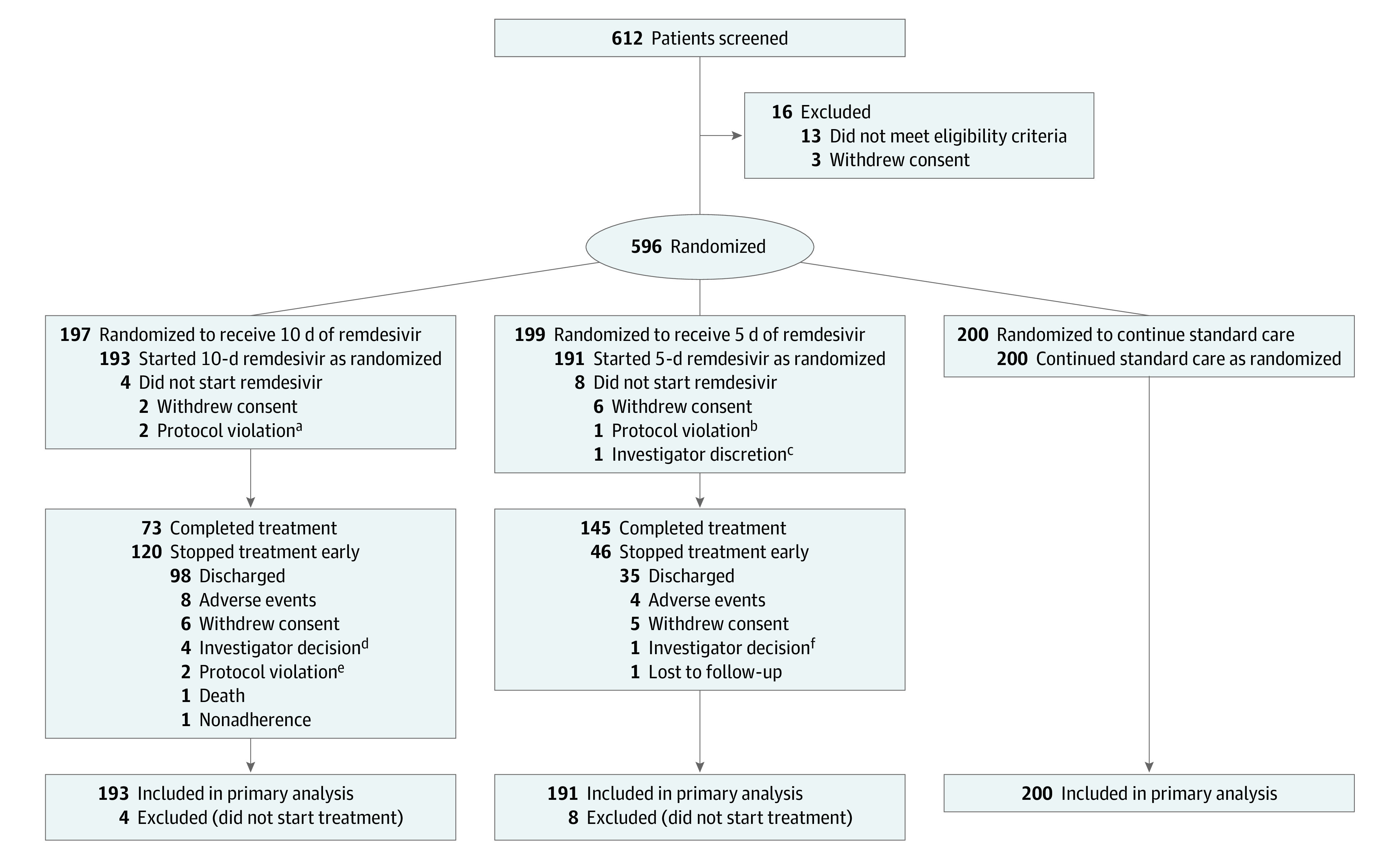
aBoth patients met the eligibility criteria for creatinine clearance at screening but not on the day of randomization.
bPatient tested negative for severe acute respiratory syndrome coronavirus 2.
cPatient’s oxygen saturation was less than 94% on the day of randomization.
dThree patients improved enough for discharge in the judgment of the investigator; 1 patient discontinued because of episodes of hypotension.
eOne patient discontinued because of use of a prohibited medication and 1 patient was found to have been enrolled in error (creatinine clearance <50 mL/min).
fPersistent difficulty with intravenous access led investigator to decide to stop treatment in this patient.
Patients in the 3 groups were balanced in demographics and disease characteristics (Table 1). Overall, 56% of patients had cardiovascular disease, 42% had hypertension, 40% had diabetes, and 14% had asthma. Although all patients had an oxygen saturation above 94% while breathing room air at screening, 12% of patients in the 10-day remdesivir group, 16% in the 5-day remdesivir group, and 19% in the standard care group used supplemental oxygen on day 1 because of deteriorating clinical status or for breathing comfort. Patients in all groups had been hospitalized a median of 2 days (interquartile range [IQR], 1-3 days) before study day 1. The median duration of symptoms before day 1 in the standard care group was 9 days (IQR, 6-11 days) compared with 8 days (IQR, 5-11 days) for both groups receiving remdesivir. Patients randomized to the standard care group were more commonly prescribed other agents with putative activity against COVID-19 (Table 1).
Table 1. Demographics and Baseline Disease Characteristics.
| Characteristics | 10-Day remdesivir (n = 193) | 5-Day remdesivir (n = 191) | Standard care (n = 200) |
|---|---|---|---|
| Age, median (IQR), y | 56 (45-66) | 58 (48-66) | 57 (45-66) |
| Sex, No. (%) | |||
| Male | 118 (61) | 114 (60) | 125 (63) |
| Female | 75 (39) | 77 (40) | 75 (38) |
| Race, No./total (%) | |||
| White | 107/188 (57) | 109/186 (59) | 112/193 (58) |
| Black | 37/188 (20) | 35/186 (19) | 27/193 (14) |
| Asian | 31/188 (16) | 34/186 (18) | 37/193 (19) |
| Othera | 13/188 (7) | 8/186 (4) | 17/193 (9) |
| Hispanic or Latino ethnicity, No./total (%)b | 42/186 (23) | 25/187 (13) | 34/186 (18) |
| Body mass index, median (IQR)c | 28 (25-32) | 27 (24-30) | 27 (24-31) |
| Day 1 clinical status on 7-point scale, No. (%) | |||
| 3: Hospitalized, requiring noninvasive ventilation or high-flow oxygen | 1 (1) | 2 (1) | 2 (1) |
| 4: Hospitalized, requiring low-flow supplemental oxygen | 23 (12) | 29 (15) | 36 (18) |
| 5: Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care | 163 (84) | 160 (84) | 160 (80) |
| 6: Hospitalized, not requiring supplemental oxygen or ongoing medical cared | 6 (3) | 0 | 2 (1) |
| Coexisting conditions, No. (%) | |||
| Cardiovascular disease | 111 (58) | 111 (58) | 107 (54) |
| Hypertension | 85 (44) | 82 (43) | 81 (41) |
| Diabetes | 85 (44) | 71 (37) | 76 (38) |
| Asthma | 31 (16) | 22 (12) | 28 (14) |
| Duration of hospitalization before first dose of remdesivir, median (IQR), d | 2 (1-3) | 2 (1-3) | 2 (1-3) |
| Duration of symptoms before first dose of remdesivir, median (IQR), d | 8 (5-11) | 8 (5-11) | 9 (6-11) |
| Concomitant medications, No. (%)e | |||
| Steroids | 29 (15) | 33 (17) | 38 (19) |
| Hydroxychloroquine/chloroquine | 22 (11) | 16 (8) | 89 (45) |
| Lopinavir-ritonavir | 11 (6) | 10 (5) | 43 (22) |
| Tocilizumab | 1 (1) | 1 (1) | 10 (5) |
| Azithromycin | 41 (21) | 35 (18) | 62 (31) |
| Aspartate aminotransferase, median (IQR), U/L | 34 (23-48) | 32 (25-48) | 34 (24-49) |
| Alanine aminotransferase, median (IQR), U/L | 28 (21-47) | 30 (19-51) | 30 (19-49) |
| Estimated glomerular filtration rate, median (IQR), mL/minf | 110 (86-143) | 99 (75-130) | 103 (78-130) |
Abbreviation: IQR, interquartile range.
Includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Arab, unknown, and not specified.
In patients with available ethnicity data.
Calculated as weight in kilograms divided by height in meters squared.
Some patients remained hospitalized for quarantine purposes or other social issues even if they did not require medical care.
Includes medications taken between first and last dose of remdesivir (or after day 1 for the standard care group).
Glomerular filtration rate estimated by the Cockcroft-Gault formula.
Of 191 patients in the 5-day remdesivir group, 145 (76%) completed the assigned treatment duration (median, 5 doses; range, 1-5) (eTable 2 in Supplement 3). Reasons for discontinuing treatment included hospital discharge (35 patients [18%]), withdrawal of consent (5 [3%]), and adverse events (4 [2%]). Of the 193 patients in the 10-day remdesivir group, 73 (38%) completed the assigned treatment duration; the median number of doses for the group was 6 (range, 1-10). Reasons for discontinuing treatment included hospital discharge (98 patients [51%]), adverse events (8 [4%]), and withdrawal of consent (6 [3%]) (Figure 1). Five hundred thirty-three patients (91%) completed the study through day 28.
There were 37 patients (6.3%) who had not died, had not been discharged, and did not have a clinical status reported on day 11: 27 were transferred to another facility before day 11, 6 withdrew consent, 1 was withdrawn for a protocol violation, and 3 were discharged prior to day 11 but rehospitalized after day 11. The last available clinical status was used to impute clinical status on day 11 for these 37 patients.
Efficacy
Primary End Point
On day 11, patients randomized to the 5-day remdesivir group had significantly higher odds of a better clinical status distribution on the 7-point ordinal scale compared with those randomized to standard care (odds ratio, 1.65; 95% CI, 1.09-2.48; P = .02) (Table 2 and Figure 2). The difference in clinical status distribution on day 11 between the 10-day remdesivir and standard care groups was not statistically significant (P = .18 by Wilcoxon rank sum test; the proportional odds assumption was not met for this comparison).
Table 2. Clinical Outcomes.
| Outcomes | 10-Day remdesivir (n = 193) | 5-Day remdesivir (n = 191) | Standard care (n = 200) |
|---|---|---|---|
| Day 11 clinical status on 7-point scale, No. (%) | |||
| 1: Death | 2 (1) | 0 | 4 (2) |
| 2: Hospitalized, requiring invasive mechanical ventilation or extracorporeal membrane oxygenation | 1 (1) | 0 | 4 (2) |
| 3: Hospitalized, requiring noninvasive ventilation or high-flow oxygen | 0 | 5 (3) | 7 (4) |
| 4: Hospitalized, requiring low-flow supplemental oxygen | 12 (6) | 7 (4) | 11 (6) |
| 5: Hospitalized, not requiring supplemental oxygen but requiring ongoing medical care | 44 (23) | 38 (20) | 46 (23) |
| 6: Hospitalized, not requiring supplemental oxygen or medical care | 9 (5) | 7 (4) | 8 (4) |
| 7: Not hospitalized | 125 (65) | 134 (70) | 120 (60) |
| Primary end point: difference in clinical status distribution vs standard care, odds ratio (95% CI)a | 1.65 (1.09-2.48) | 1 [Reference] | |
| P value | .18 | .02 | |
| Clinical improvement, No. (%)b | |||
| Day 5 | 72 (37) | 61 (32) | 66 (33) |
| Day 7 | 92 (48) | 106 (56) | 94 (47) |
| Day 11 | 126 (65) | 134 (70) | 121 (61) |
| Difference in percentage vs standard care at day 11 (95% CI) | 4.8 (−5.0 to 14.4) | 9.7 (0.1-19.1) | |
| Day 14 | 148 (77) | 146 (76) | 135 (68) |
| Day 28 | 174 (90) | 171 (90) | 166 (83) |
| Recovery, No. (%)c | |||
| Day 5 | 74 (38) | 67 (35) | 71 (36) |
| Day 7 | 94 (49) | 114 (60) | 101 (51) |
| Day 11 | 132 (68) | 141 (74) | 128 (64) |
| Difference in percentage vs standard care at day 11 (95% CI) | 4.4 (−5.0 to 13.8) | 9.8 (0.3-19.0) | |
| Day 14 | 153 (79) | 153 (80) | 145 (73) |
| Day 28 | 178 (92) | 175 (92) | 170 (85) |
The odds ratio and P value for the 5-day remdesivir treatment group comparison were estimated using the proportional odds model. The proportional odds assumption was not met for the 10-day remdesivir group comparison, so no odds ratio is presented; the P value was calculated using the Wilcoxon rank sum test.
An improvement of at least 2 points from baseline on the 7-point ordinal scale.
An improvement from a baseline score of 2 to 5 to a score of 6 or 7 or from a baseline score of 6 to a score of 7.
Figure 2. Clinical Status on a 7-Point Ordinal Scale on Study Days 11, 14, and 28 by Treatment Group.
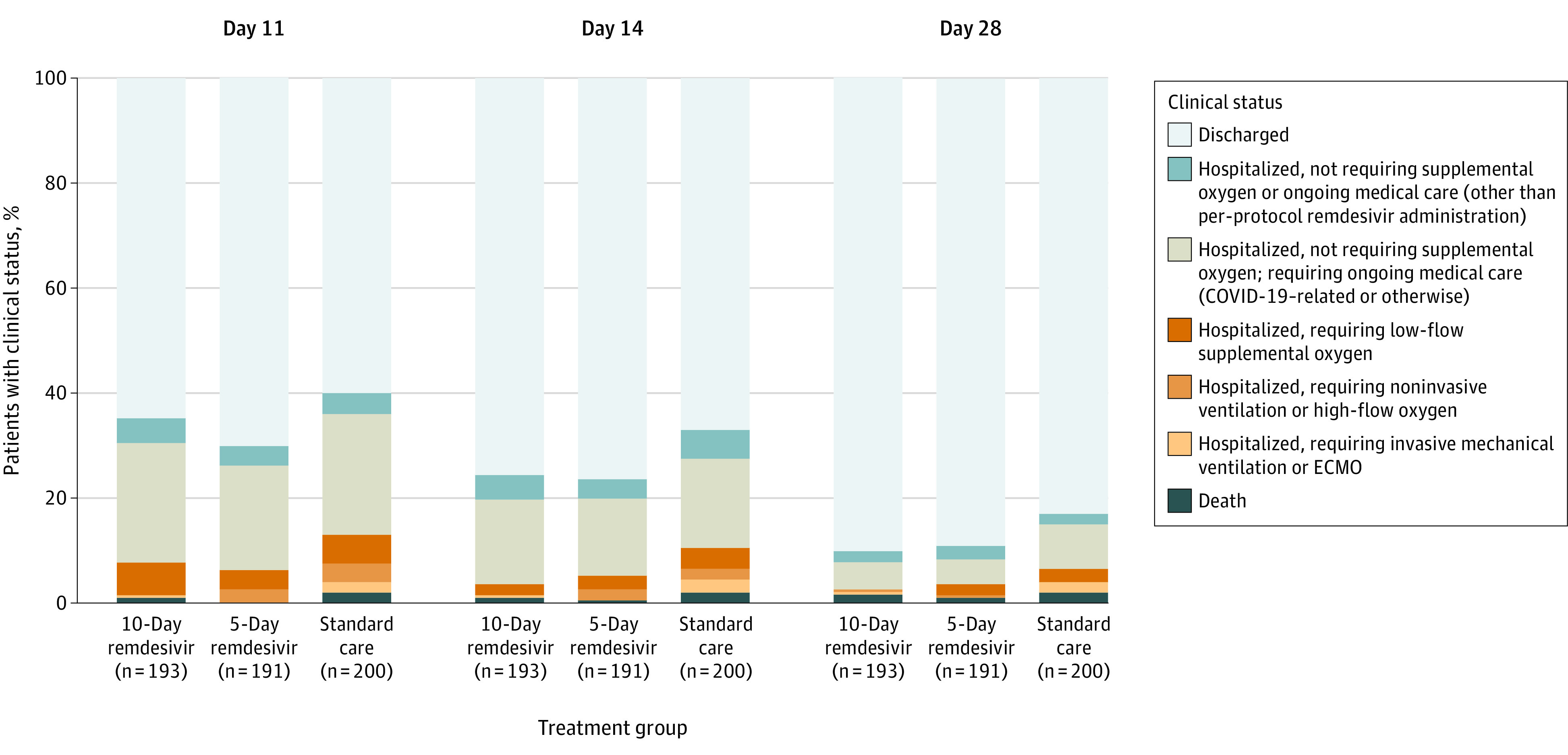
COVID-19 indicates coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation. All percentage values in each point category are provided in eTables 5 and 6 in Supplement 3. At day 11, P = .18 for comparison of the distribution of the 10-day remdesivir group vs standard care and P = .02 for 5-day remdesivir vs standard care (Table 2). At day 14, P = .03 for comparisons of both the 5-day and 10-day remdesivir groups vs standard care (eTable 5 in Supplement 3). At day 28, P = .03 for comparison of the 10-day remdesivir group vs standard care and P = .08 for 5-day remdesivir vs standard care (eTable 6 in Supplement 3). P values were calculated with the Wilcoxon rank sum test comparing the distribution of the groups.
Exploratory Efficacy End Points
There were no significant differences between the 5-day or 10-day remdesivir groups and standard care for any of the exploratory end points—time to 2-point or greater improvement in clinical status, time to 1-point or greater improvement in clinical status, time to recovery, time to modified recovery, and time to discontinuation of oxygen support (eTable 3 in Supplement 3). There were no significant differences between the remdesivir and standard care groups in duration of oxygen therapy or hospitalization. The Kaplan-Meier estimates of all-cause mortality at day 28 were 1% (95% CI, 0.0%-2.6%) for the 5-day remdesivir group (log-rank P = .43 vs standard care), 2% (95% CI, 0.0%-3.6%) for the 10-day remdesivir group (log-rank P = .72 vs standard care), and 2% (95% CI, 0.1%-4.1%) for the standard care group.
Post Hoc Analyses
Sensitivity analyses of the primary end point adjusting for day 1 clinical status score, symptom duration, imputing patients with missing status as dead, and using the intention-to-treat population produced similar results (eTable 4 in Supplement 3).
By day 14, the clinical status of the 5-day and 10-day remdesivir groups was significantly different than that of the standard care group (P = .03 for both groups) (Figure 2 and eTable 5 in Supplement 3). By day 28, clinical status remained significantly different in the 10-day remdesivir group (P = .03) compared with standard care (eTable 6 and eFigure 1 in Supplement 3). No clear subgroup differences were noted between the remdesivir groups in proportions of patients with 1-point or greater improvement in clinical status (eFigure 2 in Supplement 3).
To explore the possible effect of the open-label design on study outcomes, rates of hospital discharge over time by treatment group were tabulated (eFigure 3 in Supplement 3). Peaks in discharge rates were observed in the remdesivir groups following the end of their assigned duration of treatment (ie, there were increased rates of discharge on day 6 in the 5-day remdesivir group and on day 11 in the 10-day group), suggesting that discharges were delayed for some patients to allow them to complete full courses of assigned remdesivir treatment.
Adverse Events (Secondary End Point)
Adverse events were experienced by 51% of patients in the 5-day remdesivir group, 59% in the 10-day remdesivir group, and 47% in the standard care group (Table 3). The difference in proportions between the 5-day remdesivir group and standard care was not statistically significant (4.8%; 95% CI, –5.2% to 14.7%; P = .36), but the difference between the 10-day remdesivir group and standard care was significant (12.0%; 95% CI, 1.6%-21.8%; P = .02). Adverse events that were more common in the remdesivir groups than in the standard care group include nausea, hypokalemia, and headache (Table 3).
Table 3. Adverse Event Summarya.
| Adverse events | No./total (%) | ||
|---|---|---|---|
| 10-Day remdesivir (n = 193) | 5-Day remdesivir (n = 191) | Standard care (n = 200) | |
| Any adverse event | 113 (59) | 98 (51) | 93 (47) |
| Any grade ≥3 adverse event | 24 (12) | 20 (10) | 24 (12) |
| Any serious adverse event | 10 (5) | 9 (5) | 18 (9) |
| Discontinuation of treatment because of adverse event | 8 (4) | 4 (2) | NA |
| Deathb | 3 (2) | 2 (1) | 4 (2) |
| Adverse events occurring in >5% of participants in any treatment group | |||
| Nausea | 18 (9) | 19 (10) | 6 (3) |
| Diarrhea | 10 (5) | 12 (6) | 14 (7) |
| Hypokalemia | 13 (7) | 10 (5) | 4 (2) |
| Headache | 10 (5) | 10 (5) | 5 (3) |
| Laboratory abnormalities | |||
| Any grade | 128/179 (72) | 131/180 (73) | 136/186 (73) |
| Grade 3 | 25/179 (14) | 18/180 (10) | 25/186 (13) |
| Grade 4 | 4/179 (2) | 5/180 (3) | 9/186 (5) |
| Alanine aminotransferase increase | |||
| Any grade | 57/177 (32) | 61/179 (34) | 71/182 (39) |
| Grade 3 (>5 to 10 times ULN) | 6/177 (3) | 4/179 (2) | 11/182 (6) |
| Grade 4 (>10 times ULN) | 0 | 0 | 3 (2) |
| Aspartate aminotransferase increase | |||
| Any grade | 56/175 (32) | 56/177 (32) | 60/182 (33) |
| Grade 3 (>5 to 10 times ULN) | 2/175 (1) | 3/177 (2) | 6/182 (3) |
| Grade 4 (>10 times ULN) | 0 | 1/177 (1) | 5/182 (3) |
| Creatinine clearance decrease | |||
| Any grade | 45/176 (26) | 26/178 (15) | 55/183 (30) |
| Grade 3 (30 to <60 mL/min or 30% to <50% decrease from baseline) | 7/176 (4) | 4/178 (2) | 9/183 (5) |
| Grade 4 (<30 mL/min, ≥50% decrease from baseline, or dialysis needed) | 2/176 (1) | 0 | 5/183 (3) |
Abbreviations: NA, not applicable; ULN, upper limit of normal.
All safety analyses are inclusive of all available data for patients through the data cutoff time point.
Through day 28 of the trial.
Serious adverse events were less common in the remdesivir groups (5% in both) than in the standard care group (9%), differences of −4.3% (95% CI, −9.7% to 0.9%; P = .11) for the 5-day remdesivir group vs standard care and −3.8% (95% CI, −9.3% to 1.4%; P = .17) for the 10-day remdesivir group vs standard care. All 9 deaths through day 28 (2 [1%] in the 5-day remdesivir group, 3 [2%] in the 10-day remdesivir group, and 4 [2%] in the standard care group) occurred in patients aged 64 years or older, and none was attributed to remdesivir treatment.
Discussion
In this clinical trial of patients with moderate COVID-19 pneumonia, those who were randomized to remdesivir treatment for up to 5 days had significantly higher odds of having a better clinical status distribution on day 11 than those receiving standard care, but with an effect size of uncertain clinical importance. The difference in the distribution of clinical status on day 11 between the 10-day remdesivir and standard care groups was not significant.
Several factors may account for the lack of difference in clinical status observed in the 10-day remdesivir group, although the median length of treatment was 6 days in this group. Given the open-label design of the study and the requirement for intravenous dosing of remdesivir, discharge decisions may have been influenced by the assigned duration of remdesivir therapy. Rates of discharge peaked on the day after the end of dosing in both groups: on day 6 for the 5-day group and on day 11 for the 10-day group (eFigure 1 in Supplement 3). However, when outcomes at days 14 and 28 were evaluated, similar distributions of clinical status were observed between patients in the remdesivir groups, possibly pointing to differences compared with standard care. Patients were not stratified by site at enrollment, which may have led to imbalances in patient care and discharge practices. In addition, the possibility that additional days of hospitalization and remdesivir treatment for patients in the 10-day group had a negative effect on outcomes cannot be excluded, although the rates of grade 3 or higher adverse events and serious adverse events were not higher in the 10-day remdesivir group than in the 5-day remdesivir and standard care groups.
There was no a priori knowledge of the trajectories of patients hospitalized with moderate COVID-19 disease—those with confirmed infiltrates by radiology, but with room-air oxygen saturations greater than 94% at rest. The majority of these patients were enrolled within 2 days of hospitalization, yet 15% received supplemental oxygen on day 1. This is a relevant observation for future trials as the ordinal clinical status scales used to date do not take into account oxygen saturation values or oxygen supplies and patterns of use in different health systems.17 In the ACTT-1 and RECOVERY trials, mortality and treatment effects were strongly influenced by clinical status at randomization, confirming a spectrum of severity among hospitalized patients with COVID-19.11,17 Future trials should consider studying individual severity strata incorporating further clarification and refinements in their definitions. Factors that contribute to patients progressing to severe and critical COVID-19 remain to be elucidated. The risk of rapid disease progression described to date points to the potential benefit of earlier intervention with an effective antiviral.6,18
Limitations
This study has several limitations. First, the original protocol was written when COVID-19 cases were largely confined to Asia and the clinical understanding of disease was limited to case series.19,20 This led to a change in the primary end point on the first day of study enrollment as it became clear that hospital discharge rates varied greatly across regions and the ordinal scale had become standard for interventional COVID-19 studies.11,21 Second, the study used an open-label design, which potentially led to biases in patient care and reporting of data. Third, because of the urgent circumstances in which the study was conducted, virologic outcomes such as effect of remdesivir on SARS-CoV-2 viral load were not assessed. Fourth, other laboratory parameters that may have aided in identifying additional predictors of outcomes were not routinely collected. Fifth, the ordinal scale used to evaluate outcomes was not ideal for detecting differences in patients with moderate COVID-19, especially for a clinical situation in which discharge decisions may be driven by factors other than clinical improvement.
Conclusions
Among patients with moderate COVID-19, those randomized to a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment. Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Supplementary Methods
eAppendix 2. GS-US-540-5774 Principal Investigators and Research and Support Teams
eTable 1. Ordinal Scale of Clinical Status
eTable 2. Remdesivir Doses Received
eTable 3. Competing Risk and Kaplan-Meier Analysis of Time to Clinical Events
eTable 4. Post-Hoc Sensitivity Analyses for the Primary Endpoint
eTable 5. Clinical Status at Day 14
eTable 6. Clinical Status at Day 28
eFigure 1. Change in Clinical Status Over Time
eFigure 2. Subgroup Analysis
eFigure 3. Hospital Discharges Over Time
eReferences
Data Sharing Statement
References
- 1.World Health Organization Coronavirus Disease (COVID-19) Situation Report—202 Published August 9, 2020. Accessed August 10, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200809-covid-19-sitrep-202.pdf
- 2.Johns Hopkins Coronavirus Resource Center. Accessed August 10, 2020. https://coronavirus.jhu.edu/map.html
- 3.Cohen PA, Hall LE, John JN, Rapoport AB. The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95(6):1124-1126. doi: 10.1016/j.mayocp.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings MJ, Baldwin MR, Abrams D, et al. . Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheahan TP, Sims AC, Graham RL, et al. . Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzorno A, Padey B, Dubois J, et al. . In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antiviral Res. 2020;104878. doi: 10.1016/j.antiviral.2020.104878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson BN, Feldmann F, Schwarz B, et al. . Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. Published online June 9, 2020. doi: 10.1038/s41586-020-2423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang D, Du G, et al. . Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members . Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. Published online May 22, 2020. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 12.Goldman JD, Lye DCB, Hui DS, et al. ; GS-US-540-5773 Investigators . Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. Published online May 27, 2020. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. Published May 1, 2020. Accessed August 10, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
- 14.European Medicines Agency First COVID-19 treatment recommended for EU authorization. Published June 25, 2020. Accessed August 10, 2020. https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Peterson RL, Vock DM, Powers JH, et al. ; INSIGHT FLU-IVIG Study Group . Analysis of an ordinal end point for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials. 2017;14(3):264-276. doi: 10.1177/1740774517697919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. Published online July 17, 2020. doi: 10.1056/NEJMoa2020143632678530 [DOI] [Google Scholar]
- 18.Richardson S, Hirsch JS, Narasimhan M, et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization COVID-19 Therapeutic Trial Synopsis Published February 18, 2020. Accessed 24 July 2020. http://www.who.int/publications-detail/covid-19-therapeutic-trial-synopsis
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Supplementary Methods
eAppendix 2. GS-US-540-5774 Principal Investigators and Research and Support Teams
eTable 1. Ordinal Scale of Clinical Status
eTable 2. Remdesivir Doses Received
eTable 3. Competing Risk and Kaplan-Meier Analysis of Time to Clinical Events
eTable 4. Post-Hoc Sensitivity Analyses for the Primary Endpoint
eTable 5. Clinical Status at Day 14
eTable 6. Clinical Status at Day 28
eFigure 1. Change in Clinical Status Over Time
eFigure 2. Subgroup Analysis
eFigure 3. Hospital Discharges Over Time
eReferences
Data Sharing Statement


