Abstract
A male bias in mortality has emerged in the COVID-19 pandemic, which is consistent with the pathogenesis of other viral infections. Biological sex differences may manifest themselves in susceptibility to infection, early pathogenesis, innate viral control, adaptive immune responses or the balance of inflammation and tissue repair in the resolution of infection. We discuss available sex-disaggregated epidemiological data from the COVID-19 pandemic, introduce sex-differential features of immunity and highlight potential sex differences underlying COVID-19 severity. We propose that sex differences in immunopathogenesis will inform mechanisms of COVID-19, identify points for therapeutic intervention and improve vaccine design and increase vaccine efficacy.
Subject terms: Immunogenetics, SARS-CoV-2
Why are males more susceptible to severe COVID-19 than females? In this Perspective, Sabra Klein and colleagues consider the sex differences in the immune system that may contribute to this sex bias.
Introduction
The COVID-19 pandemic, caused by the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in millions of infections and hundreds of thousands of deaths worldwide. Human biological sex plays a fundamental role in heterogeneous COVID-19 outcomes. Sex, defined as male, female or intersex on the basis of sex chromosome complement, reproductive tissues (ovaries or testes) and sex steroid hormone (oestrogen, progesterone and testosterone) concentrations, is a multidimensional biological characteristic that shapes infectious disease pathogenesis. We discuss how sex differences in basic molecular and cellular mechanisms can be leveraged to define the immune response to infection with SARS-CoV-2.
Sex differences in COVID-19 severity
The precise drivers of death, regardless of sex, in COVID-19 remain unknown. There appears to be a subset of patients in whom high levels of dysregulated inflammation lead to severe multisystem organ pathology1,2. A postviral inflammatory syndrome has also emerged in children with COVID-19 (refs3,4). As a result, research on therapeutics has focused on both antiviral and immunomodulatory pathways2,5 with the goal of achieving an optimized balance in immune response induction and resolution. Unfortunately, most studies fail to consider the sex of the patients, which may mask therapeutic targets.
Evidence of sex differences in COVID-19 severity emerged in China, where hospital admissions and mortality were higher among males than females6–8. In South Korea, where community testing was widespread, females represented ~60% of those testing positive for SARS-CoV-2, suggesting that females acquire infection, despite having a lower case fatality rate (CFR)9,10. In the United States, where testing was prioritized for people with symptomatic disease, the diagnosis rates were similar in males and females, but males had 1.5 times higher mortality11.
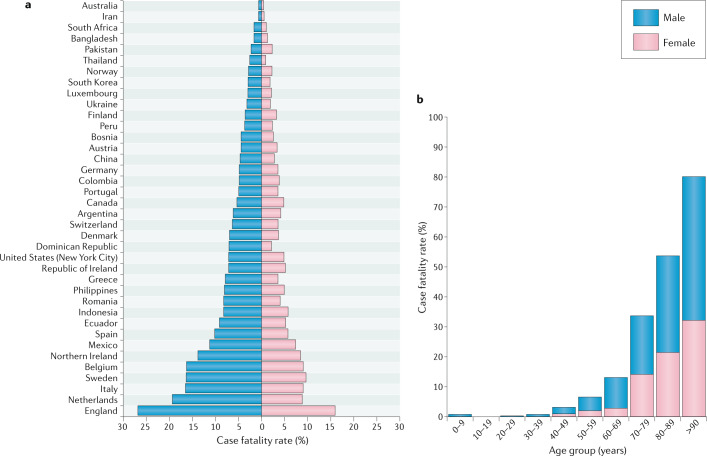
A male bias in COVID-19 mortality is currently reported in 37 of the 38 countries that have provided sex-disaggregated data (Fig. 1a). Our analyses show that the average male CFR across 38 countries is 1.7 times higher than the average female CFR (P < 0.0001) (male CFR 7.3 (95% CI 5.4–9.2); female CFR 4.4 (95% CI 3.4–5.5)), which is consistent with other reports12,13. There is increased risk of death for both sexes with advancing age, but at all ages above 30 years males have a significantly higher risk of death than females (P < 0.05) (Fig. 1b). A male predominance of deaths from COVID-19 is consistent with what was observed in the prior SARS14,15 and Middle East respiratory syndrome (MERS)16 epidemics (caused by SARS-CoV and MERS-CoV, respectively). Although gender-related social factors, including smoking, health care-seeking behaviours and some co-morbid conditions, may impact the outcomes of COVID-19 (refs6,17) and contribute to male–female differences in disease severity, the cross-cultural emergence of increased risk of death for males points to biological risk determinants. In animal models of SARS-CoV infection, differences in mortality between male and female mice were observed and were attributed to steroid hormones18. Multiple dimensions of biological sex, including sex steroids, sex chromosomes and genomic and epigenetic differences between males and females, impact immune responses19–26 and may affect responses to SARS-CoV-2 infection27.
Fig. 1. Comparative analyses of COVID-19 case fatality rates by country, sex and age.
a | COVID-19 case fatality rates (CFRs) for males and females across 38 countries or regions reporting sex-disaggregated data on COVID-19 cases and deaths. CFR was calculated as the total number of deaths divided by the total number of cases for each sex multiplied by 100. The male CFR is higher than the female CFR in 37 of the 38 regions, with an average male CFR 1.7 times greater than the average female CFR (P < 0.0001, Wilcoxon signed rank test). b | Average COVID-19 CFRs for males and females stratified by age. The data represent 12 countries currently reporting sex- and age-disaggregated data on COVID-19 cases and deaths (Australia, Columbia, Denmark, Italy, Mexico, Norway, Pakistan, Philippines, Portugal, Spain, Switzerland and England). The COVID-19 CFR increases for both sexes with advancing age, but males have a significantly higher CFR than females at all ages from 30 years (P < 0.05, Wilcoxon signed rank test). The data were obtained from Global Health 50/50 and official government websites of each respective country on 7 May and 8 May 2020. For more information on the data source for a specific country, please contact the corresponding author.
Ageing, sex and COVID-19
Although advancing age is associated with greater risk of death in both sexes, the male bias remains evident (Fig. 1b). An analysis of COVID-19 data from Italy, Spain, Germany, Switzerland, Belgium and Norway reveals that among all age groups older than 20 years, fatality rates are greater for males than females28. By contrast, male–female differences in the rate of confirmed SARS-CoV-2 infections are age dependent in all countries, being greater among females aged between 10 and 50 years and greater among males before the age of 10 years and after the age of 50 years28. The age-related male–female differences in confirmed cases of SARS-CoV-2 infections are consistent with reported confirmed cases of seasonal and pandemic influenza A virus infections in Australia and Japan29,30. We interpret these data to suggest that biological sex differences contribute to male-biased death, but gender-associated risk of exposure may affect rates of infection differently for males and females.
With a focus on biology, the impact of age on susceptibility to severe COVID-19 needs to be parsed, with both immunosenescence and dysregulation of innate immune responses as potential mechanisms31,32. Biological sex differentially affects ageing of the immune system33, in part through changing concentrations of sex steroids34. In addition to reduced concentrations of sex steroids, an age-related mosaic loss of chromosome Y in leukocytes can alter transcriptional regulation of immunoregulatory genes35. Whether sex differences in the genomic signatures of aged immune cells translate to functional differences in the response to SARS-CoV-2 infection requires attention.
Sex differences in immune responses
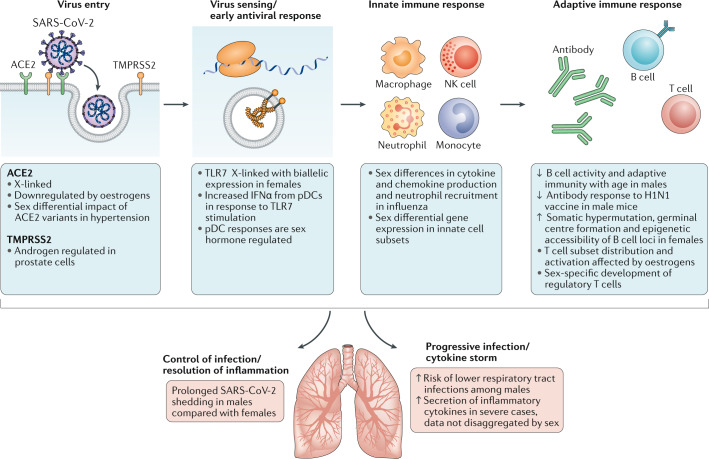
Biological sex affects innate and adaptive immune responses to self and foreign antigens, resulting in sex differences in autoimmunity as well as in responses to infections and vaccines36,37. Immune cell subsets have sex-specific patterns of gene expression, with most differentially expressed genes found on autosomes, demonstrating sex-specific regulation of shared genetic material26. The sex chromosomes also directly contribute. Males are at higher risk of diseases caused by deleterious X-linked alleles. Incomplete inactivation of immunoregulatory genes on the X chromosome can also occur in females, which results in a gene dosage imbalance between sexes38,39. Incomplete X chromosome inactivation has been implicated in female-biased autoimmune diseases40 and in vaccine efficacy41. The Y chromosome has immunoregulatory function, broadly impacting immune transcriptional profiles linked to autoimmune disease42 and impacting outcomes of influenza virus and coxsackie virus infection in animals43,44. Sex-specific features of epigenomic organization also dictate differential availability of transcriptional targets21,45. Superimposed on these genomic elements is the direct effect of sex steroid exposure. Oestrogens46,47, progesterone48–52 and testosterone53 have direct effects on immune cell function that are driven by the signalling of these hormones through their respective cellular receptors. The variation in sex steroid concentrations that occurs over the life course contributes to differences in immune profiles and disease susceptibility patterns at different ages20,52. Consistent with this variation, both sex and age contribute to unique transcriptional signatures of immune cells both at the baseline and after exposure to immunostimulants19,21,22. The summative effect is a sex-specific transcriptional regulatory network of genetic variants, epigenetic modifications, transcription factors and sex steroids that leads to a functional difference in the immune response25,54. Figure 2 highlights intersections between SARS-CoV-2 infection and sources of sex bias in pathophysiology that warrant further investigation.
Fig. 2. Known sex differences that may impact immune responses to SARS-CoV-2 and COVID-19 progression.
An illustrative summary of the sequence of events in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the associated immune responses. Broadly speaking (from left to right), there are the initial steps of virus entry, innate recognition of the virus with activation of antiviral programmes, the recruitment of innate immune cells and induction of an adaptive immune response. These major steps culminate either in successful control of infection and pathogen elimination or in a pathological inflammatory state. Sex differences that may be operative at multiple points along these pathways are indicated in the blue boxes. ACE2, angiotensin-converting enzyme 2; H1N1, H1N1 influenza virus; IFNα, interferon-α; NK, natural killer; pDC, plasmacytoid dendritic cell; TLR7, Toll-like receptor 7; TMPRSS2, transmembrane protease serine 2.
Sex bias in SARS-CoV-2 infection
Virus entry receptors
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as an entry receptor, with virus entry enhanced by cellular transmembrane serine protease 2 (TMPRSS2), which primes the spike protein of the virus55,56. ACE2 is an X chromosome-encoded gene that is downregulated by oestrogens57 and exhibits tissue-specific expression patterns39. Differences in ACE2 expression may be driven by sex-differential expression of ACE2 variants58–60. ACE2 is associated with interferon gene expression61,62, which in turn shows sex-specific regulation. The cell-intrinsic regulation of ACE2 expression may change with age, in response to changing levels of sex steroids, or following viral challenge. TMPRSS2 is regulated by androgen receptor signalling in prostate cells63. Initial investigations have not demonstrated a significant difference in TMPRSS2 mRNA expression in lung tissue from males and females, but it is unknown whether androgens may impact expression in the setting of infection with SARS-CoV-2 (refs63,64) or whether the level of expression has an impact on SARS-CoV-2 burden. Further research is needed to determine whether sex-biased expression of ACE2, coupled with the regulation of TMPRSS2 by androgens, increases SARS-CoV-2 susceptibility of males compared with females.
Interferons
Innate sensing of viruses, production of interferons and activation of the inflammasome are the first line of defence against viruses65. In the case of SARS-CoV-2, where there is no pre-existing adaptive immune memory, the success of this early antiviral response may be a determinant of disease outcome. Innate sensing of viral RNA by the pattern-recognition receptor Toll-like receptor 7 (TLR7) is sex biased, as TLR7 escapes X chromosome inactivation, resulting in greater expression in female immune cells; this has also been linked to sex differences in autoimmunity40,66 and vaccine efficacy41. There is greater production of interferon-α (IFNα) from plasmacytoid dendritic cells from adult females than from adult males67,68, an effect modulated by sex steroids69–71. In animal models of SARS-CoV infection, pretreatment with pegylated IFNα was associated with protection of lung tissue72 but without consideration of biological sex. In SARS-CoV-2, emerging data suggest that there is aberrant activation of interferon responses but preserved chemokine signalling, which has been postulated to contribute to immunopathology73. Studies are needed to determine whether differences in the magnitude or kinetics of the interferon response may contribute to a sex bias in the early control or severity of SARS-CoV-2 infection and may inform considerations of interferons as therapies for COVID-19 (ref.74). Early data suggest that male sex may be associated with a longer duration of viral detection, even within families75,76, raising the question of whether females have more efficient clearance of the virus. The rate of virus clearance will need to be assessed in evaluating the efficacy of innate and adaptive immune responses.
Adaptive immunity
Females generally mount greater antibody responses to viral infection and vaccination, albeit with higher levels of autoreactivity77. The mechanisms for sex differences in antibody production include oestrogenic enhancement of somatic hypermutation78, less stringent selection against autoreactive B cells77,79–82 and sex differences in germinal centre formation83 and in the epigenetic accessibility of B cell loci21. It is still unknown whether sex has an impact on antibody generation in SARS-CoV-2 infection. Early studies suggest that titres of antibodies to some viral epitopes are higher in patients with severe COVID-19 and that seroconversion may not be tightly linked to declining virus titres84,85. Ongoing studies evaluating the infusion of convalescent serum may provide answers as to the protective capacity of these antibodies86, but these studies are currently not considering biological sex. Generation of protective, neutralizing antibodies is a goal of vaccine development, with the cautionary note that in models of SARS-CoV vaccination some antibody responses induced potent inflammatory responses57. Persistence of antibodies, epitope targeting and non-neutralizing Fc-mediated antibody characteristics should be assessed with sex-stratified analyses. As vaccines are developed, the female bias towards both potent responses and adverse effects should be considered and sex-specific dosing should be tested, where appropriate87.
Sex impacts the development of regulatory T cells88–91, the distribution of lymphocyte subsets92 and the overall quality of T cell responses93,94. In T cells, overexpression of X-encoded immune genes, including CD40LG and CXCR3, has been linked to incomplete X chromosome inactivation and T cell-specific epigenetic modifications of the X chromosome95,96. It is unknown whether T cell phenotypes contribute to COVID-19; data from the prior SARS outbreak did not link T cell responses to outcomes in humans97, but mouse models suggest a role for CD4+ T cells98. In patients with MERS, T cell responses were dysregulated99, but sex differences were not analysed. In the current pandemic, lymphopenia is associated with severe disease100,101, and early evidence suggests that the clinical markers of lymphocyte count may be lower in males and neutrophil–lymphocyte ratios may be higher17. Further work is needed to define the sex-differential role of T cells in acute infection, in acute lung injury phenotypes102 and as potential vaccine targets.
Severe infection and acute respiratory distress syndrome
Severe cases of COVID-19 are typically marked by acute respiratory distress syndrome (ARDS), with respiratory failure requiring oxygen support and mechanical ventilation. The infection is primarily characterized by diffuse alveolar damage without a consistent pattern of cell infiltration75,103–105. The pathogenesis of ARDS involves the disruption of normal barrier function, inflammation and subsequent tissue repair. Whether there are sex-specific risks for ARDS and death from other causes, such as trauma, remains unknown106,107, although there is a suggestion of a higher risk of lower respiratory tract infections among males108 and that steroid hormones modulate the immune response to respiratory viral pathogens109. In one cohort of patients with COVID-19, severe respiratory failure was associated with a pattern of inflammation, macrophage activation and depletion of lymphocytes that was distinct from bacterial infection110. There was a sex bias for severe COVID-19 not observed in the comparator group with bacterial infections110. Sex-differential production of IL-6 (ref.111), monocyte transcriptional patterns and inflammatory set point19,21,22 could contribute to an enhanced risk of death in males and highlight the importance of sex-stratified analyses to guide deployment of safe and effective immunomodulatory therapeutics for males and females112.
Conclusions
Emerging data demonstrating more favourable outcomes for community-dwelling adult females across age strata offer an immediate opportunity for comparative biology experiments to define features of COVID-19 pathogenesis and the associated immune response. The research pipeline should integrate sex as a biological variable in all stages, from fundamental research to preclinical drug development, clinical trials and epidemiological analyses113. Considering the role of intersecting factors — including, but not limited to, gender, age, race and other identifying characteristics — is critical to understanding the biological and sociocultural factors contributing to heterogeneous COVID-19 outcomes. Sex is a driver of discovery and innovation114, and taking a sex-informed approach to COVID-19 research115 and medicine116 will uncover novel features of the host immune response to SARS-CoV-2 and ultimately result in more equitable health outcomes.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks E. Fish, P. McCombe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Global Health 50/50: http://globalhealth5050.org/covid19/
Change history
6/15/2020
Figure 1 contained a minor spelling error which has now been corrected.
References
- 1.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 3.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). CDChttps://emergency.cdc.gov/han/2020/han00432.asp (2020).
- 5.Vabret N, et al. Immunology of COVID-19: current state of the science. Immunity. 2020 doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Dudley JP, Lee NT. Disparities in age-specific morbidity and mortality from SARS-CoV-2 in China and the Republic of Korea. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health and Welfare of South Korea. Domestic occurrence status. MOHWhttp://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun= (2020).
- 11.NYC. COVID-19: data. NYC.govhttps://www1.nyc.gov/site/doh/covid/covid-19-data.page (2020).
- 12.Jin J-M, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peckham, H. et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. Preprint at ResearchSquare10.21203/rs.3.rs-23651/v2 (2020).
- 14.Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong HN, et al. SARS in Singapore–predictors of disease severity. Ann. Acad. Med. Singap. 2006;35:326–331. [PubMed] [Google Scholar]
- 16.Alghamdi IG, et al. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Y, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLOS Pathog. 2020;16:e1008520. doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Channappanavar R, et al. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J. Immunol. 2017;198:4046–53.. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bongen E, et al. Sex differences in the blood transcriptome identify robust changes in immune cell proportions with aging and influenza infection. Cell Rep. 2019;29:1961–73 e4. doi: 10.1016/j.celrep.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Klein RS. Sex drives dimorphic immune responses to viral infections. J. Immunol. 2017;198:1782–90.. doi: 10.4049/jimmunol.1601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez EJ, et al. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piasecka B, et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc. Natl Acad. Sci. USA. 2018;115:E488–E497.. doi: 10.1073/pnas.1714765115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurz H, et al. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmiedel BJ, et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell. 2018;175:1701–15.e16. doi: 10.1016/j.cell.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein, S. L. et al. Biological sex impacts COVID-19 outcomes in the United States. PLoS Pathog. (in the press). [DOI] [PMC free article] [PubMed]
- 28.Marina S. & Piemonti L. Gender and age effects on the rates of infection and deaths in individuals with confirmed SARS-CoV-2 infection in six European countries. Lancet10.2139/ssrn.3576790 (2020).
- 29.Wong KC, Luscombe GM, Hawke C. Influenza infections in Australia 2009–2015: is there a combined effect of age and sex on susceptibility to virus subtypes? BMC Infect. Dis. 2019;19:42. doi: 10.1186/s12879-019-3681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eshima N, et al. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS ONE. 2011;6:e19409. doi: 10.1371/journal.pone.0019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koff WC, Williams MA. Covid-19 and immunity in aging populations — a new research agenda. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 32.Nikolich-Zugich J, et al. SARS-COV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng SX, Margolick JB. Aging, sex, inflammation, frailty, and CMV and HIV infections. Cell Immunol. 2020;348:104024. doi: 10.1016/j.cellimm.2019.104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potluri T, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumanski, J. P. et al. Immune cells lacking Y chromosome have widespread dysregulation of autosomal genes. Preprint at bioRxiv10.1101/673459 (2020).
- 36.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 37.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35:97–104. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Carrel L, Brown CJ. When the Lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20160355. doi: 10.1098/rstb.2016.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tukiainen T, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souyris M, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018;3:eaap8855. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 41.Fink AL, Engle K, Ursin RL, Tang WY, Klein SL. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl Acad. Sci. USA. 2018;115:12477–82.. doi: 10.1073/pnas.1805268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Case LK, et al. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23:1474–1485. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krementsov DN, et al. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc. Natl Acad. Sci. USA. 2017;114:3491–3496. doi: 10.1073/pnas.1620889114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson DP, et al. Sex chromosome complement contributes to sex differences in Coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex. Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golden LC, et al. Parent-of-origin differences in DNA methylation of X chromosome genes in T lymphocytes. Proc. Natl Acad. Sci. USA. 2019;116:26779–26787. doi: 10.1073/pnas.1910072116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Straub RH. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 47.Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L415–L425. doi: 10.1152/ajplung.00398.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10:1097–107.. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 49.Hall OJ, et al. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12:e1005840. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall OJ, et al. Progesterone-based contraceptives reduce adaptive immune responses and protection against sequential influenza A virus infections. J. Virol. 2017;91:e02160-16. doi: 10.1128/JVI.02160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vom Steeg LG, Klein SL. Sex steroids mediate bidirectional interactions between hosts and microbes. Horm. Behav. 2017;88:45–51. doi: 10.1016/j.yhbeh.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vom Steeg LG, Klein SL. Sex and sex steroids impact influenza pathogenesis across the life course. Semin. Immunopathol. 2019;41:189–94.. doi: 10.1007/s00281-018-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furman D, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl Acad. Sci. USA. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuyama S, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl Acad. Sci. USA. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol. Sex. Differ. 2010;1:6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, et al. Association of angiotensin-converting enzyme 2 gene polymorphism and enzymatic activity with essential hypertension in different gender: a case-control study. Medicine. 2018;97:e12917. doi: 10.1097/MD.0000000000012917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asselta, R., Paraboschi, E. M., Mantovani, A. & Duga, S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Preprint at medRxiv10.1101/2020.03.30.20047878 (2020). [DOI] [PMC free article] [PubMed]
- 60.Gibson, W. T., Evans, D. M., An, J. & Jones, S. J. ACE 2 coding variants: a potential x-linked risk factor for COVID-19 disease. Preprint at bioRxiv10.1101/2020.04.05.026633 (2020).
- 61.Ziegler, C. G. K. et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is enriched in specific cell subsets across tissues. Cell181, 1016–1035 (2020). [DOI] [PMC free article] [PubMed]
- 62.Sungnak W, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baratchian, M. et al. No evidence that androgen regulation of pulmonary TMPRSS2 explains sex-discordant COVID-19 outcomes. Preprint at bioRxiv10.1101/2020.04.21.051201 (2020).
- 65.Iwasaki A. A virological view of innate immune recognition. Annu. Rev. Microbiol. 2012;66:177–196. doi: 10.1146/annurev-micro-092611-150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souyris M, Mejia JE, Chaumeil J, Guery JC. Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019;41:153–64.. doi: 10.1007/s00281-018-0712-y. [DOI] [PubMed] [Google Scholar]
- 67.Berghofer B, et al. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 68.Meier A, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griesbeck M, et al. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J. Immunol. 2015;195:5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laffont S, et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J. Immunol. 2014;193:5444–5452. doi: 10.4049/jimmunol.1303400. [DOI] [PubMed] [Google Scholar]
- 71.Seillet C, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 72.Haagmans BL, et al. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blanco-Melo, D. N.-P. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell10.1016/j.cell.2020.04.026 (2020). [DOI] [PMC free article] [PubMed]
- 74.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu, K. et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin. Infect. Dis.10.1093/cid/ciaa351 (2020). [DOI] [PMC free article] [PubMed]
- 76.Zheng S, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 78.Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. J. Exp. Med. 2009;206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naïve B cells. Proc. Natl Acad. Sci. USA. 2000;97:2703–2708. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Invest. 2002;109:1625–1633. doi: 10.1172/JCI0214873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17β-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J. Immunol. 2006;176:2703–2710. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 82.Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Mol. Med. 2011;17:211–220. doi: 10.2119/molmed.2010.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao R, et al. A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature. 2020;577:416–20.. doi: 10.1038/s41586-019-1873-0. [DOI] [PubMed] [Google Scholar]
- 84.To KK, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–74.. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature10.1038/s41586-020-2196-x (2020). [DOI] [PubMed]
- 86.Shen C, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33:577–99.. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 88.Dragin N, et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 2016;126:1525–1537. doi: 10.1172/JCI81894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Polanczyk MJ, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 90.Tai P, et al. Induction of regulatory T cells by physiological level estrogen. J. Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 91.Zhu ML, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 2016;7:11350. doi: 10.1038/ncomms11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Page ST, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am. J. Physiol. Endocrinol. Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 93.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes. Immun. 2009;10:509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yee Mon KJ, et al. Differential sensitivity to IL-12 drives sex-specific differences in the CD8+ T cell response to infection. Immunohorizons. 2019;3:121–132. doi: 10.4049/immunohorizons.1800066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qu K, et al. Individuality and variation of personal regulomes in primary human T cells. Cell Syst. 2015;1:51–61. doi: 10.1016/j.cels.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc. Natl Acad. Sci. USA. 2016;113:E2029–E2038. doi: 10.1073/pnas.1520113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janice Oh HL, Ken-En Gan S, Bertoletti A, Tan YJ. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 2012;1:e23. doi: 10.1038/emi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alosaimi B, et al. MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract. Cytokine. 2020;126:154895. doi: 10.1016/j.cyto.2019.154895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481.. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halter S, et al. T regulatory cells activation and distribution are modified in critically ill patients with acute respiratory distress syndrome: a prospective single-centre observational study. Anaesth. Crit. Care Pain. Med. 2020;39:35–44. doi: 10.1016/j.accpm.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 103.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153:725–33.. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian S, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian, S. et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol.10.1038/s41379-020-0536-x (2020). [DOI] [PMC free article] [PubMed]
- 106.Heffernan DS, et al. Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J. Trauma. 2011;71:878–883. doi: 10.1097/TA.0b013e31822c0d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu T, et al. The influence of sex on outcomes in trauma patients: a meta-analysis. Am. J. Surg. 2015;210:911–921. doi: 10.1016/j.amjsurg.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 108.Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respir. Med. 2007;101:1845–1863. doi: 10.1016/j.rmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 109.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J. Virol. 2014;88:4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe10.1016/j.chom.2020.04.009 (2020). [DOI] [PMC free article] [PubMed]
- 111.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 112.Bischof, E., Wolfe, J. & Klein, S. L. Clinical trials for COVID-19 should include sex as a variable. J. Clin. Invest.10.1172/JCI139306 (2020). [DOI] [PMC free article] [PubMed]
- 113.Canadian Institutes of Health Research. Why sex and gender need to be considered in COVID-19 research. CIHRhttps://cihr-irsc.gc.ca/e/51939.html (2020).
- 114.Klein SL, et al. Opinion: sex inclusion in basic research drives discovery. Proc. Natl Acad. Sci. USA. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L. Sex and gender analysis improves science and engineering. Nature. 2019;575:137–46.. doi: 10.1038/s41586-019-1657-6. [DOI] [PubMed] [Google Scholar]
- 116.Bartz D, et al. Clinical advances in sex- and gender-informed medicine to improve the health of all: a review. JAMA Intern. Med. 2020;180:574–583. doi: 10.1001/jamainternmed.2019.7194. [DOI] [PubMed] [Google Scholar]