Abstract
Background
The recent SARS‐CoV‐2 pandemic, which has recently affected Italy since February 21, constitutes a threat to normal subjects, as the coronavirus disease‐19 (COVID‐19) can manifest with a broad spectrum of clinical phenotypes ranging from asymptomatic cases to pneumonia or even death. There is evidence that older age and several comorbidities can affect the risk to develop severe pneumonia and possibly the need of mechanic ventilation in subjects infected with SARS‐CoV‐2. Therefore, we evaluated the outcome of SARS‐CoV‐2 infection in patients with inborn errors of immunity (IEI) such as X‐linked agammaglobulinemia (XLA).
Methods
When the SARS‐CoV‐2 epidemic has reached Italy, we have activated a surveillance protocol of patients with IEI, to perform SARS‐CoV‐2 search by nasopharyngeal swab in patients presenting with symptoms that could be a manifestation of COVID‐19, such as fever, cough, diarrhea, or vomiting.
Results
We describe two patients with X‐linked agammaglobulinemia (XLA) aged 34 and 26 years with complete absence of B cells from peripheral blood who developed COVID‐19, as diagnosed by SARS‐CoV‐2 detection by nasopharyngeal swab, while receiving immunoglobulin infusions. Both patients developed interstitial pneumonia characterized by fever, cough, and anorexia and associated with elevation of CRP and ferritin, but have never required oxygen ventilation or intensive care.
Conclusion
Our report suggests that XLA patients might present with high risk to develop pneumonia after SARS‐CoV‐2 infection, but can recover from infection, suggesting that B‐cell response might be important, but is not strictly required to overcome the disease. However, there is a need for larger observational studies to extend these conclusions to other patients with similar genetic immune defects.
Keywords: BTK, immunoglobulins, SARS-CoV-2, X linked agammaglobulinemia
Key Message.
Patients with agammaglobulinemia can recover from SARS‐Cov2 infection despite lack of B cells.
1. INTRODUCTION
Since February 21, 2020, several cases of coronavirus disease 2019 (COVID‐19), caused by the β‐coronavirus associated with human severe acute respiratory syndrome (SARS) officially named SARS‐CoV‐2, were identified in northern Italy. 1 The disease has a broad spectrum of clinical phenotypes ranging from mild symptomatic cases characterized by influenza‐like manifestations, which occur in the majority of subjects (about 80%), to severe pneumonia requiring admission to intensive care units in 5% or invasive mechanical ventilation, or even death. 2 Patients with severe disease usually present with comorbidities and are older than those with non‐severe disease. Most of the COVID‐19 patients exhibit lymphopenia, which is more pronounced in severe cases; thrombocytopenia; and elevation of C‐reactive protein. The disease has an incubation period of 5‐14 days and manifests with fever, cough, vomiting, and diarrhea lasting up to 2‐3 weeks.
Little is known about the mechanism of immune response against the SARS‐CoV‐2, but the first reports suggest that active immunity requires the formation of antigen‐specific cytotoxic T cells and synthesis of neutralizing antibodies directed against the virus. 3 , 4 There is no specific antiviral treatment for patients with this infection, but many antiviral or immunomodulatory drugs are under evaluation. 5 , 6 Indeed, patients with severe COVID‐19 have been successfully treated with immune plasma derived from convalescent patients, suggesting that virus‐specific neutralizing antibodies are important for disease recovery. 7 , 8
2. METHODS
After the identification of patients with SARS‐CoV‐2 infection in Italy, we started a survey protocol on patients with chronic diseases who presented with COVID‐19 to identify conditions that could constitute a risk factor for an unfavorable outcome. SARS‐CoV‐2 infection was confirmed in all patients by the detection of viral RNA by real‐time reverse transcription polymerase chain reaction (RT‐PCR) assay in nasopharyngeal swab specimens obtained during the clinical manifestations. Patient care and research were conducted in compliance with the Case Report guidelines and the Declaration of Helsinki. This study was performed after ethics approval (Ethics Committee of Brescia, protocols NP4000 and NP4047).
3. RESULTS
Herein we describe two patients with agammaglobulinemia and absence of circulating B cells due to BTK mutations who live in northern Italy and have developed COVID‐19 during the 2020 outbreak.
Patient 1 is a 34‐year‐old man who was diagnosed with agammaglobulinemia at 4 months of age because of perianal abscess associated with low levels of immunoglobulins (IgG 161 mg/dL, IgA 6.5 mg/dL, IgM 10 mg/dL as compared to IgG 222‐846 mg/dL, IgA 6‐60 mg/dL, IgM 28‐39 mg/dL in age‐matched children) and lack of B cells in peripheral blood (Figure 1). He started intravenous immunoglobulin treatment at the time of diagnosis and switched to subcutaneous immunoglobulins in March 2013. BTK genetic analysis revealed a non‐sense mutation in exon 2 (K19X) which will cause termination of protein synthesis prematurely. Chest computed tomography (CT) scan performed in January 2010 did not reveal bronchiectasis. On January 14, 2020, he performed outpatient blood tests (IgG 860 mg/dL) and received immunoglobulin treatment; then, he continued immunoglobulin therapy at home every 28 days.
FIGURE 1.
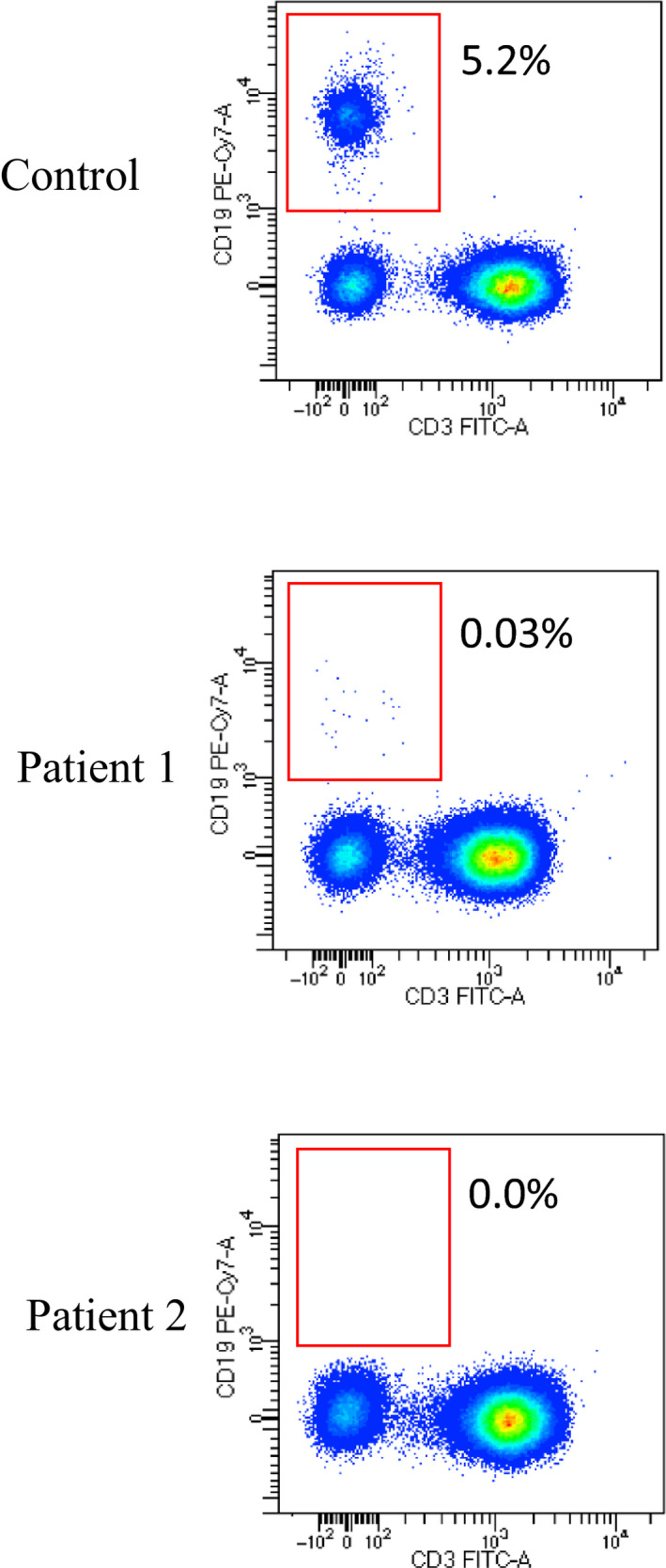
Flow cytometry dot plot of T‐ and B‐cell subsets in two XLA patients and one control subject
On March 5, he presented with fever and cough, which required medical evaluation and following treatment with oral antibiotics. Because patient general conditions were maintained with normal breathing and unremarkable chest X‐ray, he was advised to remain at home in self‐isolation to reduce contacts with potential sources of SARS‐CoV‐2 infection. However, on March 13, fever and cough reappeared and he was admitted to the hospital suspecting SARS‐CoV‐2 infection, which was confirmed by nasopharyngeal swab. Because chest radiography revealed interstitial pneumonia with bilateral infiltrates (Figure 2A), we started empirical treatment with lopinavir/ritonavir‐associated hydroxychloroquine, and subcutaneous infusion of immunoglobulins (400 mg/kg), but he never needed oxygen ventilation. During his stay in the hospital, he developed leukopenia (WB cells: 2910 cells/ul) and an increase in CRP and ferritin (Table 1), which gradually improved in the following days. He was always able to breathe spontaneously and was discharged on March 27 in good health. Analysis of lymphocyte subpopulation during the infection revealed normal T‐lymphocyte subsets and low levels expression of the activation marker HLA‐DR on both CD4 and CD8 subsets (Table 2), while B cells were about 0.03% of total lymphocytes (Figure 1).
FIGURE 2.
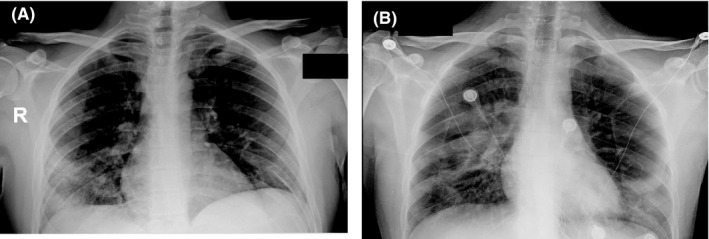
Chest radiography of two XLA patients (Patient 1 in panel A and Patient 2 in panel B) with COVID‐19
Table 1.
Clinical chemistry values of two XLA patients with COVID‐19
| Patient 1 | Patient 2 | Normal values | |||
|---|---|---|---|---|---|
| March 19 | March 27 | April 3 | April 10 | ||
| C‐reactive protein (mg/L) | 26 | 78 | 3.6 | 1.5 | (<5) |
| Lactate dehydrogenase (U/L) | 170 | 194 | 235 | 248 | (135‐225) |
| Fibrinogen (mg/dL) | 598 | 737 | 424 | 517 | (170‐410) |
| Ferritin (µg/L) | 362 | 469 | 603 | 774 | (30‐400) |
| Aspartate transaminase (U/L) | 24 | 22 | 30 | 65 | (18‐54) |
| Alanine transaminase (U/L) | 17 | 19 | 26 | 230 | (10‐50) |
Table 2.
Lymphocyte subsets of XLA patients
| Patient 1 | Patient 2 | Normal values | |
|---|---|---|---|
| CD3+ T lymphocytes (%) | 88.4 | 94.7 | (57.1‐87.6) |
| CD3+ (cells/µL) | 1040 | 1791 | (721‐2562) |
| CD4+ T cells (%) | 42.1 | 44.9 | (28.5‐65.6) |
| CD4+ (cells/µL) | 495 | 849 | (273‐1882) |
| CD8+ T cells (%) | 43.3 | 30.6 | (10.5‐37.7) |
| CD8+ (cells/µL) | 509 | 578 | (177‐783) |
| γδ+ T cells (%) | 3.8 | 32.4 | (0.9‐11.2) |
| B cells CD19+ (%) | ‐ | ‐ | (5.8‐22.1) |
| CD19+ (cells/µL) | ‐ | ‐ | (86‐684) |
| NK cells (CD3‐CD56+. CD3‐CD16+, %) | 11.6 | 5.3 | (3.4‐28.4) |
| CD3+CD4+ (%) | |||
| HLADR+ | 3.1 | 2.4 | (1.6‐12.2) |
| Naïve | 68 | 63.7 | (20.4‐63.6) |
| RTE | 41.2 | 46.9 | (11.4‐48.1) |
| RTE abs | 204 | 398 | (115‐913) |
| Central memory | 10.4 | 22.1 | (18.7‐46.2) |
| Effector memory | 17.2 | 12.2 | (7.1‐38.0) |
| Terminally differentiated | 4.4 | 2 | (0.3‐9.1) |
| CD3+CD8+ (%) | |||
| HLADR+ | 7.2 | 7.2 | (2.7‐31.7) |
| Naïve | 29.7 | 30.6 | (13.1‐66.5) |
| Central memory | 0.5 | 4.2 | (2.6‐24.5) |
| Effector memory | 15.5 | 14 | (10.1‐47.4) |
| Terminally differentiated | 54.3 | 51.2 | (5.2‐63.5) |
Abbreviation: RTE, recent thymic emigrants.
Patient 2 is a 26‐year‐old man who was diagnosed with agammaglobulinemia at 16 months of age because of respiratory tract infections and low immunoglobulins (IgG 63 mg/dL, IgA 7 mg/dL, IgM 12 mg/dL as compared to IgG 264‐1509 mg/dL, IgA 17‐178 mg/dL, IgM 48‐337 mg/dL in age‐matched children), virtual absence of B cells in the blood, and BTK mutation (S578Y). Since then, he received regular intravenous immunoglobulin infusions every 28 days and stayed in good health as chest CT scan in June 2010 did not reveal bronchiectasis. In the following years, he moved to London for work reasons and returned occasionally to see his family in northern Italy. In February 2020, he visited his family living in Milan just a few weeks before the SARS‐CoV‐2 epidemics. On March 23, he performed blood test to determine IgG levels, which were normal (IgG 923 mg/dL), before receiving immunoglobulin infusion. On April 1, he presented with anorexia, asthenia, and vomiting, without fever or diarrhea. Execution of nasopharyngeal swab at a local hospital in Milan area revealed SARS‐CoV‐2 infection; chest X‐ray showed an interstitial pneumonia (Figure 2B), while IgG levels were 863 mg/dL. In the following days, the patient presented with fever, but he never required oxygen supplementation. We started treatment with hydroxychloroquine, azithromycin, and ceftriaxone and administered immunoglobulin infusion at 400 mg/kg. In the following days, his conditions have been improving. Flow cytometry analysis of lymphocyte subpopulations, which was performed 7 days before the appearance of the first symptom, showed normal distribution of T and NK subsets (Table 2).
4. DISCUSSION
We describe two patients with XLA who have been exposed to SARS‐CoV‐2 and developed interstitial pneumonia and lymphopenia. Despite the absence of B cells, both subjects could recover without the need for intensive care or oxygen ventilation. This observation suggests that T‐cell response is probably important for immune protection against the virus, while B‐cell response might be unessential. This is in agreement with preliminary studies that have shown that in normal subjects infected by SARS‐CoV‐2, the number of cytotoxic T cells expressing activation markers such as HLA‐DR and CD38 increases during infection. 4 However, the risk to develop pneumonia after SARS‐CoV‐2 infection is quite low in young adults such as the two XLA patients, suggesting that the lack of antibody production is probably contributing to disease severity. Moreover, there is also evidence that passive antibody administration through transfusion of convalescent plasma constitutes a feasible strategy to confer immunization to patients with severe COVID‐19 and contributes to improve the clinical manifestations and the outcome of these subjects, suggesting that production of neutralizing antibodies might also be an important step for disease recovery. 7 , 8 Therefore, the lack of neutralizing antibodies in XLA patients might place these subjects at higher risk of disease relapse, suggesting the need for careful monitoring during convalescence.
The observation that patients with X‐linked agammaglobulinemia can recover from SARS‐CoV‐2 infection suggests that human immune system could use multiple paths to counteract viral infection and that a normal T‐cell immune response can be sufficient to defeat the virus in subjects who cannot synthesize antigen‐specific immunoglobulins.
However, BTK is expressed not only in B cells, but also in myeloid cells. In these cell types, BTK is involved in Toll‐like receptor–mediated production of pro‐inflammatory cytokines, such as IL‐6 and TNF‐α, which are produced in large amount in patients with SARS‐CoV‐2 infection. 9 Therefore, the lack of BTK in myeloid cells of XLA patients might provide a subordinate advantage for these patients by preventing the development of the inflammatory stage of the disease which has been associated with the possible fatal outcome of COVID‐19.
However, we cannot draw final conclusions from the description of these two cases of XLA, because the clinical and immunologic features of patients with this disease can be very heterogeneous. Indeed, Bruton's tyrosine kinase is required for many checkpoints during B‐cell differentiation and BTK mutations might also have variable effects on B‐cell precursor survival. 10 In one of the two agammaglobulinemia patients, we detected an extremely low number of B cells at various stages of differentiation, suggesting that these cells might play a role in the immune response against the virus. Moreover, both subjects have received immunoglobulin infusions before the infection and have been further supplemented with immunoglobulins at the time of infection. Infusion of polyclonal immunoglobulins derived from pools of normal donors has been used to treat COVID‐19 patients, 11 but there is limited evidence of efficacy. One might speculate that immunoglobulin pools might contain antibodies that could cross‐react with SARS‐CoV‐2 proteins by exerting a priming effect on host immune response. Alternatively, immunoglobulins could provide an immunomodulatory action on monocytes and tissue‐resident macrophages that are involved in the so‐called “cytokine storm” in the advanced phases of the infection in patients with severe COVID‐19. 12
Overall, our report provides evidence that B‐cell response can be dispensable in the immune response against SARS‐CoV‐2 infection, but clinical decisions should be based on data derived from a more broad survey of patients with IEI.
AUTHOR CONTRIBUTION
Annarosa Soresina: Conceptualization (lead); Investigation (lead); Writing‐review & editing (supporting). Daniele Moratto: Data curation (equal); Formal analysis (equal); Investigation (equal); Visualization (equal). Marco Chiarini: Data curation (equal); Investigation (equal). Ciro Paolillo: Investigation (equal); Validation (equal). Giulia Baresi: Data curation (equal); Formal analysis (equal); Investigation (supporting). Emanuele Foca': Formal analysis (supporting); Investigation (equal). Michela Bezzi: Data curation (equal); Formal analysis (equal); Investigation (supporting). Barbara Baronio: Data curation (supporting); Formal analysis (equal). Mauro Giacomelli: Data curation (equal); Formal analysis (equal); Investigation (equal). Raffaele Badolato: Conceptualization (lead); Supervision (lead); Writing‐original draft (lead).
Soresina A, Moratto D, Chiarini M, et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565–569. 10.1111/pai.13263
The peer review history for this article is available at https://publons.com/publon/10.1111/PAI.13263
REFERENCES
- 1. Tuite AR, Ng V, Rees E, Fisman D. Estimation of COVID‐19 outbreak size in Italy. Lancet Infect Dis. 2020;20(5):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non‐severe. Nat Med. 2019;26:453‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir‐Ritonavir in Adults Hospitalized with Severe Covid‐19. N Engl J Med. 2020. 10.1056/NEJMoa2001282. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabby MII. Current drugs with potential for treatment of COVID‐19: a literature review. J Pharm Pharm Sci. 2020;23:58‐64. [DOI] [PubMed] [Google Scholar]
- 7. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bloch Evan M., Shoham Shmuel, Casadevall Arturo, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. Journal of Clinical Investigation. 2020. 10.1172/jci138745. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marron TU, Martinez‐Gallo M, Yu JE, Cunningham‐Rundles C. Toll‐like receptor 4‐, 7‐, and 8‐activated myeloid cells from patients with X‐linked agammaglobulinemia produce enhanced inflammatory cytokines. J Allergy Clin Immunol. 2012;129:184.e4‐190.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vihinen M, Mattsson PT, Smith CI. Bruton tyrosine kinase (BTK) in X‐linked agammaglobulinemia (XLA). Front Biosci. 2000;5:D917‐D928. [DOI] [PubMed] [Google Scholar]
- 11. Yang Xiaobo, Yu Yuan, Xu Jiqian et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475‐481. 10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;6736:19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]


