Abstract
An increase of Ct values was 0.9 per day in 2 cases of COVID-19 treated with lopinavir/ritonavir (LPV/r), an increase was 1.0 per day in 3 cases without LPV/r through illness day 1–10, indicating that LPV/r did not shorten the duration of SARS CoV-2 shedding.
Keywords: COVID-19, SARS CoV-2, Lopinavir/ritonavir (LPV/r), Shedding
Introduction
COVID-19 soon became an unstoppable disease since an outbreak occurred in December in Wuhan, China.1 The number of cases has increased dramatically, and no approved medications or vaccines are currently available to treat human coronavirus infection. Wang et al.2 reported 124 patients (89.9%) in China who received antiviral therapy with oseltamivir, and 36 of these patients (26.1%) were transferred to the intensive care unit (ICU) and 6 died (4.3%). Holshue et al.3 presented the first case to receive intravenous remdesivir in the United States, and the outcome was satisfactory. Lopinavir and ritonavir were associated with improved clinical outcomes in patients with SARS in a non-randomized open-label trial.4 Here, we report five confirmed cases of COVID-19 infection in Taiwan; two were prescribed LPV/r as two tablets twice daily for empirical therapy, and three were not prescribed LPV/r. We describe their diagnosis, clinical course, and novel coronavirus (SARS CoV-2) viral loads during the treatment course.
Methods
Oropharyngeal swabs and sputum samples were obtained from the admitted patients for SARS CoV-2 testing every other day and were tested assay using primers and probes targeting the E and RdRP gene as described by the WHO for diagnostic detection of SARS CoV-2 by real-time RT-PCR.5 Amplification and detection are accomplished using TaqMan chemistry on the ABI 7500, and an internal positive amplification control (IPC) is included with each specimen. A sample can be interpreted as positive when the analysis of the IPC indicates that amplification has occurred in the reaction tube and signal from target reporter dye has been detected from E/RdRp1/RdRp2/N genes. Cycle threshold (Ct) values are inversely related to the viral RNA copy number, and Ct values of 30.76, 27.67, 24.56, and 21.48, correspond to 1.5 × 104, 1.5 × 105, 1.5 × 106, and 1.5 × 107 copies per milliliter. Negative samples have a Ct value of 40, which is the limit of detection. E/RdRp1/RdRp2/N genes (Ct value of ≤34 for all the three genes was considered as a positive result).
Results
Case presentations
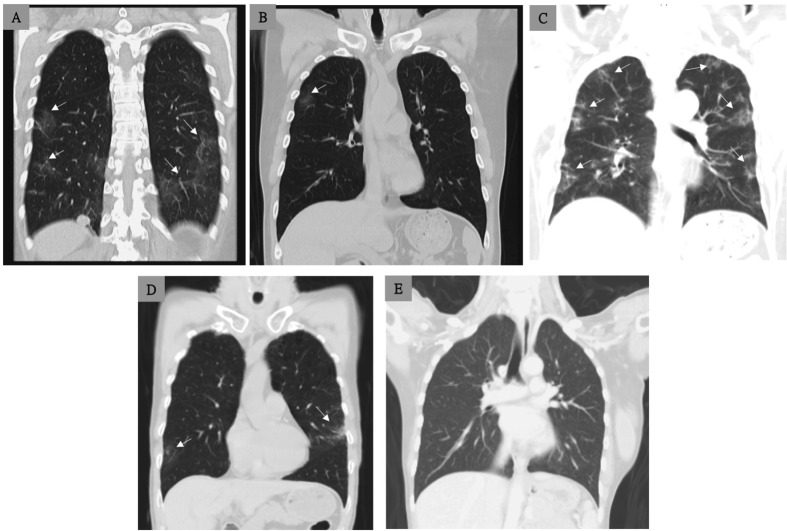
Case 1: A 56-year-old Taiwanese woman was admitted with chief complaint of 2-day history of subjective fever and dry cough. On admission (illness day 3), the patient reported that she had no shortness of breath, nausea or diarrhea. Oropharyngeal swab and sputum specimens were positive for COVID-19 (SARS CoV-2) by real-time reverse transcription–polymerase-chain-reaction (RT-PCR) assays with primers and probes targeting the E genes of SARS CoV-2. She received lopinavir (200 mg)/ritonavir (50 mg) (LPV/r) as two pills twice daily since illness day 5. On illness day 6–8, episodes of vomiting and severe watery diarrhea developed which was consistent with the side effects of LPV/r, and LPV/r was discontinued on illness day 8. The tests of SARS CoV-2 remained positive until illness day 23. On illness day 28, a chest CT was performed (Fig. 1 , Panel A) and the patient, who was afebrile, was discharged.
Figure 1.
Chest CT findings, without intravenous contrast, of the five study cases with COVID-19. Panel A (case 1) shows small areas of ground glass (arrows) in the peripheral zones of the lower lobes of the bilateral lungs on illness day 28 (before discharge). Panel B (case 2) shows patchy ground glass infiltrates (arrows) in the right upper lung on illness day 24. Panel C (case 3) shows multiple patchy ground glass infiltrates (arrows) in the bilateral lung on illness day 25. Panel D (case 4) shows a few patchy ground glass infiltrates (arrows) in the bilateral lungs on illness day 25. Panel E (case 5) shows normal finding in the bilateral lungs on day 32. CT scans were performed after at least two consecutive tests were negative in these 5 cases.
Case 2: A 53-year-old man was diagnosed COVID-19 with chief complaint of a body temperature of 38.5 °C before admission. Chest radiography of the bilateral lungs showed normal findings. The patient was prescribed LPV/r, as two tablets twice daily since day 3. He did not receive oxygen therapy during hospitalization. On day 23, chest CT was performed (Fig. 1, Panel B), and he was discharged with two consecutive negative result of SARS CoV-2 on illness day 24.
Case 3: A 52-year-old woman presented to the emergency department due to acute onset of fever, and COVID-19 was impressed due to recent travel history in Wuhan in January 2020. On day 1 of admission, supportive care, without oxygen therapy, was provided. She had persistent fever on illness days 1–9, but she did not receive oxygen therapy during hospitalization. Serial oropharyngeal swabs and sputum samples were collected, and two consecutive tests were negative on illness days 17 and 19., and she was discharged after chest CT scan was performed on illness day 25 (Fig. 1, Panel C).
Case 4: A 50-year-old man complained of mild rhinorrhea for one day, and his wife was diagnosed COVID-19 recently. He was requested to home quarantine due to a history of SARS CoV-2 exposure, and was confirmed to have COVID-19 the following day. Both oropharyngeal swabs and sputum were negative on three consecutive testing days (illness days 23, 25, and 27). He was discharged on illness day 25 in stable condition, and a chest CT was performed (Fig. 1, Panel D). During hospitalization, he received only supportive care, without any antiviral or antibiotic therapy and no oxygen therapy.
Case 5: A 46-year-old woman complained of intermittent fever and severe headache for 3 days, and her husband who just returned from Wuhan, China on 2020, January, 12. She was then diagnosed COVID-19 by RT-PCR. During admission, she received dexamethasone 4 mg 3 doses as 12 h apart due to hypersensitivity to NSAIDs. Her fever and headache subsided after 5 days under supportive observation. Three consecutive tests were negative, and she was discharged on illness day 32 in stable condition and a chest CT was performed (Fig. 1, Panel E).
SARS CoV-2 viral RNA copies (Ct values) and LPV/r treatment
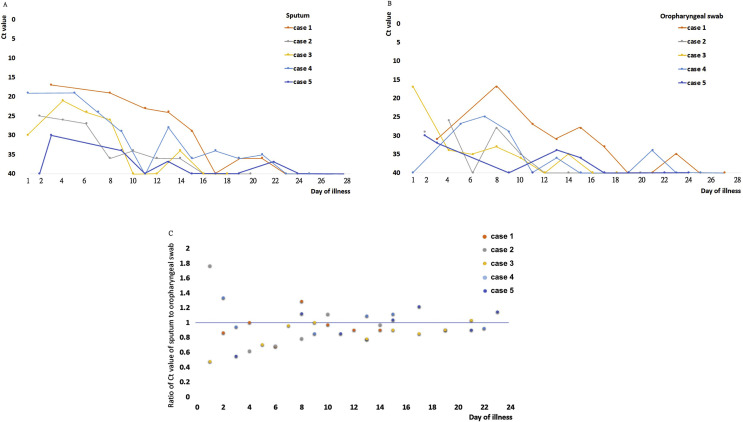
In five cases, oropharyngeal swabs and sputum samples were obtained simultaneously on every other day, and staring the Ct values are shown in Fig. 2 A and B. In total, there were 40 testing points with detectable virus before the four patients were de-quarantined (after two consecutive tests with Ct values of 40), and the data showed that viral loads of detecting SARS CoV-2 in sputum was higher. At 67.5% (27/40) of the testing time points, the ratio of the Ct value for sputum to the Ct value of the oropharyngeal swab was less than or equal to one, indicating higher viral loads of detection in sputum, compared to 32.5% (13/40) for oropharyngeal swabs. Therefore, the viral loads of sputum detection is speculated to be higher than the viral loads of oropharyngeal swab detection (Fig. 2C).
Figure 2.
Viral loads detected in oropharyngeal swabs and sputum samples obtained from the five patients with COVID-19. Panel A shows the cycle threshold (Ct) values obtained by RT-PCR assays using primers and probes targeting the E gene and sputum samples from the four cases. Panel B shows the Ct values obtained using oropharyngeal swabs. Case 1 received LPV/r since illness day 5–8, and case 2 received LPV/r since illness day 2–14. Panel C shows the ratios of the Ct values for sputum and oropharyngeal swab samples, and for 67.5% (27/40) of the samples, the ratios were less than or equal to one.
In cases 1 to 5, the mean Ct values of the initial sputum samples, collected on illness days 1–3, were 17, 25, 30, 19, and 31 respectively, which increased to 19, 35, 33, 29, and 40 respectively, in samples collected on illness days 8–10. LPV/r was administered to case 1 on illness days 5–8 and to case 2 on illness days 2–14, and an increase of Ct values between these two time points was 0.9 per day in case 1 and 2, compared to an increase of 1.0 per day between these time points in case 3, 4 and 5 which were not administered LPV/r (Fig. 2A).
Discussion
This analysis of five cases of COVID-19 in Taiwan showed that SARS CoV-2 detection in sputum specimens was more sensitive than detection in oropharyngeal swabs, as 67.5% of the Ct value ratios of sputum to oropharyngeal swabs were less than or equal to one. Therefore, the viral loads of sputum detection is speculated to be higher than that of oropharyngeal swab detection. In addition, an increase of Ct values between these two time points was 0.9 per day in case 1 and 2, compared to an increase of 1.0 per day in the non-LPV/r-treated cases (3, 4 and 5); therefore, we concluded that LPV/r did not shorten the duration of SARS CoV-2 viral shedding in patients with mild pneumonia.
No effective medical treatment against SARS CoV-2 infection has been identified. Ribavirin is a synthetic nucleoside antiviral agent that has been widely used in SARS epidemics. However, various studies have shown no definitive effectiveness and serious toxicities.6 Until now, no data have been published on the use of ribavirin for SARS CoV-2 infection.
Chloroquine is an anti-malarial drug that was shown to act at both entry and post-entry stages of SARS CoV-2 infection in Vero E6 cells.7 Chloroquine is an immune-modifier that can be distributed throughout the body, including the lungs. Hydroxychloroquine has in vitro activity against SARS-CoV-2 and may have immunomodulating properties. Mechanisms may include inhibition of viral enzymes or processes such as viral DNA and RNA polymerase, viral protein glycosylation, virus assembly, new virus particle transport, and virus release. Other mechanisms may also involve ACE2 cellular receptor inhibition, acidification at the surface of the cell membrane inhibiting fusion of the virus, and immunomodulation of cytokine release. Pre-clinical in vitro data suggest hydroxychloroquine has activity against SARSCoV-2.7 , 8 Hydroxychloroquine (EC50 = 0.72 μM) was found to be more potent than chloroquine (EC50 = 5.47 μM) to inhibit SARS-CoV-2 in vitro in Yao X's study.8 In case 2, hydroxychloroquine was added since illness day 18 after virus was not detected two days later, so it could not be concluded the efficacy of hydroxychloroquine. An open-label, non-randomized clinical trial compared hydroxychloroquine treatment (n = 26) to an untreated negative control group.9 Preliminary data showed the proportion of patients that had negative PCR results significantly differed between treated patients and untreated controls. On day 6, 70% of hydroxychloroquine-treated patients were virologically cured compared to 12.5% in the untreated control group.
Remdesivir is an adenosine analogue that has broad spectrum antiviral activity against numerous viruses, including Ebola virus.10 In vitro evidence showed that remdesivir had superior antiviral activity against MERS-CoV.11 In mice, remdesivir treatment reduced the viral load in the lungs and prevented acute lung injury. In contrast, therapeutic lopinavir/ritonavir/interferon-beta-1b preserved pulmonary function but did reduce viral replication in vivo. 11 The first reported case in the USA showed a prompt reduction in fever and much-improved clinical symptoms after administration of remdesivir on day 12 of illness after administration of remdesvir starting on day 10 of illness.3 Thereafter, the effectiveness of remdesivir garnered much attention worldwide. However, chest roentgenography of the aforementioned US case showed only relatively mild infiltrations, and the rRT-PCR assay of a oropharyngeal swab was negative on illness day 12, soon after remdesivir was first administered. Thus, the suppression of SARS CoV-2 activity by remdesivir needs to be further investigated, and as of now, there have been only a few clinical experiences.
Lopinavir is an antiretroviral agent, boosted by ritonavir, that is widely used for treating HIV. Experiences using LPV/r to treat SARS-CoV have been reported in several studies. Cao and co-workers showed 199 adult patients with severe COVID-19, no benefit was observed with lopinavir–ritonavir treatment beyond standard care, and mortality at 28 days was similar in LPV/r group and the standard-care group (19.2% vs. 25.0%; 95% CI, −17.3 to 5.7).12 Chu and co-workers assessed the effects of LPV/r compared to historic controls treated with ribavirin only.13 Adverse events, including the development of acute respiratory distress syndrome (ARDS) or death within 21 days, were significantly lower in the LPV/r group than in the historic controls (2.4% vs. 28.8%, p < 0.001). In addition, a significant reduction in the need for rescue pulsed steroids (p < 0001) and significantly lower nosocomial infections were also noted in patients treated with LPV/r (p = 0.043). Chan and colleagues demonstrated that the addition of LPV/r as an initial treatment was associated with a statistically significant reduction in the overall death rate (2.3% vs 15.6%) and intubation rate (0% vs 11.0%) when compared with matched controls (both p < 0.05).14 In a nonhuman primate model of common marmosets, subjects with MERS-CoV treated with LPV/r or interferon-beta-1b had better prognoses than untreated subjects.15 In addition, a randomized placebo-controlled trial of MERS-CoV treated with a combination of LPV/r/interferon-beta-1b has been ongoing since 2016.16 Based on the similarity between SARS-CoV and SARS CoV-2, we prescribed LPV/r for our first two cases and presumed that it would suppress the viral load. Unfortunately, there was no obvious shortening effect on viral shedding in our limited dataset. In the future, peptides or small compounds that bind to carboxypeptidase angiotensin-converting enzyme 2 (ACE2), a cellular receptor for both SARS viruses, may be potential treatment agents.17 The effective treatment is lacking, and some clinical trials investigating the efficacy are conducting globally. Currently, effective infection control intervention is the only way to prevent the spread of SARS-CoV-2.18
There are some limitations to this study. First, the study population was small because there were only a few confirmed and recovered cases of COVID-19 in Taiwan. Second, no documented effective medication could be used in a parallel group. However, the strength of our study is the complete dataset of Ct values obtained during hospitalization, and sputum and oropharyngeal swab specimens collected every 2 or 3 days to document the serial change in SARS CoV-2 viral shedding.
In conclusion, LPV/r may not be recommended for COVID-19 patients with mild pneumonia, and additional medications should be studied in scaled-up observation studies.
Declaration of Competing Interest
All authors report no conflicts of interest.
Acknowledgements
The authors would like to thank the Taiwan Centers for Disease Control for technical support with the RT-PCR assay.
Contributor Information
Chien-Yu Cheng, Email: s841060@gm.ym.edu.tw.
Yu-Lin Lee, Email: leeyulin@gmail.com.
Shu-Hsing Cheng, Email: shuhsingcheng@gmail.com.
References
- 1.World Health Organization . 2020 Mar 07. Coronavirus disease 2019 (COVID-19) Situation Report–47.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200307-sitrep-47-covid-19.pdf?sfvrsn=27c364a4_4 [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 Jan 31 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diagnostic detection of 2019-nCoV by real-time RT-PCR. 2020 Jan 17. https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn= a9ef618c_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groneberg D.A., Poutanen S.M., Low D.E., Lode H., Welte T., Zabel P. Treatment and vaccines for severe acute respiratory syndrome. Lancet Infect Dis. 2005;5:147–155. doi: 10.1016/S1473-3099(05)01307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Feb 4 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautret P., Lagier J., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020 Mar 18 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicenter retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 15.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]