Abstract
The study of the intestinal microbiota has begun to shift from cataloging individual members of the commensal community to understanding their contributions to the physiology of the host organism in health and disease. Here, we review the effects of the microbiome on innate and adaptive immunological players from epithelial cells and antigen-presenting cells to innate lymphoid cells and regulatory T cells. We discuss recent studies that have identified diverse microbiota-derived bioactive molecules and their effects on inflammation within the intestine and distally at sites as anatomically remote as the brain. Finally, we highlight new insights into how the microbiome influences the host response to infection, vaccination and cancer, as well as susceptibility to autoimmune and neurodegenerative disorders.
An astounding number and diversity of microorganisms coexist with mammalian organisms1. Recent years have seen an increase in understanding of the complexity and sophistication of the host–microbiota relationship and its effects on human health2–4. Several technological advances have bolstered the study of mammalian microbiomes. Sequencing of 16S-rRNA-encoding genes has identified the constituent bacterial species of the human intestinal microbiota as belonging predominantly to the Bacteroidetes and Firmicutes phyla. Deep sequencing of the internal-transcribed-spacer regions ITS1 and ITS2 of the fungal ribosomal DNA and improved downstream analyses5,6 have unveiled the presence of rich fungal communities, dubbed the mycobiome, within the mammalian intestinal tract7. Sequencing of total DNA, the metagenome, from fecal specimens has enabled systematic studies on the virome and has yielded valuable information about the complex interaction of these commensals with their host. Large-scale endeavors have been launched to characterize the human microbiome: the US National Institutes of Health (NIH)-funded Human Microbiome Project (HMP) and the European Metagenomics of the Human Intestinal Tract (MetaHIT)8,9. Concurrently, gnotobiotic resources and treatment of mice with antibiotics have shown how specific compositions of the mouse or human gut microbiota contribute to disease development and have enabled mechanistic dissection of host–microbiota interactions. Targeted phenotypic culturing by subjecting fecal samples to selection for a desired phenotype and subsequent whole-genome sequencing and phylogenetic analysis has revealed that almost 75% of the intestinal microbiota is culturable10. Selection for sporulation has indicated that 50–60% of intestinal bacterial genera produce resilient spores adapted for survival and dispersal10, thus potentially explaining why, in humans, the intestinal microbiota of family members with close contact have Ruminococcaceae and Lachnospiraceae spore-forming bacteria in common11. Ex vivo organ cultures of the mouse intestine have allowed for the introduction of molecules and microbes into the gut lumen in a setting that recapitulates luminal flow and features spontaneous peristaltic-like contractions and an intact tissue architecture and cellular network12.
Microbiome-wide studies have revealed important correlations between specific microbes and a range of diseases including inflammatory bowel disease (IBD), autoimmune disease13, cancer14 and metabolic4 and neurodegenerative disorders15. Chronic inflammation is a driver of many of these conditions. Here, we focus on the most recent insights into the molecular underpinnings of host–microbiota interactions that influence inflammation within the intestine and distal organs. We consider the properties of the microbiota that most critically affect the immune response, including its biogeography, metagenome and metabolome, and how the microbiome modulates the host response to infection, autoimmunity, neuroinflammation, vaccination and tumor immunotherapy.
Toward identification of an immune-modulatory microbiota
Physical and biochemical barriers anatomically segregate the microbiota from mammalian immune cells in the intestine3,16. This ‘demilitarized zone’ is essential to limit inappropriate immune activation16. On the host side of this zone lies the intestinal epithelium17, which comprises a single layer of intestinal epithelial cells whose frequent cycles of apoptosis and renewal18 maintain cellular fitness and orchestrate intestinal immune homeostasis19.
The demilitarized zone is not impermeable, and certain commensals, such as segmented filamentous bacteria (SFB), Acinetobacter spp., Bacteroides fragilis and Proteobacteria, can associate with the intestinal epithelium20. Proximity to the epithelium evokes the strongest effects on the host. For example, the capsular polysaccharide A of the human commensal B. fragilis stimulates production of the anti-inflammatory cytokine IL-10 by Foxp3+ regulatory CD4+ T (Treg) cells, thus facilitating colonization while promoting beneficial immunosuppression in the intestine20. Outer-membrane vesicles produced by B. fragilis activate noncanonical autophagy (involving the autophagy-related protein ATG16L1 and the receptor Nod2), thereby inducing Treg cells and suppressing mucosal inflammation21. Intestinal SFB colonization induces a response by IL-17-producing helper T (TH17) cells positive for the transcription factor RORγt, thus protecting mice from infection with the enteric rodent pathogen Citrobacter rodentium3. Similarly, Clostridium spp. and the human symbiont Clostridium ramosum are potent inducers of colonic Treg cells3,12. T cell–dependent immunoglobulin A (IgA) production is activated by epithelium-associated commensal bacteria, such as Mucispirillum and SFB22. These observations highlight the importance of defining the immunologically relevant microbiome, especially because many of the mucosal responses regulated by the microbiota are critical for intestinal homeostasis and are disrupted in IBD.
The mouse circadian clock is synchronized according to diurnal oscillations in the composition and activities of the microbiota23–25. The numbers and species of epithelial-associated commensals in mice fluctuate almost tenfold in the dark phase compared with the light phase, and diurnal oscillations in species such as Mucispirillum schae-dleri, Lactobacillus reuteri and Bacteroides acidifaciens are associated with the feeding cycle24. Bacterial adherence to the epithelium controls reprogramming of transcriptional oscillations not only in the colon but also at distant sites, such as the liver, through rhythmic chromatin remodeling and the activity of promoter and enhancer regions24. The diurnal detoxification of acetaminophen, regulated by circadian liver functions, is disrupted by changes in the microbiota24.
The aforementioned immunologically relevant microbiome includes several keystone pathosymbionts identified through sorting and sequencing of IgA-coated microbiota (a technique termed IgA-seq or Bug-FACS)22,26,27. During the first two years of life in humans and gnotobiotic mice, age-specific bacterial taxa define distinct temporal patterns of mucosal IgA responses28. IgA can cross-link bacteria in the mammalian intestine, thereby inhibiting bacterial pathogenesis or the genetic spread of antimicrobial resistance29. Fecal IgA varies independently of bacterial phylogeny and can be perturbed during disease30. Enrichment of Enterobacteriaceae and Lachnospiraceae in IgA-coated and IgA-negative microbiota, respectively, in both Crohn’s disease–associated spondyloarthritis31 and malnutrition26, suggest that a potential core IgA response may exist in various inflammatory conditions.
Keystone pathosymbionts may similarly affect mucosal T cell responses. Human-derived adherent-invasive Escherichia coli and Bifidobacteria adolescentis induce both mucosal and systemic inflammatory TH17 cells31,32. Although both of these pathosymbionts recapitulate the close epithelial adherence that has been observed for SFB, B. adolescentis triggers an epithelial transcriptional response distinct from that of SFB, thus suggesting the potential for shared and distinct pathways in microbial induction of TH17 cells. Whereas cluster IV, cluster XIVa and cluster XVIII Clostridium support Treg induction33, nearly one-quarter of the 53 species recently profiled similarly induce colonic Treg cells. This potential redundancy by a diverse group of bacteria may serve to ensure consistency in mucosal homeostasis. However, the immunomodulatory properties of different bacterial species do not necessarily cluster by phylum or genus, thus highlighting the importance of considering strain-specific traits when assessing immunological phenotypes.
The subset of microbes that colonize lymphoid tissues are known as lymphoid-tissue-resident commensal (LRC) bacteria and include alpha- and betaproteobacteria, such as Alcaligenes, Achromobacter, Bordetella and Ochrobactrum species34–37. LRC bacteria selectively colonize the Peyer’s patches, isolated lymphoid follicles and mesenteric lymph nodes in healthy humans, nonhuman primates and mice, and their entry to these tissues depends in part on M cells, IgA and the cytokine IL-22 (refs. 34,36,38). LRC bacteria colonize dendritic cells and uniquely modulate cytokines that promote responses by local TH17 cells and group 3 innate lymphoid cells (ILC3)34. Innate lymphoid cells are ubiquitously distributed in humans and mice but are enriched at mucosal surfaces and rapidly respond to cytokine milieus after colonization with microbes37. Among subsets of innate lymphoid cells, ILC3 are most heterogeneous, uniquely express RORγt and broadly comprise two subsets on the basis of expression of the chemokine receptor CCR6 or the transcription factor T-bet. CCR6+ ILC3 lymphoid-tissue-inducer-like cells persist after birth in secondary lymphoid tissues, cryptopatches and isolated lymphoid follicles. CCR6+ ILC3 promote gut-associated lymphoid-tissue maturation and IgA production, and contribute to the innate host defense to enteric pathogens37. CCR6+ ILC3 are also antigen-presenting cells that regulate homeostasis with beneficial microbes by limiting the development of microbiota-specific CD4+ T cell–effector responses in the intestine37. In contrast, T-bet+ ILC3 are localized diffusely in the intestinal lamina propria, require the aryl hydrocarbon receptor (AHR) and expand after microbiota colonization37,39. AHR protects mucosal sites from pathogenic infection and inflammation40. T-bet+ ILC3 are responsive to microbial sensing by mononuclear phagocytes positive for the chemokine receptor CX3CR1, and subsequent ILC3 production of IL-22 has been linked to intestinal-tissue repair and barrier function by acting directly on intestinal epithelial stem cells37,41. IL-22 production by ILC3 also regulates epithelial fucosylation and supports diverse microbiota colonization37.
LRC bacteria also induce IL-10 production by dendritic cells and provide tissue-protective functions in the context of intestinal-barrier damage34. ILC3 promote anatomical containment of LRC bacteria, because ILC3 depletion results in systemic bacterial dissemination and chronic immunological activation37. Additional research is required to define the mechanisms by which LRC bacteria colonize dendritic cells and mammalian lymphoid tissue, as well as to interrogate the functional potential and compositional changes of LRC bacteria in the context of chronic inflammatory diseases.
Interaction with symbiotic fungi, protozoa, worms and viruses
Rich and diverse fungal communities (mycobiota) colonize the mammalian barrier surfaces. Mycobiota diversity increases in the lower gastrointestinal tract, and several genera such as Candida, Saccharomyces, Aspergillus, Cryptococcus, Malassezia, Cladosporium, Galactomyces and Trichosporon have the potential to colonize the intestines7,42–44. Fungal-community changes with outgrowth of Candida spp. have been documented in people with IBD43,45–47. Deficiencies in the receptor Dectin-1 (also known as CLEC7A) and the downstream adaptor protein CARD9 lead to susceptibility to more severe experimental colitis as well as fungal and bacterial dysbiosis6,7,48. Clec7a–/– mice colonized with Candida tropicalis show aggravated experimental colitis, whereas the absence of Candida leads to less severe disease6,49. Fungi and bacteria share similar niches in the intestine, and these communities influence each other. Antibiotic treatment promotes gut Candida col-onization7,50, which can have immunological outcomes at distant sites such as the lung7,51. Bacteria affect fungal colonization both directly and indirectly. Bacteroidetes thetaiotamicron, which induces the production of the antimicrobial peptide CRAMP by the transcription factor HIF-1α, prevents Candida albicans gut colonization52. In addition to fungi, the common mouse protozoan Tritrichomonas musculis is a transmissible microorganism in mice that increases susceptibility to T cell–dependent intestinal inflammation while providing protection from intestinal infections through inflammasome activation and production of the cytokine IL-18 by intestinal epithelial cells53,54.
The mammalian gastrointestinal tract is also colonized with eukaryotic viruses, which may substantially affect intestinal health and disease. Colonization with common murine norovirus is able to compensate for several, but not all, functional and immunological defects in germ-free or antibiotic-exposed mice55. In the presence of a diverse microbiota, several enteric eukaryotic viruses interact with the commensal microbiota and consequently induce immunological- evasion pathways and ensure their own replication and transmission56,57. Although the contributions of colonizing eukaryotic viruses and bacteriophages to human health are only beginning to be interrogated, early analyses have suggested substantial changes in these populations in the context of IBD and progressing HIV infection58–60. Finally, intestinal worms or helminths have long been known to influence intestinal immune responses and physiology, and may be an ancient intestinal symbiont lost in industrialized nations. In the developing world, helminths affect bacterial composition and colonization resistance61, and independently impair host immunity to eukaryotic viruses62,63 through induction of intestinal type 2 immune responses. These data highlight intestinal symbionts other than bacteria and the importance of considering multiple cross-kingdom interactions in future basic and translational studies of the microbiota.
Microbiota small molecules mediate interspecies interaction
The gut microbiota is influenced in part by long-term dietary habits and is responsive to daily variations in food4, and it contributes to the metabolite profile in the plasma64. Bacterial metabolites exhibit rhythmicity, owing to the oscillation of several bacterial biosynthetic pathways, such as those for biotin and proline24. Concordantly, homeostatic circadian oscillations in the serum levels of amino acids and polyamines are sensitive to dysbiosis and dietary polyamine content24. Dietary-fiber deficiency promotes the proliferation of mucus-degrading bacteria, thus leading to colonic mucus erosion, association of luminal bacteria with the intestinal epithelium and increased susceptibility to Citrobacter65 (Fig. 1). Short-chain fatty acids (SCFA) derived from the anaerobic fermentation of nondigestible polysaccharides such as dietary fiber, particularly by Clostridia spp., counter inflammation and maintain gut homeostasis66 (Fig. 1). Among SCFAs, butyrate uniquely inhibits intestinal stem-cell and progenitor- cell proliferation during mucosal injury, and this inhibition is likely to prevent their potential transformation under genotoxic stress in response to luminal contents67. Colonocyte localization at the crypt mouth ensures the preferential consumption of butyrate before it reaches stem cells at the crypt base67 (Fig. 1). Microbiota-derived butyrate promotes colonic oxygen consumption stabilizing the transcription factor HIF-1 and its target barrier-protective genes68.
Figure 1.
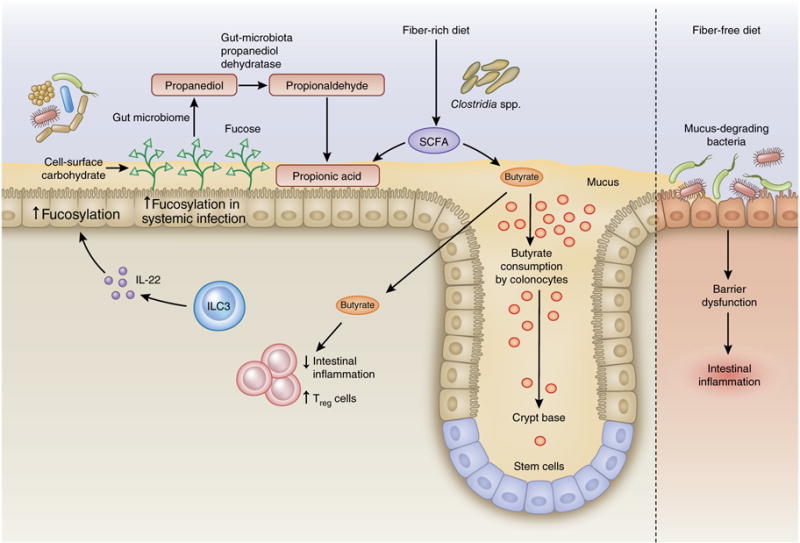
Dietary fiber and SCFAs in intestinal homeostasis. Anaerobic fermentation of dietary fiber by members of the commensal microbiota, particularly by Clostridia spp., serves as a source of SCFAs, which help to maintain Treg cell expansion, immunosuppressive function and overall intestinal homeostasis. Butyrate is the preferred metabolic energy source for colonocytes but is detrimental to stem cells, inhibiting their proliferation and wound-repair functions67. The strategic positioning of colonocytes and stem cells within the colon mirrors the concentration gradient of butyrate: colonocytes are positioned at the location of highest concentrations near the lumen, where they consume butyrate, thus decreasing the concentration to which distally located stem cells within the colonic crypts are exposed. Propionate is the end product of fucose metabolism by the microbiota. The host increases fucosylation of epithelial-cell carbohydrates during infection, thereby protecting its gut commensals116. Fucose-using B. acidifaciens increase in abundance and elevate their metabolism of fucose, thus leading to propanediol formation. Propanediol dehydratase converts propanediol to propionaldehyde, thereby generating propionic acid77, which in turn tempers inflammation and protects host tissues from collateral damage during the immune response to infection.
Gut bacteria are also an important source of potent anti- inflammatory polyamines such as putrescine and spermine. Ingestion of the probiotic Bifidobacteria LKM512 by elderly people increases intestinal polyamine concentrations and inhibits intestinal inflammation, particularly when it is administered with arginine69. Importantly, microbiota-derived histamine, putrescine and spermine suppress cleavage of the protease caspase-1 and secretion of IL-18 as well as the colonic expression of antimicrobial peptides that predispose the colon to inflammation70 (Fig. 2). The suppressive activity of these polyamines is countered by the bile-acid conjugate taurine, which induces activation of the NLRP6 inflammasome and production of IL-18 after intestinal microbial colonization and promotes microbial diversity and intestinal homeostasis70 (Fig. 2).
Figure 2.

Examples of mechanisms mediating host–microbiota interactions. A diverse microbiota provides two signals for NLRP6 inflammasome activation in intestinal epithelial cells: signal 1 is in the form of LPS, and signal 2 is in the form of metabolites such as the bile-acid conjugate taurine. Together, these signals activate the NLRP6 inflammasome in intestinal epithelial cells and lead to the production of epithelial IL-18 and downstream antimicrobial peptides (AMP). Under dysbiotic conditions, such as those in mice lacking the inflammasome adaptor ASC, microbiota-derived histamine, putrescine and spermine are increased, thus suppressing NLRP6 inflammasome signaling in intestinal epithelial cells, decreasing production of epithelial IL-18 and AMP in the colon and promoting intestinal inflammation70. B. thetaiotamicron induces the transcription factor HIF-1α in intestinal epithelial cells, thereby activating transcription and production of the antimicrobial peptide LL-37 (CRAMP in mice), which in turn promotes resistance to C. albicans colonization52. Gut anaerobic Firmicutes from the class Clostridia, and several clusters in Gram-negative Bacteroides and Desulfovibrio, express nonribosomal peptide synthetase–encoding gene clusters that mediate the synthesis of dipeptide aldehydes79. The dipeptide aldehyde Phe-Phe-H is cell permeable and has been shown to inhibit cathepsins in macrophages, an activity that might modulate antigen processing and innate immune function79. The generation of the nonproteinogenic amino acid trans-4-hydroxy-L-proline (t4L Hyp) is one of the most common post-translational modifications in eukaryotic cells but is rare in bacteria. Intestinal commensals such as Clostridiales and human pathogens such as C. difficile chemically reverse proline hydroxylation through the activity of the GRE trans-4-hydroxy-L-proline dehydratase, which generates L-proline77. Many Clostridiales then use L-proline as an electron acceptor in amino acid fermentation.
Trimethylamine-N-oxide generated through the metabolism of diet-derived choline, phosphatidylcholine and carnitine, sequentially by gut microbes and the liver, increase platelet hyper-responsiveness and thrombosis risk73 and accelerate heart and liver disease74–76. Despite abundant representation of the glycyl radical enzyme (GRE) superfamily that catalyze this enzymatic conversion by the human microbiota, little is known about the activity and roles of GREs in health and disease. The use of chemically guided functional profiling– coupled protein sequence-similarity networks combined with quantitative metagenomics has allowed for the discovery and functional characterization of trans-4-hydroxy-l-proline dehydratase77, the most abundant GRE in the NIH HMP stool microbiota. This enzyme allows the microbiota to chemically reverse C4-hydroxylation of l-proline (the most common eukaryotic post-translational modification), thereby acquiring additional sources of carbon and nitrogen (Fig. 2). Chemically guided functional profiling has also led to the functional characterization of novel coenzyme B12–independent propanediol dehydratase, which converts l-fucose to SCFA (Fig. 1). Although propanediol dehydratase might be the major contributor to propionate production at steady state, coenzyme B12–dependent propanediol dehydratase is required for TH17 induction by adherent-invasive E. coli31.
A survey of biosynthetic-gene clusters from stool samples from the NIH HMP has identified thousands of biosynthetic loci with no known functions78. Nonribosomal peptide synthetase–encoding gene clusters have been identified as an abundant gene cluster exclusive to gut-associated bacterial species, predominantly in anaerobic Firmicutes from the class Clostridia, and several clusters in Gram-negative Bacteroides and Desulfovibrio78. Their absence in free-living or nonintestinal niche-colonizing microorgansims suggests adaptation to intestinal colonization79. Heterologous expression combined with quantitative and unbiased chemical proteomics has led to the discovery of dipeptide aldehydes79. The dipeptide aldehyde Phe-Phe-H is stable and acts as a cell-permeable inhibitor of cathepsins (Fig. 2), thus suggesting active blockade of innate and adaptive immunity by microbiota-derived dipeptide aldehydes, given that cathepsins are important for antigen processing and presentation, as well as endosomal activation of the Toll-like receptor TLR9 (ref. 80). There is great potential for the discovery of novel mechanisms of immune modulation through the functional characterization of yet-undiscovered microbiota-derived molecules.
Gut microbiota modulate inflammation at distant sites
Both bacterial and fungal dysbiosis have been linked to autoimmune and immune-mediated diseases13,51,81. The prevalence of Bacteroides spp. within Finnish and Estonian infants is associated with early-onset autoimmune disease82 (Fig. 3). Relative to Russia, Finland has an incidence two- to sixfold higher for allergies and five- to sixfold higher for type 1 diabetes and other autoimmune disorders. Compared with the hexa-acylated lipopolysaccharide (LPS) expressed by the more abundant E. coli in Russian infants, the less stimulatory tetra- and penta-acylated LPS characteristic of Bacteroides spp. impairs endotoxin tolerance, thereby leading to a propensity for higher immunological stimulation82 (Fig. 3). These data are concordant with the hygiene hypothesis, in which early-life exposure to specific microbes and parasites confers protection against allergic and autoimmune disease83,84, and they highlight how perinatal environmental influences on the microbiota can determine susceptibility to immune-mediated disease later in life.
Figure 3.

Associations between the intestinal microbiota and autoimmune disorders. Infants from Russia have more abundant E. coli species expressing stimulatory hexa-acylated LPS, whereas infants from Finland and Estonia have more abundant Bacteroides spp. expressing the less stimulatory tetra- and penta-acylated LPS82. Hexa-acetylated LPS induces greater Immunological stimulation but also endotoxin tolerance thought to dampen the capacity for immunological education in early life. However, the less stimulatory LPS from Bacteroides spp. impairs LPS tolerance, thus increasing susceptibility to immunological disease later in life. Enrichment of adherent-invasive E. coli in the IgA-coated microbiota in patients with Crohn’s disease–associated spondyloarthritis correlates with E. coli seroreactivity and systemic TH17 cell activation31.
The effects of commensal microbiota on mucosal and systemic immunity highlight a potential role for keystone species in autoimmunity. Antigen-specific TH17 responses develop to the intestinal microbiota in mice3 and in people with Crohn’s disease85 as well as to the intestinal epithelium during mouse colonic infection associated with apoptosis of intestinal epithelial cells86. Severe gastrointestinal infection in mice leads to loss of T cell tolerance to commensal antigens and results in long-lived inflammatory effector T cells that drive chronic intestinal and extraintestinal inflammatory pathology87. In mice, infection-induced apoptosis of intestinal epithelial cells triggers the loss of CD4+ T cell tolerance to self-antigen derived from intestinal epithelial cells. Under these conditions, self-reactive CD4+ T cells differentiate into TH17 cells alongside pathogen-specific CD4+ T cells and mediate intestinal inflammation86,88. Notably, the TH17 response to SFB is not disrupted by concurrent infection with the TH1-cell inducer Listeria monocytogenes89. SFB-induced TH17 cells are sufficient to induce extraintestinal inflammatory disease including inflammatory joint disease90 and experimental autoimmune encephalomyelitis91.
A role for mucosa-associated microbiota is coming to light in systemic autoimmunity. IgA-coated mucin-degrading Akkermansia muciniphila are enriched in an HLA-B27-antigen transgenic rat model of inflammatory arthritis92. An enrichment in adherent-invasive E. coli in the IgA-coated microbiota has also been found in people with Crohn’s disease–associated spondyloarthritis, and this observation correlates with systemic TH17 cell activation and E. coli seroreactivity31 (Fig. 3). Adherent and invasive bacteria are enriched in ileal biopsies from people with HLA-B27+ ankylosing spondylitis93. Induction and egress of intestinal T follicular helper cells enable the gut microbiota to regulate systemic autoimmunity94, but additional models are needed to understand the contribution of microbe-specific autoimmunity to the pathophysiology of inflammatory disease.
Both the gut microbiome and the immune system are integral parts of gut–brain communication, which relies on neuroendocrine and autonomic nervous systems95,96. Enteric afferent neurons communicate intestinal conditions to intestinal muscularis macrophages via β2-adrenergic receptors97 and to the brain through the vagus nerve95,96. Intestinal infections of mice with C. rodentium, Campylobacter jejuni or Salmonella enterica var. Typhimurium increase levels of the transcription factor c-Fos in visceral and vagal neurons in select brain regions, events requiring an intact vagus nerve98. Multiple members of the microbiota such as Escherichia, Lactobaccillus, Bifidobacterium, Enterococcus and Truchuris produce neurotransmitters and neuropeptides including dopamine, acetylcholine, gamma-aminobutyric acid, serotonin (5-hydroxytryptamine) and brain-derived neurotrophic factor98 (Fig. 4). These metabolites induce mouse intestinal epithelial cells to release molecules that modulate signaling within the enteric nervous system. Spore-forming bacteria, primarily Clostridium spp., modulate the colonic luminal metabolome, including SCFAs, thereby inducing serotonin biosynthesis by enterochromaffin cells—the major producers of serotonin—and consequently affecting intestinal motility and platelet function in mice99,100 (Fig. 4). Serotonin has a wide range of physiological effects including the development and function of the immune system101, and it will be important to determine its role in intestinal inflammation and to elucidate how serotonin control by the microbiota affects function and inflammation in distal tissues including the brain. Microbiota-dependent signals also stimulate enteric-nervous-system nociceptors known to regulate inflammation12. Immunomodulatory colonic RORγt+ Treg–inducing C. ramosum represses neuronal-specific transcripts, particularly those encoding nociceptive neuropeptides, in microfluidics-supported mouse intestinal organ cultures (Fig. 4), thus suggesting an unappreciated inverse functional link between neuronal activation and Treg cell differentiation12.
Figure 4.

Links between the intestinal microbiota and neuroinflammation. Multiple members of the microbiota, such as Escherichia, Lactobaccillus, Bifidobacterium, Enterococcus and Truchuris, produce neurotransmitters and neuropeptides including dopamine, acetylcholine, gamma-aminobutyric acid, serotonin and brain-derived neurotrophic factor98. Spore-forming bacteria, primarily Clostridium spp., modulate the colonic luminal metabolome, including SCFAs, thus inducing serotonin biosynthesis by enterochromaffin cells—the major producers of serotonin—and thereby affect intestinal motility and platelet function in mice99,100. In the colon, C. ramosum induces RORγt+ Treg cells but also represses neuronal-specific transcripts, particularly those encoding nociceptive neuropeptides12. Afferent neurons within the enteric nervous system (ENS) can communicate intestinal conditions to intestinal muscularis macrophages via β2-adrenergic receptors97 and also to the brain via the vagus nerve95,96. Intestinal colonization by the microbiota increases blood–brain tight junctions and barrier function, although microbiota-derived SCFAs can gain access to the brain and promote microglia differentiation and function102,103. Microbiota-dependent metabolism of tryptophan into AHR ligands engages AHR on astrocytes, thus leading to an increase in astrocyte expression of the inhibitor protein SOCS2 and consequently inhibiting activation of the transcription factor NF-κB and thereby limiting inflammation105
The blood–brain barrier and brain lymphatic vasculature allow the passage of various immune cells, macromolecules and metabolites into the brain96. Disruption or absence of the microbiota in mice impairs the function of the blood–brain barrier (Fig. 4), alters cortical myelination and hippocampal neurogenesis, decreases cognitive function and memory formation, and decreases social and anxiety-like behavior96. Microbiota-derived SCFAs promote the differentiation and function of microglia, the resident macrophages in the brain102,103 (Fig. 4), and play a significant role in accelerating the appearance of motor deficits mediated by the neuronal protein α-synuclein as well as brain pathology in a mouse model of Parkinson’s disease104. Gut microbiota from people with Parkinson’s disease induce enhanced motor dysfunction when they are transplanted into α-synuclein transgenic mice104, thus suggesting that Parkinson’s disease–associated microbes can trigger disease symptoms in this genetically susceptible mouse model. However, microbiota-dependent metabolism of tryptophan into AHR ligands targets AHR on astrocytes, which are critical in neuronal transmission and development and repair of the central nervous system, thereby limiting central-nervous-system inflammation in mice105 (Fig. 4). Dietary supplementation with tryptophan ameliorates autoimmune encephalomyelitis scores, whereas treatment of mice with ampicillin worsens disease105.
Microbiota-driven modulation of the host immune response
Significant associations between fungal- and bacterial-induced cytokine responses and specific gut bacterial species and genera have been found through the Human Functional Genomics Project106. For example, production of the cytokines IFN-γ and TNF by peripheral blood mononuclear cells is more strongly associated with the micro-biome than are the cytokines IL-6 and TH17-derived IL-17 and IL-22. Staphylococcus aureus–induced IL-17 is positively associated with five genera, including species from Clostridium clades IV and XIV, and is negatively associated with Fecalibacterium, including Fecalibacterium prausnitzii; however, multiple diet-sensitive bacteria, such as Alistipes spp., Clostridium spp. and Bilophila spp., are negatively associated with LPS-induced TNF production106. Although these findings have identified targetable regulators of systemic inflammation, analysis of the metabolic pathways and gene-ontology categories explaining the cytokine variation has indicated that microbiome functions have a greater effect on the cytokine response than do taxonomic classifications; for example, IFN-γ and TNF are strongly modulated by microbial palmitoleic acid metabolism and degradation of tryptophan to tryptophol106.
Microbiota-driven variations in the inflammatory response have been predicted to regulate the host response to infection106. The intestinal microbiota can mediate colonization resistance against enteric pathogens. The conversion of primary to secondary bile salts in Clostridium scindens is associated with resistance to Clostridium difficile infection in mice and humans107. In Caenorhabditis elegans, the peptidoglycan hydrolase activity of the secreted antigen A from the commensal Enterococcus faecium protects against Salmonella pathogenesis108. In Drosophila, gut-microbiota-derived peptidoglycans, particularly from the commensal Acetobacter pomorum, prime intestinal induction of a secreted factor that is released after enteric viral infection and stimulates antiviral signaling by extracellular- signal-regulated kinases in intestinal epithelial cells109. Colonization resistance by the intestinal microbiota can be extended to systemic infections or pathogens infecting distant sites such as the lung. In the absence of the microbiota, hematopoietic defects in tissue-resident myeloid cells confer susceptibility to intravenous infection with L. monocytogenes110. Gut-microbiota-derived products prep-rime inflammasome-dependent cytokines that promote dendritic-cell migration from the lung during respiratory influenza A virus infection111 and enhance innate immune responses of neutrophils in a manner dependent on the receptor Nod1 (ref. 112). The protection afforded by intestinal microbiota against enteric pathogens such as C. difficile has paved the way toward therapeutic development of probiotics that enhance host resistance against life-threatening antibiotic-resistant pathogens, such as vancomycin-resistant enterococci. These bacteria expand not because of their antibiotic resistance but because the antibiotic kills the protective commensal bacterial species that provide colonization resistance113.
Beyond conferring resistance, endosymbionts confer disease tolerance to infection in insects and in mice114. Disease tolerance does not target the infecting pathogen but instead protects against physiological damage such as cachexia, muscle wasting or endotoxic shock in response to infection114. The endosymbiont E. coli O21:H+ protects mice against muscle wasting and loss of fat during enteric S. Typhimurium or respiratory Burkholderia thailandensis infections by activating the NLRC4 inflammasome115. Subsequent IL-18 sustains production of the growth factor IGF-1, which in turn activates signaling by the PI3K–AKT kinase pathway in skeletal muscle, thereby countering muscle wasting115. Increased fucosylation of the intestinal epithelium during systemic exposure to Toll-like-receptor ligands is sensed by the intestinal microbiota, thus leading to an abundance of fucose-using B. acidifaciens116 (Fig. 1). Fucose is a substrate for microbial production of propionate117 and may thus promote host-protective SCFA-mediated effects. This adaptation of the intestinal microbiota to conditions of host stress confers host tolerance to C. rodentium but notably without affecting colonic bacterial burdens116. The microbiota can also contribute to negative outcomes after acute infection with Yersinia pseudotuberculosis, in which sustained intestinal inflammation and lymphatic leakage after pathogen clearance is mediated by the microbiota118. Distinct readouts are necessary to identify whole-microbiome associations with interindividual variations in disease tolerance.
Evidence in mice has suggested that the microbiota can modulate vaccine responses. Differentiation of T follicular helper cells and plasma cells in response to intranasal immunization is promoted by the nasal microbiota of mice, particularly Staphylococcus sciuri, via signaling by Nod2 and the kinase RIPK2 in CD11c+ phagocytes119. Toll-like-receptor stimulation by microbiota-derived signals conditions IgA class-switching in mouse-lung CD103+ dendritic cells after intranasal immunization120. Treatment of mice with antibiotics diminishes specific antibody and CD8+ T cell responses to a trivalent inactivated influenza vaccine111,121. Sensing of the microbiota by the Toll-like receptor TLR5 promotes plasma-cell differentiation after parenteral administration of trivalent inactivated influenza vaccine, probably through flagellin detection121. In humans, early TLR5 expression directly correlates with the magnitude of the antibody response to the trivalent inactivated influenza vaccine122. Numerous vaccines and boosters are administered to children within the first 15 months of life, when the microbiota is highly sensitive to environmental factors such as hygiene, breast milk versus formula diet, and vaginal versus Caesarean-section delivery123,124. Emerging considerations in determining vaccination efficacy are the microbiota composition and diversity, as well as the therapeutic potential of the critical perinatal period to imprint protective host defenses in adult life. A concomitant assessment of the microbiome in prospective vaccination studies in babies and older humans will be necessary to establish and mechanistically understand the link between commensal microbial communities and vaccine effectiveness.
The microbiota plays a complex role in modulating both pro- and antitumor responses. Microbial translocation and chronic inflammation secondary to the loss of intestinal barrier function enhances intestinal tumor progression125,126 and may account for an increased risk of colorectal cancer in people with IBD127. Inflammation also facilitates the expansion of microbes with oncogenic potential, including Fusobacterium nucleatum, enterotoxigenic B. fragilis or genotoxic E. coli128–130. The microbiota is also essential for the efficacy of antitumor immunity after chemotherapy or immunotherapy14,131. In mouse models, antitumor immunity induced by chemotherapy or blockade with antibodies to the checkpoint inhibitors CTLA-4 and PD-1 is abrogated after dysbiosis or in the absence of intestinal microbiota. Chemotherapy and checkpoint-inhibitor blockade may induce microbial translation or outgrowth of immunostimulatory microbiota such as Bacteriodes or Bifidobacterium species, which can enhance dendritic-cell function and tumor-specific CD8+ T cell responses132,133. These data are provocative and suggest that in some contexts, modulating the microbiota may enhance cancer immunotherapies.
Perspectives and future directions
Host and commensal microbiota interactions follow rules of engagement different from those between host and pathogen. Future studies will undoubtedly yield exciting new insights into how the commensal microbiota modulate immune-cell function and inflammation within the intestine and at distal-tissue sites. It will be important to gain a full understanding of the composition and characteristics of the microbiome that affect vaccine efficacy as well as modulate susceptibility not only to IBD but also to neurological, metabolic and autoimmune diseases. More studies are also needed to define the microbiota constituents that promote health as well as the environmental factors early in life that favor colonization with such microbiota. Such studies should inform new approaches for manipulating the microbiome to alter disease susceptibility and improve vaccine efficacy.
Acknowledgments
The authors thank all their past and present laboratory members for their contributions. We thank our funding agencies for their support to our laboratories: NIH grants DK072201, DK111862, AI073899, AI123284 and AI127658, the Searle Scholars Program, the Burroughs Wellcome Fund, the American Cancer Society and the Leukemia & Lymphoma Society to J.M.B.; NIH grant DK099381, the Crohn’s and Colitis Foundation Senior Research Award 346814 and the Charina Foundation to R.S.L.; NIH grants DK098310 and AI123819, and Kenneth Rainin Foundation Innovator and Breakthrough awards to I.D.I.; NIH grants DP5OD012116, AI123368 and DK110262, and the Crohn’s and Colitis Foundation, the Searle Scholars Program and the American Asthma Foundation Scholar Award to G.F.S.; NIH grants AI061570, AI087990, AI074878, AI083480, AI095466, AI095608, AI102942 and AI097333, Burroughs Wellcome Fund and the Crohn’s & Colitis Foundation of America to D.A.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert JA, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 3.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittinger K, et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol. 2014;15:487. doi: 10.1186/s13059-014-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliev ID, et al. Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.55. http://dx.doi.org/10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed]
- 8.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Human Microbiome Project Consortium. A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browne HP, et al. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schloss PD, Iverson KD, Petrosino JF, Schloss SJ. The dynamics of a family’s gut microbiota reveal variations on a theme. Microbiome. 2014;2:25. doi: 10.1186/2049-2618-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yissachar N, et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell. 2017;168:1135–1148.e12. doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longman RS, Littman DR. The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity. Curr Opin Rheumatol. 2015;27:381–387. doi: 10.1097/BOR.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 15.Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 16.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 18.Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–2730. doi: 10.1111/febs.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings RJ, et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu H, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunker JJ, et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone V, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaiss CA, et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167:1495–1510.e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Thaiss CA, Zeevi D, Levy M, Segal E, Elinav E. A day in the life of the meta-organism: diurnal rhythms of the intestinal microbiome and its host. Gut Microbes. 2015;6:137–142. doi: 10.1080/19490976.2015.1016690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kau AL, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7:276ra24. doi: 10.1126/scitranslmed.aaa4877. This defining study used Bug-FACS to identify IgA-reactive microbiota from mice colonized with human microbiota from twins discordant for malnutrition. This study illustrates the utility of Bug-FACS in identifying immunologically relevant microbiota in human disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palm NW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. This study, along with ref. 22, describes the method of IgA-seq to sort and sequence IgA-coated microbiota. Culture libraries created from IgA-sorted microbiota were used to evaluate the effects of these microbiota in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planer JD, et al. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534:263–266. doi: 10.1038/nature17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moor K, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498–502. doi: 10.1038/nature22058. [DOI] [PubMed] [Google Scholar]
- 30.Geva-Zatorsky N, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168:928–943.e911. doi: 10.1016/j.cell.2017.01.022. In this study, a systematic approach using both immunological phenotyping and transcriptional profiling was used to define the effects of 53 human-gut commensal bacteria on a wide range of gut immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viladomiu M, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med. 2017;9:eaaf9655. doi: 10.1126/scitranslmed.aaf9655. Using IgA-seq to provide insight into microbiota that might have systemic inflammatory effects, this study analyzed samples from people with Crohn’s disease–associated spondyloarthritis and has identified the ability of adherent-invasive E. coli to induce inflammatory TH17 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan TG, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA. 2016;113:E8141–E8150. doi: 10.1073/pnas.1617460113. This study used a gnotobiotic mouse platform to screen 39 human-gut symbionts and has identified the ubiquitous symbiont Bifidobacteria adolescentis as a notable inducer of TH17 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 34.Fung TC, et al. Lymphoid-tissue-resident commensal bacteria promote members of the IL-10 cytokine family to establish mutualism. Immunity. 2016;44:634–646. doi: 10.1016/j.immuni.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunisawa J, Kiyono H. Alcaligenes is commensal bacteria habituating in the gut-associated lymphoid tissue for the regulation of intestinal IgA responses. Front Immunol. 2012;3:65. doi: 10.3389/fimmu.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obata T, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci USA. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato S, et al. Transcription factor Spi-B-dependent and-independent pathways for the development of Peyer’s patch M cells. Mucosal Immunol. 2013;6:838–846. doi: 10.1038/mi.2012.122. [DOI] [PubMed] [Google Scholar]
- 39.Satoh-Takayama N, et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776–788. doi: 10.1016/j.immuni.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 41.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2016;8:352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liguori G, et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J Crohns Colitis. 2016;10:296–305. doi: 10.1093/ecco-jcc/jjv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suhr MJ, Banjara N, Hallen-Adams HE. Sequence-based methods for detecting and evaluating the human gut mycobiome. Lett Appl Microbiol. 2016;62:209–215. doi: 10.1111/lam.12539. [DOI] [PubMed] [Google Scholar]
- 45.Hoarau G, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. MBio. 2016;7:e01250–16. doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, et al. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J Clin Gastroenterol. 2014;48:513–523. doi: 10.1097/MCG.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokol H, et al. Fungal microbiota dysbiosis in IBD. Gut. 2016;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamas B, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang C, et al. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18:183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Lewis JD, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18:489–500. doi: 10.1016/j.chom.2015.09.008. This study shows that inflammation, antibiotics and diet independently affect the gut microbiota in people with Crohn’s disease and provides evidence of an association between antibiotic use and fungal overgrowth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wheeler ML, et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. This study shows that targeted fungal-community dysbiosis has local and systemic effects on immunity and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan D, et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21:808–814. doi: 10.1038/nm.3871. This study shows that commensal bacteria can promote resistance to C. albicans colonization by increasing the H1F-1α-mediated expression of the antimicrobial peptide LL-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chudnovskiy A, et al. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell. 2016;167:444–456.e14. doi: 10.1016/j.cell.2016.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escalante NK, et al. The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility. J Exp Med. 2016;213:2841–2850. doi: 10.1084/jem.20161776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–98. doi: 10.1038/nature13960. This paper demonstrated that colonization with a single symbiotic eukaryotic virus can reverse some of the physiological and immunological defects observed in germ-free or antibiotic-exposed mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuss SK, et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kane M, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monaco CL, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19:311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norman JM, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Handley SA, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramanan D, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science. 2016;352:608–612. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborne LC, et al. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science. 2014;345:578–582. doi: 10.1126/science.1256942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reese TA, et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science. 2014;345:573–577. doi: 10.1126/science.1254517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu GD, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72. doi: 10.1136/gutjnl-2014-308209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desai MS, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaiko GE, et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165:1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelly CJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kibe R, et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4:4548. doi: 10.1038/srep04548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levy M, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zelante T, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Schiering C, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu W, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumas ME, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levin BJ, et al. A prominent glycyl radical enzyme in human gut microbiomes metabolizes trans-4-hydroxy-l-proline. Science. 2017;355:eaai8386. doi: 10.1126/science.aai8386. This study describes a novel chemically guided functional profiling–coupled protein sequence-similarity network with quantitative metagenomics analysis, which enabled the discovery and functional characterization of the GRE superfamily in the microbiome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donia MS, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158:1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo CJ, et al. Discovery of reactive microbiota-derived metabolites that inhibit host proteases. Cell. 2017;168:517–526.e18. doi: 10.1016/j.cell.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manoury B. Proteases: essential actors in processing antigens and intracellular toll-like receptors. Front Immunol. 2013;4:299. doi: 10.3389/fimmu.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim YG, et al. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe. 2014;15:95–102. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vatanen T, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. Bacteroides species in the microbiota of children from Finland and Estonia with high susceptibility to autoimmunity produce a type of LPS that inhibits innate immune signaling and endotoxin tolerance. These properties may interfere with early immunological education and contribute to the development of type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bach JF, Chatenoud L. The hygiene hypothesis: an explanation for the increased frequency of insulin-dependent diabetes. Cold Spring Harb Perspect Med. 2012;2:a007799. doi: 10.1101/cshperspect.a007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 85.Calderon-Gomez E, et al. Commensal-specific CD4+ cells from patients with Crohn′s disease have a T-helper 17 inflammatory profile. Gastroenterology. 2016;151:489–500.e3. doi: 10.1053/j.gastro.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 86.Campisi L, et al. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat Immunol. 2016;17:1084–1092. doi: 10.1038/ni.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hand TW, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blander JM, Torchinsky MB, Campisi L. Revisiting the old link between infection and autoimmune disease with commensals and T helper 17 cells. Immunol Res. 2012;54:50–68. doi: 10.1007/s12026-012-8311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu HJ, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108(Suppl. 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asquith MJ, et al. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis Rheumatol. 2016;68:2151–2162. doi: 10.1002/art.39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciccia F, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76:1123–1132. doi: 10.1136/annrheumdis-2016-210000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teng F, et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity. 2016;44:875–888. doi: 10.1016/j.immuni.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 96.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gabanyi I, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rieder R, Wisniewski PJ, Alderman BL. & Campbell, S.C. Microbes and mental health: a review. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.01.016. http://dx.doi.org/10.1016/j.bbi.2017.01.016. [DOI] [PubMed]
- 99.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. Indigenous spore-forming microbes from the gut microbiota produce metabolites that promote host serotonin biosynthesis in the gastrointestinal tract and affect gastrointestinal motility and hemostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reigstad CS, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 102.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matcovitch-Natan O, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 104.Sampson TR, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. SCFAs from gut microbes modulate microglia, are required for neuroinflammatory responses. They are also required for the hallmark a-synuclein-dependent motor and gastrointestinal deficits and brain pathology in a model of Parkinson’s disease. The microbiota from people with Parkinson’s disease induces motor dysfunction in this model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothhammer V, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schirmer M, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. This study investigates how differences in the microbiome contribute to variations in the human inflammatory response and demonstrates that TNF and IFNγ responses are associated with microbial palmitoleic acid and tryptophan metabolism. This study also provides a database for microbial mediators that influence human cytokine responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buffie CG, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rangan KJ, et al. A secreted bacterial peptidoglycan hydrolase enhances tolerance to enteric pathogens. Science. 2016;353:1434–1437. doi: 10.1126/science.aaf3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sansone CL, et al. Microbiota-dependent priming of antiviral intestinal immunity in Drosophila. Cell Host Microbe. 2015;18:571–581. doi: 10.1016/j.chom.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khosravi A, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ichinohe T, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clarke TB, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science. 2016;352:535–538. doi: 10.1126/science.aad9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17:83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- 115.Schieber AM, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science. 2015;350:558–563. doi: 10.1126/science.aac6468. This study elegantly demonstrates that a strain of E. coli naturally colonizing the intestine in mice is sufficient to prevent wasting after infections, owing to the sustained inflammasome-dependent activation of the IGF1-PI3K-AKT pathway in skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reichardt N, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fonseca DM, et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim D, et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22:524–530. doi: 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruane D, et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J Exp Med. 2016;213:53–73. doi: 10.1084/jem.20150567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh JZ, et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakaya HI, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity. 2017;46:350–363. doi: 10.1016/j.immuni.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35:526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 125.Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huber S, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: apopulation-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 128.Arthur JC, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kostic AD, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu S, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pitt JM, et al. Fine-tuning cancer immunotherapy: optimizing the gut microbiome. Cancer Res. 2016;76:4602–4607. doi: 10.1158/0008-5472.CAN-16-0448. [DOI] [PubMed] [Google Scholar]
- 132.Vétizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sivan A, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. Refs. 132 and 133 show that the intestinal microbiota affects the outcome of checkpoint-blockade-based cancer immunotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]


