Abstract
Mechanistic target of rapamycin (mTOR, also known as mammalian target of rapamycin) is a ubiquitous serine/threonine kinase that regulates cell growth, proliferation and survival. These effects are cell-type-specific, and are elicited in response to stimulation by growth factors, hormones and cytokines, as well as to internal and external metabolic cues. Rapamycin was initially developed as an inhibitor of T-cell proliferation and allograft rejection in the organ transplant setting. Subsequently, its molecular target (mTOR) was identified as a component of two interacting complexes, mTORC1 and mTORC2, that regulate T-cell lineage specification and macrophage differentiation. mTORC1 drives the proinflammatory expansion of T helper (TH) type 1, TH17, and CD4−CD8− (double-negative, DN) T cells. Both mTORC1 and mTORC2 inhibit the development of CD4+CD25+FoxP3+ T regulatory (TREG) cells and, indirectly, mTORC2 favours the expansion of Tfollicular helper (TFH) cells which, similarly to DN T cells, promote B-cell activation and autoantibody production. In contrast to this proinflammatory effect of mTORC2, mTORC1 favours, to some extent, an anti-inflammatory macrophage polarization that is protective against infections and tissue inflammation. Outside the immune system, mTORC1 controls fibroblast proliferation and chondrocyte survival, with implications for tissue fibrosis and osteoarthritis, respectively. Rapamycin (which primarily inhibits mTORC1), ATP-competitive, dual mTORC1/mTORC2 inhibitors and upstream regulators of the mTOR pathway are being developed to treat autoimmune, hyperproliferative and degenerative diseases. In this regard, mTOR blockade promises to increase life expectancy through treatment and prevention of rheumatic diseases.
Mechanistic target of rapamycin (mTOR) serves as a sensor of metabolic cues and as a regulator of growth, proliferation, and survival in eukaryotic cells. mTOR was initially identified as the molecular target of an antifungal macrolide antibiotic produced by the bacterium Streptomyces hygroscopicus. This bacterium was discovered in a soil sample from Easter Island, known to its inhabitants as Rapa Nui, from which the name rapamycin was derived1. Rapamycin is a potent inhibitor of antigen-induced proliferation of T cells2 and, owing to this activity, has been developed as a medication to prevent organ transplant rejection3. Rapamycin was approved by the FDA to preserve renal allografts under the generic name sirolimus4.
The potency of sirolimus in blocking T-cell activation was first found to be beneficial in the treatment of rheumatic diseases in the context of systemic lupus erythematosus (SLE), both in animal models5 and in patients6. Rapamycin and its analogues (rapalogues) were also efficacious in animal models7 and patients with rheumatoid arthritis (RA)8, juvenile idiopathic arthritis (JIA)9 and Sjögren syndrome10. In patients with systemic sclerosis (SSc), mTOR activation contributes to type I collagen production by dermal fibroblasts11,12, and rapamycin improves skin fibrosis13 and reduces osteopenia in a mouse model of the disease14. A pilot study of rapamycin in patients with SSc showed limited efficacy, but the treatment was determined safe15. Rapamycineluting endovascular stents have also been used to treat large-vessel vasculitis, aortitis16 and Takayasu arteritis17, but the drug has limited clinical efficacy in patients with granulomatosis with polyangiitis (GPA)18, despite lowering titers of myeloperoxidase and perinuclear anti-neutrophil cytoplasmic antibodies (pANCA)19. By stimulating autophagy, rapamycin improves the survival of human articular chondrocytes and, thereby, benefits patients with osteoarthritis (OA)20.
A long list of studies supports a role for the mTOR pathway in the pathogenesis of both inflammatory and degenerative rheumatic diseases. In this Review, I critically evaluate the mechanisms of mTOR activation and the means for its pharmacological blockade, which have broad implications for the pathogenesis, diagnosis and management of rheumatic diseases.
Biology of mTOR complexes
As a serine/threonine kinase, mTOR phosphorylates four signature substrates: S6K (ribosomal S6 kinase), which controls protein translation via ribosome bio-genesis21; 4E–BP1 (eukaryotic translation initiation factor 4E-binding protein 1), which regulates mRNA translation22; signal transducer and activator of transcription (STAT)3 (on Ser727) in response to amino acid excess23; and AMBRA1 (activating molecule in BECN1 (beclin-1)-regulated autophagy protein 1), which prevents serine/threonine-protein kinase ULK1/ATG1 from binding to membranes and starting autophagosome formation24,25 (FIG. 1). mTOR-dependent growth signals are triggered by metabolic cues (FIG. 1) such as amino acid sufficiency26,27 and oxidative28,29 or nitrosative stress30. In turn, activation of mTOR executes cell-type-specific commands for growth, proliferation and survival via two interactive complexes — mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (FIG. 1).
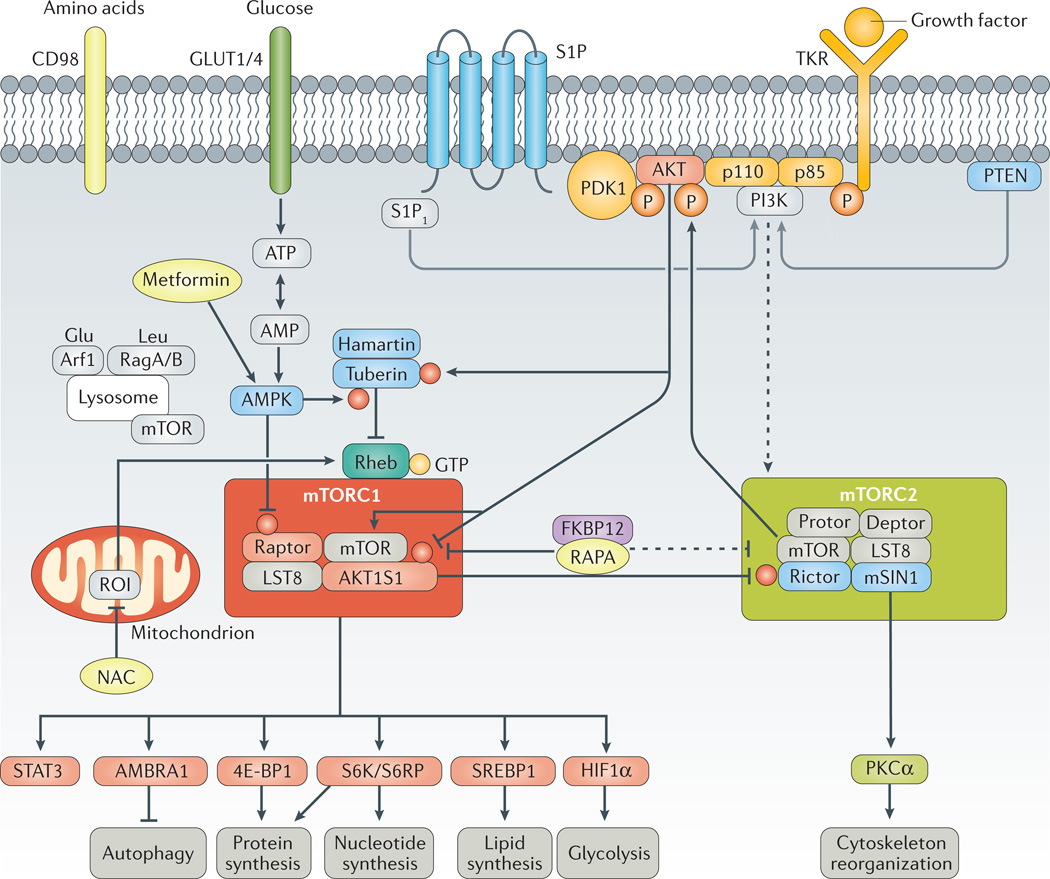
Figure 1. mTOR pathway activation.
Mechanistic target of rapamycin (mTOR) is regulated by metabolic cues, primarily glucose and amino acids, as well as by growth factors, hormones and cytokines. Glucose and amino acids enter cells via surface receptors such as glucose transporter type 1, erythrocyte/brain (GLUT1) or glucose transporter type 4, insulin-responsive (GLUT4) and CD98, respectively. Growth factors stimulate tyrosine kinase receptors (TKRs), which are activated through phosphorylation of tyrosine residues. In turn, TKR signals are transmitted to mTOR through phosphati-dylinositide 3-kinase (PI3K), phosphoinositide-dependent kinase-1 (PDK1) and RAC serine/threonine-protein kinase (AKT). Signalling through sphingosine 1-phosphate receptor 1 (S1P1) also activates mTOR complex 1 (mTORC1) via PI3K. Downstream, AKT phosphorylates mTOR, which forms two interacting complexes, mTORC1 and mTORC2. mTORC1 is composed of mTOR, regulatory-associated protein of TOR (raptor), TORC subunit LST8 (LST8), DEP-domain-containing mTOR-interacting protein (deptor) and proline-rich AKT1 substrate 1 (AKT1S1)230. mTORC2 is comprised of mTOR, rapa-mycin-insensitive companion of mTOR (rictor), stress-activated protein kinase interacting protein 1 (mSIN1, also known as TORC2 subunit MAPKAP1), protein observed with rictor-1 (protor-1), deptor and LST8. mTORC1 integrates growth signals reflecting the availability of nutrients and energy to promote either proliferation when conditions are favourable or autophagy when conditions are unfavorable; mTORC2 promotes cellular survival by activating AKT82. Pharmacologically targetable checkpoints are highlighted in yellow. 4E–BP1, eukaryotic translation initiation factor 4E–binding protein 1; AMBRA1, activating molecule in BECN1-regulated autophagy protein 1; AMPK, 5’-AMP-activated protein kinase; Arf1, ADP-ribosylation factor 1; FKBP12, peptidyl-prolyl cis-trans isomerase FKBP12; HIF1α, hypoxia-inducible factor 1α; NAC, N-acetylcysteine; PKCα, protein kinase C α type; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN; RAPA, rapamycin; Rag-A/B, Ras-related GTP-binding protein A/B; ROI, reactive oxygen intermediate; S6K, ribosomal protein S6 kinase; SREBP1, sterol regulatory element-binding protein 1; STAT3, signal transducer and activator of transcription 3.
Intracellular localization and trafficking
The presence of mTOR in several cellular compartments could be key to its ability to sense stress and execute cell-growth signals. mTORC1 was initially detected in the cytoplasm as a stimulator of protein translation by its ability to phosphorylate S6K21 and 4E-BP1 (REF. 31). Later, observations showing that mTOR was associated with endosomal compartments brought the trafficking-regulatory small Rab GTPases Rab7 and Rab4A, as well as the Rag family of Ras-related GTP-binding proteins, to the forefront of the cell-signalling field30,32. mTOR was also reported to translocate to the outer mitochondrial membrane, where it senses changes in the mitochondrial transmembrane potential, ATP depletion and oxidative stress33. Additionally, mTOR can also be detected inside the nucleus, where it might have a role in the transcription of RNA polymerase I-dependent and RNA polymerase III-dependent genes34,35. Under conditions of cell stress, mTORC1 is sequestered in an astrin (also known as sperm-associated antigen 5)-dependent manner within stress granules, limiting cell growth and promoting cell survival29.
Consensus is increasing that mTORC1 activation occurs at the surface of the lysosomal membrane in response to changes in amino acid sufficiency27, which is transduced via the Rag family of small GTPases to mediate the translocation of mTORC1 from the cytoplasm to the surface of the lysosome, where mTORC1 is activated by GTP-binding protein Rheb (FIG. 2).
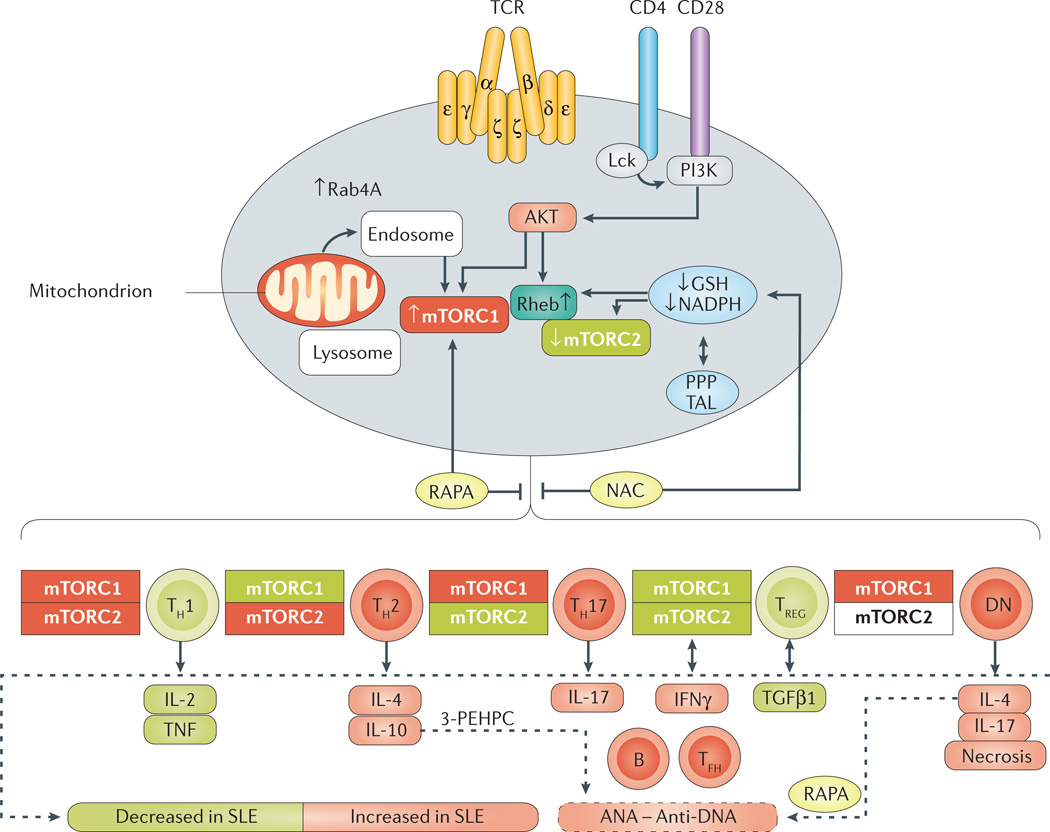
Figure 2. mTOR-mediated lineage specification in T cells.
Mechanistic target of rapamycin (mTOR) is a sensor of metabolic stress and integrator of environmental cues. Activation of mTOR complex 1 (mTORC1) is triggered by oxidative stress, amino-acid levels and endosomal traffic to the lysosome by small GTPases such as Rab4A. In turn, mTORC1 promotes inflammation by skewing T-cell development. Oxidative stress is promoted by mitochondrial electron transport and balanced by the production of reduced glutathione (GSH) and its regeneration by NADPH via the pentose phosphate pathway (PPP). Oxidative stress and activation of mTORC1 inhibit the expression of FoxP3, leading to contraction of the T regulatory (TREG) cell population, and expansion of proinflammatory T-cell lineages such as T helper (TH) 1, TH17, T follicular helper (TFH) and double-negative (DN) T cells. Pharmacological interventions by rapamycin (RAPA), N-acetylcysteine (NAC) and 3-(3-pyridyl)-2-hydroxy-2-phosphonopropanoic acid (3-PEHPC) are highlighted in yellow. AKT, RAC serine/threonine-protein kinase; ANA, antinuclear antibodies; GTP-binding protein Rheb; IFNγ, interferon γ; Lck, tyrosine-protein kinase Lck; PI3K, phosphatidylinositide 3-kinase; Rheb, SLE, systemic lupus erythematosus; TAL, transaldolase; TCR, T-cell receptor; TGFβ1, transforming growth factor β1.
The translocation of mTOR to the lysosomal membrane occurs via endosome traffic that is regulated by Rab7 (REF. 32), Rab5 (REFS 36–38) and Rab4A. Each of these GTPases also regulates autophagy37,39–42 (FIG. 2). Rab4A forms a positive feedback loop with mTORC1 and negative feedback loop with mTORC2 (REF. 43), and binds to the p85 regulatory subunit of phosphatidylinositide 3-kinase (PI3K), altering its ability to control endocytic recycling44–47. Notably, Rab4A and Rab5 are overexpressed in T cells of patients with SLE30 and in mouse models of this disease41; thus, these small GTPases are possible regulators of abnormal T-cell signal transduction in SLE. Furthermore, overexpression of Rab4A, but not Rab5, also precedes the production of antinuclear antibodies (ANA) and SLE onset41. Importantly, pharmacological inhibition of Rab GTPases by 3-(3-pyridyl)-2-hydroxy-2-phosphono propanoic acid (3-PEHPC) prevents T-cell dysfunction, ANA production, and nephritis in lupus-prone mice41 (FIG. 2).
The cell-type-specific activation of mTORC1, and its localization to the lysosome during autophagy, are subject to intricate control by metabolic regulatory net-works48. Moreover, the traffic of endosomes to lysosomes has long been implicated in interferon production and inflammation, because these structures also carry Toll-like receptors, which are able to sense nucleic acids, particularly in dendritic cells (DCs)49.
Metabolic regulatory networks
Signalling pathways that control the proliferation, survival and differentiation of cells in the immune system also regulate the metabolic processes that provide the nutrients required to support these specialized lymphocyte functions50. Although mTOR drives proinflammatory lineage specification in the T-cell compartment51, it might also have anti-inflammatory effects driven by shifting macrophage polarization from a proinflammatory M1 phenotype to an anti-inflammatory M2 phenotype (REFS 52,53). Nevertheless, M2 macrophages can also contribute to inflammation, for example by serving as hosts to cytomegalovirus (CMV), which might explain the potent anti-CMV effects of mTOR inhibitors after organ transplantation54.
Outside the immune system, mTOR activation contributes to type I collagen production by dermal fibroblasts and to fibrosis in patients with SSc11,12. In chondrocytes, genetic inactivation of mTOR prevented the development of OA induced by peroxisome proliferator-activated receptor-γ (PPARγ) deficiency55. Furthermore, mTOR blockade with N-acetylcysteine (NAC), an amino acid precursor of glutathione, reduced cognitive attention deficit hyperactivity disorder symptoms in patients with SLE56. Given all the evidence for a pathogenetic role for mTOR in rheumatic diseases and associated comorbidities, the metabolic pathways sensed and regulated by this signalling molecule are important to understand.
Amino acids
The branched chain amino acids leucine and isoleucine are stimulators of mTOR57, and glutamine, a particularly important amino acid in cell-growth control, can activate mTORC1 (REF. 58). Unlike leucine, which relies on RagA and RagB, glutamine utilizes a different GTPase, ADP-ribosylation factor 1, to activate mTORC1 on the lysosome59 (FIG. 1). A comprehensive metabolome analysis identified a redox-dependent accumulation of kynurenine, an aminoacid metabolite of tryptophan, in lymphocytes from patients with SLE60; kynurenine also triggers mTORC1 activation in T cells60.
Pentose-phosphate pathway
Upon activation, mTORC1 promotes the transcription of genes involved in glycolysis, in the pentose-phosphate pathway (PPP) and in de novo lipogenesis61. Upregulation of glycolysis is mediated via the transcription factor hypoxia-inducible factor 1α (HIF1α)62,63 (FIG. 1). As shown in a 2013 metabolomic study, most of the mTORC1-regulated metabolites are part of the PPP64. Notably, mTORC1-dependent activation of the PPP was found to be dependent on oestrogen65, which promotes surface expression of GLUT1 (glucose transporter type 1, also known as solute carrier family 2, facilitated glucose transporter member 1) and GLUT4 (solute carrier family 2, facilitated glucose transporter member 4) — two proteins that are required for glucose uptake to fuel the PPP65. This finding could be associated with the increased prevalence of SLE in women, who display increases in both expression and activity of the PPP enzyme transaldolase, and increased activation of mTORC1 (REF. 30).
cAMP
The second messenger cAMP regulates a diverse array of biological processes, mostly via its downstream effector, protein kinase A (PKA)66. Ample evidence supports the existence of crosstalk between the PKA and mTOR pathways: for example, cAMP can stimulate mTORC1 (REFS 67,68) or inhibit both mTORC1 and mTORC2 in a cell-type-dependent manner60,69. Importantly, blockade of mTORC1 activation in T cells reduces cAMP levels in peripheral blood lymphocytes (PBL) from patients with SLE after treatment with NAC in vivo70.
Fatty acid synthesis
De novo fatty-acid synthesis is essential for the proliferation and differentiation of T helper (TH) type 17 cells, whereas fatty-acid catabolism via β-oxidation is important for the development of CD8+ memory T cells71 and CD4+ T regulatory (TREG) cells72. In addition to serving as a source of energy, lipids contribute to cellular structures and signalling. Sphingolipids, particularly sphingosine-1-phosphate (S1P), are emerging as vital lipid mediators73 (FIG. 1). S1P signals through five known G-protein–coupled receptors, S1P receptors 1–5 (S1P1 to S1P5)74. S1P1, the main S1P receptor that facilitates the egress of T cells from lymphoid organs75, exerts a negative control of the thymic generation and suppressive activity of natural TREG cells, a process which is dependent on the Akt–mTOR axis76. Transgenic overexpression of S1P1 in T cells inhibits the differentiation of TREG cells in favour of the development of TH1 cells77 (FIG. 1).
Oxidative stress
Oxidative stress activates the mTOR pathway in most cells28–30,33 by a process that involves cysteine oxidation of Rheb78 and raptor (regulatory-associated protein of mTOR)28,79. With escalation of oxidative stress, astrin recruits the mTORC1 component raptor to stress granules, thereby preventing mTORC1-hyperactivation in HeLa cells78. Whether astrin is expressed and capable of similarly controlling mTORC1 activation in primary cells is currently unknown, but such a mechanism could be important in the survival of CD4−CD8− (double-negative, DN) T cells in SLE, and possibly in other proinflammatory cells, such as fibroblasts or osteo clasts, which are exposed to oxidative stress in patients with rheumatic diseases.
Stimulatory and inhibitory signal transducers
The mTOR pathway is largely controlled by upstream checkpoints at three levels: receptor tyrosine kinases and G-protein-coupled receptors, which detect growth factors; the PI3K–PDK1 (phosphoinositide-dependent kinase-1)–AKT (RAC-α serine/threonine-protein kinase) axis, which channels stimulatory signals towards mTORC1 activation; and the key negative regulators PTEN, AMPK (5′-AMP-activated protein kinase catalytic subunit α2), TSC1 and TSC2 (the latter two are also known as hamartin and tuberin, respectively).
The PI3K–AKT–mTOR axis
At the level of the organism, the PI3K–AKT–mTOR signalling network enables the development of cell-type-specific responses that integrate changes in intracellular metabolism and exposure to a variety of growth factors, as well as transmitting signals from intercellular receptor–ligand interactions.
The upstream enzyme of this signalling network is class I PI3K. PI3K is activated by receptor tyro sine kinases and autophosphorylation in response to extracellular signals such as ligand binding (FIG. 1). Upon activation, PI3K generates phosphatidylinositol-3,4,5-trisphosphate (PIP3)80, which recruits pleckstrin homology domain-containing signalling proteins such as AKT to the plasma membrane81. PDK1, which also contains a pleckstrin homology domain and can bind PIP3, is then recruited to the plasma membrane upon activation of PI3K and leads to the phosphorylation of AKT and its partial activation. Full activation of AKT occurs upon phosphorylation of Ser473 by mTORC2 and other kinases82 (FIG. 1).
Class II PI3K interacts with Rab5 and thereby controls endosomal activation of AKT83. The class III PI3K vacuolar protein sorting-associated protein 34 (VPS34, also known as PI3K catalytic subunit type 3), is tethered to endosomal membranes and directs the trafficking of proteins and vesicles, thus contributing to phagocytosis and autophagy81. The activity of VPS34 during autophagy is regulated by AMPK in response to nutrient stress responses84.
PTEN
PTEN dephosphorylates membrane phosphatidylinositols and, thereby, can operate as an inhibitor of the AKT–mTORC1 axis (FIG. 1). PTEN directly antagonizes PI3K by dephosphorylating PIP3 and has important roles in chromosome stability, DNA repair, and cell-cycle arrest in the nucleus85. Mutations of PTEN cause Cowden syndrome, which has been associated with inflammatory arthritis86 and SLE87.
The role of PTEN in pathogenesis of rheumatic diseases is also supported by mechanistic studies. PTEN is essential for the maintenance of functional capacity of TREG cells, which restrain the expansion of proinflammatory TH1, TH17, and T follicular helper (TFH) cells in autoimmune diseases88–90. In B cells from patients with SLE, increased levels of microRNA (miR)-7, miR-21 and miR-22 inhibit PTEN expression91. Loss of PTEN also increases the osteoclastogenic potential of myeloid cells, leading to enhanced local inflammation and bone destruction92.
LKB1–AMPK axis
The axis formed by liver kinase B1 (LKB1) and 5´-AMP-activated protein kinase (AMPK) is an important regulator of the mTORC1-dependent translation process. AMPK activation by LKB1 inhibits the mTORC1 pathway via phosphorylation of TSC2 (REF. 93) and Raptor94. Under energy starvation conditions, an increase in AMP levels stimulates the activity of AMPK95 and inhibits mTORC1 signalling93 (FIG. 1). In activated T cells, AMPK serves as a glucose-sensitive metabolic checkpoint that regulates mRNA translation and glutamine-dependent mitochondrial metabolism: T cells lacking AMPKα1 display reduced mitochondrial bioenergetics and cellular ATP levels in response to glucose deprivation in vitro or pathogenic challenge in vivo93. AMPKα1 is also essential for TH1 and TH17 cell development and for primary T-cell responses to viral and bacterial infections in vivo; AMPKα1-deficient macrophages and DCs exhibit heightened inflammatory function, an enhanced capacity for antigen presentation and favour TH1 and TH17 responses96. Further studies are necessary to examine whether the effects of AMPK on T-cell and B-cell development are independent from its inhibitory effect on the mTORC1 pathway.
AMPK also has a key role in activating proautophagic VPS34 complexes84 by phosphorylating beclin-1. This initial step of autophagy can be blocked by mTOR through phosphorylation of AMBRA1; conversely, mTORC1 inhibition leads to phosphorylation of beclin-1 and propagates autophagosome formation97. PBLs from patients with SLE exhibit increased mTORC1 activity30 and profoundly diminished beclin-1 expression, a state that is reversed by rapamycin treatment in vivo41. Remarkably, mechanical stress, which is the primary inducer of OA, reduces beclin-1 expression and autophagy in chondrocytes, and treatment with rapamycin reverses these changes98. Thus, mTORC1-dependent beclin-1 expression is a cell-type-specific regulator of autophagy in rheumatic diseases.
Cell-type-specific activation of mTOR
The mTOR pathway has critical roles in the development and function of the innate and adaptive arms of the immune system. An essential role of mTOR in T-cell development was uncovered51 when mTORC1 was found to be required for the differentiation of TH1 and TH17 cells, whereas TH2-cell development depends on mTORC2 (REF. 51). mTORC1 also inhibits the survival of CD8+ memory T cells, an effect that can be reversed by rapamycin treatment99,100. Both mTORC1 and mTORC2 seem to interfere with the differentiation and function of CD4+CD25+FoxP3+ TREG cells51,77,101, but mTORC1 might also support TREG-cell function102 by inhibiting the mTORC2 pathway103.
Despite its complex role in TREG-cell function, the largely proinflammatory changes elicited by mTORC1 activation in the adaptive immune system are in apparent contrast with its anti-inflammatory effects on the innate immune system (for example, mTORC1 favours M2 over M1 polarization of macrophages52). The cell-type-specific activation of mTORC1 also varies between rheumatic diseases, as mTORC1 is activated in T cells of patients with SLE30, but not in those of patients with RA104. These divergent effects of mTOR activation in T cells might be connected to alternative use of glucose between glycolysis and the PPP105.
mTOR — biomarker and pathogenetic factor
mTORC1 activation in SLE
In patients with SLE, mTORC1 is activated30,70 and mTORC2 is inhibited106 (FIG. 2). A central role for mTORC1 in abnormal T-cell lineage specification and function in SLE30,107,108 is consistent with its effects on shaping T-cell development51.
The involvement of mTOR in SLE was initially suggested by the successful blockade of T-cell hyperactivity and nephritis in rapamycin-treated lupus-prone MRL/lpr mice5. Later, rapamycin was shown to block T-cell activation in patients with SLE30, with remarkable therapeutic efficacy6,109. Interestingly, activation of mTORC1 preceded disease flares by 4 months, and was reduced by therapeutic intervention with rapamycin109. Thus, measurement of mTORC1 and mTORC2 activity by intracellular staining for phosphorylated 40S ribosomal protein S6 (at Ser235 and Ser236) and phosphorylated AKT (at Ser473), respectively, can be used as biomarkers for pathogenesis, prediction of flares and monitoring of treatment efficacy of mTOR blockade in SLE70,106,109. Measuring the activity of mTORC1 and mTORC2 in cells of patients with SLE and other autoimmune diseases could be another step in achieving personalized or precision medicine110.
Rapamycin blocks the proinflammatory, necrotic death of DN T cells and the depletion of TREG cells109, and mTORC1 inhibition by NAC had similar effects111 (FIG 1,FIG 2; TABLE 1). Diminished TREG-cell frequency has been linked to mTORC1-sensitive methylation of the Foxp3 promoter112, whereas rapamycin treatment reduced the production of IL-4 by DN T cells in vivo109, accounting for an increased production of anti-DNA autoantibodies by B cells113,114. The benefits of rapamycin can also be attributed to TREG-cell expansion109 via activation of mTORC2, at least in patients with SLE106. Given the role of mTORC2 signalling in TREG cells and in regulating the development of TFH cells89,90,115, which are expanded in mouse models of lupus116 and in patients with active SLE117, the impact of rapamycin on the proinflammatory DN T-cell subset merits further investigation. The documentation of the first clinical case of fulminant SLE in a patient with tuberous sclerosis also support a fundamental role of mTOR pathway activation in the pathogenesis of both these diseases118: in accordance with a negative regulatory role for the TSC1–TSC2 complex, all lymphocyte subsets of this patient exhibited robust mTORC1 activation118. mTORC1 is also activated in B cells119 and nephritic kidneys of lupus-prone mice120 (FIG. 3).
Table 1.
Pharmacological blockade of mTOR pathway activation in rheumatic diseases
| Drug | Mechanism of action | Molecular target | Disease |
|---|---|---|---|
| Rapamycin | FKBP12/allosteric | mTORC1 | SLE6,109, lupus nephritis5,120,176,231,232, IgA nephropathy233,234, interstitial nephritis235, SSc13,15 RA and JIA8,9, Sjögren syndrome10, osteoarthritis55,159 |
| Everolimus | FKBP12/allosteric | mTORC1 | Pulmonary hypertension236 |
| OSI-027 | ATP-competitive | mTORC1/mTORC2 | SSc, RA237,238 |
| NAC | Antioxidant | GSH | SLE56,70, RA and CIA229,239, Sjögren syndrome240,241 ILD202,242 |
| Metformin | Antioxidant | ETC complex I | SLE128, CIA98,243 |
| Fingolimod | Receptor modulator | S1P receptor | SLE214,215 |
| KN-93 | Kinase inhibitor | CamK-II/CamK-IV | SLE216–218 |
| Rimacalib | Kinase inhibitor | CamK-II/CamK-IV | RA219,220 |
CaMK-II/IV, calcium/calmodulin-dependent protein kinase type II/IV; CIA, collagen-induced arthritis; ETC, electron transport chain; GSH, reduced glutathione; ILD, interstitial lung disease; JIA, juvenile idiopathic arthritis; mTOR, mechanistic target of rapamycin; mTORC1/2, mechanistic target of rapamycin complex 1/2; NAC, N-acetylcysteine; SSc, systemic sclerosis; S1P, sphingosine-1-phosphate; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis.
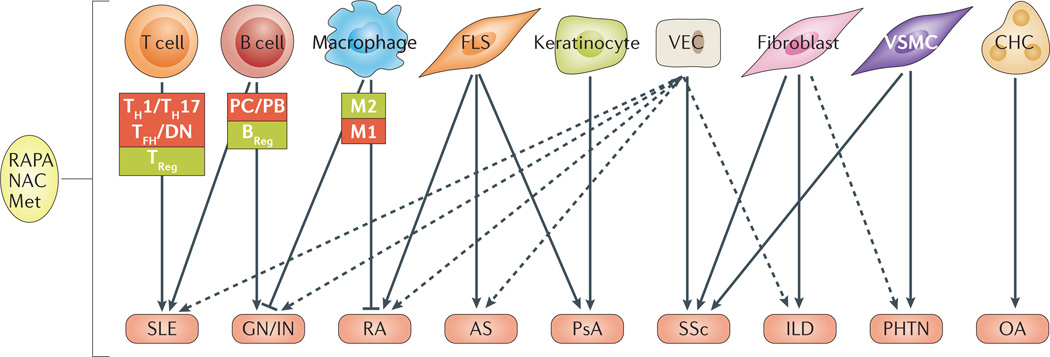
Figure 3. Cell type-specific mTOR pathway activation in rheumatic diseases.
The mechanistic target of rapamycin (mTOR) pathway is functional in several types of immune cells, including T cells, B cells and macrophages, but is also present in structural cells such as fibroblasts and keratinocytes. Activation of mTOR in these cells is associated with several rheumatic diseases. AS, ankylosing spondylitis; BREG, B regulatory cell; DN, CD4/CD8-double-negative cell; CHC, chondrocyte; FLS, fibroblast-like synoviocyte; GN/IN, glomerulonephritis/interstitial nephritis; ILD, interstitial lung disease; Met, metformin; NAC, N-acetylcysteine; OA, osteoarthritis; PB, plasmablast; PC, plasma cell; PHTN, pulmonary hypertension; PsA, psoriatic arthritis; SSc, systemic sclerosis; RA, rheumatoid arthritis, RAPA, rapamycin; SLE, systemic lupus erythematosus; TFH, T folicular helper cell; TH1, T helper cell type 1; TH17, T helper cell type 17; TREG, T regulatory cell; VEC, vascular endothelial cell; VSMC, vascular smooth-muscle cell.
Antiphospholipid antibodies (aPL) trigger considerable pathology and are one of the diagnostic criteria for SLE121–123. Moreover, aPL can elicit antiphospholipid syndrome (APS) in patients with or without SLE124. As shown in a 2014 study, seven of ten patients with APS nephropathy treated with rapamycin (70%) had a functioning allograft 144 months after transplantation, versus only three of 27 patients who were not treated with rapamycin (11%)125. Notably, the majority of the patients with APS had also been diagnosed with SLE (20 of 32 patients, 62%)125. Unfortunately, the researchers did not document how many of the seven patients who benefited from rapamcyin125 also had SLE. mTORC1 activity is not elevated in major PBL subsets of patients with SLE who also have APS, relative to those without APS126. Therefore, rapamycin might have benefited renal transplant recipients with underlying SLE125.
The mTORC1 inhibitor NAC reversed the depletion of reduced glutathione (GSH) in PBLs of patients with SLE in a double-blind, placebo-controlled, phase I–II clinical trial70. Of relevance to its mechanism of action, NAC also reversed the strong activation of mTORC1 in DN T cells, which is consistent with a role of oxidative stress as a regulatory checkpoint. Oxidative stress originates from increased mitochondrial oxygen consumption by complex I of the electron transport chain in T cells from patients with SLE127. Along this line, inhibition of mitochondrial oxidative stress with metformin also reduced mTORC1 activity and prevented nephritis in lupus-prone mice128. Therefore, these findings support a role for oxidative stress in both activation of mTORC1 and the pathogenesis of lupus nephritis in mice28,29.
In addition to oxidative stress, traffic to the lysosomal membrane and the detection of amino-acid sufficiency have been implicated in the activation of mTORC1 (REF. 27). A 2015 metabolome analysis unveiled a NAC-responsive accumulation of kynurenine in SLE PBLs60 as the strongest metabolic predictor of SLE when comparing patients with matched healthy individuals60. Kynurenine was also the strongest predictor of the NAC effect on the mTOR pathway in patients with SLE. In addition to lowering kynurenine levels, NAC greatly augmented the levels of NADPH, an effect thought to occur via sparing of NADPH through enhancement of de novo GSH synthesis129,130 (FIG. 2). Importantly, kynurenine also triggered mTORC1 activation, particularly in DN T lymphocytes60. Therefore, NAC-responsive accumulation of kynurenine is a biomarker of oxidative stress and a trigger of mTORC1 activation — two mechanistically connected metabolic checkpoints in SLE pathogenesis60. This comprehensive metabolomic study also revealed a depletion of cysteine in SLE PBLs60, further supporting the use of NAC treatment in SLE.
Rheumatoid arthritis
The role of mTOR is far less thoroughly characterized in RA than it is in SLE131. Unlike CD4 T cells in SLE, CD4 T cells from patients with RA show no significant changes in mTOR activity104,132. Of note, RA T cells exhibit diminished glycolytic activity, thus depriving them of the energy required to generate oxidative stress or to execute autophagy104. Despite the lack of changes in mTOR activity in RA T cells, rapamycin and rapalogues have shown efficacy in reducing joint inflammation in animal models of arthritis7 and in patients with RA8 or JIA9. Such clinical benefits might accrue from the inhibition of mTORC1 activation in fibroblast-like synoviocytes (FLS)133 (FIG. 3). In support of this notion, rapamycin decreased the invasive properties of RA FLS133. Additional evidence of the benefits of mTORC1 inhibition in RA includes the ability of IL-17 to induce mTORC1-dependent proliferation of RA FLS134, the increase in mTORC1 activity in osteoclasts from patients with RA and in arthritic transgenic mice7, and the downregulation of extracellular matrix digestive enzymes and induction of apoptosis in osteoclasts elicited by mTOR inhibition7 (FIG. 3). Taken together, these observations suggest a therapeutic benefit from mTOR blockade in RA that might involve the intra-articular cells that mediate erosive joint destruction.
Ankylosing spondylitis
Ankylosing spondylitis (AS) is a chronic inflammatory disease that predominantly affects the axial skeleton, with variable involvement of the peripheral joints and nonarticular structures, such as the heart valves and the eyes135. HLA-B27 has been recognized as the most important genetic risk factor for AS, but genome-wide association studies (GWAS) have now identified two AS-associated chromosomal loci independent of HLA-B27 that encode four endoplasmic reticulum aminopeptidases (ERAPs), which are involved in peptide processing before MHC class I presentation136–138. Although MHC class I peptides are primarily presented to CD8 T cells, IL-12+ and IL-23+ CD4 T cells (which can be converted to TH17 cells), have been also implicated as responders to antigen-presenting macrophages and DCs in the inflamed spine of patients with AS139.
The upregulation of let-7i miRNA, which contributes to IFNγ production, might also account for T-cell hyper-reactivity in patients with AS140, as insulin-like growth factor-1 receptor (IGF-1R) is a direct target of let-7i in T cells from these patients141. IGF-1R mediates its effect by activation of the PI3K–AKT–mTOR path-way140–143. Interestingly, let-7i overexpression increases autophagy, but inhibits apoptosis; thus, let-7i contributes to T-cell dysfunction in patients with AS141. Mo r e o v e r, unbiased proteomics studies found evidence for activation of PI3K, fatty-acid oxidation and insulin signalling in fibroblast-like ligament cells of patients with AS144. Given all these findings, mTOR-controlled metabolic pathways are likely to shape the repertoire of both adaptive and innate inflammatory cells in AS (FIG. 3).
Psoriatic arthritis
TH17 cells have pivotal roles in orchestrating the inflammation in the skin and joints of patients with psoriatic arthritis (PsA)145. Regarding the prominent expansion of TH17 cells, a paucity of studies have examined its association with mTOR activation in PsA or psoriasis. Importantly, GWAS in patients with psoriasis (20–30% of whom also have PsA) uncovered a link with a single-nucleotide polymorphism in Raptor, a component of mTORC1 (REF. 146). Whereas the functional consequences of this genetic association have not been evaluated, the involvement of mTORC1 in PsA is independently supported by mechanistic studies. As is also observed in patients with RA, the proliferation of FLSs (which can be triggered by IL-22) is mediated by the PI3K–Akt–mTOR cascade in patients with PsA147 (FIG. 3). Unlike RA T cells, however, PsA T cells exhibit increased signalling through the AKT– mTOR axis148; this observation may result from increased expression of programmed death-1 (PD-1) in T cells from patients with PsA148.
The mTOR pathway has also been implicated in keratinocyte hyperproliferation (FIG. 3), as rapamycin was found to arrest human keratinocyte stem cells in the G1 phase149. Evidence of mTORC1 activation is also provided by the increased phosphorylation of mTOR and S6K in punch biopsy samples of psoriatic skin lesions150,151.
Systemic sclerosis
Activation of the mTOR pathway has a central role in the proliferation of fibroblasts (FIG. 3), the cells that mediate skin lesions in the limited cutaneous form of SSc and in other involved organs in progressive SSc. Signalling through the PI3K–AKT–mTOR axis can be initiated by transforming growth factor β (TGFβ) and its proximal effectors, Smad2 and Smad3 (REFS 152,153). Supporting a critical role for mTOR in these diseases, rapamycin abrogates TGFβ-induced fibroblast growth154. As mentioned previously, mTORC1 activation also contributes to type I collagen production by fibroblasts, the process that leads to fibrosis in SSc skin lesions11,12 and is inhibited by rapamycin13. In a pilot study of patients with progressive SSc, rapamycin was safe, although it had limited efficacy15. TGFβ also activates mTORC2 in skin fibroblasts, as evidenced by increased phosphorylation of AKT at Ser473154, and mTORC2 activity also contributes to kidney fibrosis155.
Osteoarthritis
The pathogenesis of OA originates from excessive mechanical stress and a systemic susceptibility156. The disease process is characterized by progressive cartilage loss, subchondral bone remodelling, osteophyte formation and synovial inflammation. Notably, cartilage loss might be therapeutically targeted via mTOR blockade. Mechanical stress activates the mTOR pathway in chondrocytes during chondrogenesis and cartilage development157 (FIG. 3). In turn, treatment with rapamycin blocks the mechanical-stress-induced proliferation of chondrocytes in vitro157. Thus, blockade of mTORC1 in chondrocytes might not itself be beneficial, but human articular chondrocytes also employ the mTOR pathway to control autophagy, which initiates the release of articular cartilage vesicles (ACVs) into the cartilage matrix20. Interestingly, rapamycin promotes autophagy and consequently increases the release of ACVs by normal chondrocytes, whereas OA chondrocytes fail to increase ACV release in response to mTOR inhibition. These findings indicate that the autophagy defects in OA are downstream of mTOR activation20. Nevertheless, intervening at a critical step in the regulation of autophagy could potentially slow the adverse effects of ageing on cartilage homeostasis, which increase the risk of developing OA158. Indeed, cartilage-specific inactivation of mTOR enhances autophagy and protects against meniscus-ablation-induced OA; treated animals show a substantial reduction in articular cartilage degradation, apoptosis and synovial fibrosis159. This substantial effect of rapamycin treatment on OA prevention is remarkable, given that it also extends lifespan in mice160. Moreover, the inhibition of OA by rapamycin might also improve the quality of life of patients with this disease.
mTOR as a therapeutic target in rheumatic disease
As mentioned previously, sirolimus was initially approved by the FDA for preservation of renal allograft function owing to its capacity to inhibit T-cell proliferation4. As its molecular target, mTOR161, is involved in many cellular processes (including protein translation, growth, proliferation, survival and autophagy) that function abnormally in autoimmune, inflammatory and degenerative diseases (as well as in cancer) in a cell-type-specific manner, >1,800 clinical trials have been registered162 to test this drug in diverse disease conditions; this number does not include studies of sirolimus analogues (for example, everolimus), dual mTORC1 and mTORC1 inhibitors, PI3K or AKT inhibitors163–167.
Rapamycin, rapalogues and mTORC1/2 inhibitors
Rapamycin is an allosteric inhibitor of mTOR that acts by forming a high-affinity complex with the 12 kD intracellular protein FKBP12 (peptidylprolyl cis-trans isomerase FKBP1B)168 (FIG. 1). The resulting complex of rapamycin and FKBP12 blocks mTOR activation in yeast169 and mammalian cells161. mTOR blockade by rapamycin seems to primarily affect mTORC1 (REFS 170,171), and might cause a secondary activation of mTORC2 (REF. 82).
The duration of treatment with rapamycin is critical for mTORC1 selectivity. Whereas 2 weeks of treatment in mice blocks only mTORC1, leading to detrimental metabolic effects, 6 weeks of treatment leads to a state of metabolic transition, and 20 weeks of treatment improves metabolic profiles and insulin sensitivity by also reducing activity of mTORC2 (REF. 172). In T cells from healthy individuals and patients with SLE, rapamycin treatment in vitro reduced mTORC1 and enhanced mTORC2 activity106. Although the activity of mTORC2 is reduced in DN T cells from these patients, as evidenced by diminished phosphorylation of AKT at Ser473106, whether long-term treatment with rapamycin also activates mTORC2 in vivo is currently unknown. Therefore, whether the therapeutic benefit of rapamycin in patients with SLE originates solely from blockade of mTORC1, or whether it also involves activation of mTORC2, is also unclear. Given the role of mTORC2 in control of TREG-cell survival and function88–90, studies should be pursued in patients with SLE and other autoimmune diseases to dissect the exact mechanism of action of rapamycin. In the event that mTORC2 is activated by rapamycin in vivo, ATP-competitive, dual mTORC1 and mTORC2 inhibitors (such as torin 1 or sapanisertib) could be even more effective173. Importantly, the ATP-competitive dual inhibitor torkinib reduces the expression of cholesterol biosynthesis genes in a 4E–BP1-dependent manner174. Dual inhibitors are currently in clinical trials in cancer patients.
Rapamycin has shown great potency in blocking the development of SLE in the MRL/lpr5 and NZB/W F1 animal models41,175, in which autoimmunity, T-cell hyper-reactivity, titres of ANA and anti-DNA antibodies, glomerular immunoglobulin deposition, proteinuria, nephritis, and overall survival were all improved. Importantly, rapamycin also attenuates the severity of established nephritis176. In patients with SLE, rapamycin improved clinical outcomes as measured by the reduction of overall SLE Disease Activity Index (SLEDAI) and BILAG (British Isles Lupus Assessment Group) scores6,109. More over, a retrospective study showed diminished protein uria and improved renal function in six patients with lupus nephritis treated with rapamycin177. A prospective open-label clinical trial of rapamycin in 40 patients with SLE is due for completion in 2015, and should provide additional information about the organ systems that respond to mTOR blockade178.
Beyond its effects on T cells in SLE, rapamycin blocks fibroblast proliferation, collagen production and dermal fibrosis in the skin11,12 and holds promise for the treatment of patients with SSc15 (TABLE 1). Interestingly, mTORC2 mediates kidney fibrosis155, which could explain the limit ed efficacy of mTORC1 blockade by rapamycin in this setting, and suggests that dual ATP-competitive mTOR inhibitors might show improved efficacy (TABLE 1). As noted previously in this Review, rapamycin treatment enhances the survival of human OA chondrocytes in vitro, whereas genetic inactivation of mTOR prevents OA in mice55,159. These studies suggest that mTOR blockade and stimulation of autophagy in chondrocytes could have therapeutic benefits in OA. Conversely, rapamycin showed limited efficacy in a controlled study of patients with severe psoriasis179. The newer agent everolimus provided clinical benefit for patients with RA when used in combination with methotrexate, and had an acceptable safety and tolerability profile8. Thus, everolimus might offer a new treatment option in patients with RA who have an inadequate response to methotrexate8.
PI3K inhibitors
The PI3K–AKT–mTOR pathway is activated in CD4 T cells from mice with lupus-like symptoms induced by graft-versus-host disease180, or as a consequence of the MRL/lpr genetic background181, and in those collected from patients with SLE182. Accordingly, pharmacological blockade of PI3Kγ with AS605240 blocks T-cell activation, autoantibody production, renal infiltration and TNF release by macrophages, and ameliorates glomerulonephritis, thus extending lifespan in mouse models of lupus181,183. Additionally, the broad PI3K inhibitor LY294002 improves the survival of mesenchymal stem cells (MSCs), which exhibit enhanced senescence in patients with SLE184. PI3Kδ is highly expressed in the synovium and cultured FLS of patients with RA185, and its expression is selectively induced over other PI3K isoforms by TNF. Moreover, a novel PI3Kδ inhibitor, INK007, blocks TNF-induced AKT activation185 (TABLE 1).
N-acetylcysteine
The therapeutic use of NAC in SLE has been based on increasing evidence of oxidative stress being involved in the pathogenesis of this disease186, including effects on abnormal T-cell activation187,188, the antigenicity of DNA189 and self proteins190, and production of ANA191 (TABLE 1). The rationale for reversing oxidative stress with NAC is supported by several observations. Intracellular GSH levels are decreased in PBLs and T cells from patients with SLE30,192–195, and GSH depletion and oxidative stress contribute to T-cell dysfunction in these patients189,192,196. Additionally, NAC and GSH-sparing antioxidants improve the clinical outcome of lupus in mice197–199, and the fact that large dosages (up to 8 g daily) of NAC can be safely administered to humans200 (and that similar dosages of NAC improve fatigue in healthy individuals201) further supports the use of NAC in this context. NAC also limits the myelotoxicity of immunosuppressive medications202, which are also commonly used in patients with SLE.
NAC is inexpensive and widely accessible to patients in health-food stores, but is currently unavailable as an oral medication by prescription. Therefore, we initiated a randomized, double-blind, placebo-controlled phase I–II study to evaluate the safety and tolerability of NAC, as well as its metabolic, immunological and therapeutic effects, in 36 patients with SLE. The results of this study indicate that NAC is safe and reduces disease activity and fatigue over 3 months70. The therapeutic action of NAC is mediated by the reversal of GSH depletion and blockade of mTORC1 (REF. 70), which is a sensor of oxidative stress28–30,33,79. The clinical efficacy of NAC in patients with SLE is hypothesized to occur through a newly identified molecular mechanism: disruption of the mitochondrial hyperpolarization (MHP)–oxidative stress–mTOR axis in T cells186. DN T cells exhibit the greatest mTORC1 activation70,109 and are predisposed to proinflammatory necrotic cell death70,109; consequently, along with the blockade of their mTORC1 activity, proliferation of these cells is reversed by NAC treatment70. Thus, elimination of DN T cells can contribute to the efficacy of NAC.
The use of NAC instead of rapamycin to block mTOR might have additional benefits. Rapamycin induces hyperlipidaemia, at least in renal transplant recipients203, and infections are common adverse events in these patients, with pneumonia and sepsis being most frequent204. These unwanted consequences of rapamycin are of concern, because infections and cardiovascular disease are the leading causes of mortality in patients with SLE205. Thus, given that oxidative stress contributes to cardiovascular disease206, patients might have additional benefit from treatment with NAC instead of rapamycin, at least those with end-stage renal disease207. Future studies should focus on whether GSH depletion and activation of mTORC1 predict responsiveness to treatment by NAC and mTOR inhibitors, and whether selective or non-selective mTORC1, mTORC2 or dual mTORC1/mTORC2 inhibitors deliver differential clinical benefits (TABLE 1).
Targets under development
Metformin activates AMPK and, thus, inhibits mTORC1 (REF. 208; TABLE 1). Metformin also reduces mTORC1 activity by moderating mitochondrial oxidative stress, which has been implicated in the prevention of nephritis in lupus-prone mice128, and inhibits inflammatory arthritis in the K/B × N model of lupus209. Beyond inhibiting mTORC1-dependent inflammation, metformin might also reduce metabolic comorbidities such as hyperlipidaemia and atherosclerosis in patients with SLE or RA210.
Fingolimod, a S1P–receptor antagonist with effects upstream of mTORC1 (REFS 211,212), has been introduced for the treatment of multiple sclerosis213. Fingolimod sequesters lymphocytes in the secondary lymphoid organs by inducing S1P receptor internalization and degradation213, and blocks the expansion of DN T cells and prevents nephritis in MRL/lpr mice214,215. Given their potentially synergistic mechanism of action, fingolimod, metformin, NAC and rapamycin might be combined for the treatment of these autoimmune diseases.
Among the pharmacological interventions currently in preclinical development for the treatment of rheumatic diseases, targeting of calcium/calmodulin-dependent protein kinase type IV (CaMK-IV) seems to be promising (TABLE 1). Overexpression of CaMK-IV in T cells from lupus-prone mice216 might act upstream of mTORC1; but given that rapamycin treatment in vivo restores T-cell activation-induced Ca2+ fluxing6, CaMK-IV might also act downstream of mTORC1. Similarly to mTORC1 blockade, pharmacological inhibition of CaMK-IV with KN-93 expanded TREG cells217, decreased the expression of the co-stimulatory molecules CD86 and CD80 on B cells, decreased ANA production and suppressed nephritis and skin disease in MRL/lpr mice218. KN-93 also inhibited CaMK-II in vitro, and blocked the proliferation of FLSs from patients with RA219. Another CaMK-II inhibitor, rimacalib, which has anti-inflammatory effects on macrophages220, has entered clinical trials for the treatment of RA (TABLE 1).
Conclusions
The activation of mTORC1 observed in patients with rheumatic diseases is particularly relevant in light of the fact that these individuals have reduced life expectancy. mTORC1 blockade with rapamycin increases lifespan in mice160, and enhanced mTORC1 activation221 and related autophagy defects are implicated in endothelial inflammation, atherosclerosis and vascular ageing222. Remarkably, rapamycin also inhibits vascular ageing222,223. These preclinical studies provided a rationale for new clinical trials aimed at improving cardiovascular outcomes and overall survival in elderly individuals. Such clinical benefit could be highly relevant for patients with SLE or RA, in whom cardiovascular disease is a major cause of increased mortality205,224–226. Therefore, mTOR blockade might be highly beneficial for patients with these rheumatic diseases by reducing mortality due to cardiovascular disease, arthritis, nephritis or neurological disease.
Despite these potential advantages, the blockade of mTORC1 with rapamycin or everolimus has unique adverse effects227. Mucositis, rash, metabolic derangements such as hyperglycaemia, hyperlipidaemia and hypophosphataemia227, and an increased risk of infections204 are among the most common adverse effects of rapamycin or everolimus in patients who had a renal transplant203. The latter are of particularly concern, as infections are a leading cause of increased mortality in patients with SLE205 and are more common in patients with RA than in the general population228. Notably, inhibition of mTORC1 by agents that act at metabolic checkpoints — such as the reversal of GSH depletion and oxidative stress by NAC70 — can reduce the toxicity of this therapeutic approach, and has the added benefit of reducing cardiovascular disease207. NAC might also benefit patients with RA by moderating the toxicity of methotrexate229, which remains a mainstay of treatment in these patients. Future studies should determine whether oxidative stress, GSH depletion and activation of mTORC1 predict responsiveness to treatment with NAC, metformin and other mTOR inhibitors, alone or in combination, in patients with rheumatic diseases.
Key points.
The mechanistic target of rapamycin (mTOR) is a serine/threonine kinase that regulates growth, proliferation, survival and autophagy in a cell-type-specific manner
mTOR forms two interacting complexes, mTORC1 and mTORC2
mTORC1 drives the proinflammatory expansion of T helper (TH) type 1, TH17, and CD4−CD8− double-negative T cells, which collectively orchestrate the pathogenesis of autoimmune diseases
mTORC1 contributes to erosive arthritis by mediating the proliferation of fibroblasts-like synoviocytes and osteoclasts, and contributes to osteoarthritis by restraining autophagy in chondrocytes
Blockade of the mTOR pathway offers new treatments and prevention strategies for rheumatic diseases
Acknowledgments
The author’s research work is supported in part by grants AI 048079 and AI 072648 from the National Institutes of Health and the Central New York Community Foundation, and Investigator-Initiated Research Grant P0468×1-4470/ WS1234172 from Pfizer.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 2.Sehgal SN, Bansback CC. Rapamycin: in vitro profile of a new immunosuppressive macrolide. Ann. NY Acad. Sci. 1993;685:58–67. doi: 10.1111/j.1749-6632.1993.tb35852.x. [DOI] [PubMed] [Google Scholar]
- 3.Collier DSJ, et al. Rapamycin in experimental renal allografts in dogs and pigs. Transplant. Proc. 1990;22:1674–1675. [PubMed] [Google Scholar]
- 4.Calne RY, et al. Rapamycin for immunosuppression in organ allografting. Lancet. 1989;334:227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- 5.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cejka D, et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62:2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 8.Bruyn GAW, et al. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann. Rheum. Dis. 2008;67:1090–1095. doi: 10.1136/ard.2007.078808. [DOI] [PubMed] [Google Scholar]
- 9.Foroncewicz B, Mucha K, Pàczek L, Chmura A, Rowin´ski W. Efficacy of rapamycin in patient with juvenile rheumatoid arthritis. Transpl. Int. 2005;18:366–368. doi: 10.1111/j.1432-2277.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah M, et al. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjögren’s syndrome. J. Control. Release. 2013;171:269–279. doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponticos M, et al. Failed degradation of JunB contributes to overproduction of type I collagen and development of dermal fibrosis in patients with systemic sclerosis. Arthritis Rheumatol. 2015;67:243–253. doi: 10.1002/art.38897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaki Z, et al. Effects of the immunosuppressant rapamycin on the expression of human α2(I) collagen and matrix metalloproteinase 1 genes in scleroderma dermal fibroblasts. J. Dermatol. Sci. 2014;74:251–259. doi: 10.1016/j.jdermsci.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Yoshizaki A, et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010;62:2476–2487. doi: 10.1002/art.27498. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, et al. mTOR inhibition rescues osteopenia in mice with systemic sclerosis. J. Exp. Med. 2015;212:73–91. doi: 10.1084/jem.20140643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su TI, et al. Rapamycin versus methotrexate in early diffuse systemic sclerosis: results from a randomized, single-blind pilot study. Arthritis Rheum. 2009;60:3821–3830. doi: 10.1002/art.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terasawa A, et al. Sirolimus-eluting stent implantation for ostial stenosis of left main coronary artery after Bentall operation in aortitis syndrome. J. Cardiol. 2010;55:147–150. doi: 10.1016/j.jjcc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa Y, et al. Sirolimus-eluting stent for in-stent restenosis of left main coronary artery in Takayasu arteritis. Circ. J. 2005;69:752–755. doi: 10.1253/circj.69.752. [DOI] [PubMed] [Google Scholar]
- 18.Koening CL, Hernandez-Rodriguez J, Molloy ES, Clark TM, Hoffman GS. Limited utility of rapamycin in severe, refractory Wegener’s granulomatosis. J. Rheumatol. 2009;36:116–119. doi: 10.3899/jrheum.080664. [DOI] [PubMed] [Google Scholar]
- 19.Constantinescu AR, Liang M, Laskow DA. Sirolimus lowers myeloperoxidase and p-ANCA titers in a pediatric patient before kidney transplantation. Am. J. Kidney Dis. 2002;40:407–410. doi: 10.1053/ajkd.2002.34544. [DOI] [PubMed] [Google Scholar]
- 20.Lopez de Figueroa P, Lotz MK, Blanco FJ, Carames B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheum. 2015;67:966–976. doi: 10.1002/art.39025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauvin C, et al. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene. 2014;33:474–483. doi: 10.1038/onc.2012.606. [DOI] [PubMed] [Google Scholar]
- 22.Gingras AC, et al. Regulation of 4E–BP1 phosphorylation: a novel two step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J. Biol. Chem. 2009;284:35425–35432. doi: 10.1074/jbc.M109.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazio F, et al. MTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell. Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 25.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoncu R, et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Peled L, et al. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J. Biol. Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 29.Thedieck K, et al. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell. 2013;154:859–874. doi: 10.1016/j.cell.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez DR, et al. Activation of mTOR controls the loss of TCRζ in lupus T cells through HRES-1/ Rab4-regulated lysosomal degradation. J. Immunol. 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sancak Y, et al. The Rag GTPases bind Raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc. Natl Acad. Sci. USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shor B, et al. Requirement of the mTOR kinase for the regulation of Maf1 phosphorylation and control of RNA polymerase III-dependent transcription in cancer cells. J. Biol. Chem. 2010;285:15380–15392. doi: 10.1074/jbc.M109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang CK, Liu H, Zheng XS. mTOR binds to the promoters of RNA polymerase I- and III-transcribed genes. Cell Cycle. 2010;9:953–957. doi: 10.4161/cc.9.5.10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J. Cell. Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su WC, et al. Rab5 and class III phosphoinositide 3-kinase Vps34 are involved in hepatitis C virus NS4B-induced autophagy. J. Virol. 2011;85:10561–10571. doi: 10.1128/JVI.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia W, Pua HH, Li QJ, He YW. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J. Immunol. 2011;186:1564–1574. doi: 10.4049/jimmunol.1001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 40.Stein MP, Feng Y, Cooper KL, Welford AM, Wandinger-Ness A. Human VPS34 and p150 are Rab7 interacting partners. Traffic. 2003;4:754–771. doi: 10.1034/j.1600-0854.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 41.Caza TN, et al. HRES-1/RAB4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann. Rheum. Dis. 2014;73:1887–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talaber G, et al. HRES-1/Rab4 promotes the formation of LC3+ autophagosomes and the accumulation of mitochondria during autophagy. PLoS ONE. 2014;9:e84392. doi: 10.1371/journal.pone.0084392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telarico T, et al. HRES-1/Rab4 lupus susceptibility gene selectively regulates mammalian target of rapamycin complexes 1 and 2 in T lymphocytes. Arthritis Rheum. Abstr. 2011;63(Suppl. 10):2358. [Google Scholar]
- 44.Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85α subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J. Biol. Chem. 2004;279:48607–48614. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- 45.Chamberlain MD, et al. Disrupted RabGAP function of the p85 subunit of phosphatidylinositol 3-kinase results in cell transformation. J. Biol. Chem. 2008;283:15861–15868. doi: 10.1074/jbc.M800941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamberlain MD, et al. Deregulation of Rab5 and Rab4 proteins in p85R274A–expressing cells alters PDGFR trafficking. Cell. Signal. 2010;22:1562–1575. doi: 10.1016/j.cellsig.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Kausch C, et al. Association of impaired phosphatidylinositol 3-kinase activity in GLUT1-containing vesicles with malinsertion of glucose transporters into the plasma membrane of fibroblasts from a patient with severe insulin resistance and clinical features of Werner syndrome. J. Clin. Endocrin. Metab. 2000;85:905–918. doi: 10.1210/jcem.85.2.6347. [DOI] [PubMed] [Google Scholar]
- 48.Kim SG, et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell. 2013;49:172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 50.Caro-Maldonado A, Gerriets VA, Rathmell JC. Matched and mismatched metabolic fuels in lymphocyte function. Semin. Immunol. 2012;24:405–413. doi: 10.1016/j.smim.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011;12:295–304. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercalli A, et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140:179–190. doi: 10.1111/imm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat. Commun. 2014;5:4696. doi: 10.1038/ncomms5696. [DOI] [PubMed] [Google Scholar]
- 54.Poglitsch M, et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am. J. Transplant. 2012;12:1458–1468. doi: 10.1111/j.1600-6143.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 55.Vasheghani F, et al. PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann. Rheum. Dis. 2015;74:569–578. doi: 10.1136/annrheumdis-2014-205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia RJ, et al. Attention deficit and hyperactivity disorder scores are elevated and respond to NAC treatment in patients with SLE. Arthritis Rheum. 2013;65:1313–1318. doi: 10.1002/art.37893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Proud CG. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- 58.Duran R, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 59.Jewell JL, et al. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perl A, et al. Comprehensive metabolome analyses reveal N -acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics. 2015;11:1157–1174. doi: 10.1007/s11306-015-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu G, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1-a dependent glycolysis. Cancer Res. 2014;74:727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y, et al. Estradiol promotes pentose phosphate pathway addiction and cell survival via reactivation of Akt in mTORC1 hyperactive cells. Cell Death Dis. 2014;5:e1231. doi: 10.1038/cddis.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim. Biophys. Acta. 2013;1834:1271–1278. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Joussineau C, et al. mTOR pathway is activated by PKA in adrenocortical cells and participates in vivo to apoptosis resistance in primary pigmented nodular adrenocortical disease (PPNAD) Hum. Mol. Genet. 2014;23:5418–5428. doi: 10.1093/hmg/ddu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo SE, et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52:1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie J, et al. CAMP inhibits mammalian target of rapamycin complex-1 and −2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity. Cell. Signal. 2011;23:1927–1935. doi: 10.1016/j.cellsig.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai Z-W, et al. N -acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pearce EL, et al. Enhancing CD8 T cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 74.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 76.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P1 -mTOR axis directs the reciprocal differentiation of TH 1 and Treg cells. Nat. Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh CT, Chuang JH, Yang WC, Yin Y. & Lin, Y Ceramide inhibits insulin-stimulated Akt phosphorylation through activation of Rheb/mTORC1/ S6K signaling in skeletal muscle. Cell. Signal. 2014;26:1400–1408. doi: 10.1016/j.cellsig.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida S, et al. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J. Biol. Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol- 3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 81.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat. Rev. Mol. Cell. Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 82.Lamming DW, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braccini L, et al. PI3K–C2y is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 2015;6:7400. doi: 10.1038/ncomms8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Worby CA, Dixon JE. PTEN. Annu. Rev. Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- 86.Sagar V, Bond JR, Chowdhary VR. A 50-year- old woman with Cowden syndrome and joint pains. Arthritis Care Res. 2015;67:1604–1608. doi: 10.1002/acr.22616. [DOI] [PubMed] [Google Scholar]
- 87.Lee T, Le EN, Glass DA, Bowen CD, Dominguez AR. Systemic lupus erythematosus in a patient with PTEN hamartoma tumour syndrome. Brit. J. Dermatol. 2014;170:990–992. doi: 10.1111/bjd.12767. [DOI] [PubMed] [Google Scholar]
- 88.Shrestha S, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huynh A, et al. Control of PI3 kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ray JP, Craft J. PTENtiating autoimmunity through Treg cell deregulation. Nat. Immunol. 2015;16:139–140. doi: 10.1038/ni.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu XN, et al. Defective PTEN regulation contributes to B cell hyperresponsiveness in systemic lupus erythematosus. Sci. Transl. Med. 2014;6:246ra99. doi: 10.1126/scitranslmed.3009131. [DOI] [PubMed] [Google Scholar]
- 92.Bluml S, et al. Loss of phosphatase and tensin homolog (PTEN) in myeloid cells controls inflammatory bone destruction by regulating the osteoclastogenic potential of myeloid cells. Ann. Rheum. Dis. 2015;74:227–233. doi: 10.1136/annrheumdis-2013-203486. [DOI] [PubMed] [Google Scholar]
- 93.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 94.Gwinn DM, et al. AMPK phosphorylation of Raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardie DG, Carling D, Carlson M. The AMP- activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Ann. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 96.Carroll KC, Viollet B, Suttles J. AMPKα1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J. Leukoc. Biol. 2013;94:1113–1121. doi: 10.1189/jlb.0313157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Russell RC, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang KY, et al. Metformin downregulates TH17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 2013;16:85–92. doi: 10.1016/j.intimp.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Procaccini C, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 2015;17:29. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kato H, Perl A. Mechanistic taret of rapamycin complex 1 expands TH17 and IL-4+ double negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J. Immunol. 2014;192:4134–4144. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fernandez DR, Perl A. mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov. Med. 2010;9:173–178. [PMC free article] [PubMed] [Google Scholar]
- 108.Moulton VR, Tsokos GC. Abnormalities of T cell signaling in systemic lupus erythematosus. Arth. Res. Ther. 2010;13:207. doi: 10.1186/ar3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai Z-W, et al. mTOR activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus eryhthematosus. J. Immunol. 2013;191:2236–2246. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann. NY Acad. Sci. 2015;1346:33–44. doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tai TS, Pai SY, Ho IC. GATA-3 regulates the homeostasis and activation of CD8+ T cells. J. Immunol. 2013;190:428–437. doi: 10.4049/jimmunol.1201361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomasoni R, et al. Rapamycin-sensitive signals control TCR/CD28-driven Ifng, Il4 and Foxp3 transcription and promoter region methylation. Eur. J. Immunol. 2011;41:2086–2096. doi: 10.1002/eji.201041130. [DOI] [PubMed] [Google Scholar]
- 113.Sieling PA, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J. Immunol. 2000;165:5338–5344. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- 114.Shivakumar S, Tsokos GC, Datta SK. T cell receptor α/β expressing double-negative (CD4/CD8) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J. Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- 115.Umekawa M, Klionsky DJ. Ksp1 kinase regulates autophagy via the target of rapamycin complex 1 (TORC1) pathway. J. Biol. Chem. 2012;287:16300–16310. doi: 10.1074/jbc.M112.344952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Coz C, et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS ONE. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh N, Birkenbach M, Caza T, Perl A, Cohen PL. Tuberous sclerosis and fulminant lupus in a young woman. J. Clin. Rheumatol. 2013;19:134–137. doi: 10.1097/RHU.0b013e318289c033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu T, et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J. Clin. Invest. 2007;117:2186–2196. doi: 10.1172/JCI30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stylianou K, et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol. Dial. Transplant. 2011;26:498–508. doi: 10.1093/ndt/gfq496. [DOI] [PubMed] [Google Scholar]
- 121.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 122.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 123.Petri M, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyakis S, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 125.Canaud G, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N. Engl. J. Med. 2014;371:303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 126.Lai ZW, Marchena-Mendez I, Perl A. Oxidative stress and Treg depletion in lupus patients with anti- phospholipid syndrome. Clin. Immunol. 2015;158:148–152. doi: 10.1016/j.clim.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Doherty E, Oaks Z, Perl A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antiox. Redox Signal. 2014;21:56–65. doi: 10.1089/ars.2013.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yin Y, et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015;7:274ra18. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanczko R, et al. Prevention of hepatocarcinogenesis and acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J. Clin. Invest. 2009;119:1546–1557. doi: 10.1172/JCI35722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011;7:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med. Chem. 2015;7:1137–1147. doi: 10.4155/fmc.15.55. [DOI] [PubMed] [Google Scholar]
- 132.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J. Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 133.Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol. Med. 2010;16:352–358. doi: 10.2119/molmed.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saxena A, Raychaudhuri SK, Raychaudhuri SP. Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis Rheum. 2011;63:1465–1466. doi: 10.1002/art.30278. [DOI] [PubMed] [Google Scholar]
- 135.Reveille JD. Biomarkers for diagnosis, monitoring of progression, and treatment responses in ankylosing spondylitis and axial spondyloarthritis. Clin. Rheumatol. 2015;34:1009–1018. doi: 10.1007/s10067-015-2949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.International Genetics of Ankylosing Spondylitis Consortium (IGAS) et al. Identification of multiple risk variants for ankylosing spondylitis through high- density genotyping of immune-related loci. Nat. Genet. 2013;45:730–738. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robinson PC, et al. ERAP2 is associated with ankylosing spondylitis in HLA-B27-positive and HLA-B27-negative patients. Ann. Rheum. Dis. 2015;74:1627–1629. doi: 10.1136/annrheumdis-2015-207416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Evans DM, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Appel H, et al. In situ analysis of interleukin-23 and interleukin-12 positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. 2013;65:1522–1529. doi: 10.1002/art.37937. [DOI] [PubMed] [Google Scholar]
- 140.Lai NS, et al. Aberrant expression of microRNAs in T cells from patients with ankylosing spondylitis contributes to the immunopathogenesis. Clin. Exp. Immunol. 2013;173:47–57. doi: 10.1111/cei.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hou C, Zhu M, Sun M, Lin Y. MicroRNA let-7i induced autophagy to protect T cell from apoptosis by targeting IGF1R. Biochem. Biophys. Res. Commun. 2014;453:728–734. doi: 10.1016/j.bbrc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 142.Kooijman R, Lauf JJ, Kappers AC, Rijkers GT. Insulin-like growth factor induces phosphorylation of immunoreactive insulin receptor substrate and its association with phosphatidylinositol-3 kinase in human thymocytes. J. Exp. Med. 1995;182:593–597. doi: 10.1084/jem.182.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kooijman R, Scholtens LE, Rijkers GT, Zegers BJ. Differential expression of type I insulin-like growth factor receptors in different stages of human T cells. Eur. J. Immunol. 1995;25:931–935. doi: 10.1002/eji.1830250411. [DOI] [PubMed] [Google Scholar]
- 144.Xu WD, et al. Up-regulation of fatty acid oxidation in the ligament as a contributing factor of ankylosing spondylitis: a comparative proteomic study. J. Proteom. 2015;113:57–72. doi: 10.1016/j.jprot.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 145.Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr. Opin. Rheumatol. 2015;27:127–133. doi: 10.1097/BOR.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 146.Helms C, et al. A putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nat. Genet. 2003;35:349–356. doi: 10.1038/ng1268. [DOI] [PubMed] [Google Scholar]
- 147.Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/ Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 148.Peled M, et al. Analysis of programmed death-1 in patients with psoriatic arthritis. Inflammation. 2015;38:1573–1579. doi: 10.1007/s10753-015-0132-2. [DOI] [PubMed] [Google Scholar]
- 149.Javier AF, et al. Rapamycin (sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J. Clin. Invest. 1997;99:2094–2099. doi: 10.1172/JCI119382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buerger C, Malisiewicz B, Eiser A, Hardt K, Boehncke WH. Mammalian target of rapamycin and its downstream signalling components are activated in psoriatic skin. Br. J. Dermatol. 2013;169:156–159. doi: 10.1111/bjd.12271. [DOI] [PubMed] [Google Scholar]
- 151.Young CN, et al. Reactive oxygen species in tumor necrosis factor-a-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J. Invest. Dermatol. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilkes MC, et al. Transforming growth factor-(3 activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–10440. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 154.Rahimi RA, et al. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-p. Cancer Res. 2009;69:84–93. doi: 10.1158/0008-5472.CAN-08-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li J, et al. Rictor/mTORC2 signaling mediates TGF(31-induced fibroblast activation and kidney fibrosis. Kidney Int. 2015;88:515–527. doi: 10.1038/ki.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med. Clin. North Am. 2009;93:1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 157.Guan Y, Yang X, Yang W, Charbonneau C, Chen Q. Mechanical activation of mammalian target of rapamycin pathway is required for cartilage development. FASEB J. 2014;28:4470–4481. doi: 10.1096/fj.14-252783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lotz M, Carames B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and osteoarthritis. Nat. Rev. Rheumatol. 2011;7:579–587. doi: 10.1038/nrrheum.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Y, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis. 2015;74:1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 160.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin- dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 162.US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov
- 163.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharm. Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 164.Thoreen CC, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin- resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dancey JE. Therapeutic targets: mTOR and related pathways. Cancer Biol. Ther. 2006;5:1065–1073. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 166.Peng L, Zhou Y, Ye X, Zhao Q. Treatment-related fatigue with everolimus and temsirolimus in patients with cancer meta-analysis of clinical trials. Tumor Biol. 2014;36:643–654. doi: 10.1007/s13277-014-2669-3. [DOI] [PubMed] [Google Scholar]
- 167.Markman B, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann. Oncol. 2012;23:2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 168.Aghdasi B, et al. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc. Natl Acad. Sci. USA. 2001;98:2425–2430. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dolinski K, Muir S, Cardenas M, Heitman J. All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA. 1997;94:13093–13098. doi: 10.1073/pnas.94.24.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Houde VP, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fraenkel M, et al. mTOR inhibition by rapamycin prevents (3-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- 172.Fang Y, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell. Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schenone S, Brullo C, Musumeci F, Radi M, Botta M. ATP-competitive inhibitors of mTOR: an update. Curr. Med. Chem. 2011;18:2995–3014. doi: 10.2174/092986711796391651. [DOI] [PubMed] [Google Scholar]
- 174.Wang BT, et al. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl Acad. Sci. USA. 2011;108:15201–15206. doi: 10.1073/pnas.1103746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ramos-Barron A, et al. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus. 2007;16:775–781. doi: 10.1177/0961203307081401. [DOI] [PubMed] [Google Scholar]
- 176.Lui SL, et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol. Dial. Transplant. 2008;23:2768–2776. doi: 10.1093/ndt/gfn216. [DOI] [PubMed] [Google Scholar]
- 177.Yap DY, Ma MK, Tang CS, Chan TM. Proliferation signal inhibitors in the treatment of lupus nephritis: preliminary experience. Nephrology (Carlton) 2012;17:676–680. doi: 10.1111/j.1440-1797.2012.01646.x. [DOI] [PubMed] [Google Scholar]
- 178.US National Library of Medicine. NCT00779194. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/results?term=NCT00779194. 2015
- 179.Reitamo S, et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. Brit. J. Dermatol. 2001;145:438–445. doi: 10.1046/j.1365-2133.2001.04376.x. [DOI] [PubMed] [Google Scholar]
- 180.Niculescu F, et al. Pathogenic T cells in murine lupus exhibit spontaneous signaling activity through phosphatidylinositol 3-kinase and mitogen- activated protein kinase pathways. Arthritis Rheum. 2003;48:1071–1079. doi: 10.1002/art.10900. [DOI] [PubMed] [Google Scholar]
- 181.Barber DF, et al. PI3Ky inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat. Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 182.Suarez-Fueyo A, Barber DF, Martinez-Ara J, Zea-Mendoza AC, Carrera AC. Enhanced phosphoinositide 3-kinase 8 activity is a frequent event in systemic lupus erythematosus that confers resistance to activation-induced T cell death. J. Immunol. 2011;187:2376–2385. doi: 10.4049/jimmunol.1101602. [DOI] [PubMed] [Google Scholar]
- 183.Suarez-Fueyo A, et al. Inhibition of PI3Ky reduces kidney infiltration by macrophages and ameliorates systemic lupus in the mouse. J. Immunol. 2014;193:544–554. doi: 10.4049/jimmunol.1400350. [DOI] [PubMed] [Google Scholar]
- 184.Chen H, et al. Leptin and NAP2 promote mesenchymal stem cell senescence through activation of PI3K/Akt pathway in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:2383–2393. doi: 10.1002/art.39196. [DOI] [PubMed] [Google Scholar]
- 185.Bartok B, et al. PI3 kinase 8 is a key regulator of synoviocyte function in rheumatoid arthritis. Am. J. Pathol. 2012;180:1906–1916. doi: 10.1016/j.ajpath.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 186.Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev Rheumatol. 2013;9:674–686. doi: 10.1038/nrrheum.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gergely PJ, et al. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J. Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li Y, Gorelik G, Strickland FM, Richardson BC. Oxidative stress, T cell DNA methylation and lupus. Arthritis Rheum. 2014;66:1574–1582. doi: 10.1002/art.38427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gehrke N, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 190.Scofield RH, et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radic. Biol. Med. 2005;38:719–728. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 191.Otaki N, et al. Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies. J. Biol. Chem. 2010;285:33834–33842. doi: 10.1074/jbc.M110.165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gergely PJ, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shah D, Aggarwal A, Bhatnagar A, Kiran R, Wanchu A. Association between T lymphocyte subsets apoptosis and peripheral blood mononuclear cells oxidative stress in systemic lupus erythematosus. Free Radic. Res. 2011;45:559–567. doi: 10.3109/10715762.2011.555765. [DOI] [PubMed] [Google Scholar]
- 194.Shah D, Kiran R, Wanchu A, Bhatnagar A. Oxidative stress in systemic lupus erythematosus: relationship to TH1 cytokine and disease activity. Immunol. Lett. 2010;129:7–12. doi: 10.1016/j.imlet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 195.Li KJ, et al. Deranged bioenergetics and defective redox capacity in T lymphocytes and neutrophils are related to cellular dysfunction and increased oxidative stress in patients with active systemic lupus erythematosus. Clin. Dev Immunol. 2012;2012:548516. doi: 10.1155/2012/548516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nambiar MP, et al. Oxidative stress is involved in the heat stress-induced downregulation of TCR( chain expression and TCR/CD3-mediated [Ca2+]i response in human T-lymphocytes. Cell. Immunol. 2002;215:151–161. doi: 10.1016/s0008-8749(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 197.Suwannaroj S, Lagoo A, Keisler D, McMurray R. W Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 198.Bergamo P, et al. Conjugated linoleic acid enhances glutathione synthesis and attenuates pathological signs in MRL/MpJ-Faslpr mice. J. Lipid Res. 2006;47:2382–2391. doi: 10.1194/jlr.M600187-JLR200. [DOI] [PubMed] [Google Scholar]
- 199.Bergamo P, Maurano F, Rossi M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus- associated oxidative stress. Free Radic. Biol. Med. 2007;43:71–79. doi: 10.1016/j.freeradbiomed.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 200.Herzenberg LA, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl Acad. Sci. USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Travaline JM, et al. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am. J. Resp. Crit. Care Med. 1997;156:1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 202.Demedts M, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 203.Brattstrom C, et al. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin) Transplantation. 1998;65:1272–1274. doi: 10.1097/00007890-199805150-00023. [DOI] [PubMed] [Google Scholar]
- 204.Qi WX, Huang YJ, Yao Y, Shen Z, Min DL. Incidence and risk of treatment-related mortality with mTOR inhibitors everolimus and temsirolimus in cancer patients: a meta-analysis. PLoS ONE. 2013;8:e65166. doi: 10.1371/journal.pone.0065166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2001;13:345–351. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 206.Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr. Med. Chem. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
- 208.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yan H, Zhou HF, Hu Y, Pham CT. Suppression of experimental arthritis through AMP-activated protein kinase activation and autophagy modulation. J. Rheum. Dis. Treat. 2015;1:5. doi: 10.23937/2469-5726/1510005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martinet W, De Loof H, De Meyer GR. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis. 2014;233:601–607. doi: 10.1016/j.atherosclerosis.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 211.Balasubramaniam S, et al. Novel heterozygous mutations in TALDO1 gene causing transaldolase deficiency and early infantile liver failure. J. Pediatr. Gastroenterol. Nutr. 2011;52:113–116. doi: 10.1097/MPG.0b013e3181f50388. [DOI] [PubMed] [Google Scholar]
- 212.Taniguchi M, et al. Regulation of autophagy and its associated cell death by ‘sphingolipid rheostat’: reciprocal role of ceramide and sphingosine 1-phosphate in the mammalian target of rapamycin pathway. J. Biol. Chem. 2012;287:39898–39910. doi: 10.1074/jbc.M112.416552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 214.Okazaki H, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J. Rheumatol. 2002;29:707–716. [PubMed] [Google Scholar]
- 215.Wenderfer SE, Stepkowski SM, Braun MC. Prolonged survival and reduced renal injury in MRL/ lpr mice treated with a novel sphingosine-1-P receptor agonist. Kidney Int. 2008;74:1319–1326. doi: 10.1038/ki.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Koga T, et al. CaMK4-dependent activation of AKT/ mTOR and CREM-α underlies autoimmunity- associated TH17 imbalance. J. Clin. Invest. 2014;124:2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koga T, et al. KN-93, an inhibitor of calcium/ calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3+ regulatory T cells in MRL/lpr mice. AutoImmunity. 2014;47:445–450. doi: 10.3109/08916934.2014.915954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Inhibition of calcium/calmodulin- dependent protein kinase type IV results in suppression of autoimmunity and organ pathology in lupus-prone mice. Arthritis Rheum. 2011;63:523–529. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fujikawa K, et al. Calcium/calmodulin-dependent protein kinase II (CaMKII) regulates tumour necrosis factor-related apoptosis inducing ligand (TRAIL)- mediated apoptosis of fibroblast-like synovial cells (FLS) by phosphorylation of Akt. Clin. Exp. Rheumatol. 2009;27:952–957. [PubMed] [Google Scholar]
- 220.Westra J, et al. Expression and regulation of HIF-1α in macrophages under inflammatory conditions; significant reduction of VEGF by CaMKII inhibitor. BMC Musculoskelet. Disord. 2010;11:61. doi: 10.1186/1471-2474-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yepuri G, et al. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 2012;11:1005–1016. doi: 10.1111/acel.12001. [DOI] [PubMed] [Google Scholar]
- 222.Xiong Y, et al. ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy. 2014;10:2223–2238. doi: 10.4161/15548627.2014.981789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Elloso MM, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am. J. Transplant. 2003;3:562–569. doi: 10.1034/j.1600-6143.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 224.Manzi S, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am. J. Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 225.Mackey RH, et al. Rheumatoid arthritis, anti-CCP positivity, and cardiovascular disease risk in the Women’s Health Initiative. Arthritis Rheumatol. 2015;67:2311–2322. doi: 10.1002/art.39198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Avina-Zubieta JA, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Care Res. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 227.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 2011;6:125–129. doi: 10.1007/s11523-011-0174-9. [DOI] [PubMed] [Google Scholar]
- 228.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 229.Paul M, et al. Methotrexate promotes platelet apoptosis via JNK-mediated mitochondrial damage: alleviation by N-acetylcysteine and N-acetylcysteine amide. PLoS ONE. 2015;10:e0127558. doi: 10.1371/journal.pone.0127558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lui SL, et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus. 2008;17:305–313. doi: 10.1177/0961203307088289. [DOI] [PubMed] [Google Scholar]
- 232.Kshirsagar S, et al. Akt-dependent enhanced migratory capacity of TH17 cells from children with lupus nephritis. J. Immunol. 2014;193:4895–4903. doi: 10.4049/jimmunol.1400044. [DOI] [PubMed] [Google Scholar]
- 233.Tian J, Wang Y, Liu X, Zhou X, Li R. Rapamycin ameliorates IgA nephropathy via cell cycle- dependent mechanisms. Exp. Biol. Med. (Maywood) 2015;240:936–945. doi: 10.1177/1535370214555666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cruzado JM, et al. Low-dose sirolimus combined with angiotensin-converting enzyme inhibitor and statin stabilizes renal function and reduces glomerular proliferation in poor prognosis IgA nephropathy. Nephrol. Dial. Transplant. 2011;26:3596–3602. doi: 10.1093/ndt/gfr072. [DOI] [PubMed] [Google Scholar]
- 235.Zou Y, et al. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J. Neurosci. 2014;34:15764–15778. doi: 10.1523/JNEUROSCI.2267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Seyfarth HJ, Hammerschmidt S, Halank M, Neuhaus P, Wirtz HR. Everolimus in patients with severe pulmonary hypertension: a safety and efficacy pilot trial. Pulm. Circ. 2013;3:632–638. doi: 10.1086/674311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Raychaudhuri SK, Raychaudhuri SP. mTOR signaling cascade in psoriatic disease: double kinase mTOR inhibitor a novel therapeutic target. Indian J. Dermatol. 2014;59:67–70. doi: 10.4103/0019-5154.123499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mitra A, et al. Dual mTOR inhibition is required to prevent TGF-β-mediated fibrosis: implications for scleroderma. J. Invest. Dermatol. 2015;135:2873–2876. doi: 10.1038/jid.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Biniecka M, et al. Hypoxia induces mitochondrial mutagenesis and dysfunction in inflammatory arthritis. Arth. Rheum. 2011;63:2172–2182. doi: 10.1002/art.30395. [DOI] [PubMed] [Google Scholar]
- 240.Nahir AM, et al. Effects of oral N-acetylcysteine on both ocular and oral manifestations of Sjögren’s syndrome. 1989;46:187–192. [Google Scholar]
- 241.Walters MT, Rubin CE, Keightley SJ. A double- blind, cross-over, study of oral N-acetylcysteine in Sjögren’s syndrome. 1986;15:253–258. [PubMed] [Google Scholar]
- 242.Kelly C, Saravanan V. Treatment strategies for a rheumatoid arthritis patient with interstitial lung disease. Exp. Opin. Pharmacother. 2008;9:3221–3230. doi: 10.1517/14656560802591430. [DOI] [PubMed] [Google Scholar]
- 243.Son HJ, et al. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of TH17/Treg balance and osteoclastogenesis. Mediators Inflamm. 2014;2014:973986. doi: 10.1155/2014/973986. [DOI] [PMC free article] [PubMed] [Google Scholar]