Abstract
Purpose
Diabetic retinopathy is a common microvascular complication of long-standing diabetes. Several complex interconnecting biochemical pathways are activated in response to hyperglycemia. These pathways culminate into proinflammatory and angiogenic effects that bring about structural and functional damage to the retinal vasculature. Since Zingiber officinale (ginger) is known for its anti-inflammatory and antiangiogenic properties, we investigated the effects of its extract standardized to 5% 6-gingerol, the major active constituent of ginger, in attenuating retinal microvascular changes in rats with streptozotocin-induced diabetes.
Methods
Diabetic rats were treated orally with the vehicle or the ginger extract (75 mg/kg/day) over a period of 24 weeks along with regular monitoring of bodyweight and blood glucose and weekly fundus photography. At the end of the 24-week treatment, the retinas were isolated for histopathological examination under a light microscope, transmission electron microscopy, and determination of the retinal tumor necrosis factor-α (TNF-α), nuclear factor-kappa B (NF-κB), and vascular endothelial growth factor (VEGF) levels.
Results
Oral administration of the ginger extract resulted in significant reduction of hyperglycemia, the diameter of the retinal vessels, and vascular basement membrane thickness. Improvement in the architecture of the retinal vasculature was associated with significantly reduced expression of NF-κB and reduced activity of TNF-α and VEGF in the retinal tissue in the ginger extract–treated group compared to the vehicle-treated group.
Conclusions
The current study showed that ginger extract containing 5% of 6-gingerol attenuates the retinal microvascular changes in rats with streptozotocin-induced diabetes through anti-inflammatory and antiangiogenic actions. Although precise molecular targets remain to be determined, 6-gingerol seems to be a potential candidate for further investigation.
Introduction
Diabetic retinopathy (DR) is a common microvascular complication of long-standing diabetes. According to a systematic review based on 35 population-based studies (1980–2008), the overall prevalence of DR and vision-threatening DR is 34.6% and 10.2%, respectively [1]. Another group of researchers, by extrapolating these results to global numbers, estimated that the number of people with DR will grow from 126.6 million in 2011 to 191.0 million by 2030 [2]. The poor outcomes in diabetic retinopathy are attributed to the microvascular changes caused by hyperglycemia-induced activation of the metabolic and biochemical pathways. These pathways include activation of the polyol and hexosamine pathways, activation of the protein kinase C, and the increased formation of advanced glycation end products (AGEs) [3]. Taken together, these pathways result in oxidative stress and inflammation that attenuate vascular wall integrity leading to vascular occlusion, increased permeability, and local ischemia [4,5].
AGEs have been suggested to play a significant role in the diabetic vascular injury. Exposure of vascular endothelial cells in the retina to AGEs augments inflammation, which is considered to be the key driver in the pathophysiology of DR [6]. Increased expression of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, has increasingly been implicated in DR. Increased vitreous levels of TNF-α have been detected in patients with proliferative DR [7-9] and in the diabetic rat retina [7,10,11]. TNF-α promotes leukocyte adhesion to the retinal endothelium and increases blood–retinal barrier (BRB) permeability [12]. Increased expression of proinflammatory cytokines in DR is associated with the activation of nuclear factor-kappa B (NF-κB) [13]. NF-κB is a transcription factor that plays an important role in the development of DR via transcriptional regulation of proinflammatory cytokines [14,15].
The exposure of vascular endothelial cells to AGEs is also associated with the increased expression of vascular endothelial growth factor (VEGF), which has emerged as a key mediator of BRB breakdown and neovascularization in DR [16,17]. VEGF plays a significant role in the leukocyte-mediated breakdown of the BRB and retinal neovascularization and is causally linked to the pathogenesis of DR [17]. Increased VEGF expression has been documented in streptozotocin (STZ)-induced diabetic rat retinas [18-20], and increased vitreous concentration of VEGF has been detected in the patients with proliferative DR [21].
Current therapeutic strategies in the management of DR have suboptimal efficacy, and disease progression often continues despite pharmacological and non-pharmacological interventions. Newer therapeutic options that can target key mediators of microvascular damage in DR are of significant importance. In the current study, we investigated the efficacy of ginger (Zingiber officinale) rhizome extract, standardized to 5% 6-gingerol, in attenuating retinal microvascular changes in STZ-induced diabetic rats. 6-gingerol possesses antioxidant and anti-inflammatory properties [22,23]. The antiangiogenesis potential of 6-gingerol in vivo and in vitro has also been documented [24,25]. Since inflammation and angiogenesis are considered the key targets in the treatment of DR, we investigated whether 6-gingerol ameliorates the microvascular changes of DR by targeting TNF-α, NF-κB, and VEGF activities in the diabetic rat retina.
Methods
All animal handling in this study was in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and institutional guidelines. Wistar albino rats of either sex (200–250 g), obtained from the animal facility of the institute, were housed at 21±2 °C with a 12 h:12 h light-dark cycle. All animals received food and water ad libitum. STZ was purchased from HiMedia, Mumbai, India, enzyme-linked immunosorbent assay (ELISA) kits from Diaclone (Besancon Cedex, France) and RayBiotech, Inc. (Norcross, GA), glycosylated hemoglobin (HbA1c) kits from Biosystems S.A. (Barcelona, Spain), rabbit polyclonal NF-ƙB p65 antibody from Abcam, Plc. (Cambridge, UK), and the detection system for immunohistochemistry was from Bio SB (Santa Barbara, CA). The ginger extract, prepared by extracting 100% natural dried root with an 80:20 mixture of ethanol:water, was purchased from Xi'an Aquar Technology, Xi'an, China. The extract was authenticated and standardized to 5% of 6-gingerol contents with high-performance liquid chromatography (HPLC).
Study design
Diabetes was induced in rats with a single intraperitoneal injection of STZ 45 mg/kg bodyweight, in 0.1 M citrate buffer, pH 4.5. Animals with a blood glucose concentration greater than 300 mg/dl after 48 h of STZ injection were considered diabetic and included in the study. Age-matched healthy rats that were injected with an equal volume of citrate buffer served as the normal control (NC). The diabetic rats were randomly divided into diabetic control (DC) and ginger extract treatment groups (6-G; n = 15; 30 eyes per group). The 6-G-treated group received the ginger extract (75 mg/kg/day) evenly dispersed in 0.5% Tween-80 by oral gavage in a volume of 0.5 ml/kg bodyweight. The DC group similarly received vehicle. The dose of ginger extract selected for this study was based on previous studies that investigated the effects of ginger extract on the blood glucose level in rats [23,26,27].
During the 24-week experimental period, bodyweight and blood glucose levels were monitored weekly using Accu-Chek® Active Glucose Test Strips and Accu-Chek® meters (Roche Diagnostics, Mumbai, India). Fundus photography was performed every 4 weeks, and the last photographs were used to calculate vessel diameter.
At the end of the experimental period, blood was collected for HbA1c. Subsequently, the rats were euthanized in a CO2 chamber. Eyes were enucleated, and retinas were isolated to estimate TNF-α (n = 6), VEGF (n = 6), and phosphorylated NF-κB (n = 6) using ELISA, western blot analysis for NF-kB p65 (n = 4), histopathological examination using hematoxylin and eosin (H&E) staining (n = 4), and immunohistochemistry for NF-κB (n = 4). H&E staining and immunohistochemistry were performed using the same eye. Additionally, we performed transmission electron microscopy (TEM; n = 4) for accurate measurement of the retinal vascular membrane thickness.
Fundus photography and vessel diameter
The fundus photographs were taken after pupillary dilation with 1% tropicamide (Sunways, Mumbai, India) in conscious rats using a fundus camera (Genesis-Df, Kowa, Tokyo, Japan). The arteriolar and venular diameters in the fundus photographs were estimated as described previously [28]. Briefly, the vessel diameters of the three most prominent vessels were estimated at three sites in its widest portion. Before the diameter estimation, the retinal photographs from all groups were randomized, and three independent observers performed the estimations. An average of three estimates was taken as the final vessel diameter.
Inflammatory and angiogenic parameters
For the estimation of TNF-a α and VEGF, isolated retinas were homogenized in ice cold phosphate buffer saline (PBS; pH 7.4). Subsequently, TNF-α (n = 6) and VEGF levels in retinas (n = 6) were estimated using commercially available ELISA kits according to the manufacturer’s instructions.
Histopathological studies
The retinal tissues (n = 4) were fixed in 4% phosphate buffered formalin. Paraffin-embedded tissue was cut into 4-µm sections; slides were prepared and stained with H&E. Histological features were studied by an experienced pathologist unaware of the identity of the samples.
Immunohistochemistry for NF-ƙB
For the immunohistochemistry, 4-μm formalin-fixed, paraffin-embedded sections of the retinal tissue (n = 4 retinas) were placed on polylysine coated slides. The sections were deparaffinized and rehydrated, and endogenous peroxidase activity was blocked with H2O2 in methanol. The sections were pretreated in citrate buffer (pH 6.0) for antigen retrieval in a microwave oven [29]. Incubation was performed overnight under humid conditions using NF-ƙB rabbit polyclonal antibody (dilution 1:1,000, Abcam, Cambridge, UK) at 4 °C. The slides were then washed three times in Tris buffer for 5 min each. The mouse/rabbit ImmunoDetector horseradish peroxidase/3, 3-diaminobenzidinetetrahydrochloride (HRP/DAB) detection System (Bio SB) was used in which the sections were incubated with biotinylated anti-mouse and anti-rabbit immunoglobulin solution and then with streptavidin conjugated to HRP solution and finally with DAB plus chromogen. The slides were then counterstained with hematoxylin. Immunopositivity expression was assessed under a light microscope.
Western blot for NF-ƙB and ELISA for phosphorylated NF-ƙB
The retinal tissues were homogenized in ice-cold protein extraction buffer RIPA (3M Tris-HCl, 5M NaCl, 0.01% Nonidet P40, 0.1% glycerol, 100 mM sodium vanadate, and 0.05% protease inhibitor cocktail; Sigma-Aldrich Corporation, Bangalore, India) and incubated at 4 °C for 1 h followed by centrifugation at 10,000 ×g for 30 min at 4 °C. The supernatant was collected immediately after centrifugation and stored at −80 °C. Protein concentrations of the samples were estimated with the Bradford method. Twenty-five µg of protein was loaded in each lane, along with protein markers (molecular weight range: 10–250 kDa, Bio-Rad, Hercules, CA) in 10–12% sodium dodecyl sulfate (SDS) polyacrylamide gels and electrophoresed at 80 V. The separated proteins were then transferred on the nitrocellulose membranes at 65 V for 50 min. Later, the membranes were treated with 3% non-fat milk for 1 h to block the non-specific binding and incubated with primary antibodies against NF-kB p65 (dilution: 1:1,000, rabbit polyclonal, Abcam) and β-actin (1:5,000, mouse monoclonal, Sigma-Aldrich) for 12 h at 4 °C. The blots were then incubated in the anti-mouse and anti-rabbit secondary antibodies (dilution: 1:200) followed by incubation in preformed avidin-biotin-peroxidase complex for 2 h. The protein bands were visualized by incubation in the development solution containing DAB and hydrogen peroxide. Protein extracts from the rat retina were used as a positive control, and β-actin was used as the loading control for proteins. For densitometric analysis, the blots were scanned in a gel documentation system, using Quantity 1 software (Bio-Rad). All blots were scanned simultaneously and normalized with β-actin. The activation of NF-κB was also quantified by measuring the phosphorylated NF-κB p65 levels using the phosphorylated NF-κB p65 ELISA kit (Cell Signaling Technology, Danvers, MA), according to the manufacturer’s instructions and as described previously [30].
Transmission electron microscopy
For TEM, the retinal tissues were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). After 6 h of fixation at 4 °C, the retinas were cut around 2 mm from the region of the optic nerve head and further trimmed into 1–2 mm pieces. The 1–2 mm pieces were again fixed in 1% osmium tetraoxide, dehydrated, and embedded in araldite CY 212. The semithin sections (500 nm thick) were examined under a light microscope to select the areas of interest after staining with 0.5% toluidine blue. Thin sections (70 nm) were cut using an ultramicrotome, contrasted with uranyl acetate and lead citrate and viewed under a TECNAI G20 transmission electron microscope (FEI, Eindhoven, the Netherlands).
Measurement of vascular basement membrane thickness
The capillaries from the nerve fiber layer (NFL) and the ganglion cell layer (GCL) of the retinas were selected for the evaluation of the basement membrane (BM) thickness. Five sections were selected at 500 µm from the mid-retina. A total of four rat retinas were analyzed per group. Only cross-sectioned capillaries were considered for the BM thickness, which was measured using the Siperstein method [31]. The cross-sectioned capillaries were superimposed on a vessel, and the BM thickness was measured at the points of intersection by each beam divided symmetrically into 24 clock hours [32].
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical comparisons were made using one-way ANOVA (ANOVA) with the post-hoc analysis using the Dunnett multiple comparison test (Graph Pad Prism, Version 5). A p value of less than 0.05 was considered statistically significant.
Results
Bodyweight and blood glucose
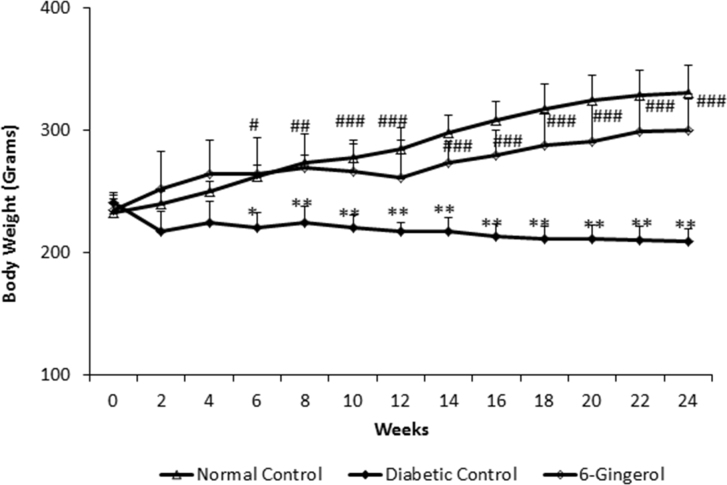
The mean bodyweight of the rats in three groups was comparable at the start of the study (p>0.05). However, at the end of the 24-week experimental period, the mean bodyweight of the animals in the DC group (210.66±11.30 g) was significantly (p<0.0001) lower than that of the NC group (328.16±20.68 g). The 6-G-treated group showed significantly greater bodyweight (298.83±29.89 g) when compared with the DC group (p<0.0001; Figure 1).
Figure 1.
The rats treated with Zingiber officinale, showed significantly lesser weight gain compared to normal rats; however it was significantly greater than diabetic controls. (n=15 for all groups). Values are mean ± standard deviation (SD). NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated. **p<0.001; *** p<0.01; ****p<0.05 versus the NC group. ##p<0.001 and ###p<0.01 versus the DC group.
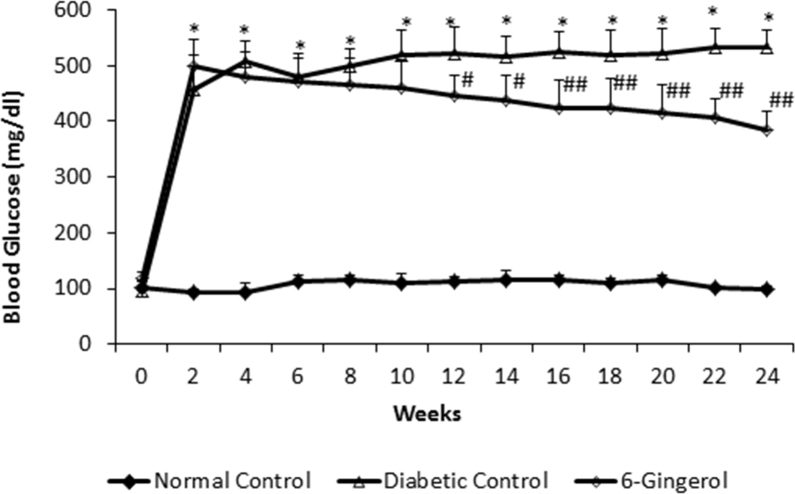
At 24 weeks, the mean blood glucose level in the DC group (533.66±113.12 mg/dl) was significantly higher (p<0.0001) than that in the NC group (99.16±8.36 mg/dl). Oral administration of the ginger extract reduced the blood glucose levels to a mean value of 361.33±93.44 mg/dl, which was significantly lower compared to that in the DC group (p<0.001) but remained significantly higher than in the NC group (p<0.001; Figure 2). In line with the blood glucose levels, HbA1c in the 6-G-treated group (6.66±1.11) was also significantly lower than in the DC group (9.91±1.56; p<0.0001) but remained significantly higher than in the NC group (4.95±0.68; p<0.01).
Figure 2.
The rats treated with Zingiber officinale, showed significantly greater blood glucose level compared to normal rats; however it was significantly lower than diabetic controls. (n=15 for all groups). All data points represent mean ± standard deviation (SD). NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated diabetic group. **p<0.001 versus the NC group. ##p<0.001 and ###p<0.01 versus the DC group.
Fundus photography and vessel diameter
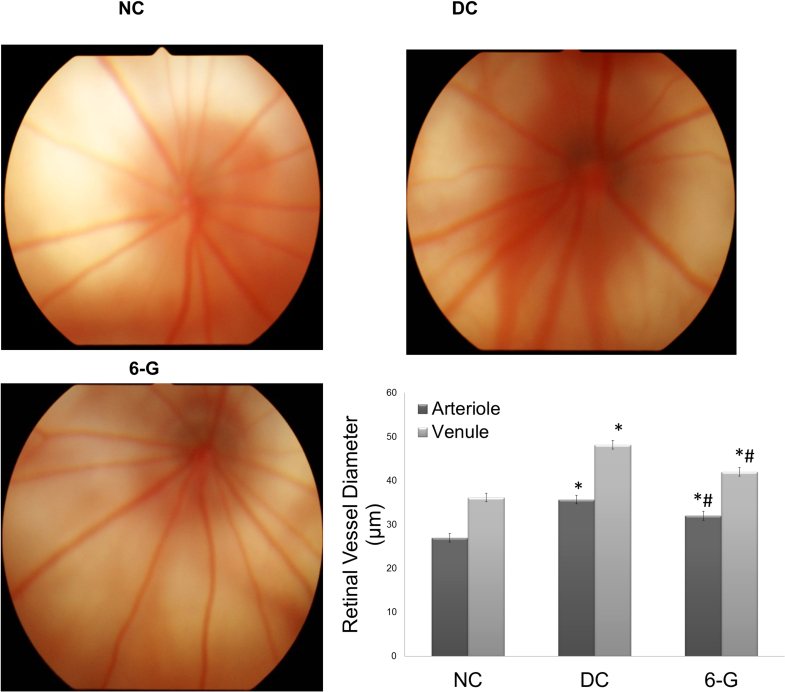
The retinal blood vessels in the DC group showed a significantly greater diameter compared to that in the NC group (p<0.0001), whereas in the 6-G-treated group the vessel diameter was significantly reduced compared to that in the DC group (arterioles p<0.001, venules p<0.0001). When compared to the NC group, the vessel diameter in the 6-G-treated group remained significantly higher (arterioles p<0.0001, venules p<0.0001; Figure 3).
Figure 3.
Fundus photographs after 24 weeks of treatment with standardized extract of Zingiber officinale in the rats with STZ-induced diabetes. Fundus photographs in the NC group show normal vascular architecture. In the DC group, the fundus photographs show dilated vessels. In the 6-G-treated group, the retinal vessel showed normalization of the vessel diameter (n=15 for all groups). Values are mean ± standard deviation (SD); *p<0.0001 versus the corresponding NC group; #p<0.0001 versus the corresponding DC group. NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated.
Proinflammatory and angiogenic markers
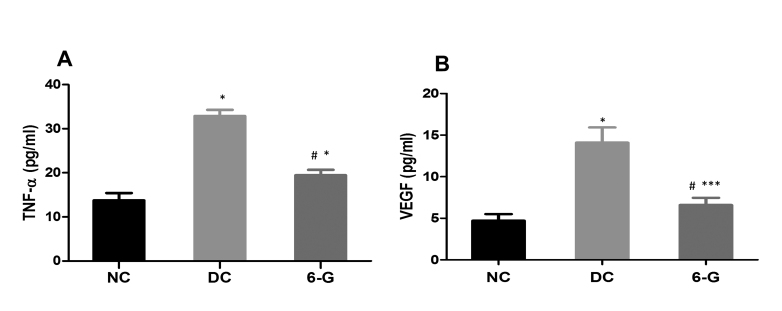
The levels of the proinflammatory marker, TNF-α, in the retinal homogenate from the DC group (32.88±1.42 pg/ml) were significantly higher compared to that in the NC rats (13.75±1.67 pg/ml). The same in the 6-G-treated group (19.47±1.23) showed significant reduction compared to the DC group; however, remained higher compared to that in the NC group (Figure 4A). The VEGF levels in the DC group (14.10±1.8 pg/ml) were significantly higher compared to that in the NC group (4.71±0.8 pg/ml), whereas the 6-G rats showed mean VEGF levels of 6.6±0.85 pg/ml, which was significantly lower than in the DC rats but remained higher than in the NC rats (p<0.0001 versus DC; p<0.01 versus NC; Figure 4B). The significantly improved TNF-α and VEGF levels in the 6-G-treated group compared to the DC group but not the NC group were in accordance with the effects observed on the vessel diameter in this group.
Figure 4.
The effect of standardized extract of Zingiber officinale on retinal TNF-α and VEGF levels after 24 weeks of treatment in rats with STZ-induced diabetes. All values are mean ± standard deviation (SD). NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated. *p<0.0001; *** p<0.01 versus the NC group; #p<0.0001 versus the DC group.
Histopathological changes
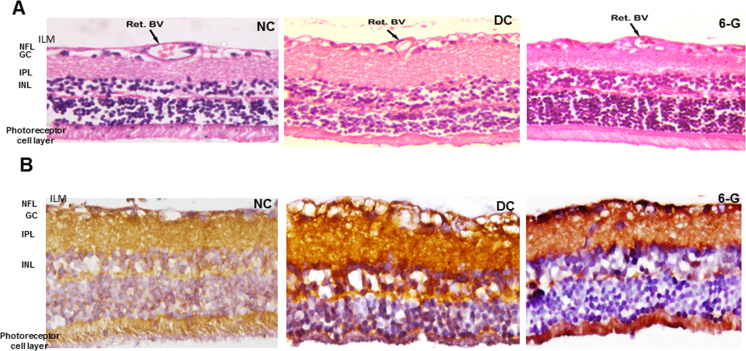
The H&E-stained sections showed well-organized retinal layers in the NC group. In the DC group, we observed edematous retinal tissue, particularly in the inner retina. Observation of the retinal blood vessel (Ret BV) was in line with the observations made earlier of vessel diameters in the fundus photography as we observed a thinner vascular basement membrane in the 6-G-treated group as well as in the NC group compared to the DC group indicating considerable thickening of the vascular basement membrane in the DC group and a reduction in the thickness after treatment with 6-G (Figure 5A).
Figure 5.
Microphotographs (40X) showing the effect of the standardized extract of Zingiber officinale on the retinal vasculature and nuclear factor-kappa B (NF-κB) expression after 24 weeks of treatment in rats with STZ-induced diabetes. A: Hematoxylin and eosin stained retinal sections showed thickened wall of the retinal blood vessel (Ret BV) in the DC group but not in the group treated with 6-G (n=4 for all groups). B: Retinal microphotographs after immunostaining for nuclear factor-kappa B (NF-κB) showed intense staining in NFL, IPL and INL. Less intense staining was observed in 6-G group (n=4 for all groups). NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated diabetic group. ILM = inner limiting membrane; NFL = nerve fiber layer; GCL = ganglion cell layers; IPL = inner plexiform layer; INL = inner nuclear layer.
Immunohistochemistry
The retinas in the DC group showed intense expression indicating higher expression of NF-kB in the nerve fiber layer (NFL), inner plexiform layer (IPL), and inner nuclear layer (INL). Less intense expression of NF-kB was visualized in the NFL, IPL, and INL in the 6-G retinas (Figure 5B).
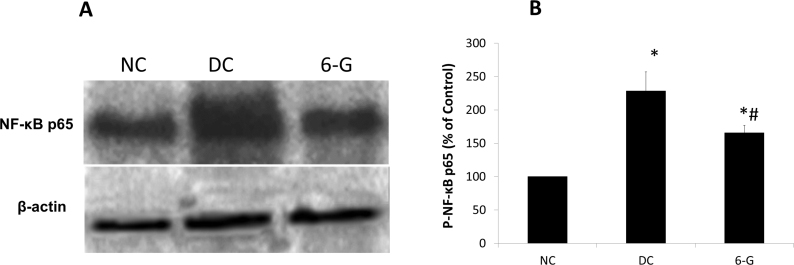
Western blot for NF-kB and ELISA for phosphorylated NF-kB
Western blot analysis was performed to assess the protein level of NF-κB p65 in the retinas of the three groups. We observed little expression of NF-κB P65 in the NC retinas whereas in the DC retinas the amount of NF-κB P65 had notably increased. However, in the retinas of the diabetic rats treated with 6-G, the expression of NF-κB p65 was markedly reduced. Quantification of P-NF-κB using ELISA showed significantly elevated levels in the DC rats compared to the NC rats, while the same was significantly lower in the 6-G-treated rats compared to the DC rats (Figure 6). These observations were in agreement with those made in the immunohistochemistry.
Figure 6.
Effect of standardized extract of Zingiber officinale on retinal NF-kB p65 expression after 24 weeks of treatment as shown with western blot. A: Intense bands were seen on western blot in DC groups but not in 6-G treated group (n=4 for all groups). B: Quantification of P-NF-kB p65 in the retinas of three groups of animals using enzyme-linked immunosorbent assay (ELISA; n=4 for all groups). Each value represents mean ± standard deviation (SD). NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated diabetic group. *p<0.0001 versus the NC group; #p<0.001 versus the DC group.
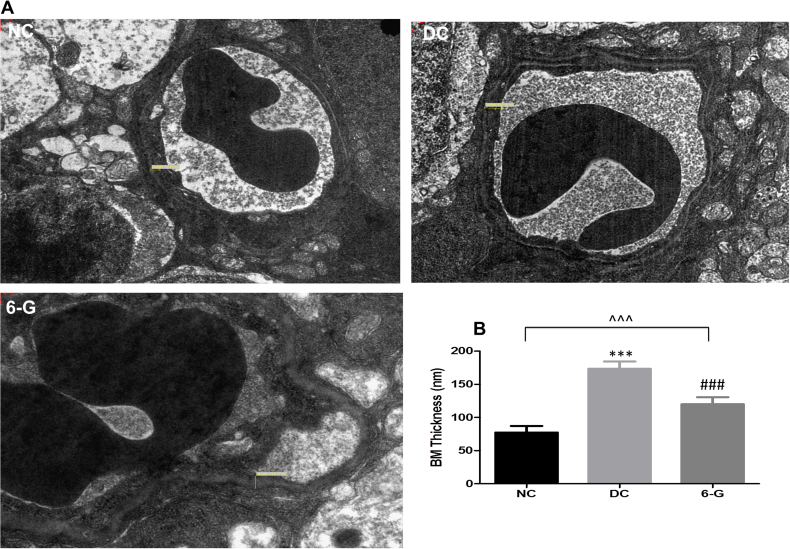
Basement membrane thickness
Electron microscopy revealed that the mean vascular basement membrane thickness in the DC group (173.64±10.85 nm) was significantly higher compared to the NC group (77.46±9.83 nm; p<0.0001). However, in the 6-G-treated group, the basement membrane thickness (120.14±10.50 nm) was significantly lower compared to the DC group (p<0.0001) but remained higher compared to the NC group (p<0.001; Figure 7). The electron microscopic measurements of the basement membrane thickness supported our observations made on the histopathological examination, which also showed that the basement membrane thickness was greater in the DC group compared to the NC group and the 6-G-treated group, and the same was comparable in the 6-G-treated group and the NC group.
Figure 7.
Transmission electron micrographs showing the retinal capillary endothelial BM. A: Thickening of the basement membrane (BM) was observed in the DC group but not in the 6-G group. B: Quantitative expression of capillary BM thickness in three groups after 24 weeks of treatment (n=4 for all groups). Values are mean ± standard deviation (SD).*p<0.0001 versus NC, #p<0.0001 versus DC. NC = normal control; DC = diabetic control; 6-G = Zingiber officinale–treated diabetic group.
Discussion
The present study demonstrated the effects of oral administration of the ginger extract containing 5% 6-G on DR in rats with STZ-induced DR. The treatment resulted in a significant reduction in the diameter of the retinal vessels and the vascular basement membrane thickness. A previous study has also shown that ginger in combination with purple waxy corn protects against retinopathy in rats with STZ-induced diabetes. In that study, the protective effect of the combined herbs was associated with reduced retinal oxidative stress [33]. In this study, the improvement in the architecture of the retinal vasculature was associated with significantly reduced activity of TNF-α, NF-κB, and VEGF in the retinas of the 6-G-treated group compared to the DC group. Additionally, we observed significant reduction in the blood glucose level compared to the DC group and better preservation of bodyweight in the 6-G-treated diabetic rats.
Several complex interconnecting biochemical pathways play a key role in the pathogenesis of microvascular complications of diabetes mellitus such as retinopathy. The role of the highly active polyol pathway that involves aldose reductase is well-established. Molecular docking studies have shown that gingerenones A, B, and C from ginger have a high docking score, binding affinity, and sustained protein-ligand interactions [34]. Thus, it is likely that inhibition of aldose reductase contributed to the reduced retinal microvascular changes in the 6-G-treated group by reducing the polyol accumulation. Excessive formation and accumulation of the AGEs is one of these biochemical pathways, and evidence from animal studies has shown that exposure to AGEs results in vascular complications [35-37]. In the current study, we did not measure the AGEs; however, their accumulation is one of the certain outcomes of prolonged hyperglycemia that was present in the STZ-injected animals. In the DC group, the presence of persistent hyperglycemia was further supported by elevated HbA1C. Treatment with 6-G resulted in a significant reduction in blood glucose levels and consequently HbA1c. Bodyweight measurement also indicated reduced catabolic influence of hyperglycemia in the 6-G-treated rats compared to the DC rats.
AGEs form covalent crosslinks between proteins, cause oxidative stress, and bring about changes in the structure and function of cellular matrices, basement membranes, and vessel-wall components [38]. They also interact with cell-surface AGE-binding receptors leading to cell activation and proinflammatory responses [35]. Additionally, hyperglycemia leads to activation of a serine/threonine kinase, protein kinase C (PKC) that brings about remodeling of the vascular structures and enhances the inflammatory responses [39-41]. In the current study, one of the hallmark features of DR in the STZ-injected animals, namely, the thickening of the basement membrane, was clearly documented using transmission electron microscopy and light microscopy. In these groups, the structural alterations in the retinal arterial and venular components were also seen by fundus photography in the form of increased vessel diameter compared to healthy vessels. Treatment of the diabetic rats with 6-G resulted in significant improvement in the microvascular structure.
In this study, the vascular structural changes in the STZ-injected rats were associated with increased retinal levels of TNF-α, NF-κB, and VEGF. Treatment with 6-G resulted in significant reduction in all three parameters. It is well-established that inflammation plays a prominent and complex role in DR. Hyperglycemia-initiated oxidative stress, AGE accumulation, and PKC activation induce an inflammatory reaction that further amplifies through increased production of inflammatory mediators. NF-κB is a transcription factor, and NF-κB signaling promotes increased expression of proinflammatory cytokines such as TNF-α. The retinas of diabetic animals and retinal cell culture in high glucose show increased NF-κB DNA binding affinity for the development of DR via proinflammatory effects [42]. Levels of TNF-α have been found to be significantly elevated in the vitreous of patients with proliferative diabetic retinopathy (PDR), and the role of TNF-α in PDR pathogenesis has been characterized [43-45]. Increased levels of TNF-α are associated with BRB breakdown, retinal leukostasis, and apoptosis [46,47]. Additionally, amplified VEGF signaling leads to BRB breakdown and increased vascular permeability.
In the current study, the beneficial effects of 6-G in attenuating hyperglycemia-induced retinal microvascular structural abnormalities could be attributed to its effects primarily on the reduction of blood glucose levels. The polyphenols from ginger have previously been shown to have hypoglycemic and insulinotropic properties [48-50]. However, ginger extract has also been shown to significantly reduce the elevated expression of NF-κB and TNF-α in the absence of hyperglycemia [51,52]. Ginger extract was shown to suppress PKC alpha and NF-κB pathways in lipopolysaccharide-stimulated mouse macrophages [53]. 6-G also inhibits VEGF-induced proliferation of human endothelial cells [24]. Thus, it is likely that in the current study, the effects of 6-G on the retinal vasculature may partially be attributed to 6-G’s direct anti-inflammatory and antiangiogenic actions resulting from the suppression of NF-κB signaling and the suppression of TNF-α and VEGF activity.
In conclusion, the current study showed that the standardized extract of Zingiber officinale attenuates retinal microvascular changes in STZ-induced diabetic rats through its anti-inflammatory and antiangiogenic actions. Although these effects could result from the antihyperglycemic effects of the ginger extract containing 5% 6-G, it is likely that this can also be attributed at least partially to the extract’s direct effects on the retinal vasculature. Although the precise molecular targets remain to be determined, 6-gingerol seems to be a potential candidate for further investigation.
Acknowledgments
The financial support provided by UKIERI and Department of Science and Technology (DST/INT/UK/P39/2012), India is gratefully acknowledged. The facilities for electron microscopy availed at SAIF (DST), All India Institute of Medical Sciences, New Delhi, are acknowledged.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease (META-EYE) Study Group Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22301125&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–31. doi: 10.4103/0301-4738.100542. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22944754&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11742414&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15919781&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol. 2012;19:52–9. doi: 10.4103/0974-9233.92116. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22346115&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–5. doi: 10.1136/bjo.86.4.363. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11914197&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–28. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19641635&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 8.Abu el Asrar AM, Maimone D, Morse PH, Gregory S, Reder AT. Cytokines in the vitreous of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1992;114:731–6. doi: 10.1016/s0002-9394(14)74052-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1463043&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 2006;20:1366–9. doi: 10.1038/sj.eye.6702138. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16284605&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Carmo A, Cunha-Vaz JG, Carvalho AP, Lopes MC. L-arginine transport in retinas from streptozotocin diabetic rats: correlation with the level of IL-1 beta and NO synthase activity. Vision Res. 1999;39:3817–23. doi: 10.1016/s0042-6989(99)00117-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10748917&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–65. doi: 10.2337/diabetes.54.5.1559. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15855346&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Ben-Mahmud BM, Mann GE, Datti A, Orlacchio A, Kohner EM, Chibber R. Tumor necrosis factor-alpha in diabetic plasma increases the activity of core 2 GlcNAc-T and adherence of human leukocytes to retinal endothelial cells: significance of core 2 GlcNAc-T in diabetic retinopathy. Diabetes. 2004;53:2968–76. doi: 10.2337/diabetes.53.11.2968. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15504978&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–6. doi: 10.1167/iovs.04-0633. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15505070&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 14.Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37:1169–80. doi: 10.1080/10715760310001604189. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14703729&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R, Jha KA, Srinivasan BP. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24952278&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998;101:1219–24. doi: 10.1172/JCI1277. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9502762&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–62. doi: 10.1167/iovs.02-0807. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12714656&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 18.Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003;52:864–71. doi: 10.2337/diabetes.52.3.864. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12606532&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Zhang SX, Ma JX, Sima J, Chen Y, Hu MS, Ottlecz A, Lambrou GN. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166:313–21. doi: 10.1016/S0002-9440(10)62255-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15632023&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2007;48:5152–9. doi: 10.1167/iovs.07-0427. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17962468&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Hernández C, Burgos R, Cantón A, García-Arumí J, Segura RM, Simó R. Vitreous levels of vascular cell adhesion molecule and vascular endothelial growth factor in patients with proliferative diabetic retinopathy: a case-control study. Diabetes Care. 2001;24:516–21. doi: 10.2337/diacare.24.3.516. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11289478&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Shukla Y, Prasad S, Tripathi C, Singh M, George J, Kalra N. In vitro and in vivo modulation of testosterone mediated alterations in apoptosis related proteins by [6]-gingerol. Mol Nutr Food Res. 2007;51:1492–502. doi: 10.1002/mnfr.200700197. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18030663&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. [[6]]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol Lett. 2012;210:34–43. doi: 10.1016/j.toxlet.2012.01.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22285432&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [[6]]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–8. doi: 10.1016/j.bbrc.2005.07.076. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16081047&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6- gingerol induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47:197–208. doi: 10.1002/mc.20374. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18058799&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang SZ, Wang NS, Mi SQ. Plasma pharmacokinetics and tissue distribution of [6]-gingerol in rats. Biopharm Drug Dispos. 2008;29:529–37. doi: 10.1002/bdd.638. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19051331&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Li CY, Wen XD, Li P, Qi LW. Plasma pharmacokinetics, tissue distribution and excretion study of 6-gingerol in rat by liquid chromatography–electrospray ionization time-of flight mass spectrometry. J Pharm Biomed Anal. 2009;49:1070–4. doi: 10.1016/j.jpba.2009.01.020. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19217234&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agarwal R, Agarwal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–30. doi: 10.1089/jop.2010.0123. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21314438&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 29.Fouad AA, Al-Sultan AI, Yacoubi MT, Gomaa W. Ameliorative effects of telmisartan in diabetic rats with indomethacin-induced gastric ulceration. Eur J Pharmacol. 2010;637:162–70. doi: 10.1016/j.ejphar.2010.04.007. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20399771&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 30.Kubota S Ozawa Y, Kurihara T, Sasaki M, Yuki K, Miyake S, Noda K, Ishida S, Tsubota K.Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011;52:9142–8. doi: 10.1167/iovs.11-8041. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22058332&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 31.Siperstein MD, Norton W, Unger RH, Madison LL. Muscle capillary basement membrane width in normal, diabetic and prediabetic patients. Trans Assoc Am Physicians. 1966;79:330–47. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=5929467&dopt=Abstract [PubMed] [Google Scholar]
- 32.Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, Jha KA. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res. 2013;87:65–74. doi: 10.1016/j.mvr.2013.01.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23376836&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 33.Thiraphatthanavong P, Wattanathorn J, Muchimapura S, Thukham-mee W, Lertrat K, Suriharn B. The combined extract of purple waxy corn and ginger prevents cataractogenesis and retinopathy in streptozotocin-diabetic rats. Oxid Med Cell Longev. 2014;2014:789406. doi: 10.1155/2014/789406. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25614778&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antony P, Vijayan R. Identification of Novel Aldose Reductase Inhibitors from Spices: A Molecular Docking and Simulation Study. PLoS One. 2015;10:e0138186. doi: 10.1371/journal.pone.0138186. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26384019&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Nat Aca Sci Am. 1991;88:11555–8. doi: 10.1073/pnas.88.24.11555. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1763069&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammes HP, Weiss A, Führer D, Krämer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996;39:251–5. doi: 10.1007/BF00418338. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8721768&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 37.Stitt A, Gardiner TA, Anderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–32. doi: 10.2337/diabetes.51.9.2826. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12196477&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 38.Nasir NAA, Agarwal R, Vasudevan S, Tripathy M, Alyautdin R, Ismail NM. Effects of topically applied tocotrienol on cataractogenesis and lens redox status in galactosemic rats. Mol Vis. 2014;20:822–35. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24940038&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 39.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9287049&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 40.Koya D, King GL. Protein kinase C activation and the developmentof diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9604860&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 41.Aiello LP, Clermont A, Arora V, Davis MD, Sheetz MJ, Bursell SE. Inhibition of PKC β by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci. 2006;47:86–92. doi: 10.1167/iovs.05-0757. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16384948&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 42.Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007;56:337–45. doi: 10.2337/db06-0789. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17259377&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 43.Adamiec-Mroczek J, Oficjalska-Młyńczak J, Misiuk-Hojło M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: analysis of vitreous samples. Cytokine. 2010;49:269–74. doi: 10.1016/j.cyto.2009.11.004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20015663&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 44.Ola MS, Nawaz MI, Siddiquei MM, Al-Amro S. Abu El-AsrarAM. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabet Complications. 2010;26:56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNF-alpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;10:1336–44. doi: 10.1167/iovs.10-5768. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21212173&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18340447&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 47.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2010;33:804–10. doi: 10.2337/dc09-1679. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20097779&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiberofficinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2004;56:101–5. doi: 10.1211/0022357022403. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14980006&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 49.Huang CN, Horng JS, Yin MC. Antioxidative and antiglycative effects of six organosulfur compounds in low-density lipoprotein and plasma. J Agric Food Chem. 2004;52:3674–8. doi: 10.1021/jf0307292. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15161248&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 50.Kondeti RS, Korivi M, Kesireddy N, Kuo CH, Kesireddy SR. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2001;124:1436–42. [Google Scholar]
- 51.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–96. doi: 10.1093/carcin/bgl241. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17151092&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 52.Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, Yusof YA. Ginger extract (Zingiberofficinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 2008;63:807–13. doi: 10.1590/S1807-59322008000600017. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19061005&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382:134–9. doi: 10.1016/j.bbrc.2009.02.160. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19268427&dopt=Abstract [DOI] [PubMed] [Google Scholar]