Abstract
Obesity is one of the major risk factors for nonalcoholic fatty liver disease (NAFLD), and NAFLD is highly associated with an increased risk of cardiovascular disease (CVD). Scholars have suggested that certain probiotics may significantly impact cardiovascular health, particularly certain Lactobacillus species, such as Lactobacillus reuteri GMNL-263 (Lr263) probiotics, which have been shown to reduce obesity and arteriosclerosis in vivo. In the present study, we examined the potential of heat-killed bacteria to attenuate high fat diet (HFD)-induced hepatic and cardiac damages and the possible underlying mechanism of the positive effects of heat-killed Lr263 oral supplements. Heat-killed Lr263 treatments (625 and 3125 mg/kg-hamster/day) were provided as a daily supplement by oral gavage to HFD-fed hamsters for eight weeks. The results show that heat-killed Lr263 treatments reduce fatty liver syndrome. Moreover, heat-killed Lactobacillus reuteri GMNL-263 supplementation in HFD hamsters also reduced fibrosis in the liver and heart by reducing transforming growth factor β (TGF-β) expression levels. In conclusion, heat-killed Lr263 can reduce lipid metabolic stress in HFD hamsters and decrease the risk of fatty liver and cardiovascular disease.
Keywords: Lactobacillus reuteri GMNL-263, transforming growth factor β, cardiovascular diseases
1. Introduction
Metabolic syndrome comprises of hypertension, dyslipidemia, obesity, glucose intolerance, and cardiovascular disease (CVD) [1,2,3,4,5]. Lipid metabolic disorders and an increase in adipose tissue accompany cardiovascular disease, particularly with obesity [6]. The main cause of obesity is excessive calorie and sugar intake [7]. An investigation into the relationship between general and central obesity revealed that all-cause and CVD-related mortality in an Asian population exhibited higher central-obesity indices, such as waist circumference (WC) [8].
In previous studies, carbon tetrachloride-induced liver injury in animal experiments showed that liver damage-induced abnormal lipid metabolism increased both cholesterol and transforming growth factor β (TGF-β) levels in blood [9,10]. Overexpression of TGF-β from a damaged liver may cause cirrhotic cardiomyopathy (CCM) [11,12]. Furthermore, lipid metabolism abnormalities in the liver also cause coagulation function disorders because many clotting factors are synthesized and secreted by the liver [13,14].
The TGF-β cytokines are pleiotropic and implicated in a wide variety of extra-cellular matrix deposition, cell proliferation, and differentiation pathways [15]. Connective-tissue growth factor (CTGF) and endothelin may also be induced and expressed as TGF-β downstream effectors [16]. TGF-β-induced CTGF expression can lead to cardiomyocyte hypertrophy and fibroblast proliferation; these changes contribute to cardiac remodeling [17,18].
Recently, certain reports have revealed the effects of certain probiotic strains on cholesterol and hypertension reduction, and these data suggest that probiotics could be more widely applied for cardiovascular health [19,20,21]. Reports show that Lactobacillus reuteri is a probiotic species with a serum cholesterol-lowering ability in humans [22]. The most accepted mechanism underlying these effects is that Lactobacillus features bile salt hydrolase (BSH) activity, which suggests that probiotics may cause deconjugation effects in primary bile acids and promote the secondary bile salts by amino acid conjugations in the gut [23]. These effects will break down the cholesterol-bile salt reabsorption and lower the cholesterol levels of the hosts [24,25,26].
Cholesterol and hypertension are risk factors associated with obesity in causing heart disease; these risks are reduced by nearly half when cholesterol and hypertension decrease [19]. Recently, two reports showed that oral Lactobacillus reuteri GMNL-263 (Lr263) administration can prevent renal fibrosis in a diabetic kidney, improve insulin resistance, and ameliorate hepatic steatosis in high fructose-fed rats [27,28]. However, probiotic administration may cause significant change to the gut biota profile in the host. Therefore heat-killed bacteria have become an attractive future strategy to simulate the effects of probiotics. Previous studies show that heat-killed Lr263 potentially improved in heart function against the effects of HFD [29]. In our previous research, a high-fat diet treatment caused obesity and cardiac fibrosis in an animal model [21,26,28]. In this work, a high-fat diet was employed to induce obesity and cardiac fibrosis in hamsters; the protective effects exerted by different doses of heat-killed Lr263 on the heart and liver were also investigated in hamsters with high-fat diet-induced obesity.
2. Results and Discussion
Epididymal adipose tissue comprises the body fat tissue of an animal [30]. After two months of experimentation, the epididymal adipose tissue mass in the HFD-only hamsters was greater than in the controls (Figure 1A). The epididymal adipose mass decreased in a dose-dependent manner for the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups. Furthermore, fatty acid synthase (FAS) is a biomarker of liver lipid metabolism [31,32]. After the RT-PCR analysis, FAS increased in the HFD-only group and decreased in the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups (Figure 1B). However, HMG-CoA reductase increased in the HFD group rats and decreased only slightly in the heat-killed Lr263 treatment groups, which was not significant (Figure 1C). LDLR and CYP7A1 are liver cholesterol metabolism biomarkers [33]. After the RT-PCR analysis, LDLR and CYP7A1 were lower in the HFD-only group and greater in the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups (Figure 1D–F).
Figure 1.
The animal epididymal adipose tissue weight and liver lipid and cholesterol metabolism biomarkers. (A) The HFD hamster epididymal adipose tissue weights were higher than in the control group, and the apididymal adipose tissue weight was lower in the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups; (B) FAS; (C) HMG-CoA reductase; (D) CYP7A1; (E) PPARγ; and (F) LDLR are liver lipid metabolism biomarkers and were analyzed using RT-PCR. FAS and HMG-CoA reductase were greater in the HFD group and lower in the heat killed Lr263 (3125 mg/kg-hamster/day) treatment groups, but the difference in HMG-CoA reductase was not significant in the heat-killed Lr263 treatment groups. LDLR and CYP7A1 was lower in the HFD group and greater in the heat killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups. (* p < 0.05 compared with the HFD group).
Nonalcoholic fatty liver disease (NAFLD) is associated with an increased risk of cirrhosis in both obese adults and children [34]. Certain studies have shown that the fibrosis severity stage in non-alcoholic fatty liver disease (NAFLD) patients was highly associated with mortality from cardiovascular causes and cardiac risk [35,36,37]. Supplementation with probiotics to reset the symbiotic obese gut microbiome may be an approach to improving outcomes [19].
One study found no drastic change in food intake in Lactobacillus rhamnosus GG treated mice when compared to control C57BL mice, and the significant weight decrease in epididymal fat tissue was not because of reduced energy intake [38]. This experimental result suggests an anti-obesity effect of Lactobacillus rhamnosus GG administration is directly through epididymal fat mass reduction. [38]. In this work, heat killed Lr263 oral gavage treatment was provided for eight weeks and exhibited a similar effect, directly decreasing epididymal fat mass in HFD hamsters (Figure 1A). Furthermore, lipid and cholesterol metabolic function in the HFD hamster liver improved with heat-killed Lr263 treatments in a dose-dependent manner (Figure 1).
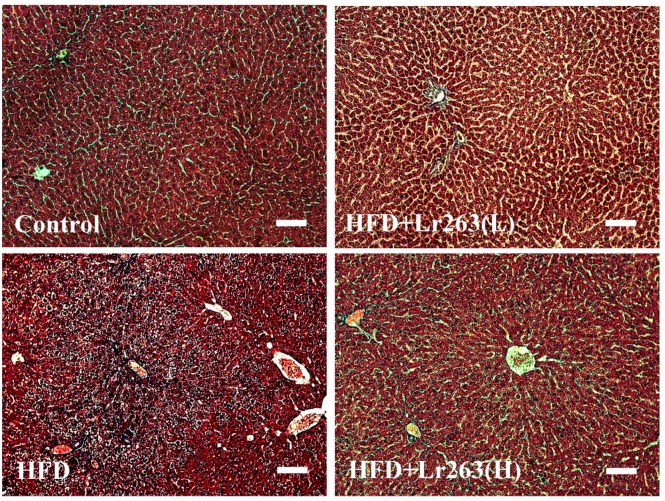
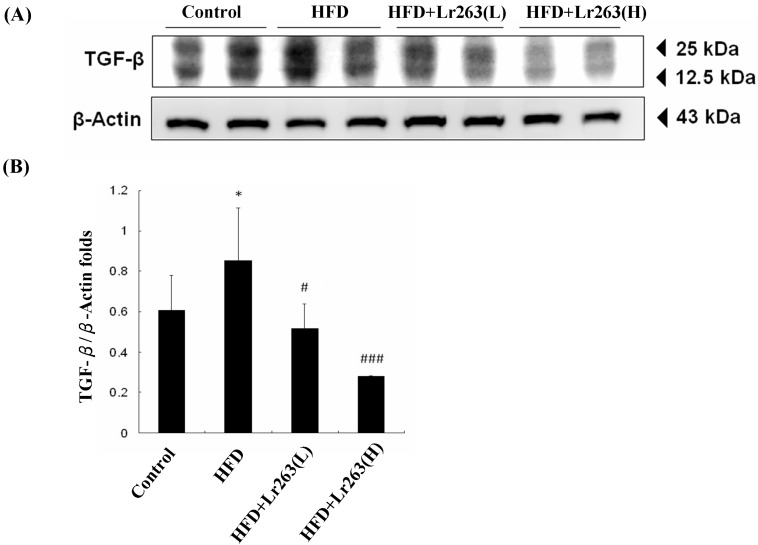
In this work, an autopsy of the HFD-induced fatty livers of the animals was used to evaluate fibrosis using Masson’s trichrome staining. For the HFD-only group hamster livers, the adipose capillaries that formed around the vacuoles are shown in Figure 2. However, collagen did not accumulate and cause fibrosis in the HFD-only group hamster livers. Furthermore, fatty liver disease and fibrosis were prevented in the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment groups (Figure 2). The TGF-β protein level was higher in the HFD-only group than in the other hamster livers (Figure 3). Heat-killed Lr263 (625 mg/kg-hamster/day) treatment decreased TGF-β protein levels in the liver to a level similar to that of the control group, and heat-killed Lr263 (3125 mg/kg-hamster/day) decreased TGF-β protein expression to a level lower than that in the HFD-only hamster livers.
Figure 2.
Fibrosis assessments. Cirrhosis was assessed using Massion’s trichrome (MS) staining assay to indicate the collagen accumulation (by the blue color) in liver tissue slides. The bar length is 100 μm.
Figure 3.
TGF-β protein levels in animal livers. (A) High TGF-β expression was observed only in the HFD group hamster livers; (B) The normalized protein expression of TGF-β with β-actin (* p < 0.05 compared with the control group, # p < 0.05, and ### p < 0.001 compared with the HFD group).
Interestingly, a possible mechanism underlying the effects of the probiotic is the interruption of cholesterol metabolism to replace the processed bile salts in the gut [24,26]. In this work, heat-killed Lr263 treatments also exerted a similar effect in the HFD hamsters. The lipid profiles of the livers and fecal analyses show a break-down in lipid absorption in the HFD hamsters after the heat-killed Lr263 treatments, as shown in Table 1. This result suggests a dose-dependent relationship between the heat-killed Lr263 treatments and lipid-elimination effects. Furthermore, the liver section results show higher TGF-β expression in the HFD hamster fatty liver (Figure 3), whereas the HFD hamster fatty liver only exhibits slight fibrosis (Figure 2).
Table 1.
The body weight, food intake, lipid profile of hamster livers and fecal analyses.
| Treatments | Control | HFD | HFD + Lr-263(L) | HFD + Lr-263(H) |
|---|---|---|---|---|
| Parameter | ||||
| Body Weight (g) | 126.5 ± 10.6 * | 138.3 ± 5.9 | 136.7 ± 3.8 | 124.7 ± 4.0 * |
| Food Intake (g) | 8.1 ± 0.8 | 8.2 ± 0.9 | 7.9 ± 1.1 | 7.8 ± 0.5 |
| Liver | ||||
| TG (mg/g) | 85.0 ± 6.5 * | 93.3 ± 5.3 | 90.7 ± 3.4 | 84.0 ± 4.3 * |
| T-CHO (mg/g) | 89.7 ± 0.4 * | 130.0 ± 4.9 | 118.3 ± 12.8 | 127.3 ± 6.0 |
| MDA (μg/g) | 4.2 ± 0.4 * | 8.6 ± 0.7 | 6.7 ± 1.1 | 5.5 ± 0.4 * |
| Fecal | ||||
| TG (mg/g) | 5.9 ± 0.7 | 4.7 ± 0.8 | 7.6 ± 0.9 * | 10.4 ± 1.7 * |
| T-CHO (mg/g) | 6.7 ± 0.7 | 8.5 ± 1.4 * | 8.5 ± 1.4 | 11.0 ± 0.8 * |
TG: triglyceride; T-CHO: total cholesterol; * p-value < 0.001 compared with the HFD group.
Another in vitro study evaluated probiotic cholesterol assimilation in culture media and under simulated intestinal conditions; the results show that most Lactobacillus strains exhibit strong cholesterol assimilation and that Lactobacillus reuteri NCIMB 701089 assimilated over 67% of the cholesterol [39]. This result is similar to our results and suggests that the cholesterol assimilation ability of a probiotic is independent of whether they are alive or in a probiotic bacterial culture. The LPS and CpG DNA potentially possess the predominant bioactivities of a bacterium even after heat attenuation. Several TLRs (A Toll-like receptor recognizes bacterial DNA) were known to be induced by some bacterial LPS and CpG DNA. [40]. Reports show that certain Lactobacillus strains can evoke immunostimulatory effects through Toll-like receptor 2 (TLR-2); TLR-2 and TLR-4 are the key mediators of the inflammatory reaction in human visceral adipose tissue [41,42]. The relationship between heat-killed and living Lr263 cardiac protective effects, as well as those of TLRs, requires more experimentation.
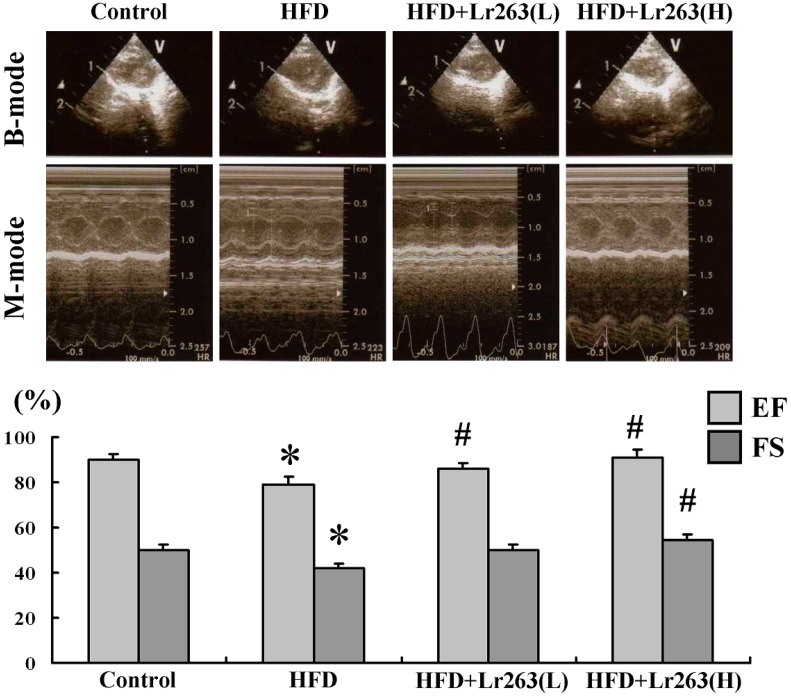
In the echocardiographic analysis results, the heart function evaluated in the control group hamsters using fractional shortening (FS) was 55.10%; the ejection fraction (EF) was 90.09%; the FS was 42.11%, and the EF was 79.02% in the HFD-only hamster group (Figure 4). After heat-killed Lr263 (625 mg/kg-hamster/day) treatment, the FS improved to 50.00%, and the EF improved to 86.22%. Moreover, after heat killed Lr263 (3125 mg/kg-hamster/day) treatment, the FS improved to 56.52%, and the EF improved to 91.03%. Based on our previous work, TGF-β expression in the liver may regulate cirrhosis cardiomyopathy (CCM) in the early stage of CCl4-induced liver fibrosis [12]. Furthermore, a previous report showed that early treatment with a neutralizing anti-TGF-β antibody increased mortality in an infarcted heart [43]. However, experimental evidence shows that late TGF-β inhibition decreases collagen deposition and suppresses the number of myofibroblasts in wound remodeling after the inflammatory phase in an infarcted heart [44]. In this work, TGF-β expression in the HFD hamster heart may result in remodeling and affect HFD hamster heart function (Figure 4).
Figure 4.
The echocardiography analysis of the hamster was performed using a 10 MHz transducer (GE 10S-RS). The B-mode was visualized for two-dimensional (long-axis and short-axis of the left ventricle) mode images and B-mode perspectives were further used to evaluate the left ventricle for the M-mode cursor. An M-mode evaluation of heart function was performed by comparing the left ventricular systolic and diastolic distances, which are shown as ejection fraction (EF) values and fractional shortening (FS) values (n = 6 in each group, * p < 0.05 compared with control group, and # p < 0.05 compared with HFD group).
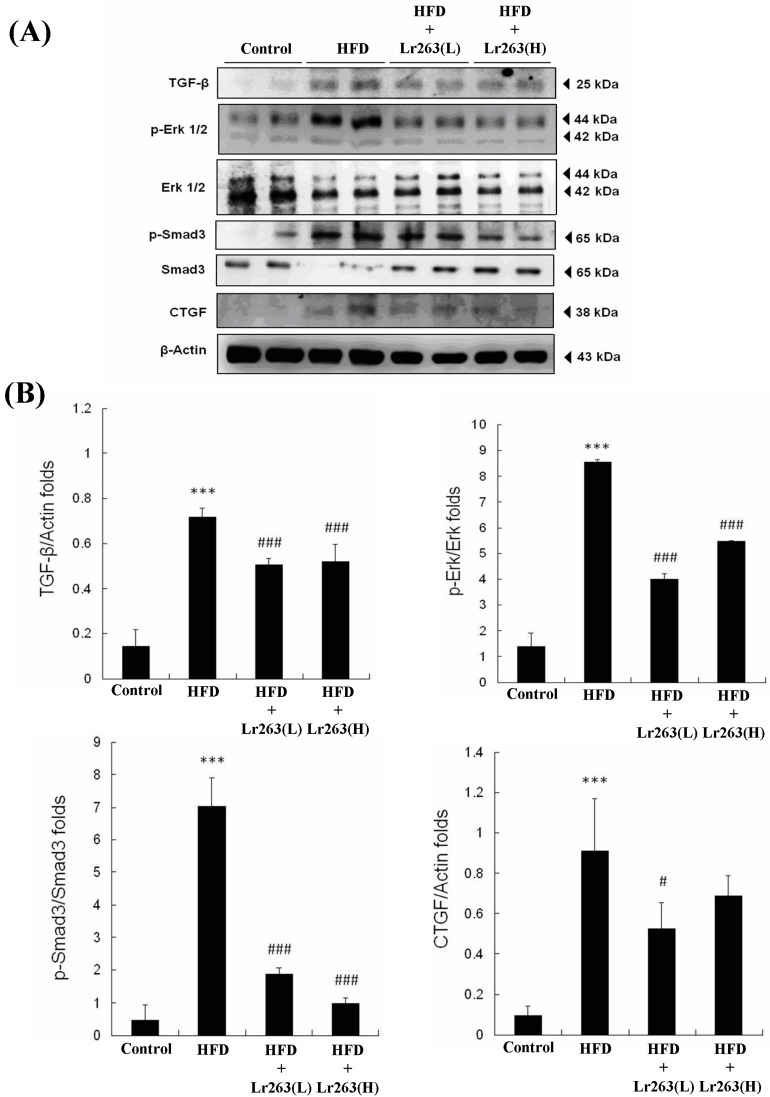
The sections with MT staining show fibrosis in portions of the HFD-only treatment hamster hearts (Figure 5). After heat-killed Lr263 (625 mg/kg-hamster/day) treatment, the fibrotic portions of the HFD treatment hamster hearts improved. Moreover, after heat-killed Lr263 (3125 mg/kg-hamster/day) treatment, the fibrotic portions in the HFD-only treatment hamster hearts exhibited more improvement. Furthermore, the TGF-β-induced fibrosis pathway was analyzed, and the TGF-β and its downstream proteins such as p-Erk, p-Smad3, and CTFG were highly expressed in the HFD-only treatment hearts (Figure 6). After heat-killed Lr263 (625 mg/kg-hamster/day) treatment, the TGF-β protein expression and downstream p-Erk, p-Smad3, and CTFG expression levels decreased in the HFD-only treatment hamster hearts.
Figure 5.
Assessment of fibrosis in the animal hearts. Cirrhosis was assessed using Masson’s trichrome (MS) staining assay to indicate collagen accumulation (the blue color indicated by the arrows) in the heart tissue slides. The bar length is 100 μm.
Figure 6.
TGF-β pathway protein expression analysis. (A) The TGF-β/Smad-3/CTGF expression levels increased in the HFD treatment hamster hearts and decreased in the heat-killed Lr263 (625 and 3125 mg/kg-hamster/day) treatment hamster hearts; (B) The normalized protein expression of TGF-β/β-actin, p-Erk/Erk, p-Smad3/Smad3, and CTGF/β-actin (*** p < 0.001 compared with the control group, # p < 0.05, and ### p < 0.001 compared with the HFD group).
Furthermore, Bujak et al. used mice with targeted disruption of Smad3, which showed no defect in inflammation resolution, but exhibited less fibrosis in the infarct; this result suggests that the Smad3-mediated fibrogenic actions of TGF-β do not regulate TGF-β anti-inflammatory functions [45]. The autopsy results show that TGF-β-induced fibrosis in certain portions of the HFD hamster hearts, which was slightly decreased by the heat-killed Lr263 (625 mg/kg-hamster/day) treatments (Figure 5). Furthermore, after heat killed Lr263 (3125 mg/kg-hamster/day) treatment, the TGF-β, p-Erk, p-Smad3, and CTFG protein levels in the HFD treatment hamster hearts decreased to levels similar to those of the control group. Only high-dose heat-killed Lr263 (3125 mg/kg-hamster/day) treatments reduced the TGF-β protein level and the expression levels of its downstream proteins; the data showed improvements in fibrosis induction in the HFD hamster heart (Figure 6).
3. Experimental Section
3.1. Preparing the Probiotic Suspensions
The Lr263 was deposited in the Bioresource Collection and Research Center, Taiwan (BCRC 910452) and China Center for Type Culture Collection, China (CCTCC M209263). The heat-killed Lr263 was provided by GenMont Biotech Inc., Tainan, Taiwan. The heat-killed Lr263 powder (8.0 × 108 cells/g) was prepared from autoclaved Lr263. Next, it was diluted to the indicated probiotic concentrations (5 × 108 and 2.5 × 109 cells/mL) in the samples prepared for animal administration.
3.2. Animals
The protocol of animal use experimental in this work was approved by the Institutional Animal Care and Use Committee (IACUC) of China Medical University (No.101-263-B). All 24 male SD hamsters (eight weeks old, 300 g weight) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and divided into four groups (n = 6 each). The control hamster group was labeled as control. The high-fat diet treatment hamster group was labeled as HFD. The hamster group with a high-fat diet combined with a low dose of heat-killed Lr263 (625 mg/kg-hamster/day) was labeled as HFD + Lr263(L). The hamster group with a high-fat diet combined with high dose of heat-killed Lr263 (3125 mg/kg-hamster/day) was labeled as HFD + Lr263(H). Oral administration of the probiotics was performed by gavage using a feeding needle, and the experiments were applied for eight weeks. All high-fat diet treatment hamsters were fed normal water ad libitum and a high-fat diet (HFD); the components of the normal diet and high-fat diet are listed in Table 2.
Table 2.
Components of the normal diet and high-fat diet.
| Components (g/kg) | Normal Diet | High-Fat Diet |
|---|---|---|
| Casein | 200 | 232 |
| l-Cystine | 3.0 | 3.0 |
| d,l-Methionine | N.D. | 3.5 |
| Corn Starch | 397.48 | 137 |
| Maltodextrin | 132 | 150 |
| Sucrose | 100 | 162.58 |
| Cellulose | 50 | 50 |
| Cholesterol | N.D. | 1.9 |
| Mineral Mix (AIN-93) | 35 | 40.60 |
| Calcium phosphate dibasic | N.D. | 4.64 |
| Vitamin Mix (AIN-93) | 10 | 16.24 |
| Choline Bitartrate | 2.5 | 5 |
| Tert-butylhydroquinone | 0.014 | 0.04 |
| Soybean oil | 70 | 40 |
| Lard | N.D. | 153.5 |
N.D.: None detectable.
3.3. Cardiac Echocardiography
The small animal M-mode echocardiography analyses were performed using the Vivid I Ultrasound System (GE Healthcare, Milwaukee, WI, USA) via the parasternal long-axis and short-axis approach. B-mode previewing offers optimal positioning for the left ventricle (LV). M-mode measurements were performed from the B-mode perspectives and immediately recorded the left ventricular internal end-diastolic dimensions (LVIDd) and left ventricular internal end-systolic dimensions (LVIDs). Fractional shortening (FS%) was presented as the calculated results using the following equation: [(LVIDd − LVIDs)/LVIDd] × 100. The ejection fraction (EF) means the percentage of the blood volume pumped out from LV.
3.4. Masson’s Trichrome Staining
The 2 μm thick paraffin sections of each group hamster hearts and livers were cut from paraffin-embedded tissue blocks. The slices were deparaffinized and rehydrated before further staining. The samples were then subjected to with Masson’s trichrome staining and investigate the histological and fibrotic changes in heart and liver sections. The photomicrographs were obtained using microscopes (Zeiss Axiophot, Oberkochen, Deutschland, Germany) under the 200× magnification.
3.5. RNA Extraction and RT-PCR
The total RNA for all liver tissues was isolated using Trizol single-step RNA isolation reagent (Invitrogen Life Technologies, BRL, Carlsbad, CA, USA) and then subjected to reverse transcription using an RT-PCR (Promega, San Luis Obispo, CA, USA). The PCR primers used for fatty acid synthase (FAS) were as follows: forward, 5′-GTGGAAGGCTGGGCTCTATG-3′; and reverse, 5′-AGGCGTCGAACTTGGACAGA-3′. The primers used for peroxisome prolifera proliferator-activated receptor γ (PPARγ) were as follows: forward, 5′-TCAGGTTTGGGCGAATGC-3′; and reverse, 5′-GGGTTCAGCTGGTCGATATCAC-3′. The primers used for 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) were as follows: forward, 5′-TGTGGGAACGGTGACACTTA-3′; and reverse, 5′-CTTCAAATTTTGGGCACTCA-3′. The primers used for LDL-cholesterol receptor (LDLR) were as follows: forward, 5′-AGCCGATGCATTCCTGACTC-3′; and reverse, 5′-AGTTCATCCGAGCCATTTTCAC-3′. The primers used for cholesterol 7α-hydroxylase (CYP7A1) were as follows: forward, 5′-ACGTGGTTGGAAGAAGCG-3′; and reverse, 5′-GAATGTGGGCAGCGAGAA-3′. The primers used for β-actin were as follows: forward, 5′-AGGGAAATCGTGCGTGACA-3′; and reverse, 5′-GTGGCCATCTCTTGCTCGAA-3′. The reaction mixtures were maintained at 48 °C for 45 min and then heated to 94 °C for 2 min for reverse transcription using the following protocol: 94 °C for 30 s of denaturation, 60 °C for 1 min of annealing, 68 °C for a 2 min extension for 40 cycles, and one cycle of 7 min for a final extension in a PerkinElmer PCR machine. Each PCR product result was normalized and expressed as the relative density to the β-actin gene. We also tested GAPDH as a reference gene and it gave similar results to β-actin.
3.6. Tissue Protein Extraction
Heart tissue protein samples were extracted and homogenized from six hamsters in each group using the lysis buffer contents 0.05 M Tris-HCl, 0.15 M NaCl, 0.25% deoxycholic acid, 1% nonyl phenoxypolyethoxylethanol, and 1 mM EDTA at pH = 7.4. The supernatants were collected form the homogenates after centrifuged at 13,000 rpm for 40 min. The protein concentration of each sample was calibrated and then stored the samples at −80 °C for further experiments.
3.7. Western Blot Assay
Heart tissue proteins were separated in a 12% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a Hybond-C membrane (GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK). Then 3% bovine serum albumin (BSA) in a tricine buffer solution was used to block the Hybond-C membrane. After 30 min BSA blocking and additional three times PBS washing, the primary antibodies were added to identify the indicated proteins. The primaries used in this work were including β-actin (sc-47778, Santa Cruz Biotechnology, Dallas, TX, USA), CTGF (sc-1745, Santa Cruz Biotechnology), p-Erk (sc-7382, Santa Cruz Biotechnology), Erk (sc-94, Santa Cruz Biotechnology), and TGF-β (sc-31609, Santa Cruz Biotechnology). After the antibodies’ reorganizations, horseradish peroxidase-labeled antibodies were used and pictures were then taken using a Fujifilm LAS-4000 (GE Healthcare UK Ltd.).
3.8. Liver and Fecal Lipid Profile Analysis
All of the liver and fecal samples were freshly collected after the experiments, and 100 mg of each sample was lyophilized, weighed, and then homogenized in 5 ml of chloroform-methanol (v/v = 2:1) solution. All of the solution samples were further analyzed using a triglyceride quantification assay kit (ab65336, Abcam, Taipei, Taiwan) and cholesterol/cholesteryl ester quantitation assay kit (ab65359, Abcam) in accordance with the protocols suggested by the manual.
3.9. Statistical Analysis
The results were obtained from six hamsters of each experimental group and are represented as the group mean ± standard deviation (SD). One-way analysis of variance was used to indicate an overall statistical significance among the means of the four experimental groups. A p-value less than 0.05 was considered significant. Statistical analyses were performed using SigmaPlot v.10.0 software (San Jose, CA, USA).
4. Conclusions
In conclusion our current findings show that Lr263 daily oral gavage treatment may reduce the lipid metabolic stress in liver, and attenuate the cardiac fibrosis by suppressing the TGF-β expression. Our results suggest that heat-killed Lr263 supplementation would potentially improve the health of the liver and heart.
Acknowledgments
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002).
Author Contributions
The authors’ contributions were as follows: Viswanadha Vijaya Padma, Yi-Hsing Chen and Chih-Yang Huang designed the experiments; Wei-Jen Ting, Wei-Wen Kuo and Dennis Jine-Yuan Hsieh acquired and analyzed the results; Wei-Jen Ting, Ya-Hui Chen, Ray-Jade Chen, Cecilia-Hsuan Day and Yu-Lan Yeh interpreted the results; and Wei-Jen Ting and Chih-Yang Huang prepared and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Casavalle P.L., Lifshitz F., Romano L.S., Pandolfo M., Caamaño A., Boyer P.M., Rodríguez P.N., Friedman S.M. Prevalence of dyslipidemia and metabolic syndrome risk factor in overweight and obese children. Pediatr. Endocrinol. Rev. 2014;12:213–223. [PubMed] [Google Scholar]
- 2.Cortez M., Singleton J.R., Smith A.G. Glucose intolerance, metabolic syndrome, and neuropathy. Handb. Clin. Neurol. 2014;126:109–122. doi: 10.1016/B978-0-444-53480-4.00009-6. [DOI] [PubMed] [Google Scholar]
- 3.El-Bassossy H.M., Shaltout H.A. Allopurinol alleviates hypertension and proteinuria in high fructose, high salt and high fat induced model of metabolic syndrome. Transl. Res. 2014;165:621–630. doi: 10.1016/j.trsl.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Geliebter A., Ochner C.N., Dambkowski C.L., Hashim S.A. Obesity-related hormones and metabolic risk factors: A randomized trial of diet plus either strength or aerobic training versus diet alone in overweight participants. J. Diabetes Obes. 2014;1:1–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Suh S., Baek J., Bae J.C., Kim K.N., Park M.K., Kim D.K., Cho N.H., Lee M.K. Sex factors in the metabolic syndrome as a predictor of cardiovascular disease. Endocrinol. Metab. 2014;29:522–529. doi: 10.3803/EnM.2014.29.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moh M.C., Sum C.F., Lam B.C., Ng X.W., Su C., Tavintharan S., Yeoh L.Y., Wong M.D., Lee S.B., Tang W.E., et al. Evaluation of body adiposity index as a predictor of aortic stiffness in multi-ethnic Asian population with type 2 diabetes. Diabetes Vasc. Dis. Res. 2014;12:111–118. doi: 10.1177/1479164114554609. [DOI] [PubMed] [Google Scholar]
- 7.Ruff R.R., Akhund A., Adjoian T., Kansagra S.M. Calorie intake, sugar-sweetened beverage consumption, and obesity among New York City adults: Findings from a 2013 population study using dietary recalls. J. Community Health. 2014;39:1117–1123. doi: 10.1007/s10900-014-9865-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang K.C., Lee L.T., Chen C.Y., Sung P.K. All-cause and cardiovascular disease mortality increased with metabolic syndrome in Taiwanese. Obesity. 2008;16:684–689. doi: 10.1038/oby.2007.112. [DOI] [PubMed] [Google Scholar]
- 9.Ma J.Q., Ding J., Zhao H., Liu C.M. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin. Pharmacol. Toxicol. 2014;115:389–395. doi: 10.1111/bcpt.12245. [DOI] [PubMed] [Google Scholar]
- 10.Tomita K., Teratani T., Suzuki T., Shimizu M., Sato H., Narimatsu K., Usui S., Furuhashi H., Kimura A., Nishiyama K., et al. Acyl-CoA: Cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J. Hepatol. 2014;61:98–106. doi: 10.1016/j.jhep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Fede G., Privitera G., Tomaselli T., Spadaro L., Purrello F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. 2015;28:31–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C.H., Ting W.J., Day C.H., Ju D.T., Yeh Y.L., Chung L.C., Tsai F.J., Tsai C.H., Tsai Y., Huang C.Y. SHSST cyclodextrin complex prevents the fibrosis effect on CCl₄-induced cirrhotic cardiomyopathy in rats through TGF-β pathway inhibition effects. Int. J. Mol. Sci. 2014;15:8037–8048. doi: 10.3390/ijms15058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens A.P., III, Passam F.H., Antoniak S., Marshall S.M., McDaniel A.L., Rudel L., Williams J.C., Hubbard B.K., Dutton J.A., Wang J., et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J. Clin. Investig. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramspoth T., Roehl A.B., Macko S., Heidenhain C., Junge K., Binnebösel M., Schmeding M., Neumann U.P., Rossaint R., Hein M. Risk factors for coagulopathy after liver resection. J. Clin. Anesth. 2014;26:654–662. doi: 10.1016/j.jclinane.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Dobaczewski M., Chen W., Frangogiannis N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 17.Dobaczewski M., Bujak M., Li N., Gonzalez-Quesada C., Mendoza L.H., Wang X.F., Frangogiannis N.G. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ. Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panek A.N., Posch M.G., Alenina N., Ghadge S.K., Erdmann B., Popova E., Perrot A., Geier C., Dietz R., Morano I., et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS ONE. 2009;4:e6743. doi: 10.1371/journal.pone.0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger G., MacDonald K., Reid G., Burton J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin P.P., Hsieh Y.M., Kuo W.W., Lin Y.M., Yeh Y.L., Lin C.C., Tsai F.J., Tsai C.H., Huang C.Y., Tsai C.C. Probiotic-fermented purple sweet potato yogurt activates compensatory IGF-IR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int. J. Mol. Med. 2013;32:1319–1328. doi: 10.3892/ijmm.2013.1524. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.F., Lin P.P., Chen C.H., Yeh Y.L., Huang C.C., Huang C.Y., Tsai C.C. Effects of lactic acid bacteria on cardiac apoptosis are mediated by activation of the phosphatidylinositol-3 kinase/AKT survival-signalling pathway in rats fed a high-fat diet. Int. J. Mol. Med. 2015;35:460–470. doi: 10.3892/ijmm.2014.2021. [DOI] [PubMed] [Google Scholar]
- 22.Sanders M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008;2:S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 23.Elkins C.A., Moser S.A., Savage D.C. Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology. 2001;147:3403–3412. doi: 10.1099/00221287-147-12-3403. [DOI] [PubMed] [Google Scholar]
- 24.Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F., Hill C., Gahan C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar M., Nagpal R., Kumar R., Hemalatha R., Verma V., Kumar A., Chakraborty C., Singh B., Marotta F., et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012;2012:902917. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai C.C., Lin P.P., Hsieh Y.M., Zhang Z.Y., Wu H.C., Huang C.C. Cholesterol-lowering potentials of lactic Acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci. World J. 2014;2014:690752. doi: 10.1155/2014/690752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh F.C., Lee C.L., Chai C.Y., Chen W.T., Lu Y.C., Wu C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. 2013;10:35. doi: 10.1186/1743-7075-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y.C., Yin L.T., Chang W.T., Huang J.S. Effect of Lactobacillus reuteri GMNL-263 treatment on renal fibrosis in diabetic rats. J. Biosci. Bioeng. 2010;110:709–715. doi: 10.1016/j.jbiosc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ting W.J., Kuo W.W., Kuo C.H., Yeh Y.L., Shen C.Y., Chen Y.H., Ho T.J., Viswanadha V.P., Chen Y.H., Huang C.Y. Supplementary heat-killed Lactobacillus reuteri GMNL-263 ameliorates hyperlipidaemic and cardiac apoptosis in high-fat diet-fed hamsters to maintain cardiovascular function. Br. J. Nutr. 2015;114:706–712. doi: 10.1017/S0007114515002469. [DOI] [PubMed] [Google Scholar]
- 30.Wang S., Miller B., Matthan N.R., Goktas Z., Wu D., Reed D.B., Yin X., Grammas P., Moustaid-Moussa N., Shen C.L., et al. Aortic cholesterol accumulation correlates with systemic inflammation but not hepatic and gonadal adipose tissue inflammation in low-density lipoprotein receptor null mice. Nutr. Res. 2013;33:1072–1082. doi: 10.1016/j.nutres.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y.J., Shin H.S., Choi H.S., Park J.W., Jo I., Oh E.S., Lee K.Y., Lee B.H., Johnson R.J., Kang D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014;94:1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 32.Yao X., Hou S., Zhang D., Xia H., Wang Y.C., Jiang J., Yin H., Ying H. Regulation of fatty acid composition and lipid storage by thyroid hormone in mouse liver. Cell Biosci. 2014;4:38. doi: 10.1186/2045-3701-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yiu W.F., Kwan P.L., Wong C.Y., Kam T.S., Chiu S.M., Chan S.W., Chan R. Attenuation of fatty liver and prevention of hypercholesterolemia by extract of Curcuma longa through regulating the expression of CYP7A1, LDL-receptor, HO-1, and HMG-CoA reductase. J. Food Sci. 2011;76:80–89. doi: 10.1111/j.1750-3841.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 34.Mansoor S., Yerian L., Kohli R., Xanthakos S., Angulo P., Ling S., Lopez R., Christine C.K., Feldstein A.E., Alkhouri N. The evaluation of hepatic fibrosis scores in children with nonalcoholic fatty liver disease. Dig. Dis. Sci. 2015;60:1440–1447. doi: 10.1007/s10620-014-3494-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim D., Kim W.R., Kim H.J., Therneau T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treeprasertsuk S., Bjornsson E., Enders F., Suwanwalaikorn S., Lindor K.D. NAFLD fibrosis score: A prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 2013;19:1219–1229. doi: 10.3748/wjg.v19.i8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz Y., Kurt R., Yonal O., Polat N., Celikel C.A., Gurdal A., Oflaz H., Ozdogan O., Imeryuz N., Kalayci C., et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Atherosclerosis. 2010;211:182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Ji Y.S., Kim H.N., Park H.J., Lee J.E., Yeo S.Y., Yang J.S., Park S.Y., Yoon H.S., Cho G.S., Franz C.M., et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28. Benef. Microbes. 2012;3:13–22. doi: 10.3920/BM2011.0046. [DOI] [PubMed] [Google Scholar]
- 39.Tomaro-Duchesneau C., Jones M.L., Shah D., Jain P., Saha S., Prakash S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. CholesteBiomed. Res. Int. 2014;2014:380316. doi: 10.1155/2014/380316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 41.Jung J.Y., Shin J.S., Lee S.G., Rhee Y.K., Cho C.W., Hong H.D., Lee K.T. Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR 2-mediated activation. Int. Immunopharmacol. 2015;28:88–96. doi: 10.1016/j.intimp.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Fusaru A.M., Stănciulescu C.E., Surlin V., Taisescu C., Bold A., Pop O.T., Baniţă I.M., Crăiţoiu S., Pisoschi C.G. Role of innate immune receptors TLR2 and TLR4 as mediators of the inflammatory reaction in human visceral adipose tissue. Rom. J. Morphol. Embryol. 2012;53:693–701. [PubMed] [Google Scholar]
- 43.Ikeuchi M., Tsutsui H., Shiomi T., Matsusaka H., Matsushima S., Wen J., Kubota T., Takeshita A. Inhibition of TGF-β signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 2004;64:526–535. doi: 10.1016/j.cardiores.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Frantz S., Hu K., Adamek A., Wolf J., Sallam A., Maier S.K., Lonning S., Ling H., Ertl G., Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res. Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 45.Bujak M., Ren G., Kweon H.J., Dobaczewski M., Reddy A., Taffet G., Wang X.F., Frangogiannis N.G. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]