Abstract
Antiviral agents are urgently needed to fight severe acute respiratory syndrome (SARS). We showed that niclosamide, an existing antihelminthic drug, was able to inhibit replication of a newly discovered coronavirus, SARS-CoV; viral antigen synthesis was totally abolished at a niclosamide concentration of 1.56 μM, as revealed by immunoblot analysis. Thus, niclosamide represents a promising drug candidate for the effective treatment of SARS-CoV infection.
Severe acute respiratory syndrome (SARS) has recently emerged as a new human disease with considerable morbidity and mortality. It was first identified in southern China in November 2002, and a serious outbreak occurred in Hong Kong in March 2003 (5). From November 2002 to June 2003, a total of more than 8,000 probable SARS cases were reported to the World Health Organization, of which approximately 800 were fatal (1). This outbreak of a life-threatening respiratory disease is associated with a newly discovered coronavirus termed SARS-CoV (4, 8). Thus, effective antiviral agents are urgently needed to fight this emerging deadly disease. Although drug screening can be designed to test a wide range of compounds in high-throughput screening assays, the process for new drug discovery and development starting with a novel chemical entity is quite long. In an attempt to provide immediate cures, one of our strategies was to quickly identify potential drug candidates. We therefore focused on screening a set of marketed drugs which have not been previously recommended for antiviral use.
A small chemical library consisting of a set of marketed drugs was evaluated for anti-SARS-CoV activities. In this study, Vero E6 cells were infected with SARS-CoV at a multiplicity of infection of 0.1. When infected at this multiplicity of infection, Vero E6 cells started to develop mild cytopathic effects (CPE) at 1 day postinfection, and typical CPE as reported by Ksiazek et al. (4) could be observed at 2 days postinfection. In the drug treatment experiments, drugs were added 1 h before viral infection. Drugs (at 10 μM concentrations) were evaluated for inhibition of SARS-CoV replication as measured by the protection of Vero E6 cells from forming CPE after virus infection. Niclosamide (2′,5-dichloro-4′-nitrosalicylanilide) (Fig. 1A) was found to be effective as a virus replication inhibitor.
FIG. 1.
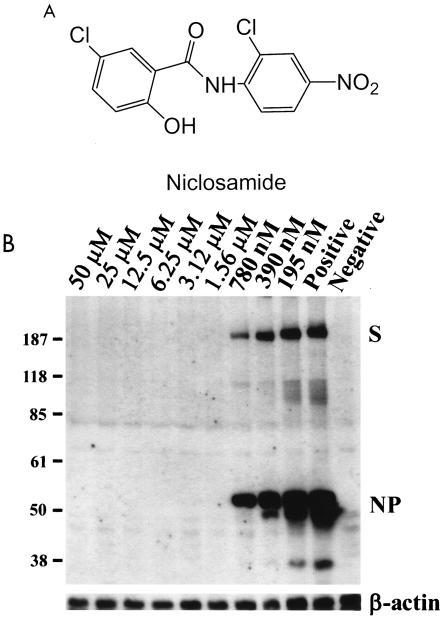
Inhibition of SARS-CoV replication by niclosamide in Vero E6 cells. (A) Chemical structure of niclosamide (2′, 5-dichloro-4′-nitrosalicylanilide); (B) dose-dependent inhibition of SARS-CoV antigen synthesis in Vero E6 cells by niclosamide. This immunoblot analysis shows that niclosamide, at a concentration of 1.56 μM or higher, completely inhibited the synthesis of viral antigens of SARS-CoV in Vero E6 cells. The bottom panel is an immunoblot stained with antiactin antibody as an internal control. The viral antigens spike protein (S) and nucleocapsid protein (NP) are indicated.
Subsequently, the effect of niclosamide was further confirmed with several alternative assays. In the immunoblot assay, Vero E6 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. In each well of 48-well plates, 4 × 104 cells were seeded to form a cell layer that is approximately 70% confluent. During virus infection, Dulbecco's modified Eagle's medium containing 2% fetal bovine serum was used. Niclosamide was prepared as a 10 mM stock solution in dimethyl sulfoxide, and the drug was soluble at concentrations up to 100 μM in culture medium. Cell lysates were harvested at 48 h postinfection and analyzed by immunoblotting with an antiserum derived from a SARS patient (Tri-Service General Hospital, Taipei, Taiwan). In this immunoblot assay, antiserum from a convalescent SARS patient was used as the primary antibody because many prominent bands could be observed in cells infected by SARS-CoV. These proteins are likely to be SARS-CoV antigens, as they were not present in noninfected cells. After the blots were stained with the primary antibody, they were treated with horseradish peroxidase-conjugated goat anti-human immunoglobulins (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) at a 1:1,000 dilution and developed with an ECL kit system (Amersham Biosciences, Piscataway, N.J.). The synthesis of viral antigens was completely inhibited at a niclosamide concentration of 1.56 μM or higher in this immunoblot analysis (Fig. 1B).
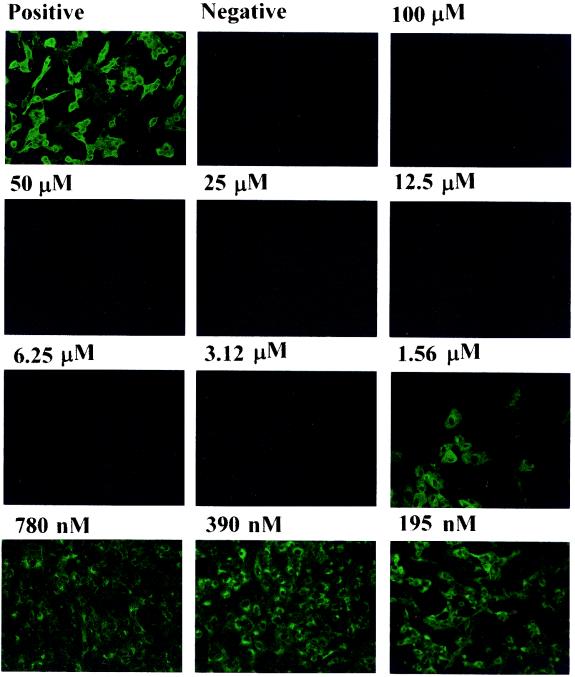
Next, we employed an immunofluorescence assay (IFA) to examine the inhibitory effect of niclosamide on viral antigen synthesis. Niclosamide was added to cells at serial dilutions. The drug was present in all of the following procedures. At 48 h postinfection, the cells were fixed and stained with antiserum from the same convalescent SARS patient described above (Fig. 1B). As shown in Fig. 2, the expression of viral antigens was inhibited by niclosamide in a dose-dependent manner. Each virus-infected cell without drug treatment emitted bright fluorescent light. At concentrations of 3.12 μM and higher, niclosamide was able to completely inhibit viral antigen synthesis. Thus, the effective concentration of niclosamide that inhibited 50% of viral antigen synthesis was estimated to be within the range of 1 to 3 μM. This experiment, done in triplicate for each drug dose, was repeated three times, and representative results are shown in Fig. 2. The concentration of compound that reduced cell viability to 50%, used to determine the cellular toxicity of niclosamide, was approximately 250 μM after 48 h of drug treatment (data not shown). Cell viability was determined by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay as previously described (2).
FIG. 2.
Inhibition of viral antigen synthesis in SARS-CoV-infected Vero E6 cells as revealed by an IFA. Vero E6 cells were treated with the various concentrations of niclosamide indicated, and an IFA was performed at 48 h postinfection. Vero E6 cells were fixed and stained with the same primary antiserum directed against SARS-CoV as that used in the immunoblot analysis. Fluorescein isothiocyanate-conjugated goat anti-human immunoglobulin (Jackson ImmunoResearch Laboratoeies, Inc.) was used as the secondary antibody. The positive panel shows cells infected by SARS-CoV but without drug treatment, while the negative panel shows cells without virus infection.
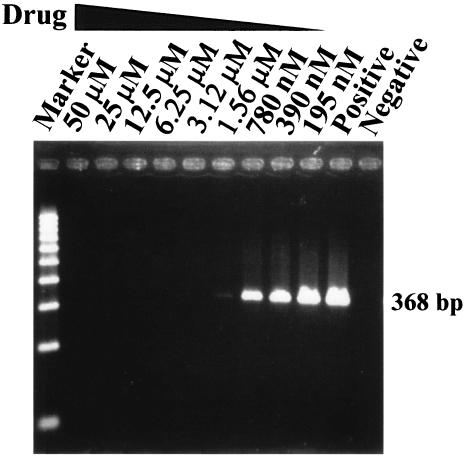
To examine whether virus yield was also inhibited by niclosamide, culture supernatants were collected, and a reverse transcriptase PCR (RT-PCR) was employed to detect viral RNA. Two days postinfection, viral RNA was isolated from 140 μl of supernatant from SARS-CoV-infected cells with a QIAamp viral RNA minikit (QIAGEN) in accordance with the manufacturer's instructions. One microliter of extracted viral RNA was prepared for RT-PCR by using a QIAGEN One Step RT-PCR kit with the SARS-specific primers Cor-p-F2 5′CTAACATGCTTAGGATAATGG3′ and Cor-p-R1 5′CAGGTAAGCGTAAAACTCATC3′ (4). This primer set was designed to amplify a region in open reading frame 1b in the genome of SARS-CoV (4). The RT-PCR conditions were as follows: 50°C for 30 min; 95°C for 15 min; 25 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and 72°C for 10 min. No specific PCR product of 368 bp could be detected in the medium of virus-infected cells when niclosamide was applied at concentrations of 3.12 μM or higher, whereas a faint band of the correct length was observed in the PCR-amplified product from the medium of cells treated with 1.56 μM niclosamide (Fig. 3 and Table 1). Apparently, there was a dose-dependent reduction of virus yield in the supernatant of infected cells treated with niclosamide.
FIG. 3.
Effect of niclosamide on reduction of the viral yields in the culture supernatants from SARS-CoV-infected Vero E6 cells. Viral RNA was isolated from culture supernatants from SARS-CoV-infected Vero E6 cells and analyzed by RT-PCR. PCR products of 368 bp were observed when Vero E6 cells were treated with niclosamide at concentrations of 1.56 μM and lower, whereas no PCR products could be detected when cells were treated with niclosamide at concentrations of 3.12 μM and higher. Representative results from five separate experiments are shown. Positive, cells infected by SARS-CoV without drug treatment; Negative, cells without virus infection. The RT-PCR products were quantitated by image analysis, and results are shown in Table 1.
TABLE 1.
Effect of niclosamide on viral yielda
| Drug concn (μM) | % Yieldb |
|---|---|
| 50 | ND |
| 25 | ND |
| 12.5 | ND |
| 6.25 | ND |
| 3.12 | ND |
| 1.56 | 42 ± 2 |
| 0.78 | 62 ± 4 |
| 0.39 | 80 ± 4 |
| 0.20 | 97 ± 5 |
| 0 | 100 |
The images of the bands in each RT-PCR gel in Fig. 3 were captured by a charge-coupled device video camera, and the intensity was quantified by the AlphaImager 2000 (Alpha Innotech Corporation, San Leandro, Calif.). Values are means ± standard deviations of results from five experiments. Percent yield is defined as the density of each band divided by that of the control (positive virus alone).
ND, not detectable.
It is important to explore the mechanism of action of niclosamide in the inhibition of SARS-CoV replication. When niclosamide was added 3 h after cells were infected with SARS-CoV, it was still active in inhibiting SARS-CoV replication, indicating that niclosamide does not interfere with the virion's attachment to and entry into cells. Further, niclosamide did not inhibit the protease activity of 3C-like protease (results not shown). Results from this study also warrant further investigation to examine the effects of niclosamide in SARS animal models or in human clinical trials to provide a proof of principle of whether niclosamide is capable of alleviating the serious sequelae caused by SARS-CoV infection. Since SARS-CoV is known to actively replicate in the intestinal tract (6), it is also important to evaluate whether niclosamide taken orally can inhibit viral replication and lower the viral load in the lumen of the intestine or in the stool. If viral replication in the intestine can be adequately inhibited by niclosamide, this drug might be considered for use in controlling the fecal-oral route that has been speculated to be one of the possible avenues of transmission (7, 9). Given that profuse watery diarrhea is a common symptom and that SARS-CoV is shed in large quantities in the stools of SARS patients, niclosamide may therefore be appropriate for treatment.
In summary, we discovered that niclosamide was able to inhibit SARS-CoV replication at a micromolar concentration. Niclosamide is an old drug used in antihelminthic treatment (3). Because niclosamide has been used for the treatment of parasite diseases in humans, this drug may be considered for immediate use in the treatment of SARS patients, alone or in combination with other drugs.
Acknowledgments
This study was funded by the National Health Research Institutes (NHRI) and the National Science Council (NSC) in Taiwan.
We thank Xin Chen at NHRI for her critical review of this manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2003. Update: severe acute respiratory syndrome—United States, June 18, 2003. Morb. Mortal. Wkly. Rep. 52:570. [PubMed] [Google Scholar]
- 2.Cory, A. H., T. C. Owen, J. A. Barltrop, and J. G. Cory. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207-212. [DOI] [PubMed] [Google Scholar]
- 3.Katz, M. 1977. Anthelmintics. Drugs 13:124-136. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 5.Lee, N., D. Hui, A. Wu, P. Chan, P. Cameron, G. M. Joynt, A. Ahuja, M. Y. Yung, C. B. Leung, K. F. To, S. F. Lui, C. C. Szeto, S. Chung, and J. J. Sung. 2003. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348:1986-1994. [DOI] [PubMed] [Google Scholar]
- 6.Leung, W. K., K. F. To, P. K. Chan, H. L. Chan, A. K. Wu, N. Lee, K. Y. Yuen, and J. J. Sung. 2003. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris, J. S., C. M. Chu, V. C. Cheng, K. S. Chan, I. F. Hung, L. L. Poon, K. I. Law, B. S. Tang, T. Y. Hon, C. S. Chan, K. H. Chan, J. S. Ng, B. J. Zheng, W. L. Ng, R. W. Lai, Y. Guan, and K. Y. Yuen. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2003. SARS virus identified. Bull. W. H. O. 81:384. [Google Scholar]
- 9.2003. World Health Organization. 16 May 2003, posting date. WHO environmental health team reports on Amoy gardens. [Online.] http://www.info.gov.hk/gia/general/200305/16/0516114.htm.