Abstract
Major Depression is a complex and severe psychiatric disorder whose symptomatology encompasses a critical shift in awareness, specifically in the balance from external to internal mental focus. This is reflected by unspecific somatic symptoms and the predominance of the own cognitions manifested in increased self-focus and rumination. We posit here that sufficient empirical data has accumulated to build a coherent biological model that links these psychological concepts and symptom dimensions to observed biochemical, cellular, regional and neural network deficits. Specifically, deficits in inhibitory gamma amino butyric acid (GABA) regulating excitatory cell input/output and local cell circuit processing of information in key brain regions may underlie the shift that is observed in depressed subjects in resting state activities between the perigenual anterior cingulate cortex (PACC) and the dorsolateral prefrontal cortex (DLPFC). This regional dysbalance translates at the network level in a dysbalance between default-mode and executive networks, which psychopathologically surfaces as a shift in focus from external to internal mental content and associated symptoms (See overview in Figure 1). We focus here on primary evidence at each of those levels and on putative mechanistic links between those levels. Apart from its implications for neuropsychiatric disorders, our model provides for the first time a set of hypotheses for cross-level mechanisms of how internal and external mental contents may be constituted and balanced in healthy subjects, and thus also contributes to the neuroscientific debate on the neural correlates of consciousness.
Keywords: gamma-Aminobutyric Acid, major depression, somatostatin, parvalbumin, prefrontal cortex, default-mode network, executive network
I. INTRODUCTION
Major depressive disorder (MDD) is a leading cause of disability worldwide.1 It is also a complex psychiatric disorder characterized by various symptoms including low affect, anhedonia, sadness, ruminations, increased self-focus, loss of appetite, and sleep disturbances.2-6 Taking a more general view, the various symptoms in depression signal a shift in mental experience, or “awareness”, reflected in the balance between internal and external mental contents and their coupling with abnormally negative affect. Indeed, rather than focusing on external mental contents in the environment like objects or events, the awareness in MDD patients is dominated by internal mental contents stemming either from the own body or thoughts. This is well reflected in the unspecific somatic symptoms (e.g., increased bodily-focus) and the strong predominance of cognitive processes such as ruminations (e.g., increased self-focus) of these patients, with this shift towards internal contents (somatic and mental) being notably associated with abnormally negative affect (see 6-9), however the physiological mechanisms underlying this abnormal balance and its association with abnormally negative affect remain unclear.
Imaging studies in humans and animal models demonstrate increased resting state activity in the perigenual anterior cingulate cortex (PACC) in depression, which may be related to abnormal GABA function in this region (See 8,10,11). Interestingly, the dorsolateral prefrontal cortex (DLPFC), especially in the left hemisphere, is characterized by an opposite pattern of decreased resting state activity.10,12 This shift in resting state balance seems to be closely related to the shift in internal and external mental contents in awareness; this will be the main focus of our paper while leaving aside the neural mechanisms related to the generation, association and cognitive regulation of the abnormally negative affect in MDD. Studies of the resting state activity in both healthy13 and MDD subjects14,15 demonstrated that internal mental contents are associated with PACC and default-mode network/DMN activity, while external mental contents induce increased activity in DLPFC and the executive network. Due to reasons of clarity, space, and focus, we set aside the potential role of other regions and networks like the insula and salience network,16-18 the mechanisms of affect generation as related to subcortical regions,19 and the neural mechanisms of cognitive emotion regulation as associated with DLPFC-amygdala down-modulation and cognitive-behavioral therapy in MDD.20,21
The model we develop here relies on direct brain-based evidence in human subjects with major depression and extends previously-articulated GABA hypotheses of emotion dysregulation in depression.22,23 The GABA hypothesis of depression was originally proposed in 1980, based on the efficacy of sodium valproate, a GABA-ergic anticonvulsant, in treatment of mania.24 This indirect level of evidence was supported by reports of low GABA levels in the plasma and cerebrospinal fluid of depressed subjects,25-28 of decreased GABA concentration in the PACC and occipital cortex (OCC) in MDD, as observed by proton magnetic resonance spectroscopy (1H-MRS) or by transcranial magnetic stimulation,11,29-31 and later by the association between GABAergic transmission and control of stress,23 the effect of monoaminergic antidepressants on GABAergic transmission,32 and genetic manipulation studies in rodents.33 However, the exact cellular, biochemical, physiological and regional mechanisms of how GABA dysregulations affect various nested and bottom-up biological levels and ultimately the different symptoms at the psychopathological level in MDD remain unclear. Furthermore, how those GABA-related cellular and biochemical changes relate to broader neural network hypotheses of depression9,34, including pathophysiological mechanisms underlying the abnormal balance between PACC/DMN and DLPFC/ executive network, in MDD has not been addressed.
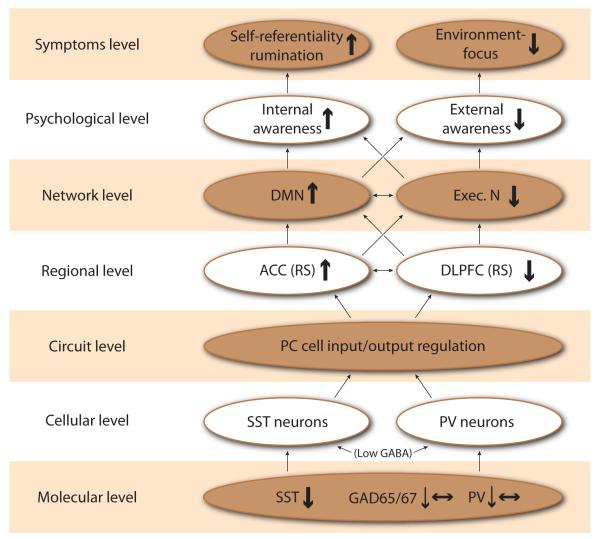
We here hypothesize that the abnormal balance between PACC/anterior DMN and DLPFC/executive network (and subsequently the abnormal balance between internal and external mental contents in awareness) in MDD may be traced back to regional and cellular distribution and changes in GABA interneurons and to their specific organization in regulating the input, output and integrity of information processing as it transits through cortical layers. More specifically, we hypothesize the following bottom-up cross-level mechanisms (See Figure 1 for an overview): (i) there are robust MDD deficits in somatostatin (SST)-positive interneurons that mostly regulate excitatory input on the dendrites of pyramidal cells (as observed in postmortem brain); (ii) there are sparser MDD deficits in parvalbumin (PV)-positive GABA-ergic interneurons that regulate the excitatory output of pyramidal cells, especially in DLPFC; (iii) these cell-specific deficits may translate into a dysbalance between input and output of pyramidal cells which, on a regional level, may translate into altered activity and a shift of the resting state balance between PACC (and the DMN) and DLPFC (and the executive network (EN)) in MDD (as it can be observed); (iv) the abnormal resting state between PACC/DMN and DLPFC/EN may be biochemically mediated by abnormal GABA function at the regional level (as supported by reduced biochemical levels and cell-specific changes) and (v) translates at the network level into a dysbalance between DMN and EN which psychopathologically surfaces in an abnormal balance between internal and external mental contents in awareness (as it is clinically evidenced).
Figure 1. Overview of biological levels and associated evidence of dysregulation related to depression.
This document describes how deficits in inhibitory gamma-amino butyric acid (GABA) regulating excitatory cell input/output and local cell circuit processing of information in key brain regions may underlie the shift in resting state activities between the perigenual anterior cingulate cortex (PACC) and the dorsolateral prefrontal cortex (DLPFC). This regional dysbalance translates at the network level in a dysbalance between default-mode and executive networks, which psychopathologically surfaces as a shift from external to internal mental content in awareness. This shift in mental content is reflected by unspecific somatic symptoms and the predominance of the own cognitions manifested in increased self-focus and rumination.
II. FROM GENES TO CELLS AND LOCAL CELL CIRUIT REGULATION
Recent findings from preclinical, clinical and human post-mortem studies point towards an altered balance of excitatory (i.e. glutamaterigic) and inhibitory (i.e. GABAergic) components of local cell circuits in cortical structures of the brain (See next section). Here we review briefly the cellular structure and connectivity of canonical local cell circuits that form the basic functional units of input/output regulation of excitatory pyramidal cells and cortical structures. The primary molecular evidence collected in postmortem subjects with depression suggest reduced expression of the inhibitory GABAergic components of those local units that regulate incoming signaling, or information input. Hence we focus on the inhibitory GABA component, but recognize that associated changes in the glutamatergic system need to occur to maintain the excitation-inhibition balance (see for instance Sanacora et al35).
From inhibitory neuron identity to local circuit regulation of excitatory pyramidal cell function
Excitatory pyramidal cells use glutamate as the main neurotransmitter to propagate excitatory signals across cortical layers and brain regions.36 Those excitatory neurons are under negative inhibitory control that is exerted mostly by local neurons that use GABA as their main inhibitory neurotransmitter.37,38 GABAergic neurons are divided into subgroups based on the molecular markers they express, the cellular compartment they target, and their electrophysiological properties.37,38 For the purpose of this report, we can describe GABA neurons in relation to a simplified pyramidal neuron two compartment model, corresponding to their input and output functions (Figure 2A).37,39
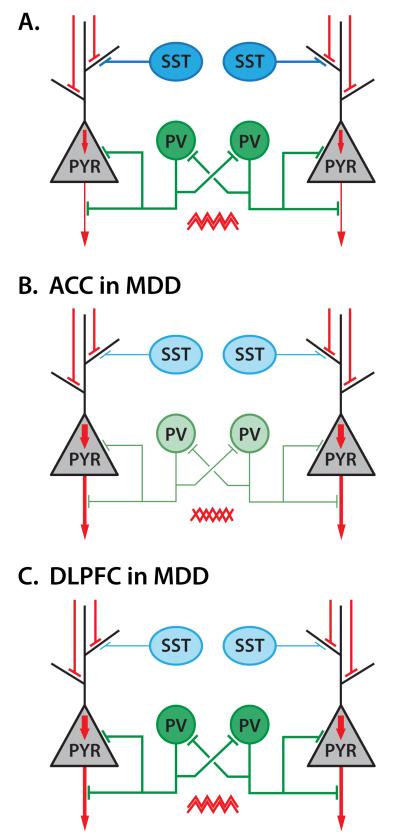
Figure 2. Inhibitory neuron identity and local circuit regulation of excitatory pyramidal cell function in controls and major depression.
(A) Pyramidal neurons (PYR) receive inhibitory input on dendrites from SST-positive GABA neurons, and in the perisomatic region from PV-positive GABA neurons. Excitatory signal input is therefore regulated by SST neurons whereas PV neurons regulate excitatory signal output. Furthermore, PV-neurons are reciprocally connected by inhibitory input, and are critical in the generation of synchronous firing in the gamma range (sawtooth symbols), mediating propagation of information across ensembles of neurons. (B) In PACC, reduced expression of markers for SST and PV neurons and of GABA synthesis genes suggests reduced inhibition of input and output of information (and potentially less synchronous) in MDD. (C) In DLPFC selective reduction in the expression of markers for SST neurons suggests reduced inhibition of input, but intact output, potentially resulting in overall increased information transfer (however see section PACC and DLPFC reciprocal inhibition for a putative overriding effect of increased PACC activity on DLPFC activity under resting state condition). Red codes for excitatory signal, and blue and green for inhibitory signals.
The input function consists of incoming excitatory signals onto the dendritic tree of pyramidal cells, and it is targeted/inhibited by GABA neurons that are characterized by the expression of the neuropeptide somatostatin (SST). The output function of pyramidal cells consists of excitatory signals generated at the level of the cell body and axon, which are targeted/inhibited by GABA neurons that mainly express the calcium-binding peptide parvalbumin (PV) (Figure 2A).37 Additional GABA neuron subtypes exist, including some that target and regulate the function of SST and PV neurons,40 but they are omitted here for simplicity and due to sparse evidence for dysregulations in MDD (See next section). To better understand the implications of selective changes in MDD, we first provide a more detailed description of the respective functions and characteristics of SST and PV neurons.
SST neurons target and inhibit non-specifically all distal dendrites of pyramidal neurons with a probability that is inversely related to their distance from pyramidal neurons.41,42 They are recruited in a feedforward manner by activated pyramidal neurons for which they also provide feedback inhibition. SST neurons are characterized by delayed, sustained and adapting firing properties.43 They also display low synchrony (due to absence of SST-SST feedback inhibition).40 Together SST neurons are specialized in customized and targeted local regulation of incoming excitatory signals, and are thus critical in maintaining the integrity of information input.37 Recent findings (albeit in rodent visual cortex) suggest that SST neurons may also provide significant inhibition to most other GABA neuron subtypes,40,44 and may thus regulate the overall inhibitory tone within local circuits in addition to regulating dendritic inhibition.
PV neurons target non-specifically the perisomatic compartment of pyramidal cells in a cell distance-dependent fashion.41 PV neurons are directly activated by thalamic projections and cortico-cortical projections, and are characterized by fast spiking and non-adapting properties. Unlike SST neurons, PV neurons are highly synchronized through dense PV-PV reciprocal inhibition.45,46 Together, PV neurons are specialized in regulating the output of targeted neurons and in the synchronization of firing of ensembles of pyramidal neurons, and thus contribute to maintaining the integrity and propagation of information output.37,45,46 This intricate local cell circuit connectivity and distribution of tasks demonstrates the close links between excitatory and inhibitory functions, highlights the necessity of simultaneously maintaining proper regulation of input and output functions to preserve the integrity of transferred information, and also suggests multiple sites of putative deregulations in the context of brain disorders. This latter point is well illustrated by the fact that many forward-genetic and pharmacological studies in rodent systems or drug studies in humans can affect the excitatory/inhibition balance and lead to altered information transfer and related behaviors, including antidepressant activity.35,47
Taken together, this evidence demonstrates various ways in which the glutamatergic or GABAergic components of local circuits can be dysregulated; however, it does not necessarily address the question as to which changes actually occur in the context of depression. Knowing the true pathogenic mechanisms occurring at the level of the local cell circuits has consequences for understanding and modeling its bottom-up contribution to dyregulated functions in upper biological scales, including neural network activity and potential symptom dimension. Note that this manuscript is on purpose based solely on results from human studies. Studies in rodents have provided supporting evidence for the local circuit basic neuroscience described here (i.e. wiring, cell types), but have with few exceptions48 so far not directly tested aspects of the neurobiology of disease hypotheses described in this report.
From the local cell circuit MDD-related pathology to ACC and DLPFC regional specificity
Surveys of the expression of genes implicated in the function and identification of the GABA/ glutamate components of local cell circuits suggest primary deficits affecting the GABA system, specifically SST neurons targeting the dendritic compartment and regulating information input.49 Human post-mortem studies have reported a down-regulation of SST gene expression in the DLPFC, PACC, and amygdala of MDD patients compared to healthy comparison subjects.50-53 These findings are consistent with earlier postmortem studies showing reduced calbindin-positive GABA interneuron numbers in the frontal cortex of MDD patients,54,55 as SST is mostly expressed in calbindin-positive interneurons [reviewed in 56]. In addition, neuropeptide Y and cortistatin, two peptides partly co-localized with SST, were found to be similarly down-regulated in the PACC and amygdala,50,53 but not in DLPFC in MDD patients. These three neuropeptides (somatostatin, neuropeptide Y, and cortistatin) are markers of the GABAergic neuron subtype described above that specifically regulate incoming excitatory signals or information input onto pyramidal cells.56,57
Regarding markers of other GABA neuron subtypes, results have been mostly negative across several brain regions investigated.50-52 In a study comprising postmortem samples from over 50 pairs of MDD and psychiatric control subjects, Tripp et al.58 reported downregulation of PV and enzymes necessary for the production of GABA (GAD65 and GAD 67), in addition to reduced SST, NPY and CORT expression. Reduced GAD67 was also reported in the DLPFC and amygdala in different studies.59,60 Zhao et al61 observed that the transcripts for the genes for GABA-A receptors (beta subunit) were significantly reduced in the PACC in MDD, whereas the DLPFC did not show any abnormalities. Postmortem findings suggest complex changes in the expression of GABA receptors and subunits in MDD, and are described in 62-64 and summarized in 10,23. These findings are important in that they suggest multiple changes in mediators of GABA signaling, although those GABA genes are expressed across different cell types and do not identify a specific cellular focus of pathology, as is the case for PV and SST markers. Finally, due to the close functional balance exerted between GABA and glutamate it is not surprising that changes in markers of glutamatergic functions have been reported in post-mortem subjects with MDD, although results on glutamate-related genes and gene products are more variable across studies and not consistent.65,66
These recent studies on neuron subtypes point to the necessity of refining the low GABA hypothesis of depression in terms of cellular origin and impact on information transfer. Specifically, the converging evidence now suggests that the low GABA phenotype observed in MDD may originate from the selective downregulation of SST-positive GABA neurons, at least across the corticolimbic areas investigated so far. Given the specialized function of these cells, the GABA dysregulation may concern deficits in information input regulation in brain regions that largely process emotionally-salient information (amygdala, PACC) and integrate it with cognitive processing (DLPFC). These postmortem studies also suggest greater changes in PACC that extend to altered PV-mediated output regulation and reduced GABA synthesis, consistent with reduced GABA levels measured in that area by MRS (see below). Figure 2 summarizes these primary findings and predicted impact on input/output regulation and information transfer in DLPFC and PACC respectively.
III. FROM THE CIRCUIT LEVEL TO THE REGIONAL LEVEL OF NEURAL ACTIVITY
How do specific changes in GABA neuron subtypes affecting input (SST) and output (PV) translate into neural activity at the regional level in MDD? Changes in GABA neuron subtypes affecting input and output are permanent and may therefore affect the generation of the ongoing neural activity, the resting state, as well as subsequent stimulus-induced or task-evoked activity. More specifically, these cellular changes should provide the most direct link to resting state activity on a regional level since the impact of the endogenous changes in excitatory (glutamatergic) and inhibitory (GABAergic) balance should be the strongest in the absence of modulation by specific tasks or stimuli. Moreover, following the cellular-biochemical findings (see above and below), we here focus on the resting state activity in PACC and DLPFC, as two key regions consistently implicated in MDD in recent imaging studies, and on the abnormal shift from external to internal mental contents in awareness (see 8-10,67-69). In contrast, we here leave aside other regions and networks like insula, salience network, amygdala, and subcortical regions that may be implicated in the generation and cognitive regulation of affect and its subsequent association with the internal (and external) mental contents.17,19-21
From local cell circuits to regional input/output structure in PACC and DLPFC
Canonical cortical circuitry
A typical cortical input structure, as apparent in the DLPFC, is characterized by cortico-cortical projections to pyramidal neurons located in layers 2/3 and by thalamic projections to deep layer 3 and layer 4.36 Input to layers 2 and 3 is characterized by robust inhibitory regulation by SST neurons that occur mostly in distal dendrites located in layer 1 (Figure 3a), and thus shows a predominance of input regulation. In contrast, the layer 4 granular neurons have smaller dendritic arbors that do not extend to layer 1 and are less sensitive to cortico-cortical processing and SST-mediated input regulation; these layer 4 neurons are densely targeted and inhibited by PV neurons, hence showing a predominance of output regulation. Deep layer 3 neurons that are also targeted by thalamic projections display more typical pyramidal cell structure, extend dendrites to layer 1 and thus show more balanced input and output regulation, and cortico-cortical processing (through extensions to layer 1) compared to layer 4 neurons (Figure 3).70
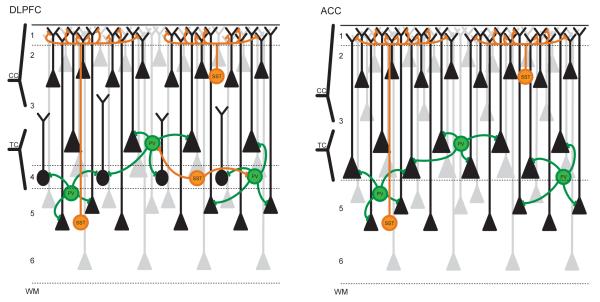
Figure 3. Local cell circuits and input/output regulation structure in DLPFC and PACC.
(A) The DLPFC cortical input structure is characterized by cortico-cortical (CC) projections to pyramidal neurons located in layers 2/3 and by thalamo-cortical (TC) projections to deep layer 3 and layer 4. Inputs to layers 2 and 3 are characterized by robust inhibitory regulation by SST neurons that occur mostly in distal dendrites located in layer 1. The layer 4 granular neurons have reduced dendritic arbors that do not extend to layer 1 and are less sensitive to cortico-cortical processing and SST-mediated input regulation. Layer 4 neurons are densely targeted and inhibited by PV neurons, hence showing a predominance of output regulation. Deep layer 3 neurons that are also targeted by thalamic projections display more typical pyramidal cell structure, extend dendrites to layer 1 and thus show more balanced input and output regulation, and cortico-cortical processing (through dendritic extensions to layer 1) compared to layer 4 neurons. (B) The PACC departs from the canonical layer structure described in (A) due to its absence of layer 4 (i.e., agranular cortex). All thalamic projections terminate in deep layer 3 and are submitted to greater processing through SST-mediated input regulation compared to DLPFC, notably by including cortico-cortical processing through layer 1 extension.
PACC
The PACC is a part of the limbic system that extends from the subcortical midline regions like raphe nucleus, locus coerulus, ventral tegmental area, amygdala, hypothalamus, periaqueductal gray, and ventral striatum (and many others) to the PACC and the insula at the cortical level.71-74 The PACC consequently receives inputs from these subcortical limbic regions stemming mostly from proprioceptive and especially interoceptive-vegetative inputs as well as from other cortical regions that are not directly implicated in exteroceptive stimulus processing. In addition to the predominantly internal input, the PACC also receives direct external inputs from all five exteroceptive sensory modalities and can therefore be considered a convergence zone between internal and external inputs.74,75
Notably the PACC departs from the canonical layer structure described above in an important way, due to its absence of layer 4 (i.e., agranular cortex) 76. This indicates a structure-based difference in how information is processed in PACC; it implies that all thalamic projections terminate in deep layer 3 and are submitted to greater processing through SST-mediated input regulation compared to DLPFC, notably by including cortico-cortical processing through layer 1 extension (Figure 3b). These structural-organizational peculiarities in PACC (when compared to DLPFC) are accompanied by specific biochemical features. The subgenual subdivision of the ACC shows high GABA-A and GABA-B receptor expression as well as benzodiazepine, 5HT1a, and alpha 1 receptors77,78 which has been confirmed in mice79 monkeys80 and humans using MRS-based measurement of GABA, glutamate and glutamine.81 A recent imaging study observed that the highest concentration of GABA and glutamate was found in PACC. Concentrations for both neurotransmitters were lower in supragenual and posterior cingulate regions, following the distribution of GABA-B receptors (see 81). Analogous regional biochemical data are not yet available for the DLPFC so we currently remain unable to directly compare both regions with regard to GABA and glutamate (and ultimately in their excitation-inhibition balance).
DLPFC
The DLPFC is located more laterally and is part of what is described as the executive network.16 The DLPFC receives strong inputs from all five exteroceptive sensory modalities and their respective sensory cortices and from the midline regions via especially the supragenual anterior cortex.74,82 Such predominance of external input is corroborated by the major input from the thalamus via thalamo-cortical loops.8,75 This robust thalamic input terminates in deep layer 3 and layer 4, which is paralleled by dense PV neurons in those layers in DLPFC. The combination of strong development of layer 4, thalamic input, and predominance of external input distinguishes the DLPFC from the PACC (Figure 3) and has implications for how that information is processed in those two regions, and that may become apparent and relevant in the context of pathological changes in MDD.
Regional specificities and putative impact of MDD cellular pathology in ACC and DLPFC
What does such input/output structure and its biochemical modulation imply for neural activity at the regional level in PACC and DLPFC and how do the MDD-related changes in GABA markers described in the previous section interact with this regional cellular and layer specificity? First, one would predict that the PACC is activated by predominantly internally-generated contents stemming from internal inputs like the strong subcortical vegetative-interoceptive input, its convergence with external inputs, and the continuous input from other cortical regions unrelated to exteroceptive processing. Second, given the integrated SST- and PV-GABA neuron mediated input/output regulation (i.e. agranular cortex; Figure 3) and the biochemical structure of the PACC with the strong concentration of GABA-A/B receptors and benzodiazepines receptors, one would expect that the internal inputs into PACC may be strongly related to GABA. Third, one would expect a different pattern in DLPFC. Due to its extended cortical connections, especially those with sensory modalities and presence of a clearly delineated layer 4 with direct and less processed external input via the thalamus (Figure 3), one would expect externally-generated contents stemming from exteroceptive inputs of the environment to predominate here. Fourth, changes in the excitation-inhibition balance in either PACC or DLPFC should lead to an abnormal balance between internally- and externally-generated contents.
Based on the MDD-related reported changes described above in markers of GABA-mediated input/output regulation, these structural differences including the differences in relative intero- and exteroceptive input processing, suggest a specific pattern of changes differentially affecting the DLFPC and PACC. In DLPFC, the prediction is of reduced inhibition and processing of cortico-cortical and thalamic input but normal (or putatively increased) output of the processed information, as mediated by reduced SST- but intact PV-related inhibition (Figure 3). In PACC, the prediction is of more profound deregulations encompassing reduced inhibition and fine-tuning of input (due to reduced SST neuron function) and reduced output inhibition (due to reduced PV neuron function). These PACC changes would occur in a context of a general decrease in GABA concentration (due to lower GAD65/67-mediated synthesis50) together leading to increased regional activation. Such increased regional activation, however, is not necessarily associated with greater synchronized output towards other regions since reduced PV cell function may also lead to reduced efficacy in synchronization of firing of ensembles of pyramidal neurons (see for instance 10,83) (Figure 3b).
From input structure to resting state activity in PACC and DLPFC
Imaging findings in healthy subjects show a reciprocal pattern of neural activity between PACC and DLPFC (Figure 4). Increased activity in DLPFC is accompanied by decreased activity levels in PACC and vice versa (see for instance 84,85). This however must be further specified in both neural and psychological regard; let us focus in the following, mainly on the neural mechanisms like the processing of the input on the regional levels of PACC and DLPFC while leaving aside for the moment the psychological issues like the balance between internal and external mental contents and their association with abnormally negative affect.
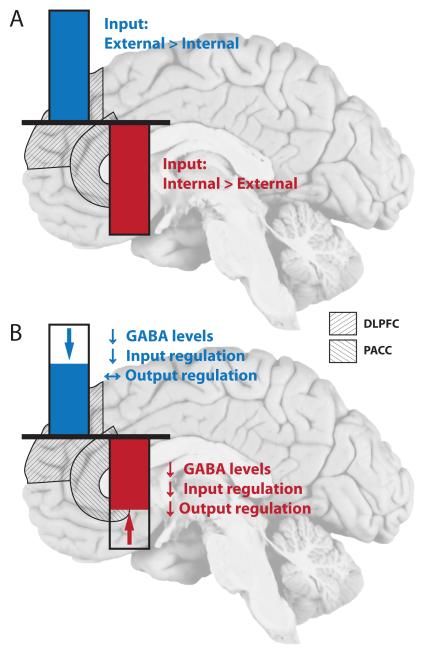
Figure 4. Resting state activity in PACC and DLPFC in controls and major depression.
(A) Imaging findings in healthy subjects show a reciprocal pattern of neural activity between PACC and DLPFC. Increased activity in DLPFC is accompanied by decreased activity levels in PACC and vice versa. The relative ratio of information content (Internal versus external) is depicted for both areas. (B) In MDD, the resting state activity of the DLPFC is reduced. In contrast the PACC shows increased resting state activity in MDD (i.e. less negative activity), resulting in an abnormal reciprocal modulation between the two regions. Underlying cellular and biochemical changes are highlighted and described in Figures 2-3.
Most imaging studies are conducted in functional magnetic resonance imaging (fMRI) that yields blood-oxygen-level dependent (BOLD) signal. The BOLD signal has been shown to be based predominantly on the input to a certain region and its local field potentials rather than its output.86,87 Most importantly, the BOLD signal shows positive signal changes, so-called activation, and negative ones that is, deactivation. While the physiological basis of the activation has been relatively well investigated, the physiological underpinnings of the deactivation remain unclear (although see 88,89).
Why is that important for PACC and DLPFC? Interestingly, the PACC shows predominantly negative BOLD response that is, deactivation in fMRI (see 85,90). This contrasts with the DLPFC that shows positive BOLD signals that is, activation. Independent and separate analyses of both regions’ neural activities reveal that both activation and deactivation seem to be reciprocally modulated between PACC and DLPFC: Greater deactivation in PACC entails increased activation in DLPFC whereas lower deactivation in PACC leads to low activation in DLPFC (Figure 4). This has been demonstrated in several studies in healthy subjects (see 8,84,85).
Most interestingly, this pattern of reciprocal modulation seems to be related to the processing of internally- and externally-generated contents. Cognitive tasks, such as working memory or executive tasks that focus strongly on external stimuli from the environment, strongly recruit neural activity and more specifically activation in the DLPFC. This contrasts with the PACC where the deactivation is rather modulated by vegetative and emotional processing.2,91 The differential pattern of neural activity, activation versus deactivation, in PACC and DLPFC consequently suggests relation to the processing of different kinds of contents, internally- versus externally-generated, that stem from different origins, interoceptive and somatic from the own body (and/or cortically from other cortical regions’ ongoing activity) or exteroceptive from the external environment.
What about the findings in both regions in MDD? The PACC (and the adjacent ventromedial prefrontal cortex) shows increased resting state activity in MDD (see 4,8,10,12,92 for recent reviews). In contrast, resting state activity in especially the left DLPFC is reduced in MDD (Figure 4). See also 93 for a confirmation of abnormal reciprocal modulation between PACC and DLPFC in MDD during task-evoked activity. Such opposite change in the resting state activity level in MDD is well compatible with their reciprocal modulation where changes in the activity level of one region are accompanied by opposite changes in the respective other region.
How is such reciprocal pattern between PACC and DLPFC in the resting state related to their different input structure and cellular/biochemical modulation? This remains unclear at this point in time, however. One may be puzzled at first glance that such question about the relationship between input structure on a cellular level and resting state activity on a regional level can be raised at all. Intuitively, one would associate the resting state activity with the absence of any input. However, this concerns only the absence of specific external inputs like particular goals or tasks as related to exteroceptive input. In contrast, the unspecific input coming from other cortical and subcortical regions, the interoceptive input from the body, and the unspecific exteroceptive input from the senses are still processed even in the resting state (which therefore can be coined resting state only in an operational sense of the term but not in a physiological meaning).94,95
Given this, one may indeed raise the question whether the opposite and thus reciprocal pattern of PACC and DLPFC resting state activity in MDD is related to a dysbalance in the unspecific inputs: even in the resting state the interoceptive input from the own body and the input from the other cortical regions’ ongoing resting state activity are processed and, due to the alleged cellular-biochemical abnormalities (see above), may predominate. Such predominance of internal inputs may, hypothetically, be caused by the decreased gating of these inputs onto excitatory neurons by the disturbed SST interneurons as well as by reduced inhibitory regulation of excitatory output by the disturbed PV interneurons in PACC in MDD (Figure 2) which, in turn, may ultimately lead to increased resting state activity in PACC.
In contrast, in DLPFC the integrity of the unspecific exteroceptive input that predominates may be affected due to reduced SST interneuron-mediated function in MDD. Although this may translate into increased regional activity, at least two mechanisms may suggest the opposite (i.e. reduced DPPFC activity as observed): (1) the unspecific exteroceptive input may increase which, in turn, would induce even stronger down-modulation by the reciprocal feedback inhibition of the PACC, or (2) increased input may induce stronger degrees of output inhibition by the preserved (and abundant) PV interneurons in DLPFC. Future investigations are necessary to determine the exact neural mechanism of DLPFC down-regulation in MDD, and also how the abnormal dysbalance between PACC and DLPFC may affect the control of the DLPFC in down-modulating amygdala activity, due to the central role of cognitive emotion regulation in cognitive behavioral therapy.20,21
Taken together, the cellular and biochemical abnormalities in the input-output gating mechanisms in PACC and DLPFC may lead to a dysbalance in the processing between internally- and externally-generated contents in the resting state in MDD. More specifically, the lack of input gating onto pyramidal neurons by the deficient SST neurons and the decreased inhibition by the deficient PV neurons may lead to an overflow of internally-generated contents in PACC which, tentatively, may be reflected in its elevated resting state activity. Since PACC and DLPFC activity levels are reciprocally coupled with each other, resting state hyperactivity in PACC entails resting state hypoactivity in DLPFC which is exactly what can be observed in especially the left DLPFC in MDD. Hence, even if the amount of externally-generated input that is processed in DLPFC remains within the ‘normal’ limits, resting state activity in DLPFC may nevertheless be down-modulated due to its reciprocal coupling with the abnormally high resting state activity in PACC. Note that we have so far only accounted for the mere processing of inputs on the regional level of neural activity in PACC and DLPFC while not touching upon how these inputs are converted into mental contents and associated with negative affect.
From GABA to resting state activity in PACC (and DLPFC)
GABA and glutamate resting state concentrations can be investigated in humans using MRS. As alluded to earlier, findings show significant reductions of GABA in occipital cortex in MDD. Analogous findings (though not fully consistent) have been observed in PACC (and/or adjacent regions) where especially GABA is reduced in MDD (see 29,31,96-98). A recent translational meta-analysis in MDD combining human imaging data and animal model-based data 10 confirmed the reduced GABA concentration in PACC with further findings suggesting reduction in both GABAA and GABA-B receptors and GAD-67, the enzyme that converts glutamate into GABA. In contrast to the PACC, MRS findings have not been as consistent in DLPFC (see for instance 15) for which reason we focus on PACC in the following, consistent with the above described decreases in SST- and PV-mediated input/output gating deficits.
How does such seemingly reduced GABA-ergic mediated neural inhibition translate into the elevated resting state activity level in PACC? To directly test this association, it is necessary to combine MRS with fMRI. One of the first such studies in this regard combined fMRI with MRS in PACC. Interestingly, the concentration of GABA in PACC predicted the degree of negative BOLD signal, or deactivation, in the same region: the higher the GABA concentration, the stronger the degree of negative BOLD response.7 In contrast, no such coupling was found for glutamate. These associations between resting state neurotransmitter concentration and BOLD signal are different in MDD. In MDD patients, the negative BOLD signal in PACC, which is reduced in MDD, is no longer predicted by resting state GABA concentration.99 Instead, the reduced negative BOLD signal in PACC is now predicted by the concentration of glutamate, suggesting an abnormally strong role of glutamatergic-mediated neural excitation in parallel to a decreased GABA-ergic mediated neural inhibition, potentially reflecting (mal)adaptive changes in excitation/inhibition balance in that region in MDD.
This is consistent with the earlier described deficit in expression of genes coding for PV, SST and GABA synthesizing enzymes in in the PACC in MDD, as the potential source of reduced GABA levels. However, it remains unclear how those GABA neuron deficits impact the intra- and extracellular concentration of GABA as it is measured with MRS. More specifically, we have to consider that MRS does not measure synaptic activity related to GABA (and Glutamate) but only intra- and extracellular concentrations100; this means that direct inference from the cellular-synaptic changes reported above to the cellular-regional level as measured with MRS remains impossible. Moreover, one has to consider that most MRS studies do not provide a separate measurement of Glutamate as distinguished from Glutamine. Therefore, we can only point out intuitive correspondence and consistency between the findings on the different levels while abstaining from any causal assumptions.
Based on the above described correspondences between cellular and regional level, we suggest the following. One would expect that SST-related deficits as well as PV and GABA abnormalities lead to an abnormal shift in the excitation-inhibition balance which then abnormally up-modulates the level of resting state activity in PACC. This could be tested in electrophysiological studies that also include measurements of SST, PV, Glutamate, and GABA, where one would expect the SST-related deficits to result in a shift of the excitation-inhibition balance towards abnormally elevated excitation and reduced inhibition in MDD. Initial testing of this hypothesis in animal models does indeed suggest long-term local and network adaptations in the excitation-inhibition balance48,101. In humans, first tentative and incomplete support for GABA-ergic modulation of the excitation-inhibition balance on the regional level comes from combined TMS-MRS studies that show changes in GABA concentration during TMS-induced increase or decrease in neural inhibition (over the motor cortex)102,103. Future studies may want to extend such approach to the above mentioned substances which could lend further support to our hypothesis. Moreover, the initial fMRI-MRS combined studies point to differential effects of Glutamate and GABA on fMRI BOLD signal which is in line with their differences on the cellular level. How this impacts the excitation-inhibition balance remains unclear, however. Since the excitation-inhibition balance results from the interaction between Glutamate and GABA, one may want to relate a combined measure of their ratio (rather than single measures) in future fMRI-MRS or TMS-MRS studies.
IV. FROM THE REGIONAL LEVEL OF NEURAL ACTIVITY TO THE NETWORK LEVEL AND THE SYMPTOMS
From regions to networks and psychological functions
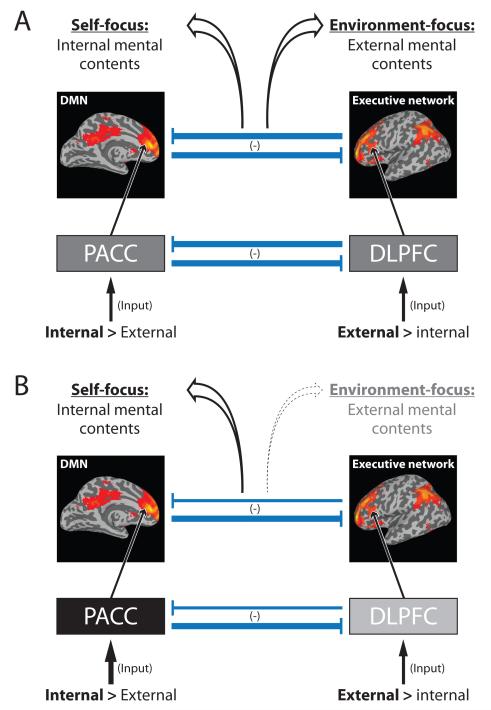
We already mentioned the default-mode network (DMN) and the executive network as two key networks in the resting state. The PACC is a key region in especially the anterior DMN 16,104,105 that is signified by low frequency fluctuations16,99,100, while the DLPFC is crucial for the executive network16. The DMN and executive networks are anti-correlated, that is, they stand in a negative inhibitory relationship to each other: e.g., increase in executive network excitation (in for instance DLPFC) decreases DMN PACC (and medial prefrontal) functional connectivity to the executive network (and the salience network) (and vice versa with a decrease in executive network excitation disinhibiting DMN functional connectivity)106 (Figure 5). Taken together, this strongly suggests that the above mentioned reciprocal modulation between PACC and DLPFC on the regional level resurfaces as negative relationship or anti-correlation between DMN and executive network on the network level. Psychologically, we assume the balance between DMN and executive network to be associated with the abnormal shift from external to internal mental contents in awareness in MDD which, in turn, is coupled to an abnormally negative affect. We here focus on the first, the neural mechanisms underlying the balance between internal and external mental contents while leaving aside the second, the association of mental contents with negative affect (for which PACC and DLPFC and their connections to subcortical regions seems to play an essential role9,19).
Figure 5. From input over regions and networks to contents in awareness.
(A) ‘Normal’ balance between default-mode network (DMN) and executive network (EN) in healthy subjects. Lower part: The figure illustrates schematically the relationship between regions (lower part; PACC: Perigenual anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex) and their respective input (PACC: stronger internal input from own body and thoughts; DLPFC: stronger external input from environment). Upper level: the PACC is the core region of the default-mode network (DMN) as visualized in the brain on the left while the DLPFC is the core region of the executive network (EN) as visualized in the brain on the right. Like PACC and DLPFC both networks, DMN and EN, stand in a reciprocal relationship to each other as signified by the negative sign symbol. The balance between DMN and EN mediates the balance between internal and external mental contents in our awareness which can be described as self-focus and environment-focus. (B) Dysbalance between default-mode network (DMN) and executive network (EN) in MDD. The mechanisms described in the healthy brain in panel A are altered in MDD. There is a dysbalance on the input level with decreased input to DLPFC which in turn implies (relatively) increased internal input in PACC. This dysbalances PACC and DLPFC and subsequently alters the balance between DMN and EN, in turn, abnormally increasing internal mental contents leading to an increased self-focus while, at the same time, external mental contents are decreased with a decreased external environment-focus.
What psychological functions are associated with the DMN and the executive network? As implied by its name, the executive network is closely related to cognitive-executive functions including generation of goal-orientation and planning and initiation of action and movements. As such it processes predominantly external stimuli stemming from exteroceptive sensory inputs which are cognitively elaborated and transformed into action in the executive network (see16). Hence, the input structure of the DLPFC where the thalamically-mediated exteroceptive input predominates seems to be translated onto the network level of the executive network. This clearly distinguishes it from the DMN that has been associated with various inner mental functions like mind wandering,107,108 random or undirected thoughts,109 consciousness110,111 and self-referential activity.105,112,113 It remains to be explored whether the mental functions associated with the DMN originate in the internal input from other cortical and subcortical regions as it may be hypothesized on the basis of the input structure of the PACC.
From GABA to neural networks
What about the biochemical modulation of these networks? A recent fMRI-MRS study demonstrated that the resting state functional connectivity within the DMN is modulated by the concentration of GABA in the posterior cingulate cortex (PCC):114 the higher the concentration of GABA in PCC, the lower the functional connectivity from PCC to other regions within the DMN. In contrast to GABA, PCC glutamate correlated positively with functional connectivity in the DMN. Another study by Duncan et al115 demonstrated that PACC glutamate levels positively predicted the strength of functional connectivity from PACC to various limbic-subcortical regions like the thalamus, ventral striatum, and PAG. These initial results suggest that functional connectivity within the DMN is modulated by GABA and glutamate and thus most likely dependent upon the excitation-inhibition neuronal balance. This is further supported by challenge studies using benzodiazepines. A recent study by Flodin et al116 showed increase in functional connectivity within especially the DMN during application of oxazepam, a benzodiazepine that modulates GABA-A receptors and thus GABA-ergic neural inhibition. An earlier challenge study using the benzodiazepine Lorazepam117 demonstrated altered balance between PACC deactivation and DLPFC activation during Lorazepam challenge, a finding which is well in accordance with the observations on the network level.
In addition to GABA-ergic challenges, recent studies have used Ketamine, a glutamatergic agent, to challenge resting state functional connectivity. Again, changes in functional connectivity in especially the DMN and dysbalance with the executive network were observed118,119 (see also 120). We have to be careful, however. While the results of these first studies demonstrate regionally- and network-specific effects of Ketamine and Benzodiazepines, we have to consider that these effects may be non-specific for several reasons. First, they are globally distributed throughout the whole brain which, in most of the studies, is corrected for by global normalization when testing for region- and/or network-specific effects. Secondly, we cannot exclude vascular rather than neuronal effects of these substances, especially given the neuro-vascular nature of the BOLD signal in fMRI. Future investigations may consequently want to include measures of vascular blood flow (like arterial spin labelling/ASL) in challenge studies with Ketamine or Benzodiazepines.
Taken together, considering methodological caveats, these studies tentatively suggest that GABA and glutamate impact not only cellular and regional, but also network-related activity that affects the balance between the DMN and executive network. One may therefore suggest that the excitation-inhibition balance may be crucial in modulating the balance between these two networks. The central role of the excitation-inhibition balance on the network level is further supported by the recent study by Chen et al106 who observed that (using transcranial magnetic stimulation/TMS) increases and decreases in the degree of neural excitation in DLPFC/EN lead to opposite changes in PACC/DMN functional connectivity (see also 120,121).
What do these findings in healthy subjects let us predict for MDD? First, given that DMN and executive network show a different input structure, one would suspect dysbalance between both networks in MDD. With the PACC as key region, the DMN network may predominate and thus be abnormally strong leading to the executive network being down-modulated, so that one would expect anti-correlation between the internal DMN functional connectivity and the internal executive network functional connectivity, reflecting network dysbalance. Second, one would expect that such network dysbalance may be accompanied by psychological dysbalance between internal and external contents: the DMN-mediated internal contents like self-referential activity, mind wandering, and random thoughts may predominate over the rather suppressed external content as processed in the executive network. Third, one would expect the biochemical dysbalance between GABA and glutamate to drive the network dysbalance; the excitation-inhibition balance in the DMN may tilt abnormally toward the excitatory pole whereas in the executive network it may be shifted more toward the inhibitory pole. There is, as we will see, strong empirical support for the first and second predictions while evidence for the third remains to be provided.
From altered networks to depressive symptoms
Recent studies in MDD indeed show changes in resting state functional connectivity. Though differing in their details and the exact regions, all studies have reported increased functional connectivity of the pre- and/or subgenual ACC with other regions of the DMN. This includes the VMPFC and other subcortical limbic regions (e.g., the amygdala) which may be central in cognitive emotion regulation and cognitive behavioral therapy in MDD.122-126 Most interestingly, these studies report increased functional connectivity in the anterior part of the DMN that includes the PACC, the VMPFC, and the DMPFC. This seems to be a trait marker of MDD that remains independent of the state; that is, depressed or non-depressed.123-125
Though not fully consistent, the data show that the functional connectivity between PACC and DLPFC is abnormally increased in MDD.124,126,127 Does this mean that the abnormally increased functional connectivity, especially within the anterior DMN enslaves the neural activity of the DLPFC? If so, one would expect decreased anti-correlation between DMN and executive networks in MDD. Unfortunately, the degree of anti-correlation has not yet been measured as such in MDD. However, decreased anti-correlation between DMN and executive network would be well in accordance with the decreased reciprocal modulation between PACC and DLPFC in MDD previously described. Finally, the psychopathological specificity of the networks findings for MDD may be questioned. Studies in for instance schizophrenia and bipolar disorder also report abnormalities in both DMN and executive network (see for instance128-130). However, the nature of changes seems to distinguish depression from both bipolar and schizophrenia: increased PACC functional connectivity to other regions both within and outside the DMN which are specifically involved in emotion processing (e.g., subcortical limbic regions) and emotion regulation (like executive network) seems to be relatively specific for MDD and distinguishes it from bipolar disorder and schizophrenia (see for instance131,132). More specifically, MDD patients show hyperactivity (and increased functional connectivity) in PACC (and ventromedial prefrontal cortex) while the same regions are hypoactive in schizophrenia which may be related to their opposite symptoms with regard to the self (see below)92.
Are specific symptoms associated with the network dysbalance? Studies demonstrated that regions of the default-mode network including the anterior regions seem to be directly related to self-referential processing.14,15,133,134 Behaviorally, MDD patients show increased degrees of self-referentiality, an increased self-focus as it has been termed,7 which they attribute to negative emotional stimuli; this is directly related to decreased deactivation and functional connectivity in the anterior midline regions like PACC, VMPFC, and DMPFC. Berman et al135 demonstrated increased functional connectivity between PACC and PCC in MDD which, most importantly, predicted especially the degree of rumination and brooding in these patients (see also136-138 for more or less similar results) (Figure 5). These results support the assumption by Kuhn and Gallinat92 who, based on meta-analysis (see above), associate increased PACC/anterior DMN activity with increased self-focus, while decreased activity in the same regions in schizophrenia is related to decreased self-focus (if not loss of self).
Taken together, these data suggest that symptoms like rumination and increased self-referentiality, as characterized by abnormally strong internal contents, are associated with changes in the DMN and specifically its anterior regions including the PACC. This inclines one to assume that the input structure, with the predominance of internal input in PACC and DMN, is also reflected at the network and most importantly at the symptom level in MDD. One may thus infer that the cellular and circuit abnormalities focusing on SST-and PV-positive GABA interneurons are integrated with regional structural specificities and together conveyed or translated into abnormal regional and network activity, where they manifest psychologically in dysbalance between internal and external contents. This manifest as increased rumination and abnormally elevated self-referentiality, and decreased external mental contents from the environment resulting in (subjective-experiential) detachment from the environment, i.e., a decreased environment-focus.7,9,139 However, it remains unclear why and how internal mental contents like ruminations or body changes are more strongly associated with negative rather than positive affect and emotions in MDD (see for instance 8). Finally, in addition to ruminations and internal self-focus, one may also want to consider other symptoms in MDD like anhedonia. Anhedonia has been associated with resting state hyperactivity and hyperconnectivity in PACC and downstream subcortical regions like the striatum (see 9,140). Most interestingly, anhedonia has also been associated with abnormal levels of GABA and Glutamate and hence with an abnormal excitation-inhibition balance in these regions in both animal models and human MDD31,99,141.
V. CONCLUSION
Molecular studies in MDD demonstrate changes in specific GABA interneurons, namely SST-positive and to a lesser extent PV-positive neurons, affecting the regulation of input/output of excitatory signals from and onto pyramidal neurons. We here hypothesize that such dysbalance at the local cell circuit affects the processing of information as it transits through cortical layers, translating, through multiple steps described here, to a dysbalance between the PACC and DLPFC as core regions of the DMN and executive network, and in turn leading to a dysbalance between internal and external mental contents in awareness in MDD as clinically observed. Accordingly, we propose a cross-level hypothesis ranging from cellular and biochemical levels to regional and neural network levels of activities and to their manifestation at the behavioral level within the gestalt of clinical symptoms (Figure 1). In addition to providing a putative molecular-based explanation of clinical symptoms, this model also supports novel opportunities to directly link molecularly-based animal models of human MDD pathology with human imaging studies of regional and network-related activity changes. Finally, our hypotheses about the cellular, biochemical, and regional mechanisms of internal and external awareness in MDD carry important implications beyond MDD. These concern other psychiatric and neurologic disorders like schizophrenia, where partly overlapping neuronal changes are observed, and where the balance between internal and external (mental) contents including their association with the self is also abnormal or disrupted (though in ways that differ from MDD). While our hypothesis addresses mainly neuroscientific mechanisms, it may nevertheless be relevant in future diagnosis and therapy of MDD. For instance, it may allow developing more specific neuronal and biochemical markers for one of the central symptoms of MDD, i.e., increased self-focus. In turn, this may enable the development of therapeutically more specific pharmacological and psychotherapeutic interventions. Finally, apart from neurologic and psychiatric disorders, our hypotheses provide for the first time a set of hypotheses and suggestions for cross-level mechanisms of how internal and external contents may be constituted and balanced in awareness in healthy subjects. This can thus be seen as a contribution to the neuroscientific debate on the neural predispositions and correlates of consciousness.94,139
Acknowledgements
This work was supported by National Institute of Mental Health MH084060 and MH077159 (ES) as well as CIHR, HDRF, and EJLB-CIHR and Michael Smith Foundation (GN). We thank both Sibille and Northoff lab members, H.Aizenstein and K.Erickson for feedback on the content of this manuscript, and B.French for careful feedback and help with the manuscript.
Abbreviations
- PACC
perigenual anterior cingulate cortex
- DLPFC
dorsolateral prefrontal cortex
- GABA
gamma amino butyric acid
- MDD
Major depressive disorder
- SST
somatostatin
- DMN
default-mode network
- EN
executive network
- PV
parvalbumin
- MRS
magnetic resonance spectroscopy
- fMRI
functional magnetic resonance imaging
- BOLD
blood-oxygen-level dependent
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.WHO . World Health Organization - The Global Burden of Disease - 2004 update. WHO Library; 2008. [Google Scholar]
- 2.Mayberg HS. Modulating limbic-cortical circuits in depression: targets of antidepressant treatments. Semin Clin Neuropsychiatry. 2002;7(4):255–268. doi: 10.1053/scnp.2002.35223. [DOI] [PubMed] [Google Scholar]
- 3.Belmaker RH, Agam G. Major depressive disorder. NEnglJ Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379(9820):1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am. 2003;13(4):805–815. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 6.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34(1):1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 8.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Northoff G, Wiebking C, Feinberg T, Panksepp J. The ‘resting-state hypothesis’ of major depressive disorder-a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35(9):1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34(4):592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16(6):604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27(5):488–492. doi: 10.1097/jcp.0b013e318151521c. [DOI] [PubMed] [Google Scholar]
- 13.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30(8):2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12(8):627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- 16.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J Biol Psychiatry. 2010;11(3):538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- 18.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 19.Panksepp J. Affective neuroscience : the foundations of human and animal emotions. Oxford University Press; New York: 1998. p. xii.p. 466. [Google Scholar]
- 20.Gross JJ. Emotion regulation: taking stock and moving forward. Emotion. 2013;13(3):359–365. doi: 10.1037/a0032135. [DOI] [PubMed] [Google Scholar]
- 21.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8(8):721–737. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 23.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emrich HM, von Zerssen D, Kissling W, Moller HJ, Windorfer A. Effect of sodium valproate on mania. The GABA-hypothesis of affective disorders. Arch Psychiatr Nervenkr. 1980;229(1):1–16. doi: 10.1007/BF00343800. [DOI] [PubMed] [Google Scholar]
- 25.Gold BI, Bowers MB, Roth RH, Sweeney DW. Gaba Levels in Csf of Patients with Psychiatric-Disorders. Am J Psychiat. 1980;137(3):362–364. doi: 10.1176/ajp.137.3.362. [DOI] [PubMed] [Google Scholar]
- 26.Petty F, Schlesser MA. Plasma GABA in affective illness. A preliminary investigation. J Affect Disord. 1981;3(4):339–343. doi: 10.1016/0165-0327(81)90003-3. [DOI] [PubMed] [Google Scholar]
- 27.Petty F, Sherman AD. Plasma GABA levels in psychiatric illness. J Affect Disord. 1984;6(2):131–138. doi: 10.1016/0165-0327(84)90018-1. [DOI] [PubMed] [Google Scholar]
- 28.Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry. 1981;138(8):1098–1101. doi: 10.1176/ajp.138.8.1098. [DOI] [PubMed] [Google Scholar]
- 29.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67(5):458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69(2):139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. AmJ Psychiatry. 2002;159(4):663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 33.Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27(14):3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61(6):729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9(3):206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 37.Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2013;19(3):228–237. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14(3):202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao R, Schummers J, Knoblich U, Lacey CJ, Van Wart A, Cobos I, et al. Influence of a subtype of inhibitory interneuron on stimulus-specific responses in visual cortex. Cereb Cortex. 2012;22(3):493–508. doi: 10.1093/cercor/bhr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16(8):1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Packer AM, McConnell DJ, Fino E, Yuste R. Axo-Dendritic Overlap and Laminar Projection Can Explain Interneuron Connectivity to Pyramidal Cells. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69(6):1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26(19):5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77(1):155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. BiochemPharmacol. 2009;78(5):431–439. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soumier A, Sibille E. Opposing Effects of Acute Versus Chronic Blockade of Frontal Cortex Somatostatin-Positive Inhibitory Neurons on Behavioral Emotionality in Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin LC, Sibille E. Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol. 2013;4:110. doi: 10.3389/fphar.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14(6):721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42(1):116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32(2):471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, et al. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67(5):465–470. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286(1-2):75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 57.de Lecea L, del Rio JA, Criado JR, Alcantara S, Morales M, Danielson PE, et al. Cortistatin is expressed in a distinct subset of cortical interneurons. J Neurosci. 1997;17(15):5868–5880. doi: 10.1523/JNEUROSCI.17-15-05868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13(4):411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Bao AM, Qi XR, Kamphuis W, Luchetti S, Lou JS, et al. Gene expression of GABA and glutamate pathway markers in the prefrontal cortex of non-suicidal elderly depressed patients. J Affect Disord. 2012;138(3):494–502. doi: 10.1016/j.jad.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24(6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64(8):645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 64.Poulter MO, Du L, Zhurov V, Palkovits M, Faludi G, Merali Z, et al. Altered Organization of GABA(A) Receptor mRNA Expression in the Depressed Suicide Brain. Front Mol Neurosci. 2010;3:3. doi: 10.3389/neuro.02.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. ProcNatlAcadSci USA. 2005;102(43):15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127(1-3):230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. AmJPsychiatry. 2001;158(9):1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 71.Reiner A. Role in Paleocerebral Functions. Paul D. MacLean; Plenum, New York: 1990. The Triune Brain in Evolution; p. xxiv.p. 672. illus. $75. [DOI] [PubMed] [Google Scholar]; Science. 1990;250(4978):303–305. doi: 10.1126/science.250.4978.303-a. [DOI] [PubMed] [Google Scholar]
- 72.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75(2):143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Morgane PJ, Mokler DJ. The limbic brain: continuing resolution. Neurosci Biobehav Rev. 2006;30(2):119–125. doi: 10.1016/j.neubiorev.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Mesulam MM. Principles of behavioral and cognitive neurology. 2nd edn Oxford University Press; Oxford ; New York: 2000. p. xviii.p. 540. [Google Scholar]
- 75.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 76.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 77.Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508(6):906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30(8):2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. Cingulate area 32 homologies in mouse, rat, macaque and human: Cytoarchitecture and receptor architecture. J Comp Neurol. 2013 doi: 10.1002/cne.23409. [DOI] [PubMed] [Google Scholar]
- 80.Palomero-Gallagher N, Zilles K, Schleicher A, Vogt BA. Cyto- and receptor architecture of area 32 in human and macaque brains. J Comp Neurol. 2013;521(14):3272–3286. doi: 10.1002/cne.23346. [DOI] [PubMed] [Google Scholar]
- 81.Dou W, Palomero-Gallagher N, van Tol MJ, Kaufmann J, Zhong K, Bernstein HG, et al. Systematic regional variations of GABA, glutamine, and glutamate concentrations follow receptor fingerprints of human cingulate cortex. J Neurosci. 2013;33(31):12698–12704. doi: 10.1523/JNEUROSCI.1758-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feinberg TE. From axons to identity: Neurological explorations of the nature of the self. W. W. Norton & Company; New York and London: 2009. [Google Scholar]
- 83.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87(1):B11–22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- 85.Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual-Leone A, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21(3):202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 87.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 88.Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron. 2012;76(3):629–639. doi: 10.1016/j.neuron.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62(2):1040–1050. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 90.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 92.Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39(2):358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bermpohl F, Walter M, Sajonz B, Lucke C, Hagele C, Sterzer P, et al. Attentional modulation of emotional stimulus processing in patients with major depression--alterations in prefrontal cortical regions. Neurosci Lett. 2009;463(2):108–113. doi: 10.1016/j.neulet.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 94.Northoff G. What the brain’s intrinsic activity can tell us about consciousness? A tri-dimensional view. Neurosci Biobehav Rev. 2013;37(4):726–738. doi: 10.1016/j.neubiorev.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45(4):1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 96.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, P MM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11(2):255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 97.Shaw A, Brealy J, Richardson H, Muthukumaraswamy SD, Edden RA, John Evans C, et al. Marked reductions in visual evoked responses but not gamma-aminobutyric acid concentrations or gamma-band measures in remitted depression. Biol Psychiatry. 2013;73(7):691–698. doi: 10.1016/j.biopsych.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 98.Plante DT, Jensen JE, Schoerning L, Winkelman JW. Reduced gamma-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: a link to major depressive disorder? Neuropsychopharmacology. 2012;37(6):1548–1557. doi: 10.1038/npp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66(5):478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 100.Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol. 2011;4(5):573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seybold BA, Stanco A, Cho KK, Potter GB, Kim C, Sohal VS, et al. Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex. Proc Natl Acad Sci U S A. 2012;109(34):13829–13834. doi: 10.1073/pnas.1205909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589(Pt 23):5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tremblay S, Beaule V, Proulx S, de Beaumont L, Marjanska M, Doyon J, et al. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J Neurophysiol. 2013;109(5):1343–1349. doi: 10.1152/jn.00704.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 106.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Christoff K. Undirected thought: neural determinants and correlates. Brain Res. 2012;1428:51–59. doi: 10.1016/j.brainres.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 110.Qin P, Di H, Liu Y, Yu S, Gong Q, Duncan N, et al. Anterior cingulate activity and the self in disorders of consciousness. Hum Brain Mapp. 2010;31(12):1993–2002. doi: 10.1002/hbm.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Z, Dai R, Wu X, Yang Z, Liu D, Hu J, et al. The self and its resting state in consciousness: An investigation of the vegetative state. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Northoff G, Panksepp J. The trans-species concept of self and the subcortical-cortical midline system. Trends Cogn Sci. 2008;12(7):259–264. doi: 10.1016/j.tics.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 113.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 114.Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, et al. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8(4):e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flodin P, Gospic K, Petrovic P, Fransson P. Effects of L-dopa and oxazepam on resting-state functional magnetic resonance imaging connectivity: a randomized, cross-sectional placebo study. Brain Connect. 2012;2(5):246–253. doi: 10.1089/brain.2012.0081. [DOI] [PubMed] [Google Scholar]
- 117.Northoff G, Witzel T, Richter A, Gessner M, Schlagenhauf F, Fell J, et al. GABA-ergic modulation of prefrontal spatio-temporal activation pattern during emotional processing: a combined fMRI/MEG study with placebo and lorazepam. J Cogn Neurosci. 2002;14(3):348–370. doi: 10.1162/089892902317361895. [DOI] [PubMed] [Google Scholar]
- 118.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18(11):1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7(9):e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeng LL, Shen H, Liu L, Hu D. Unsupervised classification of major depression using functional connectivity MRI. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 125.Li CT, Chen LF, Tu PC, Wang SJ, Chen MH, Su TP, et al. Impaired prefronto-thalamic functional connectivity as a key feature of treatment-resistant depression: a combined MEG, PET and rTMS study. PLoS One. 2013;8(8):e70089. doi: 10.1371/journal.pone.0070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye T, Peng J, Nie B, Gao J, Liu J, Li Y, et al. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol. 2012;81(12):4035–4040. doi: 10.1016/j.ejrad.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 127.Kong L, Chen K, Tang Y, Wu F, Driesen N, Womer F, et al. Functional connectivity between the amygdala and prefrontal cortex in medication-naive individuals with major depressive disorder. J Psychiatry Neurosci. 2013;38(5):120117. doi: 10.1503/jpn.120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74(6):458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, et al. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148(1-3):74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 130.Mamah D, Barch DM, Repovs G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150(2):601–609. doi: 10.1016/j.jad.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu Y, Shen H, Zeng LL, Ma Q, Hu D. Convergent and divergent functional connectivity patterns in schizophrenia and depression. PLoS One. 2013;8(7):e68250. doi: 10.1371/journal.pone.0068250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sambataro F, Wolf ND, Pennuto M, Vasic N, Wolf RC. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med. 2013:1–11. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- 133.Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Lehericy S, et al. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J Affect Disord. 2010;124(1-2):196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136(1-2):e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 135.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 138.Kuhn S, Vanderhasselt MA, De Raedt R, Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. J Affect Disord. 2012;141(2-3):352–360. doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 139.Northoff G. Unlocking the Brain. Volume II: Consciousness. Oxford University Press; Oxford, New York: 2013. vol. 2. [Google Scholar]
- 140.Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–641. e613. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Venzala E, Garcia-Garcia AL, Elizalde N, Tordera RM. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur Neuropsychopharmacol. 2013;23(7):697–708. doi: 10.1016/j.euroneuro.2012.05.010. [DOI] [PubMed] [Google Scholar]