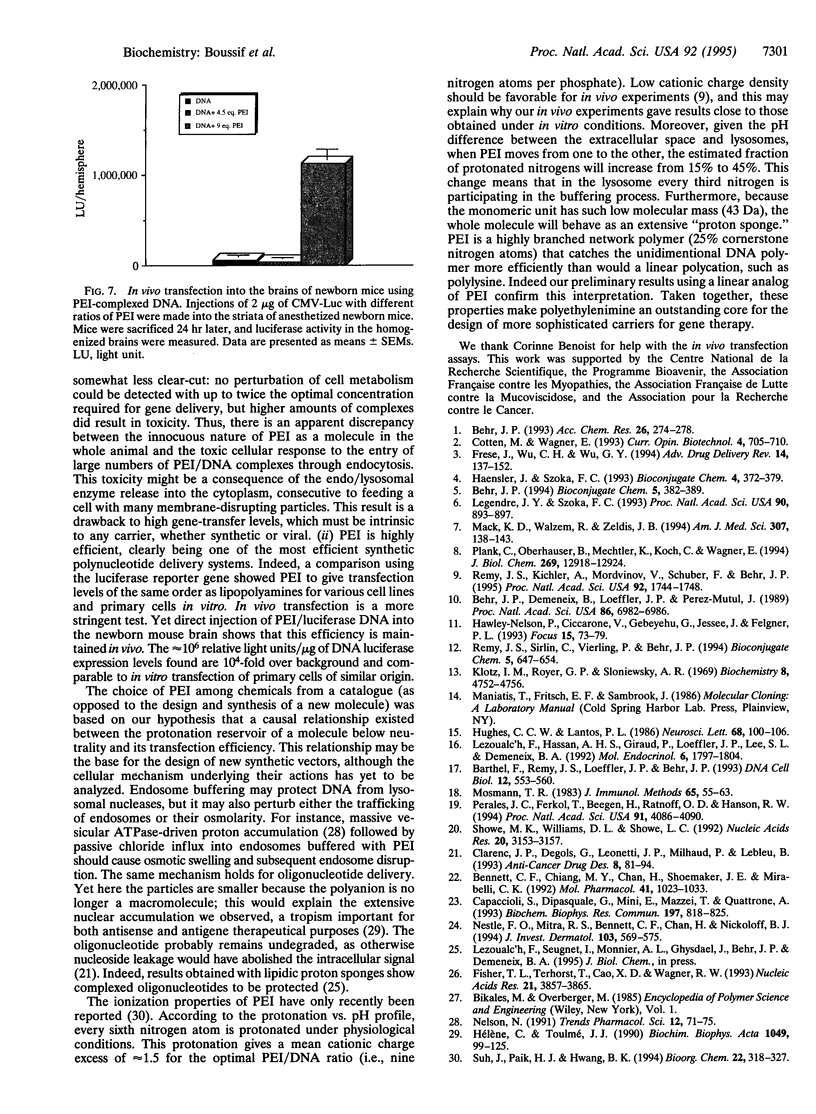
Abstract
Several polycations possessing substantial buffering capacity below physiological pH, such as lipopolyamines and polyamidoamine polymers, are efficient transfection agents per se--i.e., without the addition of cell targeting or membrane-disruption agents. This observation led us to test the cationic polymer polyethylenimine (PEI) for its gene-delivery potential. Indeed, every third atom of PEI is a protonable amino nitrogen atom, which makes the polymeric network an effective "proton sponge" at virtually any pH. Luciferase reporter gene transfer with this polycation into a variety of cell lines and primary cells gave results comparable to, or even better than, lipopolyamines. Cytotoxicity was low and seen only at concentrations well above those required for optimal transfection. Delivery of oligonucleotides into embryonic neurons was followed by using a fluorescent probe. Virtually all neurons showed nuclear labeling, with no toxic effects. The optimal PEI cation/anion balance for in vitro transfection is only slightly on the cationic side, which is advantageous for in vivo delivery. Indeed, intracerebral luciferase gene transfer into newborn mice gave results comparable (for a given amount of DNA) to the in vitro transfection of primary rat brain endothelial cells or chicken embryonic neurons. Together, these properties make PEI a promising vector for gene therapy and an outstanding core for the design of more sophisticated devices. Our hypothesis is that its efficiency relies on extensive lysosome buffering that protects DNA from nuclease degradation, and consequent lysosomal swelling and rupture that provide an escape mechanism for the PEI/DNA particles.
Full text
PDF
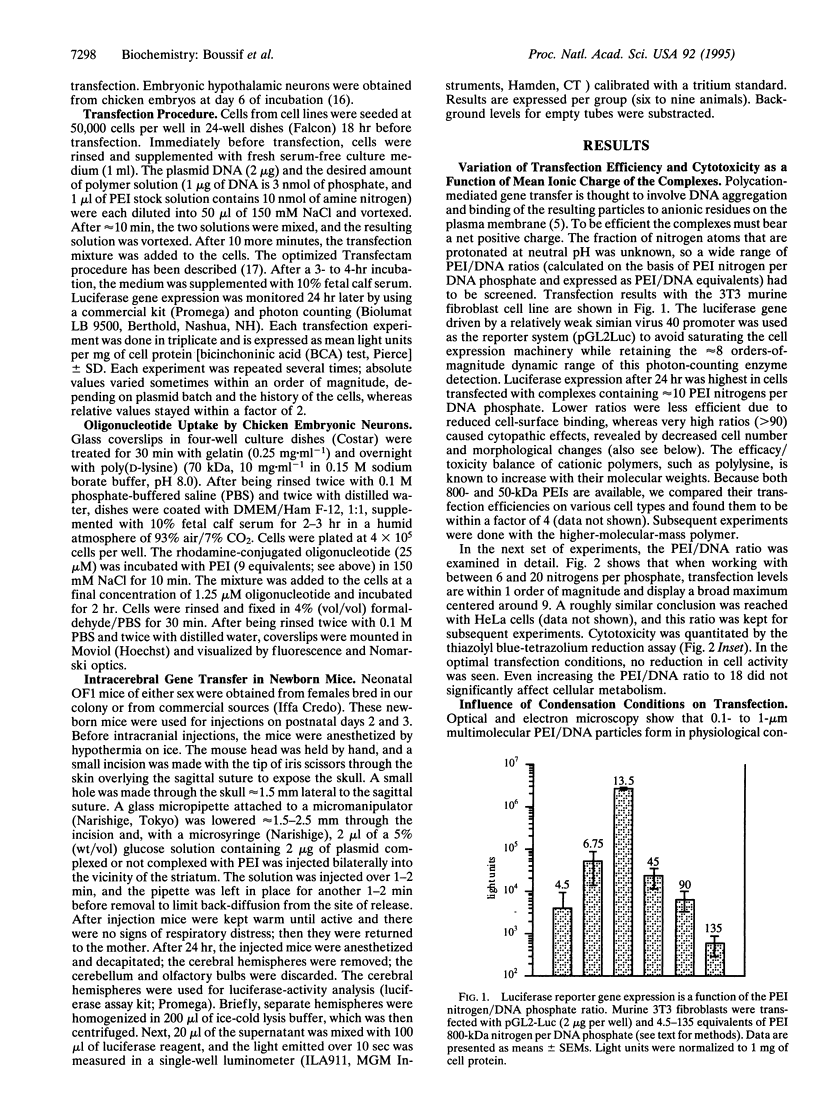
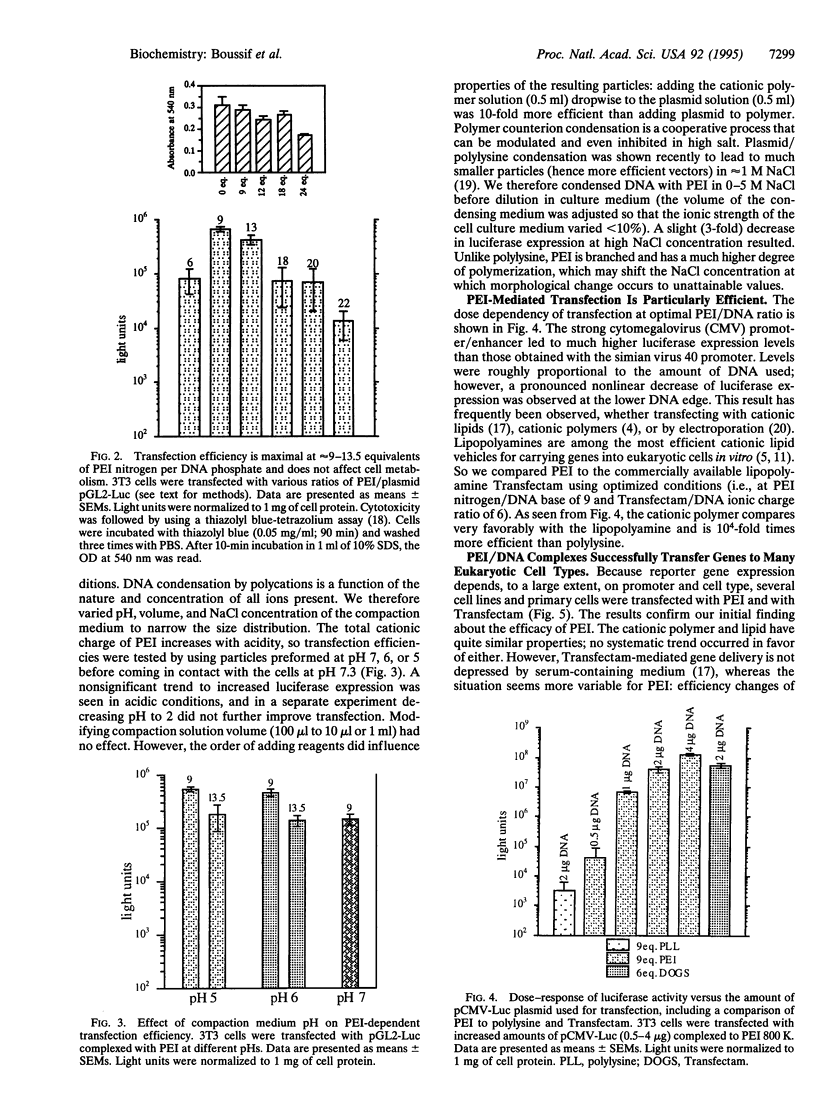
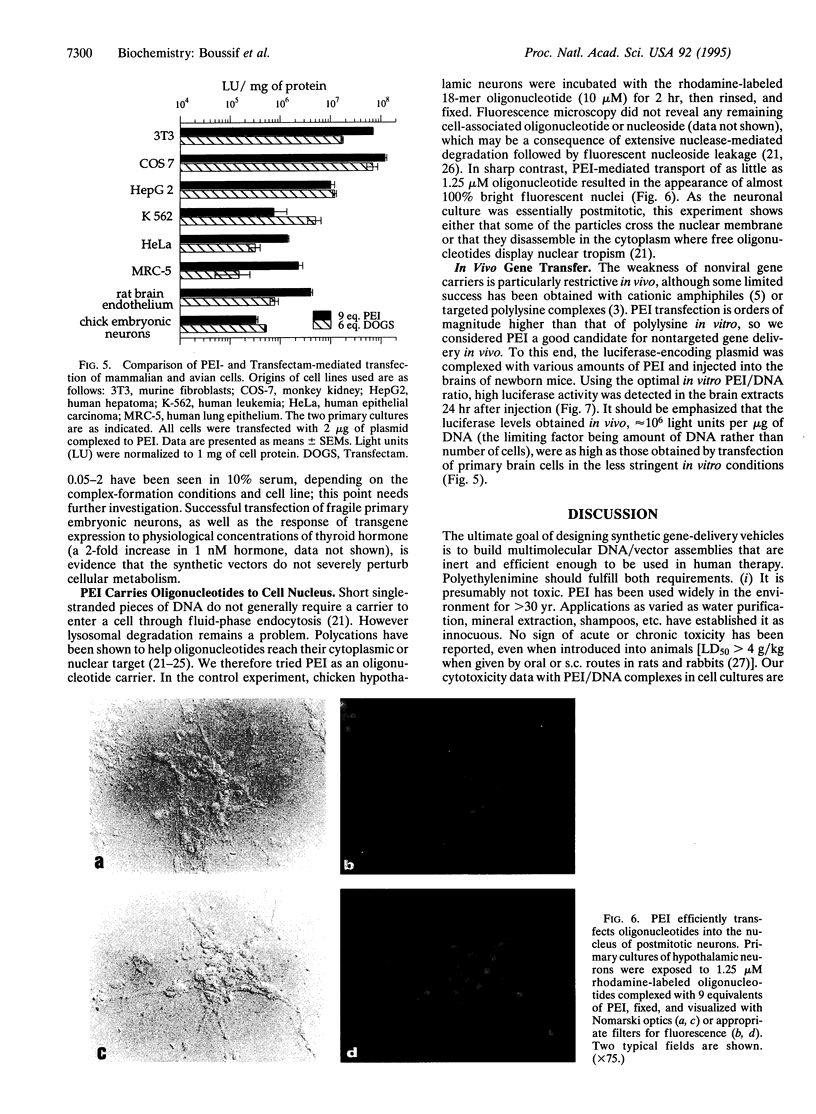
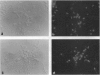
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthel F., Remy J. S., Loeffler J. P., Behr J. P. Gene transfer optimization with lipospermine-coated DNA. DNA Cell Biol. 1993 Jul-Aug;12(6):553–560. doi: 10.1089/dna.1993.12.553. [DOI] [PubMed] [Google Scholar]
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J. P. Gene transfer with synthetic cationic amphiphiles: prospects for gene therapy. Bioconjug Chem. 1994 Sep-Oct;5(5):382–389. doi: 10.1021/bc00029a002. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Chiang M. Y., Chan H., Shoemaker J. E., Mirabelli C. K. Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol. 1992 Jun;41(6):1023–1033. [PubMed] [Google Scholar]
- Capaccioli S., Di Pasquale G., Mini E., Mazzei T., Quattrone A. Cationic lipids improve antisense oligonucleotide uptake and prevent degradation in cultured cells and in human serum. Biochem Biophys Res Commun. 1993 Dec 15;197(2):818–825. doi: 10.1006/bbrc.1993.2552. [DOI] [PubMed] [Google Scholar]
- Clarenc J. P., Degols G., Leonetti J. P., Milhaud P., Lebleu B. Delivery of antisense oligonucleotides by poly(L-lysine) conjugation and liposome encapsulation. Anticancer Drug Des. 1993 Feb;8(1):81–94. [PubMed] [Google Scholar]
- Cotten M., Wagner E. Non-viral approaches to gene therapy. Curr Opin Biotechnol. 1993 Dec;4(6):705–710. doi: 10.1016/0958-1669(93)90053-y. [DOI] [PubMed] [Google Scholar]
- Fisher T. L., Terhorst T., Cao X., Wagner R. W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993 Aug 11;21(16):3857–3865. doi: 10.1093/nar/21.16.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensler J., Szoka F. C., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993 Sep-Oct;4(5):372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Lantos P. L. Brain capillary endothelial cells in vitro lack surface IgG Fc receptors. Neurosci Lett. 1986 Jul 11;68(1):100–106. doi: 10.1016/0304-3940(86)90237-5. [DOI] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Royer G. P., Sloniewsky A. R. Macromolecule--small molecule interactions. Strong binding and cooperativity in a model synthetic polymer. Biochemistry. 1969 Dec;8(12):4752–4756. doi: 10.1021/bi00840a015. [DOI] [PubMed] [Google Scholar]
- Legendre J. Y., Szoka F. C., Jr Cyclic amphipathic peptide-DNA complexes mediate high-efficiency transfection of adherent mammalian cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):893–897. doi: 10.1073/pnas.90.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezoualc'h F., Hassan A. H., Giraud P., Loeffler J. P., Lee S. L., Demeneix B. A. Assignment of the beta-thyroid hormone receptor to 3,5,3'-triiodothyronine-dependent inhibition of transcription from the thyrotropin-releasing hormone promoter in chick hypothalamic neurons. Mol Endocrinol. 1992 Nov;6(11):1797–1804. doi: 10.1210/mend.6.11.1480171. [DOI] [PubMed] [Google Scholar]
- Mack K. D., Walzem R., Zeldis J. B. Cationic lipid enhances in vitro receptor-mediated transfection. Am J Med Sci. 1994 Feb;307(2):138–143. doi: 10.1097/00000441-199402000-00013. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nelson N. Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci. 1991 Feb;12(2):71–75. doi: 10.1016/0165-6147(91)90501-i. [DOI] [PubMed] [Google Scholar]
- Nestle F. O., Mitra R. S., Bennett C. F., Chan H., Nickoloff B. J. Cationic lipid is not required for uptake and selective inhibitory activity of ICAM-1 phosphorothioate antisense oligonucleotides in keratinocytes. J Invest Dermatol. 1994 Oct;103(4):569–575. doi: 10.1111/1523-1747.ep12396876. [DOI] [PubMed] [Google Scholar]
- Perales J. C., Ferkol T., Beegen H., Ratnoff O. D., Hanson R. W. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank C., Oberhauser B., Mechtler K., Koch C., Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 1994 Apr 29;269(17):12918–12924. [PubMed] [Google Scholar]
- Remy J. S., Kichler A., Mordvinov V., Schuber F., Behr J. P. Targeted gene transfer into hepatoma cells with lipopolyamine-condensed DNA particles presenting galactose ligands: a stage toward artificial viruses. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1744–1748. doi: 10.1073/pnas.92.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy J. S., Sirlin C., Vierling P., Behr J. P. Gene transfer with a series of lipophilic DNA-binding molecules. Bioconjug Chem. 1994 Nov-Dec;5(6):647–654. doi: 10.1021/bc00030a021. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Williams D. L., Showe L. C. Quantitation of transient gene expression after electroporation. Nucleic Acids Res. 1992 Jun 25;20(12):3153–3157. doi: 10.1093/nar/20.12.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]