Abstract
Nausea and emesis are a major side effect and obstacle for chemotherapy in cancer patients. Employ of antiemetic drugs help to suppress chemotherapy-induced emesis in some patients but not all patients. Ginger, an herbal medicine, has been traditionally used to treat various kinds of diseases including gastrointestinal symptoms. Ginger is effective in alleviating nausea and emesis, particularly, for cytotoxic chemotherapy drug-induced emesis. Ginger-mediated antiemetic effect has been attributed to its pungent constituents-mediated inhibition of serotonin (5-HT) receptor activity but its cellular mechanism of action is still unclear. Emetogenic chemotherapy drugs increase 5-HT concentration and activate visceral vagal afferent nerve activity. Thus, 5-HT mediated vagal afferent activation is essential to provoke emesis during chemotherapy. In this experiment, water extract of ginger and its three major pungent constituent's effect on 5-HT-evoked responses were tested on acutely dispersed visceral afferent neurons with patch-clamp methods. The ginger extract has similar effects to antiemetic drug ondansetron by blocking 5-HT-evoked responses. Pungent constituents of the ginger, [6]-shogaol, [6]-gingerol, and zingerone inhibited 5-HT responses in a dose dependent manner. The order of inhibitory potency for these compounds were [6]-shogaol>[6]-gingerol>zingerone. Unlike well-known competitive 5-HT3 receptor antagonist ondansetron, all tested ginger constituents acted as non-competitive antagonist. Our results imply that ginger and its pungent constituents exert antiemetic effects by blocking 5-HT-induced emetic signal transmission in vagal afferent neurons.
Keywords: 5-HT receptor, Antiemetic, Chemotherapy, Shogaol, Vagal afferent nerves
INTRODUCTION
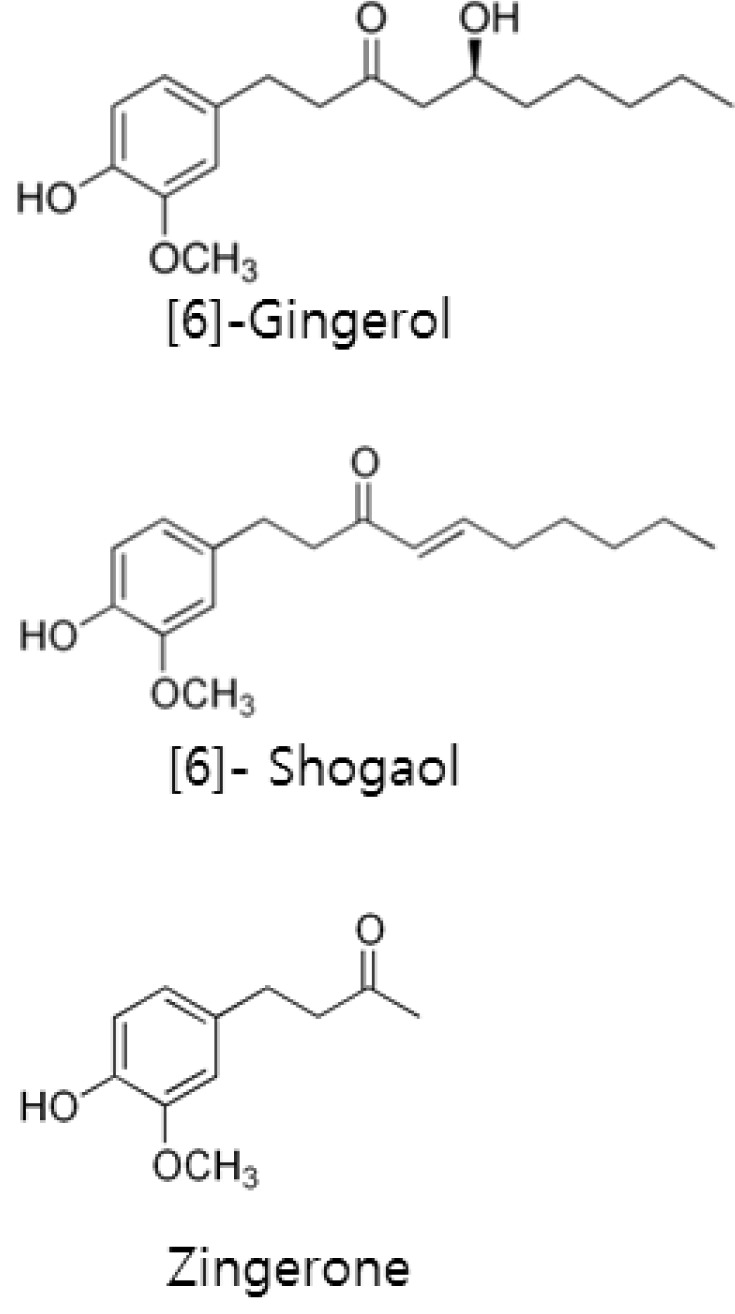
Over 70% of cancer patients, those who properly treated with antiemetic drugs, still experience emesis during chemotherapy [1]. Supplementary use of ginger further reduces chemotherapy-induced nausea in such patients [2]. The rhizome of ginger (Zingiber officinale Roscoe) has been traditionally used as herbal medicine to treat various kinds of diseases including gastrointestinal symptoms [3]. Ginger is especially effective to alleviate nausea and emesis due to various causes such as motion sickness, hyperemesis gravidarum, and chemotherapy with emetogenic drugs [4,5,6]. Among them antiemetic efficacy against chemotherapy-induced emesis has attracted much attention [1,2,5]. The cytotoxic chemotherapy drugs increases 5-HT release in the intestine and augments activity of visceral afferent vagus nerves [7,8]. Abdominal vagotomy or administration of 5-HT type 3 (5-HT3) receptors antagonists suppress cytotoxic drug-induced vomiting in an animal model [9]. Therefore, 5-HT3 receptor mediated activation of visceral afferent nerve is integral for induced emesis during chemotherapy. Recently, Abdel-Aziz et al. [10] found that pungent constituents of ginger inhibited 5-HT3 receptor function in neuroblastoma cells and suggest that the antiemetic efficacy of ginger may contribute to its 5-HT3 receptor blocking activity. In spite of these advances, ginger and its pungent constituent's effects on visceral afferent neurons have not been tested. In this study patch-clamp methods were used to test the impact of ginger extract and its three major pungent constituents (Fig. 1) on 5-HT-induced responses in acutely dispersed visceral afferent neurons.
Fig. 1.
Molecular structure of [6]-shogaol, [6]-gingerol, and zingerone.
METHODS
Nodose neuron dissociation
Visceral afferent neurons were dissociated from nodose ganglia from two to three week old Sprague-Dawley rats (Orient Bio Inc., Seongnam, Korea) of either gender as previously described [11]. All animal procedures were conducted with the approval of the institutional Animal Care and Use Committee in Kyung Hee University, Seoul, ROK, study protocol #KHUASP(SE)-13-002 (approval date: April 2, 2013). Briefly, the rats were deeply anesthetized (Zolazepam 0.3 ml/kg, Virbac Laboratories., Carros, France), and the vagus nerve from the jugular foramen was exposed for a length of 0.5 cm caudally. After the vagus nerve was carefully freed from surrounding tissue, the attached ganglion was immediately placed into a Petri dish containing chilled (4℃) artificial cerebrospinal fluid (ACSF) solution, which was composed of the following (in mM): 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 glucose, and 2 CaCl2, and bubbled with 95% O2-5% CO2. After, gently removing excess connective tissue, the bulb of the nodose ganglion was incubated for 28~40 min at 31℃ in ACSF solution containing type II-S trypsin (4~5 mg/ml at 1,310 units/mg; Sigma-Aldrich., St. Louis, MO). Following enzyme treatment, the ganglion was placed in enzyme-free ACSF for 1~3 hours at room temperature. The ganglia were then mechanically dispersed using fire-polished Pasteur pipettes in a glass bottom perfusion chamber filled with standard external recording solution containing (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH was adjusted to 7.4 with Tris-base).
Electrical measurements
For voltage-clamp recording, isolated neurons were visualized using a phase contrast microscope (IX70; Olympus, Tokyo, Japan) with a 40× objective and 10× ocular lens. Recordings utilized an EPC 9 (HEKA, Lambrecht, Germany) and pClamp 9 software (Molecular Devices, Sunnyvale, California). Electrical recordings used a modified perforated patch method using a nystatin concentration of 450 µg/ml [12]. The ionic composition of the internal patch-pipette solution for nystatin perforated patch recordings was (mM): 50 KCl, 100 K gluconate, and 10 HEPES. The pH of this solution was adjusted to 7.2 with Tris-OH. The nystatin-internal solution filled patch-pipettes has 5~7 MΩ resistance. Junction potential for these internal and standard external solutions was 11.9 mV and compensated throughout experiments. The access resistance, measured at approximately 15 min after formation of a gigaohm seal, was 8.5±1.2 MΩ. Neurons were voltage clamped to -50 mV and currents were sampled at 10 KHz with 3 KHz filtering and saved to computer. All experiments were conducted at room temperature (21~22℃).
Drugs & ginger extraction
Serotonin, CPBG (1-(3-Chlorophenyl)biguanide hydrochloride), zingerone, [6]-gingerol, and [6]-shogaol were purchased from Sigma-Aldrich (St. Louis, MO). All three pungent constituents of ginger were dissolved in DMSO and then diluted to the standard external recording solution. The final concentration of DMSO was less than 0.3%, a concentration which had no effect on the cells. 5-HT3 receptor selective antagonist ondansetron (1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one hydrochloride) was purchased from Tocris Cookson (Ballwin, MO). Ginger extract were prepared from freeze-dried Zingiberis officinalis roots. Finely grounded 10 g ginger was extracted with 400 mL water. The extract was filtered, freeze-dried and kept -20℃ until use. Each 10 g of dried ginger yielded 1.61 g dried power. All of the drugs were applied via a rapid application Y-tube microperfusion system that provided complete solution changes surrounding the recorded neurons within 0.1 seconds [13].
Data analysis
Data were analyzed off-line using Clampfit 9 software. All data are represented as mean±standard error of the mean (SEM). For statistical comparisons, one-way ANOVA with post-hoc multiple comparisons (Bonferroni/Dunn's correction, StatView, SAS Institute Inc., Cary, NC) was used. Differences were considered statistically significant for p values<0.05. The dose response curves for concentration-inhibition relationships were constructed according to the following equation (1) using a least-square fitting routine:
| I=1-[CnH/(CnH+IC50nH)] | (1) |
Where I is the current amplitude normalized to the control response, C is the corresponding ginger constituents or ondansetron concentrations. IC50 and nH denote the half-maximum inhibition concentration and the Hill coefficient, respectively. The continuous theoretical curves for the concentration response relationships were constructed according to a Hill equation (2) using a least-square fitting routine as described elsewhere [14] :
| I=Imax [CnH/(CnH+EC50nH)] | (2) |
Where I is the current amplitude normalized to the control response and C is the corresponding 5-HT concentration. EC50 and nH denote the half-maximum concentration and Hill slope, respectively.
RESULTS
Inhibition of 5-HT-induced current by ginger extract
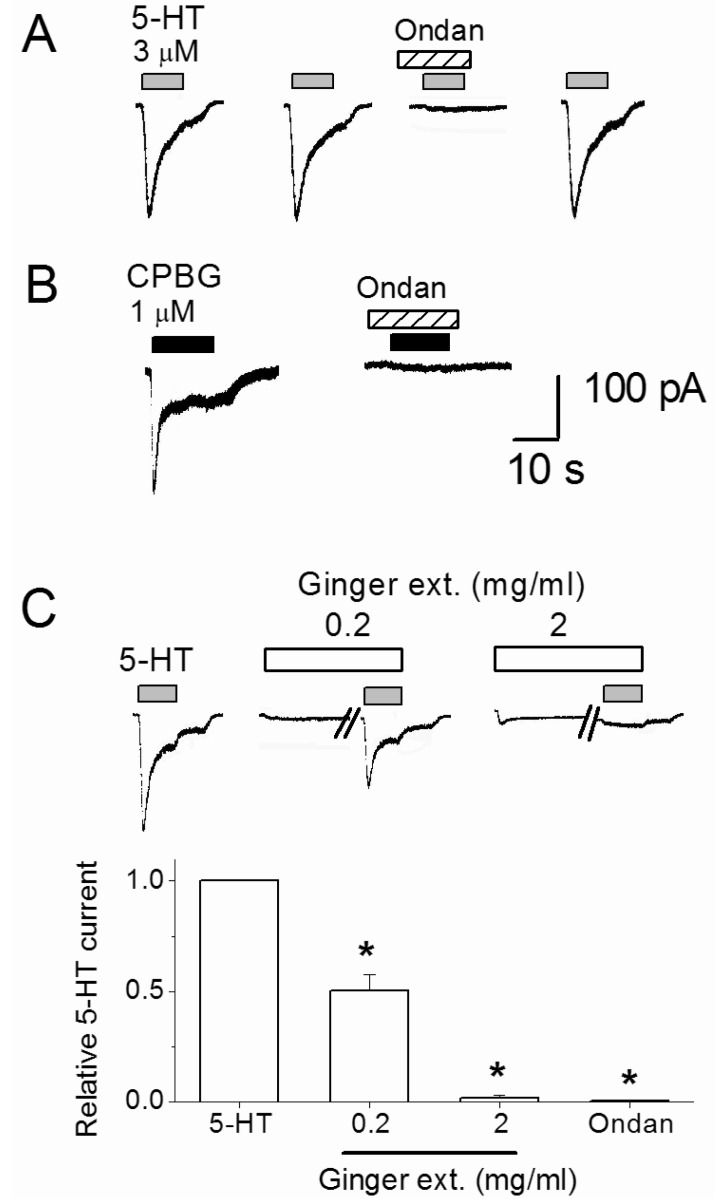
To characterize the base serotonin (5-HT) responses, whole-cell current recordings were made at a holding potential (VH) of -50 mV. Serotonin evoked inward currents in 29% (n=9/31) of assayed nodose neurons in a concentration dependent manner (0.001 to 1 mM). Three micromole 5-HT was used for subsequent control responses, at which approximately 25% of the maximal current amplitude was obtained. As shown in Fig. 2A, 4 min repeated application of the 3 µM 5-HT produced constant 5-HT responses. Co-application of 5-HT3 receptor selective antiemetic drug ondansetron (10 nM) reversibly blocked the 5-HT-evoked currents by an average of 99.5±0.4% (p=0.0006, n=7). Serotonin type 3 receptor selective agonist, CPBG (1 µM), mimicked the 5-HT effect by inducing inward current responses and ondansetron effectively inhibited CPBG responses (n=5, Fig. 2B). These results demonstrate that 5-HT-evoked current responses by activating 5-HT3 receptors. In the continuous experiments, inhibitory effects of the ginger extract on the 3 µM 5-HT-induced control responses were tested. Before the simultaneous application with 5-HT, an external solution containing 0.2 or 2 mg/ml ginger extract was pretreated for 2 min (Fig. 2C). The 5-HT current was suppressed by 49.5±7.2% and 98.2±1.2% by 0.2 and 2 mg/ml ginger extract, respectively. As shown in Fig. 2C traces, ginger extract alone evoked 37.5±17.1 pA (n=8) and 38.8±8.9 pA (n=6) currents at 0.2 mg/ml and 2 mg/ml, respectively. Those responses are not inhibited by ondansetron (p>0.05, n=6).
Fig. 2.
Inhibition of 5-HT-evoked current by ginger dry extract (Ginger ext.) and ondansetron (Ondan). (A) Serotonin (3 µM) was repeatedly applied with 4 min intervals with or without 5-HT3 receptor antagonist ondansetron (10 nM). (B) CPBG-evoked current was blocked by 10 nM ondansetron. (C) The traces show that 5-HT-evoked currents were suppressed by 0.2 and 2 mg/ml ginger dry extract. Ginger extract was pre-applied 2 min before co-application with 3 µM 5-HT. The diagram shows summary of average 5-HT current change by 0.2 and 2 mg/ml ginger extract and 10 nM ondansetron treatments. *Significant difference from control (p≤0.01). Each column and vertical lines represent the mean±SEM.
In summary, the above result shows that ginger extract and ondansetron have similar modes of action to inhibit 5-HT-evoked response in nodose ganglia neurons.
Inhibition of 5-HT current by ginger constituents
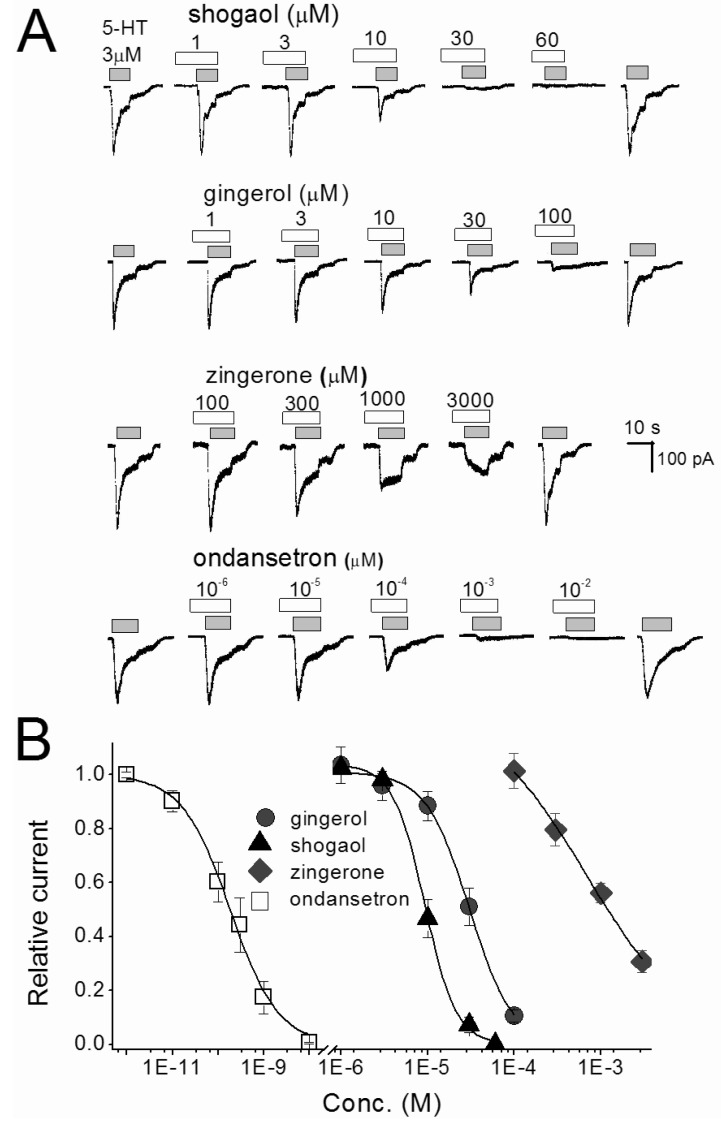
To determine which of the pungent ginger constituents, [6]-gingerol, [6]-shogaol and zingerone, block 5-HT-evoked responses in nodose neurons we tested them independently. The neurons were perfused with an external solution containing each compound from 30 seconds prior until simultaneous application with 5-HT. All constituents concentration-dependently inhibited 5-HT-evoked currents (Fig. 3). Where control 5-HT responses were significantly (p<0.05) attenuated by ≥10 µM gingerol, ≥10 µM shogaol, and ≥300 µM zingerone. For comparison, ondansetron was tested in same conditions and the 5-HT responses were significantly (p<0.0001) inhibited by ≥10-4 µM concentrations. All ginger constituents reversibly inhibited the control response in a concentration-dependent manner. The control responses were recovered by 4 to 5 min washing with ACSF. The order of inhibitory potency for these compounds were [6]-shogaol>[6]-gingerol>zingerone. The IC50 of [6]-shogaol and [6]-gingerol were 128 and 39 times lower than zingerone, respectively (Table 1). Thus, [6]-shogaol and [6]-gingerol are more effective than zingerone.
Fig. 3.
Concentration-inhibition relationships for single compounds of ginger and ondansetron in nodose ganglion neurons. (A) The traces shown are inhibition of 3 µM 5-HT-evoked current by [6]-shogaol, [6]-gingerol, zingerone and ondansetron. The traces were taken from different set of neurons. Various concentrations of reagents were pretreated 30 sec, and then co-application with 5-HT. (B) Represents the concentration-inhibition relationship of [6]-shogaol, [6]-gingerol, zingerone and ondansetron. Each point is the average of 4~7 neurons.
Table 1.
Half-maximum inhibition concentration (IC50) for zingerone, [6]-shogaol, [6]-gingerol, and ondansetron on 3 µM 5-HT evoked currents in nodose neurons
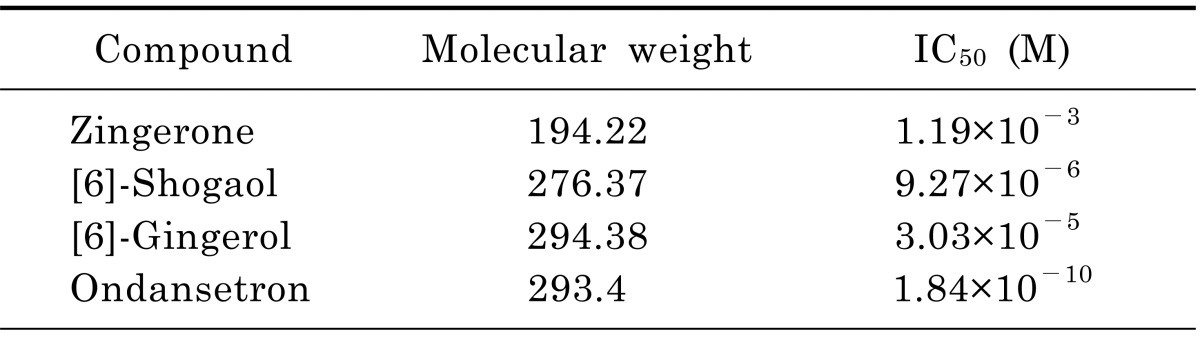
The IC50 was determined by fitting the data obtained from 4~7 cells to concentration-inhibition equation in the method using a least square fitting routine.
Pungent ginger constituents act as non-competitive antagonists
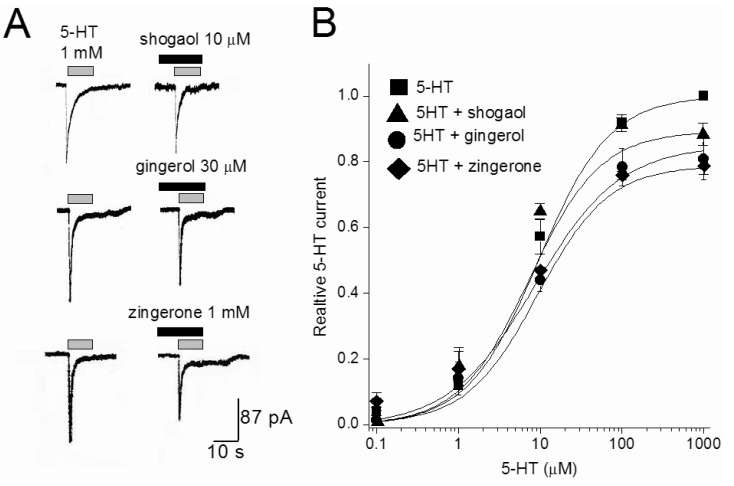
The effects of three ginger constituents were examined at different 5-HT concentrations. Fig. 4 shows the concentration-response curves of 5-HT in the absence and presence of [6]-shogaol (10 µM), [6]-gingerol (30 µM), or zingerone (1 mM). The EC50 of the 5-HT was 7.81±0.63 µM. In the presences of [6]-shogaol, [6]-gingerol, or zingerone, respective values for EC50 were 7.2±1.32, 7.9±1.7 and 6.59±2.24 µM. The EC50 value for 5-HT was not changed in the presence of three tested constituents (p>0.05, n=4-7). In contrast that the maximal response induced by 1 mM 5-HT was significantly decreased (p<0.026, n=4) to 89±3.4, 85±3.6, and 79±2.7% of control values in the presence of [6]-shogaol (10 µM), [6]-gingerol (30 µM), and zingerone (1 mM), respectively. These results support a non-competitive interaction by ginger constituents at the 5-HT site.
Fig. 4.
The effect of [6]-shogaol, [6]-gingerol, and zingerone on 5-HT concentration-response curves. (A) Representative currents traces activated by 1 mM 5-HT in the presence or absence of [6]-shogaol (10 µM), [6]-gingerol (30 µM), and zingerone (1 mM). Various concentrations of reagents were pretreated 30 sec, and then co-application with 5-HT. (B) Concentration-response relationship for 5-HT in the absence and presence of 10 µM [6]-shogaol, 30 µM [6]-gingerol, and 1 mM zingerone. The current amplitude is normalized to the current activated by 1 mM 5-HT. Data points are the mean±S.E.M. (n=4~6). The curve is the best fit of the data to the Hill equation described in methods.
DISCUSSION
Nausea and emesis are inherent defense mechanisms for avoid ingestion or expel toxic food, however, these symptoms are also a major side effect and obstacle for chemotherapy. Combined use of 5-HT3 antagonists and dexamethasone help to suppress chemotherapy-induced moderate to severe emesis in some (≤23.3%) but not all patients [1]. Ginger was found to be effective in animal experiments by suppressing cytotoxic chemotherapy drug-induced emesis [5,15]. In clinical trials, for the ethical reason, antiemetic efficacy of the ginger was only tested in the presence of antiemetic drugs. Ginger supplementation effectively reduced severity of chemotherapy-induced nausea in cancer patients [1,2]. Several studies have been conducted to determine the mechanism of gingers' antiemetic action. Based on anti-serotonergic effects of ginger extract on isolated guinea-pig ileum, Yamahara et al. [15] suggested that the antiemetic actions of ginger may be attributed to 5-HT3 receptor antagonism. In recent studies, Abdel-Aziz et al. [10] reported that ginger extract and its constituents inhibit 5-HT3 receptor function in neuroblastoma cells and rat ileum. Emetogenic chemotherapy drugs increase 5-HT concentration in the plasma and intestine [8,16] and activate vagal afferent nerves via 5-HT3 receptors [7]. Resection of afferent vagus nerve or administration of 5-HT3 receptors antagonists inhibits cisplatin-induced emesis [9]. Thus, 5-HT3 mediated activation of visceral afferent nerves underlies the mechanism for induction of emesis during chemotherapy. For the first time, we tested ginger extract and its major pungent ingredients on 5-HT-evoked currents in acutely isolated visceral afferent nodose neurons and found that all tested substances inhibited the 5-HT response in a concentration-dependent manner. In spite of the results of current experiments, the following question remains. If active ginger constituents block 5-HT3 receptors at much higher IC50 concentration than antiemetic drug ondansetron, how ginger extract exert additive antiemetic efficacy in cancer patients, who treated with antiemetic drugs? Interestingly, previous reports suggest that ginger constituents have distinct binding sites from that of 5-HT3 receptor antagonists. Unlike, 5-HT3 receptor selective antiemetic drugs which bind at the 5-HT binding site [17], the major active constituents of ginger inhibit the 5-HT3 receptor ion channel by binding to a modulatory binding site distinct from the serotonin recognition site [18]. In addition that Walstab et al. [19] showed that ginger extract and [6]-gingerol non-competitively inhibited 5-HT induced Ca2+ influx through human recombinant 5-HT3 receptors. In this experiment, we showed that [6]-shogaol, [6]-gingerol, and zingerone act as non-competitive antagonists for 5-HT3 receptors in visceral afferent neurons. Thus, overlapping inhibition to 5-HT3 receptors via combination therapy of pharmaceutical 5-HT3 antagonists and ginger may increase antiemetic efficacy for more patients. In conclusion, our results suggest that ginger and its pungent constituents may suppress chemotherapy-induced emesis by blocking 5-HT-induced emetic signal transmission in vagal afferent nerves.
ACKNOWLEDGEMENTS
This work was supported by the grant from Kyung Hee University (KHU-20090600). We are grateful to Dr. S. McDougall for helpful criticisms in preparing the manuscript.
ABBREVIATIONS
- 5-HT
serotonin
- CPBG
1-(3-Chlorophenyl) biguanide hydrochloride
- Ondansetron
1,2,3,9-Tetrahydro-9-methyl-3-[(2-methyl-1-imidazol-1-yl)methyl]-4-carbazol-4-onehydrochloride
References
- 1.Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234–238. doi: 10.1002/pbc.22778. [DOI] [PubMed] [Google Scholar]
- 2.Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, Hickok JT, Morrow GR. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479–1489. doi: 10.1007/s00520-011-1236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Fischer-Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 1991;38:19–24. doi: 10.1016/0028-2243(91)90202-v. [DOI] [PubMed] [Google Scholar]
- 5.Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol. 1997;57:93–96. doi: 10.1016/s0378-8741(97)00054-8. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JJ, Wood MJ, Wood CD, Mims ME. Effects of ginger on motion sickness susceptibility and gastric function. Pharmacology. 1991;42:111–120. doi: 10.1159/000138781. [DOI] [PubMed] [Google Scholar]
- 7.Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Schwörer H, Racké K, Kilbinger H. Cisplatin increases the release of 5-hydroxytryptamine (5-HT) from the isolated vascularly perfused small intestine of the guinea-pig: involvement of 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:143–149. doi: 10.1007/BF00167211. [DOI] [PubMed] [Google Scholar]
- 9.Hawthorn J, Ostler KJ, Andrews PL. The role of the abdominal visceral innervation and 5-hydroxytryptamine M-receptors in vomiting induced by the cytotoxic drugs cyclophosphamide and cis-platin in the ferret. Q J Exp Physiol. 1988;73:7–21. doi: 10.1113/expphysiol.1988.sp003124. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Aziz H, Nahrstedt A, Petereit F, Windeck T, Ploch M, Verspohl EJ. 5-HT3 receptor blocking activity of arylalkanes isolated from the rhizome of Zingiber officinale. Planta Med. 2005;71:609–616. doi: 10.1055/s-2005-871265. [DOI] [PubMed] [Google Scholar]
- 11.Choi MJ, Jin Z, Park YS, Rhee YK, Jin YH. Transient receptor potential (TRP) A1 activated currents in TRPV1 and cholecystokinin-sensitive cranial visceral afferent neurons. Brain Res. 2011;1383:36–42. doi: 10.1016/j.brainres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- 14.Lee BH, Choi SH, Hwang SH, Kim HJ, Lee JH, Nah SY. Resveratrol inhibits GABACρ receptor-mediated ion currents expressed in xenopus oocytes. Korean J Physiol Pharmacol. 2013;17:175–180. doi: 10.4196/kjpp.2013.17.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamahara J, Rong HQ, Naitoh Y, Kitani T, Fujimura H. Inhibition of cytotoxic drug-induced vomiting in suncus by a ginger constituent. J Ethnopharmacol. 1989;27:353–355. doi: 10.1016/0378-8741(89)90010-x. [DOI] [PubMed] [Google Scholar]
- 16.Castejon AM, Paez X, Hernandez L, Cubeddu LX. Use of intravenous microdialysis to monitor changes in serotonin release and metabolism induced by cisplatin in cancer patients: comparative effects of granisetron and ondansetron. J Pharmacol Exp Ther. 1999;291:960–966. [PubMed] [Google Scholar]
- 17.Mohan KC, Ravikumar K. Ondansetron hydrochloride: a competitive serotonin 5-HT3 receptor blocker. Acta Crystallogr C. 1995;51:2627–2629. [PubMed] [Google Scholar]
- 18.Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136–143. doi: 10.1016/j.ejphar.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Walstab J, Krüger D, Stark T, Hofmann T, Demir IE, Ceyhan GO, Feistel B, Schemann M, Niesler B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol Motil. 2013;25:439–447. doi: 10.1111/nmo.12107. [DOI] [PubMed] [Google Scholar]