Introduction
Approximately 33.3 million people worldwide are living with HIV/AIDS, and 1.8 million deaths and 2.6 million new infections occur annually [1]. Unprotected intercourse is the most common route through which HIV-1 is transmitted, and semen of HIV-infected men is an important source of infectious HIV [2]. Whereas the HIV/AIDS epidemic in Sub-Saharan Africa is generalized with approximately equal percentages of infections occurring in men and women, the epidemic in the US and many other developed countries is concentrated in men who have sex with men (MSM) [3] Recent reports suggest that MSM populations in lower and middle income countries are also disproportionately infected with HIV in several locations [4].
MSM are highly susceptible to HIV transmission. In the pre-antiretroviral therapy (ART) era, the rate of HIV transmission among MSM was estimated to be 1 in 100 acts of unprotected anal intercourse [5]), whereas the overall rate of HIV sexual transmission from men to women was much lower (<1 in 1,000 acts of unprotected vaginal intercourse [6]). High peripheral blood and genital HIV viral loads are associated with an increased rate of HIV transmission [7, 8], and ART, which reduces blood and genital viral load, has been shown to decrease HIV transmission [7, 9-11]. Yet, evidence suggests that the MSM epidemic has had a resurgence in the era of potent antiretroviral therapy [12, 13].
Genital infections with common STI pathogens including HSV-2, Neisseria gonorrhoeae and Chlamydia trachomatis have been associated with increased HIV viral load in semen and enhanced HIV transmission in ART-naïve men [14]. Several recent studies have documented the persistence of HIV virions and infected cells in semen from men on stable ART regimens despite undetectable viral loads in peripheral blood {reviewed in [15]}, and HIV transmission events have also been reported [16, 17]. Viral persistence in semen may be attributable to HIV compartmentalization in the male genital tract [18], isolated viral replication due to incomplete penetration of antiretroviral drugs [15], and/or activation of resident or infiltrating HIV-infected cells by inflammatory mediators produced locally in the genital tract in response to either symptomatic or asymptomatic infections [19]. MSM on HAART may continue to shed HIV in semen because of continued high risk sex behavior and a high prevalence of STIs and genital inflammation [20-22].
To determine the prevalence of seminal HIV shedding in sexually active HIV-infected MSM on HAART, and risk factors associated with detection of HIV in semen, we recruited participants from a Lesbian/Gay/Bisexual/Transgender (LGTB) clinic in Boston, MA, that provides ART to a substantial population of HIV-infected MSM at high risk for STIs [22]. We quantified levels of HIV in paired blood and semen samples, and examined the relationship between HIV in semen and an array of clinical, behavioral and biological variables.
Methods
Study Participants
Study participants were recruited from men receiving medical care for HIV infection at Fenway Health in Boston, Massachusetts, USA. Men were eligible for the study if they were HIV-1 infected, on a stable HAART regimen for three months or longer, and were sexually active (i.e., having had sex in the past six months).
This study was approved by the Institutional Review Boards of both Fenway Health and Boston University Medical Campus (BUMC). A research assistant directly asked study participants questions concerning demographics, sexual behavior, HIV therapy, history of STIs and substance use. Participants were asked to report the number of times they engaged in anal, oral or vaginal intercourse without and with condoms in the previous three months. Participants were also asked to report the number of sexual partners with whom they engaged for each behavior, and the HIV serostatus of those partners, if known.
Specimen Collection and Processing
Men were instructed to collect semen by masturbation after a minimum of 24 hours of sexual abstinence. Ten ml of blood were collected at the same visit by venipuncture in test tubes coated with EDTA. All samples were sent to the laboratory at BUMC, and processed within 4 hours of collection.
Semen volume was measured, and sperm and polymorphonuclear granulocytes (PMN) were counted with a microscope [23]. The remaining semen was diluted 1:1 in PBS, and semen cells were pelleted by centrifugation at 600 × g for 10 minutes. Seminal plasma and cell aliquots were stored with and without TRI Reagent/PolyAcryl Carrier at −80° C.
Whole blood was centrifuged (400 × g for 20 min) and plasma removed. Blood plasma aliquots were stored with and without TRI Reagent/PolyAcryl Carrier at −80° C.
Quantitative RT-PCR and PCR
The details of the HIV PCR assays have been described by us previously {RNA and DNA extraction from semen [24]; quantitative HIV RT-PCR and PCR assays [25]}. For HIV-1 RNA, the lowest quantitative limit was 1 copy/μl RNA (equivalent to 80 copies/ml in seminal or blood plasma, and 80 copies/sample in semen cells). For HIV-1 DNA, the lowest quantitative limit was 1 copy/2 μl DNA (equivalent to 100 copies/sample in semen cells). HSV-2 DNA was quantified as described by us previously [23].
Determination of HSV Serostatus
HSV serostatus was assessed by Focus HerpeSelect 1 & 2 Immunoblot IgG Assay (Focus Diagnostics, Cypress, CA, USA).
Assays of Cytokines and Other Immune Factors in seminal plasma
Proinflammatory cytokines IL-6, IL-8, and TNF-α, were measured by Bio-Plex Suspension Array System (Bio-Rad Laboratories, Hercules, CA, USA). Levels of innate immunity markers lysozyme (ALPCO, Salem, NH, USA) and SLPI (R & D Systems, Inc., Minneapolis, MN, USA) were determined by ELISA.
Statistical Analysis
For the initial analyses, differences in continuous variables between two independent samples were determined by the non-parametric Mann-Whitney U test, correlations between two continuous variables were determined by Spearman Rank Correlation Coefficient, and categorical variables were analyzed by Fisher's Exact test or Fisher-Freeman-Halton test. Final models used logistic regression [26] to evaluate associations of independent variables with the dichotomous outcome variable, detection of HIV RNA/DNA in semen. Simple and multiple regression models were constructed to include inflammatory biomarkers that were shown previously by descriptive analysis to be associated with our outcome variables, along with risk group indicators (see below) or self-report of unprotected anal sex. Multiple regression models were constructed by stepwise addition of covariates shown via simple regression to be significantly associated with the outcome. In the final model, covariates significantly associated with the outcome were retained along with behavioral risk factors, and presented with adjusted estimates of relative odds (odds ratios) and associated 95% confidence intervals. Additionally, p-values are presented for each adjusted measure of association. SAS (version 9.1.3), StatView (version 5.0.1) (both from SAS Institute, Cary, NC, USA) and StatXact (version 6.2.0, Cytel Software Corporation, Cambridge, MA, USA) statistical software were utilized to perform the statistical computations.
Results
Cohort Characteristics
A total of 101 men were enrolled in the study. The men were predominately Caucasian (74%) and virtually all MSM (97%) by self report; the median age was 43 years, and the median peripheral blood CD4 count was 513 cells/mm3. Eighty percent had been on HAART for >1 year and 72% had been on their current HAART regimen for >6 mos. Twenty-seven percent of the participants were classified as low-risk for STI acquisition (protected intercourse for past 3 months) and 73% were classified as high-risk for STI acquisition (unprotected intercourse in the past 3 months). Nine men, all belonging to the high-risk group, had STI/urethritis [diagnosed with Neisseria gonorrhoeae, Chlamydia trachomatis, Treponema pallidum or nongonococcal urethritis (NGU) within 7 days prior to entry into the study, or experiencing HSV-2 reactivation as detected by HSV-2 DNA in semen]. Sixty-three percent of the men were seropositive for HSV-2 antibodies (Table 1).
Table 1. Comparison of Men with and without Detectable HIV RNA in Blood Plasma (n=101).
| Variable | All Subjects (n=101) | BP HIV Positive (n=18) | BP HIV Negative (n=83) | P-valuea |
|---|---|---|---|---|
|
| ||||
| Age | 43 (24-59)b | 45 (27-55) | 43 (24-59) | 0.43 |
|
| ||||
| Race | ||||
| White | 75/101 (74%) | 14/18 (78%) | 61/83 (74%) | 0.80 |
| Black | 19/101 (19%) | 4/18 (22%) | 15/83 (18%) | |
| Native American | 1/101 (1%) | 0/18 (0%) | 1/83 (1%) | |
| Asian/Pacific Islander | 1/101 (1%) | 0/18 (0%) | 1/83 (1%) | |
| Other | 5/101 (5%) | 0/18 (0%) | 5/83 (6%) | |
|
| ||||
| Duration of ART | ||||
| >1 Year | 81/101 (80%) | 15/18 (83%) | 66/83 (80%) | >0.99 |
|
| ||||
| Duration of Current HAART Regimen | ||||
| >3 Months | 101/101 (100%) | 18/18 (100%) | 83/83 (100%) | >0.99 |
| >6 Months | 73/101 (72%) | 11/18 (61%) | 62/83 (75%) | 0.26 |
| >12 Months | 50/101 (50%) | 11/18 (61%) | 39/83 (47%) | 0.31 |
|
| ||||
| Peripheral CD4 Count (cells/mm3) | 513 (108-1,604)b | 394 (125-921) | 524 (108-1,604) | 0.08 |
|
| ||||
| HIV Detected in Semen Totalc | 30/101 (30%) | 9/18 (50%) | 21/83 (25%) | 0.049 |
|
| ||||
| Cell-Free RNA | 17/101 (17%) | 6/18 (33%) | 11/83 (13%) | 0.07 |
|
| ||||
| Cell-Associated RNA | 17/101 (17%) | 7/18 (39%) | 10/83 (12%) | 0.01 |
|
| ||||
| Cell-Associated DNA | 5/100 (5%) | 3/17 (18%) | 2/83 (2%) | 0.03 |
|
| ||||
| Sexual Orientation | ||||
| Homosexual | 90/101 (89%) | 16/18 (89%) | 74/83 (89%) | 0.64 |
| Bisexual | 8/101 (8%) | 1/18 (6%) | 7/83 (8%) | |
| Heterosexual | 3/101 (3%) | 1/18 (6%) | 2/83 (2%) | |
|
| ||||
| Circumcision | 84/101 (83%) | 14/18 (78%) | 70/83 (84%) | 0.50 |
|
| ||||
| STI Risk Group | ||||
| Low Risk Group | 27/101 (27%) | 6/18 (33%) | 21/83 (25%) | 0.56 |
| High Risk Groupd | 74/101 (73%) | 12/18 (67%) | 62/83 (75%) | |
|
| ||||
| STI Status | ||||
| Current STI/Urethritis | 9/101 (9%) | 1/18 (6%) | 8/83 (10%) | >0.99 |
| HSV-1 Seropositive | 72/101 (71%) | 15/18 (83) | 57/83 (69%) | 0.26 |
| HSV-2 Seropositive | 64/101 (63%) | 11/18 (61%) | 53/83 (64%) | >0.99 |
BP HIV Positive vs. BP HIV Negative; significant p-values in bold type
Median (Range)
Cell-Free and/or Cell-Associated HIV RNA and/or HIV DNA
Unprotected receptive and/or insertive intercourse within six months prior to study entry
Detection of HIV in Blood and Semen: Association with Clinical Characteristics
Of the 101 men, 18% had HIV RNA in blood [median HIV-RNA copy number/ml (range): 560 (80-6.4 × 105)], and 30% had HIV RNA and/or DNA in semen. Table 1 compares clinical, behavioral and seminal HIV variables of men with detectable HIV in blood plasma (BP-HIV+), and those with undetectable HIV in blood (BP-HIV−). BP-HIV+ men had a higher prevalence of HIV in semen than BP-HIV− men [9/18 (50%) vs. 21/83 (25%), p=0.049]. None of the clinical or behavioral characteristics differed between the BP-HIV+ and BP-HIV− groups except for medication adherence. Five men in the BP-HIV+ group were found to have poor adherence to their HAART regimens, and 2 had clinical indications of virologic HAART failure.
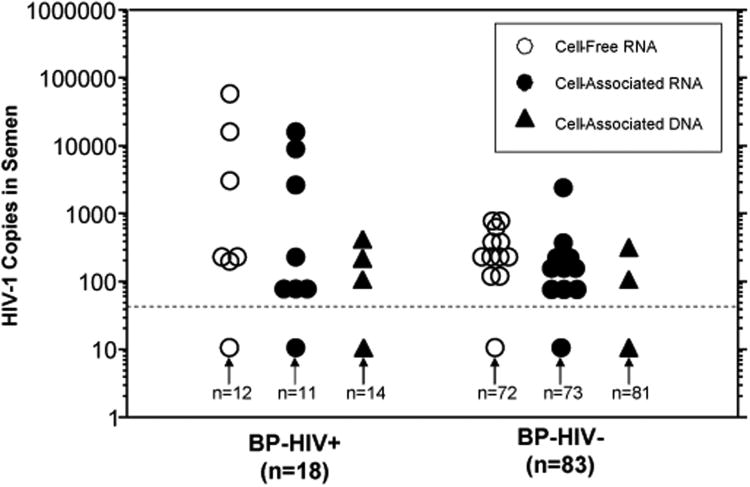
Figure 1 compares concentrations of cell-free (CF) and cell-associated (CA) HIV in semen from BP-HIV+ vs. BP-HIV− men. Of the 30 men with HIV in semen, 17 had CF HIV-RNA, 17 had CA HIV-RNA and 5 had CA HIV-DNA. Since both cell-free and cell-associated forms of HIV are potentially infectious [27], all three semen HIV variables were combined into a single variable (“any HIV in semen”) for the statistical analyses below.
Figure 1.
Cell-free and cell-associated HIV RNA and DNA in semen of men with detectable (BP-HIV+) and undetectable (BP-HIV−) HIV in blood plasma. Cell-Free HIV is expressed as HIV-1 copies per ml seminal plasma. Cell-associated HIV RNA and DNA are numbers of copies per sample. The dotted line indicates the lower limit of HIV detection, and values under this line represent samples with “undetectable” HIV. BP-HIV+ men had significantly higher concentrations of cell-free HIV RNA (mean ± SE: 4,438 ± 3,388 vs. 51 ± 17, p< 0.03), cell-associated HIV RNA (1,604 ± 1,006 vs. 50 + 31, p<0.005) and cell-associated HIV DNA (41 ± 26 vs. 5 ± 4, p< 0.009).
Univariate Analysis of Variables Associated with Seminal HIV Shedding in BP-HIV− Men
Table 2 presents a univariate analysis of variables associated with HIV in semen from BP-HIV− subjects. The prevalence of HIV in semen was significantly associated with STI risk [5% for the low-risk group vs. 32% for the high-risk group (p=0.02), and 75% for the STI/urethritis group (p=0.003)]. In addition, detection of HIV in semen was highly associated with LCS (p=0.0001) and seminal PMN count (p=0.003). The majority (57%) of SE-HIV+ men had LCS; the prevalence of seminal HIV in LCS+ men was 60% vs. 14% in LCS− men. Detection of HIV in semen was also associated with elevated concentrations of several other seminal inflammatory markers: TNF-α, IL-6, IL-8, and SLPI. Sexual behavior determinants associated with seminal HIV were unprotected insertive anal sex (UIAS), and UIAS with an HIV+ partner (UIAS-HIV). Neither UIAS nor UIAS-HIV was significantly associated with seminal PMN count, LCS, or concentrations of any other seminal inflammation markers (data not shown).
Table 2. Univariate Analysis of Variables Associated with HIV in Semen of Men with Undetectable HIV in Blood Plasma (n=83).
| Risk Factor | HIV in Semena | P-valueb | |
|---|---|---|---|
|
| |||
| Positive (n=21) | Negative (n=62) | ||
|
| |||
| Duration of ART | |||
| >1 Year (n=66) | 17/21 (81%) | 49/62 (79%) | 0.74 |
|
| |||
| Duration of Current HAART Regimen | |||
| >3 Months (n=83) | 21/21 (100%) | 62/62 (100%) | >0.99 |
| >6 Months (n=62) | 17/21 (81%) | 45/62 (73%) | 0.57 |
| >12 Months (n=39) | 9/21 (43%) | 30/62 (48%) | 0.80 |
|
| |||
| Peripheral CD4 Count (cells/mm3) | |||
| ≤200 (n=7) | 2/21 (10%) | 5/62 (8%) | 0.99 |
| >200 (n=76) | 19/21 (91%) | 57/62 (92%) | |
|
| |||
| STI Risk Group | |||
| Low Risk Group (n=21) | 1/21 (5%) | 20/62 (32%) | 0.02 |
| High Risk Groupc (n=62) | 20/21 (95%) | 42/62 (68%) | |
|
| |||
| High Risk Sexual Behavior | |||
| UIASd (n=36) | 14/21 (67%) | 22/62 (36%) | 0.02 |
| UIAS-HIVe (n=28) | 13/203 (65%) | 15/62 (24%) | 0.002 |
|
| |||
| STI Status | |||
| Current STI/Urethritis (n=8) | 6/21 (29%) | 2/62 (3%) | 0.003 |
| HSV-1 Seropositive (n=57) | 14/21 (67%) | 43/62 (69%) | 0.79 |
| HSV-2 Seropositive (n=53) | 11/21 (52%) | 42/62 (68%) | 0.29 |
|
| |||
| Genital Inflammation | |||
| Leukocytospermia | |||
| Yes (n=20) | 12/21 (57%) | 8/62 (13%) | 0.0001 |
| No (n=63) | 9/21 (43%) | 54/62 (87%) | |
| PMN (#/ml × 106) | 1.12 (ND-12)f | 0.04 (ND-6.8) | 0.003 |
| TNF-α (pg/ml) | 8 (2-77) | 6 (ND-78) | <0.0001 |
| IL-6 (pg/ml) | 105 (11-2,773) | 23 (3-1,400) | <0.0001 |
| IL-8 (pg/ml) | 1,152 (134-41,482) | 546 (42-4,270) | 0.005 |
| Lysozyme (ng/ml) | 157 (59-242) | 180 (50-413) | 0.21 |
| SLPI (ng/ml) | 10.5 (5-279) | 5.8 (4-107) | 0.009 |
ND: Not detectable
Cell-Free and/or Cell-Associated HIV RNA and/or HIV DNA
Significant p-values in bold type
Unprotected receptive and/or insertive intercourse within three months prior to study entry or ongoing STI
Unprotected insertive anal sex with HIV− or HIV+ person within three months of sample
Unprotected insertive anal sex with HIV+ person within three months of sample
Median (Range)
Study variables not associated with detection of HIV in semen in BP-HIV− men included duration of antiretroviral treatment and current HAART regimen, use of a protease inhibitor, peripheral blood CD4 counts, and circumcision (Table 2 and data not shown). Detection of HIV in semen was also not associated with HSV-1 or 2 serostatus, but 2 out of three men with HSV-DNA in semen (included in STI group) had HIV detected in semen.
Regression Models
Simple Logistic Regression
The results of simple logistic regression analysis of data from the BP-HIV− cohort indicated that a number of study variables were associated with HIV detection in semen. Specifically, both report of STI/urethritis (OR 11.5, p<0.006) and behavioral high risk of acquiring an STI (OR 9.35, p<0.017) were associated with HIV detection in semen. In addition, LCS (OR 8.68, p=0.0003) and upper quartile concentrations of seminal plasma TNF-α (OR 8.68, p=0.0003), IL-6 (OR 5.57, p<0.004), and IL-8 (OR 3.42, p<0.048) were associated with HIV detection in semen.
UIAS was also associated with HIV detection in semen (OR 3.58; p<0.03); the association was considerably stronger when UIAS occurred with an HIV+ partner (UIAS-HIV; OR 5.67; p<0.0025). Competitive analyses indicated that the association of UIAS-HIV with HIV detection in semen was not due to differential numbers of unprotected anal insertive contacts or partners in the UIAS-HIV subjects (data not shown).
Multiple Regression
Table 3 shows the final multiple regression model. Patients with STI/urethritis were more than 29 times as likely to have HIV in semen (OR: 29.03; 95% CI: 2.60, 523.53) when compared with patients without STI/urethritis. Seminal plasma TNF-α levels in the upper quartile remained highly associated with HIV in semen, with a magnitude of association of almost 14-fold when compared with lower concentrations (OR: 13.97; 95% CI: 2.85, 95.02). UIAS-HIV was the strongest behavioral risk factor associated with seminal HIV shedding. UIAS-HIV was associated with a greater than 7-fold risk of having HIV in semen (OR: 7.34; 95% CI: 1.59, 47.73) when compared with patients not reporting UIAS-HIV.
Table 3. Final Multivariate Analysis Model of Risk Factors Associated with Detection of HIV in Semen from HIV-infected MSM on HAART with Undetectable HIV VL in Blood.
| Parameter | Unadjusted OR (95% Confidence Interval) | Unadjusted P-value | Adjusted OR (95% Confidence Interval) | Adjusted P-value |
|---|---|---|---|---|
| High TNF-α levels in seminal plasma | ||||
| Yes (levels in upper quartile) | 8.68 (2.50, 32.81) | 0.0003 | 13.97 (2.85, 95.02) | 0.0003 |
| No (levels in lower three quartiles) | 1.0 (referent) | 1.0 (referent) | ||
| STI Status | ||||
| Patient with STI/urethritis | 11.52 (1.83, 127.80) | 0.006 | 29.03 (2.60, 523.53) | 0.003 |
| Patient without STI/urethritis | 1.0 (referent) | 1.0 (referent) | ||
| Unprotected Insertive Anal Sex with HIV+Person | ||||
| Yes | 5.67 (1.74, 20.24) | 0.003 | 7.34 (1.59, 47.73) | 0.007 |
| No | 1.0 (referent) | 1.0 (referent) |
Discussion
Because HIV transmission by semen is a major factor driving the AIDS epidemic, it is important to define variables that determine shedding of infectious HIV into semen. Concomitant STIs, genital inflammation and high peripheral blood viral loads have been associated with seminal HIV shedding in the past, largely in pre-HAART populations [14, 28]. As more men initiate ART and HAART, it is crucial to understand the interplay of therapy, which can suppress blood and genital HIV viral loads, with risk factors that can enhance HIV replication in the genital tract, and the potential for sexual transmission of drug-resistant HIV. Recent evidence indicates that HAART suppresses HIV transmission [7, 10]. The HPTN 052 clinical trial [9] demonstrated that early initiation of ART was associated with a 96% reduction in HIV transmission in HIV discordant heterosexual couples with a low prevalence of STIs. The only study on the effect of HAART on HIV transmission in an MSM population, an observational study conducted in San Francisco over a decade ago, concluded that the transmission rate was decreased by approximately 60% [29].
In our cross-sectional study of sexually active, ART-experienced MSM on a current HAART regimen for >3 mos, 30% of the men had detectable HIV RNA and/or DNA in semen. Eighteen percent of the men had detectable HIV in blood plasma despite being on a HAART regimen, consistent with earlier studies that have shown that substantial numbers (up to 25%) of men will experience virologic failure while on a HAART regimen [30]. These men, as expected, had a significantly higher prevalence as well as increased copy numbers of HIV in semen than did the BP-HIV− men in our study, indicating, as has been shown before [2], that HIV levels in peripheral blood are an important predictor of seminal HIV.
Among the 83 men with undetectable HIV in blood plasma, 25% had HIV in semen. This is a higher prevalence of seminal HIV shedding than has been reported from other large recent studies of BP-HIV− men on HAART, which have reported 2-3% shedding rates [31, 32]. This is likely due to the high prevalence of STIs and genital inflammation in our sexually active MSM cohort; 9.6% of the men were diagnosed with STI or urethritis, and 24% had genital inflammation (leukocytospermia). Our study provides evidence that genital infections and inflammation are common in HIV-infected MSM that engage in unprotected intercourse, and that these factors can promote compartmentalized shedding of HIV in the genital tract of men on suppressive HAART therapy. We also used a highly sensitive detection assay and measured both cell-free and cell-associated HIV. Cell-associated HIV was included because early reports suggested that HIV-infected cells persisted in semen longer than cell-free HIV after initiation of HAART [24, 33], and also because of a recent resurgence in interest in the sexual transmission of cell-associated HIV [34]. CF-RNA and HIV-infected cells were each detected in 13% of BP-HIV− cases, but only 1/21 subjects with seminal HIV was positive for both forms of virus. This provides further evidence that cell-free and cell-associated HIV in semen may arise from different sources, as has been suggested in previous reports [35].
In our study, seminal HIV viral copy numbers ranged from 80-2,560 (median 200 copies) in men with undetectable HIV in blood. These seminal HIV levels are considerably lower that those commonly detected in ART-naïve men [24, 36, 37], but could represent an infectious innoculum in MSM since rectal intercourse is an especially effective route of HIV transmission due to the thin rectal epithelium [5, 6]. A series of recent studies substantiate this possibility. A study in macaques comparing the relative transmissibility of SHIV across different mucosal surfaces demonstrated that rectal transmission could be achieved with 5-fold less SHIV than needed for vaginal transmission [38]. A theoretical paper [39] and results from a clinical study [8] both conclude that <1,000 copies of HIV RNA in semen pose a low but real risk of male-to-female HIV transmission; a five-fold reduction in this copy number for rectal transmission (<200 copies) is within the range of values detected in our study. Furthermore, Butler et al. [40] reported a median seminal plasma viral load of 4,300 HIV RNA copies/ml for men transmitting HIV to their MSM partners with a minimum and maximum of 110 and 69,000 HIV RNA copies/ml, respectively. Therefore, the data from our study in combination with these recent reports suggest that sexually active HIV–infected MSM on ART that continue to shed low levels of HIV in semen may be infectious to their sexual partners.
One concern about seminal HIV shedding in men on HAART is that the virus can develop antiretroviral resistance mutations [24, 41, 42], which may contribute to the high prevalence of antiretroviral drug-resistant HIV in ART-naïve HIV-infected individuals in the United States (reported to range from 8 to 24% [43-45]). MSM are at higher risk of acquiring drug-resistant HIV than heterosexual men or women [43, 45]. Our data suggest that HIV replication commonly occurs in the genital tract of sexually active MSM under evolutionary pressure from HAART; this could increase the prevalence of drug-resistant HIV in semen and its transmission to seronegative partners. Although early attempts to culture and sequence HIV in semen from men in this study failed due to low viral copy numbers, further studies are warranted to determine whether HIV in semen from MSM on HAART is infectious in vitro and contains drug resistance mutations..
The multivariate analysis also revealed an independent association between seminal HIV and UIAS-HIV. This raises the possibility that seminal HIV in subjects engaging in UIAS with HIV-infected partners may be attributable to urethral superinfection or contamination with HIV from rectal secretions of sex partners. Reports of high numbers of HIV-target cells in the urethra [46], and HIV-infected cells in urethral secretions from HIV-seropositive men with and without urethritis [47] indicate that the urethra may be a primary HIV infection site. Furthermore, higher concentrations of HIV RNA have been reported in rectal mucosa secretions than in blood and seminal plasma among MSM, and may be independent of ART [48]. Given that many HIV+ MSM engage in unprotected anal intercourse with other HIV-infected partners as a way to experience pleasure without having to worry about transmitting HIV to others [20], the potential of acquiring HIV from an HIV+ partner during unprotected insertive sex, and subsequently transmitting it to an HIV− partner must be considered. This finding needs to be confirmed, since at present “serosorting” (HIV-infected individuals only having unprotected sex with other infected persons), is considered by some to be a reasonable public health strategy to prevent transmission to uninfected partners [49].
In conclusion, we detected a high prevalence of seminal HIV shedding in a cohort of sexually active HIV-infected MSM on HAART. HIV in semen was associated with detection of HIV in blood (due to poor drug adherence and/or virologic HAART failure), and with STIs and genital inflammation in men who were fully suppressed (undetectable HIV in blood). In light of recent evidence that even low amounts of HIV in semen could pose a transmission risk in MSM, who are more vulnerable to HIV infection than heterosexual men, this information has potential clinical significance for the HIV epidemic in MSM. HIV-infected men who engage in unprotected intercourse may use HAART and viral load status in their sexual decision-making, and being on HAART or having an undetectable blood viral load may relax concerns about transmitting HIV [50]. Therefore, MSM at risk for transmitting HIV may believe that they have a low risk based on incorrect assumptions that HAART eliminates HIV from semen. Until more information on transmission risk in MSM is available, it would be prudent to advise sexually active HIV-infected MSM to use condoms and other risk-reduction strategies throughout all stages of HIV disease regardless of HIV treatment status, and to promote the aggressive diagnosis and treatment of STIs.
Acknowledgments
The study team thanks Rodney VanDerwarker, Marcy Gelman and Chris Grasso of Fenway Health for their assistance in getting local approval for this study and operationalizing participant recruitment. The study team also expresses their appreciation to study participants for their involvement with this demanding protocol.
Research supported by NIH grant R56 AI071909 (DA).
Footnotes
The authors have no conflicts of interest.
Research finding presented at the 2009 IAS meeting in Cape Town, South Africa.
Author Contributions: Study concept: DA, KM and JP. Study design: DA, KM, JP and SW. Clinical/experimental procedures: WO, JP, FB and XC. Data analysis: SW and JP. Initial manuscript draft: DA and JP. Manuscript revisions: KM, SW, XC, FB and WO.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. Report on the global AIDS epidemic. Geneva: UNAIDS; 2010. [Google Scholar]
- 2.Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 3.van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS. 2009;4:300–307. doi: 10.1097/COH.0b013e32832c3bb3. [DOI] [PubMed] [Google Scholar]
- 4.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000-2006: a systematic review. PLoS Med. 2007;4:e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dosekun O, Fox J. An overview of the relative risks of different sexual behaviours on HIV transmission. Curr Opin HIV AIDS. 2010;5:291–297. doi: 10.1097/COH.0b013e32833a88a3. [DOI] [PubMed] [Google Scholar]
- 7.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 8.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA Predicts Risk of Heterosexual HIV-1 Transmission. Sci Transl Med. 2011;3:77. doi: 10.1126/scitranslmed.3001888. ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bezemer D, de Wolf F, Boerlijst MC, van Sighem A, Hollingsworth TD, Prins M, et al. A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS. 2008;22:1071–1077. doi: 10.1097/QAD.0b013e3282fd167c. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PS, Hamouda O, Delpech V, Geduld JE, Prejean J, Semaille C, et al. Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996-2005. Ann Epidemiol. 2009;19:423–431. doi: 10.1016/j.annepidem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:305–310. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:335–343. doi: 10.1097/COH.0b013e32833a0b69. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zeng G, Luo J, Duo S, Xing G, Guo-Wei D, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–238. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturmer M, Doerr HW, Berger A, Gute P. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antivir Ther. 2008;13:729–732. [PubMed] [Google Scholar]
- 18.Nickle DC, Jensen MA, Shriner D, Brodie SJ, Frenkel LM, Mittler JE, Mullins JI. Evolutionary indicators of human immunodeficiency virus type 1 reservoirs and compartments. J Virol. 2003;77:5540–5546. doi: 10.1128/JVI.77.9.5540-5546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L et al. HIV-1 Populations in Semen Arise through Multiple Mechanisms. PLoS Pathog. 6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crepaz N, Marks G, Liau A, Mullins MM, Aupont LW, Marshall KJ, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS. 2009;23:1617–1629. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 21.Defraye A, Van Beckhoven D, Sasse A. Surveillance of sexually transmitted infections among persons living with HIV. Int J Public Health. 2010 doi: 10.1007/s00038-010-0209-5. [DOI] [PubMed] [Google Scholar]
- 22.Mayer KH, O'Cleirigh C, Skeer M, Covahey C, Leidolf E, Vanderwarker R, Safren SA. Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: findings from a Boston community health centre. Sex Transm Infect. 2010;86:66–70. doi: 10.1136/sti.2009.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87:1087–1097. doi: 10.1016/j.fertnstert.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer KH, Boswell S, Goldstein R, Lo W, Xu C, Tucker L, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999;28:1252–1259. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi W, Msamanga G, Antelman G, Xu C, Hertzmark E, Spiegelman D, et al. Effect of prenatal vitamin supplementation on lower-genital levels of HIV type 1 and interleukin type 1 beta at 36 weeks of gestation. Clin Infect Dis. 2004;38:716–722. doi: 10.1086/381673. [DOI] [PubMed] [Google Scholar]
- 26.Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med. 1995;14:2143–2160. doi: 10.1002/sim.4780141908. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DJ, Politch JA, Nadolsky AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse Leukocytes for HIV Prevention. AIDS. 2010;24:163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson DJ, O'Brien TR, Politch JA, Martinez A, Seage GR, Padian N, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. Jama. 1992;267:2769–2774. [PubMed] [Google Scholar]
- 29.Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, Osmond DH. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18:81–88. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Rio C. Current concepts in antiretroviral therapy failure. Top HIV Med. 2006;14:102–106. [PubMed] [Google Scholar]
- 31.Halfon P, Giorgetti C, Khiri H, Penaranda G, Terriou P, Porcu-Buisson G, Chabert-Orsini V. Semen may harbor HIV despite effective HAART: another piece in the puzzle. PLoS One. 2010;5:e10569. doi: 10.1371/journal.pone.0010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcelin AG, Tubiana R, Lambert-Niclot S, Lefebvre G, Dominguez S, Bonmarchand M, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. Aids. 2008;22:1677–1679. doi: 10.1097/QAD.0b013e32830abdc8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Dornadula G, Beumont M, Livornese L, Jr, Van Uitert B, Henning K, Pomerantz RJ. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 34.von Bubnoff A. Is HIV hitching a ride inside cells? IAVI Rep. 2011;15:8–11. [PubMed] [Google Scholar]
- 35.Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD, Smith DM. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. J Virol. 2005;79:1734–1742. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. Aids. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 38.Chenine AL, Siddappa NB, Kramer VG, Sciaranghella G, Rasmussen RA, Lee SJ, et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J Infect Dis. 2010;201:1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15:621–627. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 40.Butler DM, Smith DM, Cachay ER, Hightower GK, Nugent CT, Richman DD, Little SJ. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS. 2008;22:1667–1671. doi: 10.1097/QAD.0b013e32830bfed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor S, Sadiq ST, Weller I, Kaye S, Workman J, Cane PA, et al. Drug-resistant HIV-1 in the semen of men receiving antiretroviral therapy with acute sexually transmitted infections. Antivir Ther. 2003;8:479–483. [PubMed] [Google Scholar]
- 42.Ghosn J, Viard JP, Katlama C, de Almeida M, Tubiana R, Letourneur F, et al. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. Aids. 2004;18:447–457. doi: 10.1097/00002030-200402200-00011. [DOI] [PubMed] [Google Scholar]
- 43.Ross L, Lim ML, Liao Q, Wine B, Rodriguez AE, Weinberg W, Shaefer M. Prevalence of antiretroviral drug resistance and resistance-associated mutations in antiretroviral therapy-naive HIV-infected individuals from 40 United States cities. HIV Clin Trials. 2007;8:1–8. doi: 10.1310/hct0801-1. [DOI] [PubMed] [Google Scholar]
- 44.Shet A, Berry L, Mohri H, Mehandru S, Chung C, Kim A, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr. 2006;41:439–446. doi: 10.1097/01.qai.0000219290.49152.6a. [DOI] [PubMed] [Google Scholar]
- 45.Weinstock HS, Zaidi I, Heneine W, Bennett D, Garcia-Lerma JG, Douglas JM, Jr, et al. The epidemiology of antiretroviral drug resistance among drug-naive HIV-1-infected persons in 10 US cities. J Infect Dis. 2004;189:2174–2180. doi: 10.1086/420789. [DOI] [PubMed] [Google Scholar]
- 46.Anderson D, Politch JA, Pudney J. HIV infection and immune defense of the penis. Am J Reprod Immunol. 2011;65:220–229. doi: 10.1111/j.1600-0897.2010.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 48.Zuckerman RA, Whittington WL, Celum CL, Collis TK, Lucchetti AJ, Sanchez JL, et al. Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. J Infect Dis. 2004;190:156–161. doi: 10.1086/421246. [DOI] [PubMed] [Google Scholar]
- 49.Eaton LA, Kalichman SC, O'Connell DA, Karchner WD. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care. 2009;21:1279–1288. doi: 10.1080/09540120902803208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalichman SC, Eaton L, Cain D, Cherry C, Fuhrel A, Kaufman M, Pope H. Changes in HIV treatment beliefs and sexual risk behaviors among gay and bisexual men, 1997-2005. Health Psychol. 2007;26:650–656. doi: 10.1037/0278-6133.26.5.650. [DOI] [PubMed] [Google Scholar]