Abstract
Helios, a member of the Ikaros transcription factor family, is preferentially expressed at the mRNA level by regulatory T cells (Treg cells). We evaluated Helios protein expression using a newly generated mAb and demonstrated that it is expressed in all thymocytes at the double negative 2 stage of thymic development. Although Helios was expressed by 100% of CD4+CD8−Foxp3+ thymocytes, its expression in peripheral lymphoid tissues was restricted to a subpopulation (~70%) of Foxp3+ T cells in mice and humans. Neither mouse nor human naive T cells induced to express Foxp3 in vitro by TCR stimulation in the presence of TGF-β expressed Helios. Ag-specific Foxp3+ T cells induced in vivo by Ag feeding also failed to express Helios. Collectively, these results demonstrate that Helios is potentially a specific marker of thymic-derived Treg cells and raises the possibility that a significant percentage of Foxp3+ Treg cells are generated extrathymically.
Regulatory T cells (Treg cells) suppress immune activation in a dominant manner and, thus, play a critical role in the maintenance of self-tolerance (1–3). Treg cells are characterized by the expression of the transcription factor Foxp3; the importance of Treg cells in immune homeostasis is best illustrated by the development of severe autoimmune diseases in mice and humans with genetic deficiencies of Foxp3 (4, 5). It is believed that there are two major subsets of Treg cells: natural, or thymic-derived Treg cells, which develop in the thymus and induced Treg cells, conventional Foxp3− T cells that are converted in peripheral lymphoid tissue to cells that express Foxp3. Although it was demonstrated that Treg cells can be generated in vitro and in vivo in the periphery, specifically in the gut, the lack of a specific marker or markers has precluded the demonstration that peripherally induced Treg cells exist under physiologic conditions (3).
The Ikaros transcription factor family is made up of five DNA-binding proteins that are characterized by six highly conserved C2H2 zinc fingers. Four N-terminal zinc fingers are involved in DNA binding, whereas the two C-terminal zinc fingers are responsible for homodimeric and heterodimeric protein interactions (6). Two members, Eos and Pegasus, are expressed in a wide variety of tissues, whereas the remaining three, Ikaros, Helios, and Aiolos, are reportedly limited to the hematopoietic system. Ikaros is expressed in all hematopoietic cells, including very early hematopoietic stem cells, whereas Aiolos is expressed in most hematopoietic cells, although not in the earliest uncommitted cells (7). Aiolos is observed in committed cells of the lymphoid lineage and is very highly expressed in mature B cells. However, Helios seems to be restricted to the T lymphocyte lineage (8, 9).
Targeted mutations of the Ikaros and Aiolos genes demonstrated the importance of this family in lymphocyte development and homeostasis. Mice with a null mutation of Ikaros lack B and NK cells and have greatly reduced numbers of thymic dendritic cells (10). In addition, fetal T cells are absent; however, a small number of T cells do develop after birth but are skewed toward the CD4 subpopulation and are hyperproliferative. Mice that are homozygous for a dominant negative mutation of Ikaros also lack B and NK cells and are devoid of all T cells and early lymphoid progenitors (11). The pups fail to thrive and die within 3 wk of cannibalism or opportunistic infections. Mice that are heterozygous for the same dominant negative mutation exhibit a less severe phenotype (12). Although lymphocyte populations seem normal during the first 4 wk of life, T cells from heterozygous mice are hyperproliferative, and the mice develop leukemia and lymphoma. Finally, mice with a targeted mutation of Aiolos have elevated serum IgG and IgE and possess autoantibodies (13). These mice have more germinal centers, their B cells display an activated phenotype, B cells are hyperproliferative in vitro, and by 8–10 mo the mice develop B cell lymphomas.
Despite the clear importance of the Ikaros family, their mechanisms of action are still poorly understood. Ikaros family members are thought to regulate gene transcription through chromatin remodeling, and studies showed that Ikaros and Helios interact in the nucleosome remodeling and the DNA methylation complex, an ATP-dependent complex that has nucleosome-disruption activity and histone deacetylase activity (14, 15). Furthermore, Ikaros was shown to bind to the IL-2 promoter and repress expression, whereas reduced binding of Ikaros releases the requirement for CD28 costimulation for IL-2 expression (16).
Helios was originally cloned from a mouse thymoma line and was reportedly expressed by an undefined subpopulation of T cells (9). Subsequently, Helios has been shown to be selectively expressed in Foxp3+ Treg cells in a number of microarray comparisons of Foxp3+ T cells and conventional T cells and to be a potential Foxp3 target gene (17–19). The expression and function of Ikaros family members in Treg cells has not been extensively studied; however, a very recent study demonstrated that Eos interacts with Foxp3 to mediate gene silencing through chromatin modifications (20). To further analyze the expression and function of Helios in Foxp3+ Treg cells, we developed a hamster mAb to a large peptide from the N-terminal region of mouse Helios. In this article, we demonstrate that Helios is selectively expressed in a subset (70%) of Foxp3+ Treg cells in mice and humans and that expression of Helios is confined to the thymic-derived Treg cell population.
Materials and Methods
Animals
BALB/c and C57BL/6 mice were obtained from the National Cancer Institute (Frederick, MD). Foxp3-GFP mice (21) and MHC class II-deficient mice (Abβ−/−) were obtained by the National Institute of Allergy and Infectious Diseases (NIAID) and were maintained by Taconic Farms (Germantown, NY) under contract by NIAID. The Foxp3-GFP mice were crossed to OT-II TCR transgenic (Tg) RAG−/− mice obtained from Taconic Farms. Female heterozygous B6.Cg-Foxp3sf/J (Scurfy) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred to C57BL/6 WT male mice to generate hemizygous male B6 (Cg-Foxp3sf/Y [Scurfy]) offspring. Armenian hamsters were purchased from Cytogen Research and Development (West Roxbury, MA). Mice bearing loxP-flanked conditional (fl/fl) alleles of Helios on a C57BL/6 background were generated by Ozgene (Bentley Dc, Australia). Ikzf2fl/fl mice were bred in our laboratory to mice expressing Cre recombinase under the control of the CD4 promoter (CD4-Cre) to produce Ikzf2fl/fl × CD4-Cre mice, which will be described elsewhere (A.M. Thornton, J. Murphy, and E.M. Shevach, manuscript in preparation). CD4-Cre mice were obtained from Taconic Farms. All animal protocols used in this study were approved by the NIAID Animal Care and Use Committee.
Human cells
Peripheral blood was obtained from healthy adult donors by the Department of Transfusion Medicine at the National Institutes of Health. The acquisition of blood products was approved according to the Institutional Review Board and in accordance with the Declaration of Helsinki.
Media, Abs, and reagents
Mouse cells were grown in RPMI 1640 (Lonza, Walkersville, MD) supplemented with 10% heat-inactivated FCS, penicillin (100 μg/ml), streptomycin (100 μg/ml), 2 mM L-glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (all from Lonza), and 50 μM 2-ME (Sigma-Aldrich, St. Louis, MO). Purified anti-CD3 (2C11), PE–anti-CD25 (PC61), and FITC–anti-CD8 were purchased from BD Biosciences (San Jose, CA). Mouse Abs included tricolor–anti-CD4 (Invitrogen, Carlsbad, CA) and Alexa Fluor 647 anti-Foxp3, PE anti-FoxP3, PE-Cy7 anti-CD8, Pacific Blue anti-CD4, Alexa Fluor 750 anti-CD45.1, PE-Cy5.5 anti-CD45.2, and Alexa Fluor 700 anti-CD44 (eBioscience, San Diego, CA). Human Abs included FITC anti-CD4, PE-Cy5.5 anti-CD45RA, PE anti-CD25, allophycocyanin anti–IL-2, and allophycocyanin anti–IFN-γ (Invitrogen); Alexa Fluor 647 anti-CD127 and anti-CD28 (BD Biosciences); Alexa Fluor 647 anti-human FOXP3 (236A/E7) and Alexa Fluor 647 anti–IL-17 (eBioscience); and anti-CD3 (OKT3; Ortho Biotech Products, Bridgewater, NJ). CD8, CD4, and PE microbeads were purchased from Miltenyi Biotec (Auburn, CA). Intracellular staining was performed with the Foxp3 staining buffer kit, according to the manufacturer’s protocol (eBioscience). Flow cytometry analysis was performed using FlowJo software. Human rIL-2 was obtained from the Preclinical Repository of the Biological Resources Branch, National Cancer Institute. TGF-β1 was purchased from PeproTech (Rocky Hill, NJ).
Cell purification, activation, and RNA isolation of mouse cells
Peripheral lymph nodes (LNs) were harvested from 8-wk-old female BALB/c or C57BL/6 mice. CD4+CD25− and CD4+CD25+ cells were purified as previously described (22). The purity of CD4+CD25− and CD4+CD25+ cells was ~97%. CD8+ T cells were purified from mouse LNs using CD8 microbeads. For thymic subpopulations, cells were labeled with PE anti-CD25, tricolor anti-CD4, or FITC anti-CD8 and were purified by cell sorting. For cell activation, purified cells (106/ml) were cultured in complete RPMI 1640 supplemented with 100 U/ml IL-2 at 37°C for 16 h in 24-well plates precoated with 5 μg/ml anti-CD3. RNA was purified using TRIzol reagent (Invitrogen), according to manufacturer’s instructions.
PCR-based cDNA subtraction
Total RNA was subjected to PCR-Select cDNA Subtraction, followed by mirror-orientation selection (BD Clontech, Palo Alto, CA) (23). The CD4+ CD25+ T cell-specific library resulted in 106 clones. cDNA inserts were sequenced by Lofstrand Labs (Gaithersburg, MD).
Quantitative PCR
RNA from purified or purified and activated cells was isolated using an RNeasy Plus kit (Qiagen, Valencia, CA). RNA was converted to cDNA using SuperScript II reverse transcriptase (Invitrogen) with random primers, according to the manufacturer’s protocol. Primers and FAM-labeled probes were purchased from Integrated DNA Technologies (San Diego, CA). Helios forward: 5′-TCACAACTATCTCCAGAATGTCAGC-3′; reverse: 5′-AGGCGGTACATGGTGACTCAT-3′; probe: 5′-TGGAGGCTGCCG-GGCAGG-3′. Foxp3 forward: 5′-CCCAGGAAAGACAGCAACC-3′TT; reverse: 5′-TTCTCACAACCAGGCCACTTG-3′; probe: 5′-ATCCTACC-CACTGCTGGCAAATGGAGTC-3′. 18S rRNA (Applied Biosystems, Foster City, CA) was used as an internal control for normalization. All PCR reactions were performed in triplicate using TaqMan Universal PCR Master Mix (Applied Biosystems). An ABI prism 7700 sequence detection system (Applied Biosystems) was used for 40 cycles of PCR. Each experiment was performed at least three times, and a representative experiment is shown.
Generation of the 22F6 anti-Helios mAb
A peptide from the Helios protein (aa 51–107) was synthesized by the NIAID Peptide Synthesis Laboratory (Bethesda, MD) and was used to immunize Armenian hamsters. Spleens from immunized animals were fused with SP2/0-Ag14 mouse myeloma cells. Hybridoma supernatants were first screened by ELISA using the peptide and HRP conjugated goat anti-Armenian Hamster IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Supernatants were screened by Western blot analysis on EL-4 whole-cell extracts using HRP-conjugated goat anti-Armenian Hamster IgG or by flow cytometry using intracellular staining methods and FITC-conjugated goat anti-Armenian Hamster IgG as the secondary Ab. The mAb 22F6 was subsequently conjugated to FITC or PE by the NIAID Custom Ab Facility (Bethesda, MD) or to FITC, PE, or Alexa Fluor 647 by BioLegend (San Diego, CA).
Cell purification and activation of human cells
PBMCs were prepared over Ficoll-Paque Plus gradients (GE Healthcare, Piscataway, NJ). The CD4+ T cells were enriched by autoMACS positive selection with human CD4 microbeads. The cells were labeled with anti-CD4, anti-CD45RA, anti-CD25, and anti-CD127 and purified by cell sorting with BD Aria. The postsort purity was >95% for CD4+CD25− CD127+CD45RA+ and CD4+CD25−CD127+CD45RA− cells, and the FOXP3 purity was >85% for CD4+CD25hi cells. The cells were activated in vitro with plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (2.5 μg/ml), with or without 5 ng/ml TGF-β1 in 24-well culture plates (Corning Life Sciences, Acton, MA) at 500,000 cells per well in complete RPMI 1640 supplemented with 100 U/ml IL-2.
Proliferation and suppression assay
Purified cells were cultured as previously described (22). For human in vitro suppression assays, 50,000 FACS-sorted allogenic CD4+CD25− T cells were stimulated with 25,000 nonirradiated autologous CD3-depleted HLA-DR+ APCs and 0.25 μg/ml OKT3 alone or with varying numbers of Treg cells. The cells were cultured for 3 d in 96-well flat-bottom plates (Corning Life Sciences), pulsed with [3H]thymidine deoxyribose (1 μCi/well) for the last 6–8 h, and then quantified with a scintillation counter. The APCs were isolated by depleting CD3+ cells with CD3 microbeads over the AutoMACS, followed by positive selection with HLA-DR microbeads.
Generation of mouse-induced Treg cells
CD4+GFP− cells and CD4+GFP+ cells from the LN and spleen of Foxp3-GFP reporter mice were purified by cell sorting. For cell activation, purified cells (106/ml) were cultured in complete RPMI 1640 supplemented with 100 U/ml IL-2 at 37°C for 3 d in 24-well plates precoated with 5 μg/ml anti-CD3 in the presence or absence of 5 ng/ml TGF-β.
Oral tolerance induction
CD4+GFPneg T cells from OT-II TCR Tg × Foxp3-GFP mice were purified from the spleen and LN by cell sorting. Two million cells were transferred i.v. into B6.SJL congenic mice. The mice were given 1.5% OVA-supplemented water for 6 d; the water was changed every 2 d.
Thymocyte transfer
CD4+CD8−GFP+ thymocytes were purified from the thymus of 4-wk-old Foxp3-GFP mice. A total of 6 × 105 cells were transferred i.v. Cells were harvested 4 wk later and analyzed by flow cytometry.
Results
Helios is differentially expressed in Treg cells
To elucidate genes specific to CD4+CD25+ Treg cells, RNA isolated from CD4+CD25−and CD4+CD25+ cells was subjected to Clontech PCR Select Differential Screening, followed by mirror-oriented selection (23). Of the 106 clones specific to the CD4+CD25+ Treg cell library, 54 clones contained a fragment of the Helios gene (Supplemental Table I). Helios expression was analyzed by quantitative PCR (qPCR) to validate the differential screening (Supplemental Fig. 1A). Helios mRNA was detected in freshly isolated CD4+CD25+ cells, and it increased ~3-fold upon activation of the cells. Helios was not detected in freshly isolated or activated CD4+ CD25− cells, and it was barely detectable in CD8+ cells. We were unable to detect mRNA for Helios in B cells, dendritic cells, or cells of the monocyte/macrophage lineage (data not shown). To further characterize Helios, we examined Helios mRNA expression in various thymic subpopulations. We observed that Helios was expressed in the thymus at low levels as early as the double negative (DN) stage (Supplemental Fig. 1B). Helios continued to be expressed at low levels in double positive (DP) cells, CD8+ cells, and CD4+CD25−cells. However, there was a striking increase in Helios mRNA in thymic CD4+CD25+ cells, concurrent with the first appearance of Foxp3 mRNA (Supplemental Fig. 1C). In peripheral LNs, Helios expression correlated with Foxp3 expression and was detected exclusively in Treg cells.
Development of an anti-mouse/human Helios mAb
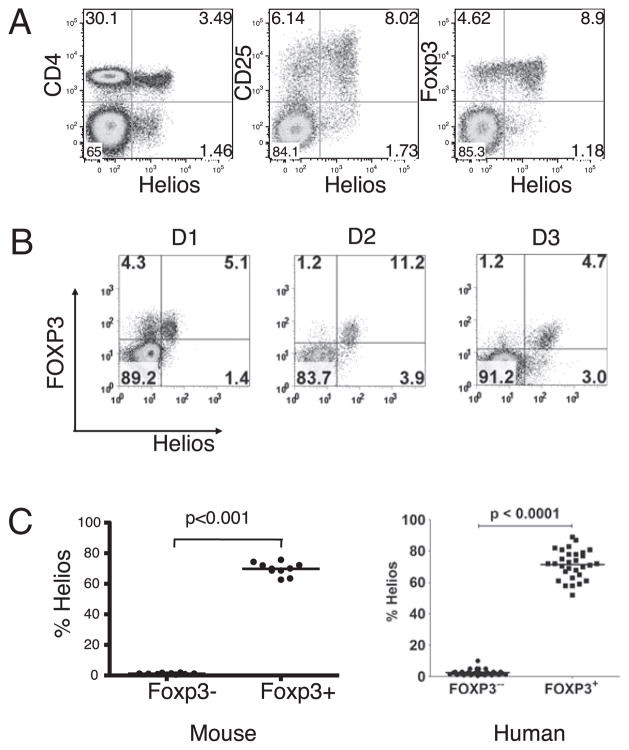
All members of the Ikaros family of transcription factors contain a large region of six highly conserved zinc fingers. However, the N terminus of the members is not conserved. To more thoroughly examine Helios expression and function, we generated a mAb (22F6) to a large peptide within the nonconserved 110-aa N terminus region of Helios. When Helios expression was analyzed by intracellular staining of permeabilized cells with this mAb, 10% of CD4+ T cells in the peripheral LN of mice were positive (Fig. 1A). In agreement with the qPCR, mAb 22F6 reacted preferentially with CD4+CD25+ Treg cells. Notably, when LN cells were gated on CD4+ cells and analyzed for Foxp3 and Helios expression, Helios was expressed on only 70% of Foxp3+ cells. A low percentage of non-CD4+ cells also reacted with the mAb (Fig. 1A): ~50% of these cells were CD8+ and 20% were TCRγδ+ (data not shown). Lineage-specific markers have not been defined for the remaining 30% of the non-CD4+ cells that reacted with the mAb.
FIGURE 1.
Helios is expressed in a subset of CD4+Foxp3+ cells. A, Mouse LN cells were analyzed by flow cytometry for Helios expression using mAb 22F6 (left panel). LN cells were gated on CD4+ cells and examined for CD25 and Helios expression (middle panel) and Foxp3 and Helios expression (right panel). B, PBMCs from normal human donors were gated on CD4+ T cells and analyzed for FOXP3 and Helios expression. Three representative FACS plots are shown. C, The percentage of Helios expression in CD4+Foxp3− and CD4+Foxp3− T cells in normal mouse LN or in PBMC of healthy adults. Analysis was performed a minimum of three times with samples from at least two independent mice.
Because 22F6 was raised to a peptide derived from the mouse sequence that differed from the human sequence by only two amino acids, we analyzed the reactivity of 22F6 in human cells. 22F6 also reacted with human PBMCs and was again specific to the CD4+ CD25hi Treg cell subset (data not shown). Although much more variable than in mouse CD4+ cells, Helios was expressed in a subset of CD4+Foxp3+ cells in humans that ranged from 50–90% (Fig. 1B). Despite this wide range of variability, analysis of a large number of normal donors demonstrated that CD4+Foxp3+Helios+ cells represent, on average, 70% of Foxp3+ cells in man, which is strikingly similar to that observed in mice (Fig. 1C).
Expression of Helios during T cell development
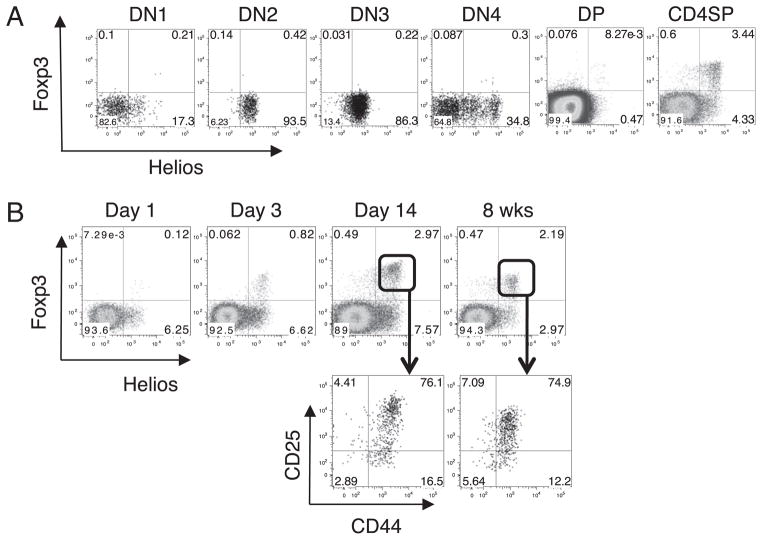
Because our qPCR studies suggested that Helios mRNA was expressed at different stages of thymic development, we used mAb 22F6 to examine the expression of Helios protein in subsets of adult mouse thymocytes. Helios was expressed at the DN stage of development, in agreement with the qPCR data (Fig. 2A). Specifically, Helios was expressed at low levels as early as DN1, but it was expressed in 100% of DN2 and DN3 cells. By DN4, Helios expression had begun to decrease. In contrast, Foxp3 was not expressed at the DN stage. Although Foxp3+Helios− T cells could be detected in the adult thymus (Fig. 2A), they were absent at day 3 of life, when Foxp3+ cells first appear in the thymus (Fig. 2B). Although Foxp3+Helios− cells can be detected in the thymus as the mice mature, the percentage of Foxp3+Helios−CD4 single positive (SP) cells in an adult remains lower (15%) than that observed in peripheral lymphocytes (30%). Lio and Hsieh (24) recently proposed a two-step process for thymic Treg cell development and demonstrated that Foxp3+ Treg cells can develop from the CD4SPFoxp3−CD25hi subset. Helios was expressed in 30% of CD4SPFoxp3−CD25hi thymocytes, but it remains to be determined whether these cells are the precursors of the CD4+ Foxp3+ cells (data not shown).
FIGURE 2.
Expression of Helios during thymic T cell development. A, Mouse thymocytes were stained for CD4, CD8, CD44, and CD25. DN, DP, and CD4SP cells were gated based on CD4 and CD8 expression and were further analyzed by flow cytometry for Helios and Foxp3. DN1–DN4 cells were gated on CD4−CD8− cells and further gated based on CD44 and CD25 expression. B, Thymocytes from two individual mice at the indicated ages were analyzed by flow cytometry for Helios and Foxp3 expression. Plots shown are gated on CD4+CD8− thymocytes. Mice were analyzed on different days but were always compared with an adult mouse. Shown are representative plots of one mouse from at least two independent experiments.
We next examined Helios expression in the developing peripheral tissue. Foxp3+Helios− cells are absent in the neonate, do not significantly appear in the spleen until between days 10 and 12 of life, and do not reach the percentage observed in an adult until after the pups have been weaned (Fig. 3A). Phenotypic analysis of the Foxp3+Helios+ cells shows that the vast majority of the cells in the thymus (Fig. 2B) and peripheral lymphoid tissue (Fig. 3B) are CD44hiCD25+ in adults and neonates. In contrast, when the Foxp3+ Helios− cells can first be detected in the periphery, the majority are CD44lowCD25− (Fig. 3C) and do not acquire the CD44hiCD25+ phenotype until after the mice have been weaned. This result is consistent with the possibility that the precursors of the Foxp3+ Helios− cells do not arise in the thymus.
FIGURE 3.
Expression of Helios during peripheral T cell development. A, Splenocytes from two individual mice at the indicated ages were analyzed by flow cytometry for Helios and Foxp3 expression. Plots shown are gated on CD4+ cells. B, Splenocytes from two individual adult or 4-d-old mice were analyzed by flow cytometry for CD25 and CD44 expression. Plots shown are gated on CD4+ cells. C, Splenocytes from two individual mice at the indicated ages were analyzed by flow cytometry for CD25 and CD44 expression. Plots shown are gated on CD4+ Foxp3+Helios− cells. Mice of different ages were analyzed on different days but were always compared with an adult mouse. Shown are representative plots of one mouse from at least two independent experiments.
Thymic Foxp3+Helios+ Treg cells are a stable lineage
Although the differential expression of CD25 and CD44 on Foxp3+ Helios− T cells during development was consistent with the development of this population in the periphery, it remained possible that some Foxp3+Helios−T cells were derived from Foxp3+Helios+ precursors. We are unable to isolate Foxp3+Helios+ and Foxp3+ Helios− populations from peripheral lymphoid tissues based on the differential expression of any other cell surface Ag. However, the thymus of young mice is almost devoid of Foxp3+Helios− cells. To examine the stability of the thymic Foxp3+Helios+ population, we sorted CD4+CD8−Foxp3−GFP+ cells (~3–5% Foxp3+Helios−) from the thymus of young mice and transferred them to CD45.1+ congenic hosts. After 4 wk, the transferred cells were analyzed for
their expression of Foxp3 and Helios. The transferred cells maintained their expression of Helios and Foxp3 1 m after transfer to normal recipients (Fig. 4), indicating that Foxp3+Helios+ cells are unlikely to be the precursors of the Foxp3+Helios− cells.
FIGURE 4.

Thymic-derived Treg cells maintain Helios and Foxp3 expression. CD4+CD8−GFP+ thymocytes were sorted from Foxp3-GFP mice, and 6 × 105 cells were adoptively transferred into congenic B6.SJL mice. Four weeks after transfer, LN and spleen cells were analyzed by flow cytometry. Shown are representative profiles of two independent experiments after gating on CD4 and CD45.1+ donor-derived cells.
Foxp3+ cells generated in vitro or in vivo are Foxp3+Helios−
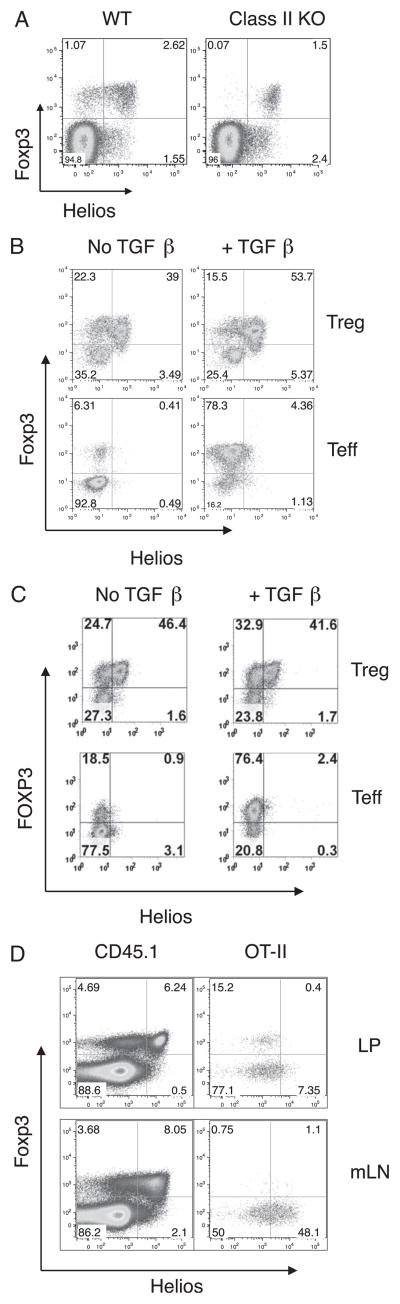
The observation that Foxp3+Helios− cells seem to arise in peripheral lymphoid tissue and do not acquire their full phenotypic profile until after weaning led us to examine the expression of Helios in MHC class II-deficient (−/−) mice. Previous studies showed that Foxp3+ T cells are readily detectable in the thymus and the periphery of MHC class II−/− mice, and the majority express CD8 (25). We reasoned that if Foxp3+Helios− cells are induced in the periphery, this induction would require TCR/MHC class II interactions. When LN cells from adult wild-type (WT) and MHC class II−/− mice were analyzed for the expression of Foxp3 and Helios, the Foxp3+Helios− cells were not observed in MHC class II−/− mice, but they were readily detectable in WT LNs (Fig. 5A).
FIGURE 5.
Peripherally induced Treg cells are Foxp3+Helios−. A, LN cells from two adult C57BL/6 or two MHC class II-deficient mice were analyzed flow cytometry. Plots shown are gated on CD4+ cells and analyzed by flow cytometry for Helios and Foxp3 expression. Shown are representative plots of one mouse. B, CD4+GFP+ or CD4+GFP− cells were purified from the LN of Foxp3-GFP mice by cell sorting. Cells were stimulated with plate-bound anti-CD3 and IL-2 in the presence or absence of TGF-β and then analyzed by flow cytometry for Foxp3 and Helios expression. Plots shown are gated on CD4+ cells. Shown is a representative experiment from three independent experiments. C, Human PBMCs were cell sorted for CD4+CD25hiCD127− (Treg) cells and CD4+CD25–CD127+ CD45RA+ (Teff) cells. The cells were stimulated in the presence or absence of TGF-β and then analyzed by flow cytometry for Foxp3 and Helios expression. Plots shown are gated on CD4+ cells. Shown is a representative experiment from at least three independent experiments. D, CD4+GFP− cells were sorted from OT-II × Foxp3-GFP mice, and 2 × 106 cells were transferred to congenic B6.SJL recipient mice. The recipient mice were given OVA-supplemented drinking water for 6 d. LP cells were isolated and analyzed for Foxp3 and Helios expression by flow cytometry. Plots shown are gated on CD4+CD45.1+ host cells or CD4+CD45.2+ cells (OT-II). A representative result of four individual mice is shown from two independent experiments.
We next analyzed Helios expression in Foxp3+ T cells that had been induced in vitro in the presence of TGF-β. CD4+GFP− and CD4+GFP+ cells were sorted from Foxp3-GFP reporter mice and activated in the presence or absence of TGF-β. Helios was not expressed in Foxp3− T cells activated in the absence of TGF-β (Fig. 5B). Strikingly, Helios was also not induced when the Foxp3− cells were cultured in the presence of TGF-β, despite the strong induction of Foxp3 expression. Expansion of CD4+Foxp3+ T cells under similar conditions led to a loss of Foxp3 expression in some of the population, but it did not change the ratio of Foxp3+Helios−/Foxp3+Helios+ cells in the cells that remained Foxp3+. Helios expression was not observed in cells that had lost expression of Foxp3. Although human CD4+Foxp3− T cells can be induced to express Foxp3 by stimulation in vitro in the presence of TGF-β, they lack regulatory function (26, 27). Nevertheless, similar results were observed when human CD4+CD25−CD127+CD45RA+ cells (<1% FOXP3+) and CD4+CD25hiCD127− cells (>85% FOXP3+) were activated in the presence or absence of TGF-β (Fig. 5C). Helios expression was never observed when human FOXP3− T cells were activated in vitro under conditions in which FOXP3 expression was readily induced.
Mucida et al. (28) showed that peripheral conversion of CD4+ Foxp3− cells to CD4+Foxp3+ Treg cells can be readily detected in vivo after oral exposure to Ag, whereas Sun et al. (29) demonstrated that this occurs specifically in the gut-associated tissues, such as the lamina propria (LP). Using this model, we adoptively transferred CD4+Foxp3− cells from OT-II TCR Tg × Foxp3-GFP mice into CD45.1+ congenic hosts and fed the recipients OVA in the drinking water for 6 d. Foxp3+Helios+ cells made up ~60% of the endogenous Foxp3+ cells in the LP and 70% of Foxp3+ cells in the mesenteric LN (mLN) (data not shown); oral administration of OVA did not alter these ratios in the host-derived CD4+ LP cells and mLN cells (Fig. 5D). As expected following feeding, OT-II+Foxp3+ T cells could be detected in the LP. However, the converted cells failed to express Helios, thus demonstrating that Foxp3+ T cells generated in vivo and in vitro do not express Helios. In the mLN, a small percentage (~2%) of OT-II cells converted to Foxp3-expressing cells. However, in the mLN, a significant percentage of Foxp3−cells expressing Helios was detected, raising the possibility that transient expression of Helios occurs in some or all activated cells upon exposure to oral Ag but that expression of Helios is silenced when Foxp3 expression is present.
Human FOXP3+Helios− T cells are enriched in cytokine-producing cells
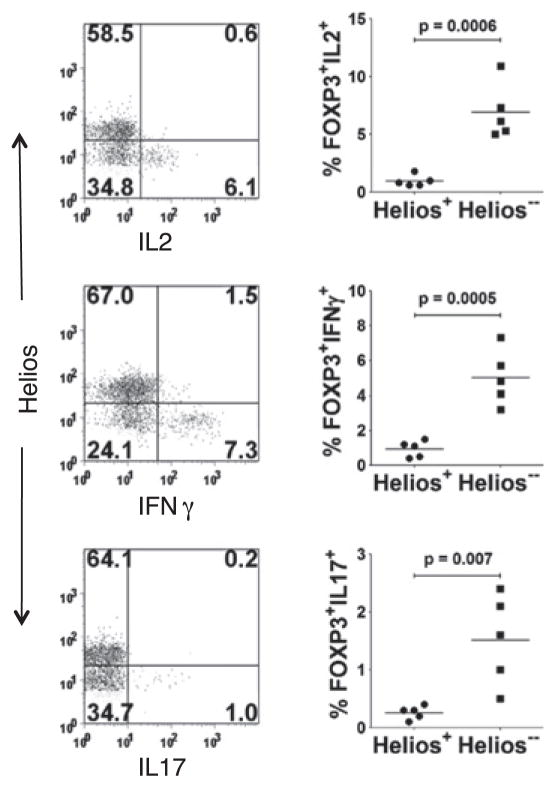
The issue of the stability of Foxp3 expression was recently questioned, and it seems likely that some Foxp3+ cells can lose expression of Foxp3 and become effector T cells (30). If Foxp3+ Helios− T cells are primarily generated from conventional T cells in the periphery, one might predict that these cells are intrinsically more prone to exhibit plasticity. In particular, a subpopulation of human FOXP3+ cells was recently shown to produce IL-17 when stimulated in the presence of proinflammatory cytokines. Therefore, it was of interest to examine the cytokine-expression profile of human FOXP3+ cells based on Helios expression. When human PBMCs were stimulated with PMA and ionomycin for 5 h and cytokine production by Foxp3+ T cells was analyzed by intracellular staining, low, but significant, numbers of IL-2– and -17–and IFN-γ–producing cells were detected; however, these were present almost exclusively within the Foxp3+Helios− population (Fig. 6). Thus, the lack of expression of Helios seems to define a Treg cell population with the potential for plasticity.
FIGURE 6.
FOXP3+Helios− T cells are enriched in cytokine-producing cells. PBLs from healthy adults were activated for 5 h with PMA/ionomycin, gated on CD4+Foxp3+ cells, and analyzed for Helios and cytokine expression. Panels on the left show a representative plot of Helios versus the indicated cytokine, whereas panels on the right show cumulative data from five healthy adults.
Expression of Helios is not required for Treg cell function
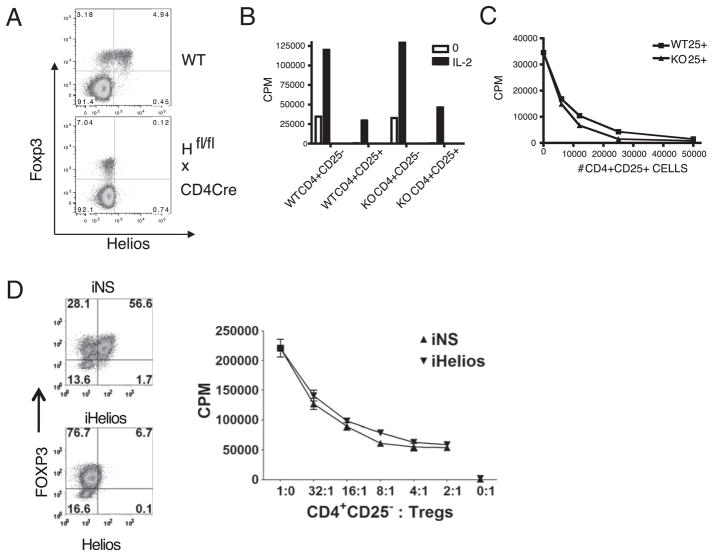
Because Helios expression in the thymus precedes Foxp3, we examined the possibility that Helios could regulate the expression of Foxp3. We generated Ikzf2fl/fl mice (Supplemental Fig. 2) and crossed them to CD4-Cre mice (Ikzf2fl/fl × CD4-Cre). Because Helios is expressed prior to CD4 in the thymus, Helios expression is only slightly reduced in DN thymocytes, but it is virtually absent by the CD4 single-positive stage (Supplemental Fig. 3). However, despite the deletion of Helios in peripheral CD4+ T cells, Foxp3 expression remains unchanged (Fig. 7A). Thus, Helios does not seem to regulate Foxp3 expression, nor does Foxp3 seem to be required for Helios expression. Because Foxp3 was reported to bind to the Helios promoter, and thus, possibly regulate its expression, we examined the expression of Helios in Scurfy mice that possess a mutation in the Foxp3 gene that results in the absence of Foxp3 protein. Because these mice die at an early age, they were examined at 4 or 17 d after birth. Despite the absence of Foxp3, Helios was expressed in the thymus and spleen of Scurfy mice (Supplemental Fig. 4).
FIGURE 7.
Helios-deficient Treg cells express Foxp3 and are anergic and suppressive. A, LN cells from two adult C57BL/6 or two Ikzf2fl/fl × CD4-Cre mice were analyzed by flow cytometry. Cells were gated on CD4+ cells and analyzed for Helios and Foxp3 expression. Shown are representative plots of one mouse from three independent experiments. B, CD4+CD25− and CD4+CD25+ cells were isolated from LN of WT C57BL/6 or Ikzf2fl/fl × CD4-Cre mice. Cells were stimulated with T-depleted splenocytes and anti-CD3 in the presence or absence of IL-2 for 3 d. C, WT CD4+CD25− cells were cocultured with the indicated number and type of CD4+CD25+ cells and were stimulated with T-depleted splenocytes and anti-CD3 for 3 d. D, Human Treg cells were activated and expanded for 14 d and then treated with nonspecific (iNS) or Helios (iHelios) siRNA and rested for 24 h. Treated Treg cells were then cultured alone or in titrated numbers with CD4+CD25−responders stimulated with HLA-DR+ APCs and soluble anti-CD3 for 3 d before pulsing with [3H]thymidine deoxyribose. Panels on the left represent analysis of Foxp3 and Helios in Treg cells at the end of the coculture. The experiments were set up in triplicate, and a representative experiment from three independent experiments is shown (B–D).
To examine the function of Helios in Treg cells, we purified CD4+ CD25+ T cells from Ikzf2fl/fl × CD4-Cre mice and assayed the cells for suppressive function in vitro. Treg cells deficient for Helios were nonresponsive to TCR stimulation and failed to respond to IL-2, but they responded to anti-CD3 and IL-2 in a manner similar to Treg cells from WT mice (Fig. 7B). In addition, Treg cells from the Helios−/− mice were as suppressive as Treg cells from WT mice (Fig. 7C). Helios expression in expanded human CD4+CD127− CD25hi Treg cells was markedly reduced following electroporation of Helios small interfering (siRNA). The loss of Helios expression did not reverse the nonresponsiveness of the Treg cells nor did it impair their suppressive capacity (Fig. 7D).
Discussion
We initially used PCR-based cDNA subtraction followed by qPCR to determine that the transcription factor Helios is preferentially expressed in Treg cells. Treg cell-specific expression of Helios was also found using microarray analyses (18, 19), and, very recently, a study by Cai et al. (31) confirmed these findings by Western blot analysis. We now extend these findings and demonstrate, by intracellular staining using a mAb to a 55-aa fragment from the nonconserved N-terminal region of Helios, that Helios is expressed in mouse and human Foxp3+ Treg cells in peripheral sites but that its expression is limited to only 70% of Foxp3+ Treg cells in either species. In addition, Helios is expressed at the DN2 stage of thymic development in the mouse, prior to Foxp3 expression.
Because the expression of Helios clearly divides the peripheral pool of Foxp3+ T cells into two defined populations, we attempted to determine whether these two populations were derived from distinct progenitors or whether they manifested distinct functions. We are unable to isolate the Foxp3+Helios− population, so our approach has been indirect and correlative. It seems that the Foxp3+Helios− population is generated in the periphery, because thymic Foxp3+ cells from young mice (3–7 d old) are exclusively Foxp3+Helios+. We observe Foxp3+Helios− cells in the thymus later during ontogeny (20%), but we believe that these cells represent activated recirculating cells that have been induced in the periphery. Although this number may seem high, no study has accurately measured the extent of Treg cell (induced or natural) recirculation to the adult thymus. Numerous studies demonstrated that activated conventional T cells preferentially recirculate to the thymus, so it is equally possible that newly generated induced Treg cells also preferentially recirculate to the thymus (32). Foxp3+Helios− cells are not observed in the spleen until day 12 and do not reach the percentage observed in an adult animal until ~1 wk after weaning. Moreover, a major phenotypic difference between Foxp3+Helios+ T cells generated in the thymus and the Foxp3+Helios− T cells generated in the periphery is the differential expression of CD44 and CD25. Neonatal thymic and splenic Foxp3+Helios+ cells express high levels of CD44 and CD25, as do Foxp3+Helios+ and Foxp3+Helios− T cells in the adult. In contrast, Foxp3+Helios− T cells in the neonate are predominantly CD25−, with the expression of variable levels of CD44. It is not until after weaning (day 25) that the levels of CD44/CD25 on splenic Foxp3+Helios− T cells resemble those seen in the adult. Thus, the development of Foxp+Helios+ and Foxp3+Helios− T cells seem to be distinct in terms of the site of generation and the time of first appearance.
We first noted that Foxp3+ T cells in MHC class II−/− mice were uniformly Helios+. The majority of Foxp3+ T cells in these mice is CD8+ and is likely to be thymic derived (25). Although the Foxp3+ cells in MHC class II−/− mice are an unusual subset, one interpretation of this finding is that TCR MHC class II interactions in the periphery are required for the generation of the CD4+ Foxp3+Helios− population. Our most striking finding is that Helios is not expressed in human or mouse T cells induced to express Foxp3 by TCR stimulation in vitro. More importantly, Ag-specific Foxp3+ T cells, generated in vivo from peripheral Foxp3− cells by the oral administration of Ag, were uniformly Helios−. Collectively, these studies strongly suggest that Foxp3+Helios− T cells are induced Treg cells that are generated in peripheral lymphoid tissues. One surprising observation in the oral administration of Ag study was that a significant proportion of the transferred Ag-specific T cells expressed Helios but failed to express Foxp3. The fate of these Helios+Foxp3− T cells is unclear. They may ultimately downregulate Helios and then upregulate Foxp3. We have not observed Helios+Foxp3− T cells under any other conditions, including following activation of conventional T cells in vitro or following immunization in vivo with Ag and adjuvant. In addition, Th1, Th2, and Th17 cell lines generated in vitro are uniformly Helios− (data not shown). Further analysis of Helios expression in other in vivo models (33–35) used for the induction of Ag-specific Foxp3+ cells is clearly warranted.
A number of studies have suggested that Foxp3 is not the major factor that controls the development of the Treg cell lineage (36, 37). Indeed, the expression of Helios in DN2/3 cells and the subsequent restriction of its expression to Foxp3+ T cells raised the possibility that Helios might play a major role in the generation of Foxp3+ Treg cells upstream of Foxp3. We have no data supporting this model for the role of Helios in the development of Foxp3+ Treg cells. The relationship, if any, between Foxp3 and Helios expression is an intriguing issue. Chromatin precipitation assays using an Ab to Foxp3 suggested that Foxp3 binds to the Helios promoter and could regulate its expression (38). However, Helios is expressed in all thymocytes at the DN2 stage, upstream of Foxp3, raising the possibility that Helios could regulate Foxp3 or influence the generation of Treg cells. Our data indicate that the absence of Foxp3 in Scurfy mice does not seem to affect Helios expression, nor does the absence of Helios in mature CD4+ T cells from Ikzf2fl/fl × CD4-Cre mice impact the expression of Foxp3. One difficulty in the interpretation of this result is that CD4-Cre does not become active until the DP stage; thus, Helios may play a role in the development of the Treg cell lineage prior to this time point. However, we very recently generated Ikzf2fl/fl × Vav-Cre mice, and Helios expression is extinguished in the thymus prior to the DN1 stage, yet thymic development of conventional and Foxp3+ Treg cells seems to be normal. This result is consistent with the recent studies that demonstrated that mice with a global deletion of Helios on the C57BL/6 background die very early in life (31) (A. Thornton, unpublished observations), but that a low percentage of Helios-deficient mice on a mixed 129/B6 background survive for a prolonged period and have normal development of conventional T cells and Treg cells (31).
We postulated that Helios might play a critical role in the function of Foxp3+ Treg cells, because Eos, another member of the Ikaros family, directly interacts with Foxp3 and mediates some aspects of Foxp3 gene silencing and because Ikaros itself binds to the IL-2 promoter and regulates its expression (16, 20). Because Treg cells do not express IL-2, it is possible that Helios/Ikaros heterodimers inhibit transcription of the IL-2 gene. Thus far, our in vitro studies with Foxp3+Helios− Treg cells from Ikzf2fl/fl × CD4-Cre mice and human Treg cells with Helios knocked down by siRNA indicate that Helios plays no role in Treg cell function in vitro. Similar results were observed in the few surviving mice with a global deletion of Helios (31). In addition, our preliminary studies in the Ikzf2fl/fl × CD4-Cre mice demonstrate that they develop experimental autoimmune encephalomyelitis in a manner similar to WT mice and respond normally to acute and chronic infection with lymphocytic choriomeningitis virus. We have not analyzed Foxp3+ Treg cells from the Ikzf2fl/fl × Vav-Cre mice in depth, but preliminary studies showed that they seem to function normally in vitro. Taken together, these studies indicate that Helios plays no role in the development of Foxp3+ Treg cells or in their function. It remains possible that the function of Helios is so critical to the development/function of Foxp3+ Treg cells that redundant pathways are in place so that other members of the Ikaros family can substitute for Helios in its absence. Of note, though, retroviral transfection of Helios into CD4+Foxp3− cells results in extremely low survival of the transduced cells (19).
It is notable that 30% of Foxp3+ T cells failed to express Helios in peripheral sites in mice and humans. We noted a modest increase in the percentage of Foxp3+Helios− cells in elderly individuals (D.Q. Tran, unpublished observations), but the percentage of Foxp3+ Helios−T cells has remained constant in mice as old as 1.5 y (A.M. Thornton, unpublished observations). Therefore, it is likely that the factors controlling the homeostasis of these two populations are similar. Several studies have compared the TCR repertoires of conventional and Foxp3+ Treg cells. By analyzing the CDR3 regions of Vα genes from mice with fixed Vβ genes, Hsieh et al. (39) reported the repertoire similarities to be 25%, whereas Pacholczyk et al. (40) reported the similarity to be between 10% and 20%, and Wong et al. (41) found a 42% similarity. One interpretation of these studies is that the overlap between the TCR repertoires represents induced Foxp3+ Treg cells, because they would presumably express the same TCR repertoire as conventional T cells. Intriguingly, Pacholczyk et al. (40) also reported that the overlap between the TCR repertoires of thymic Treg cells and peripheral LN Treg cells was 65%. If one assumes that the Treg cells isolated from thymus are primarily generated in the thymus, whereas the peripheral LN Treg cells would contain a mixture of thymic-derived and peripherally induced Treg cells, the 65% overlap in repertoires is strikingly close to the percentage (70%) of Foxp3+Helios+ T cells that we detect in peripheral sites.
Our data suggest that peripheral conversion accounts for 30% of all Treg cells. This is in stark contrast to a recent study in which extrapolated data determined that peripheral conversion accounted for 4–7% of CD4+Foxp3+ cells (42). However, Lathrop et al. (42) also emphasized that the estimate of ~4–7% conversion was based on a steady-state study that may “underestimate the role of peripheral conversion.” Indeed, a higher rate of conversion was observed after adoptive transfer into lymphopenic hosts. Our studies show that the Foxp3+Helios− T cells are preferentially generated during days 10–25 of life, a period of time when the animal is lymphopenic, and it is possible that the progeny of these cells may constitute a major percentage of the adult Foxp3+ Treg cell pool.
Recent studies have called into question the stability of the Treg phenotype and of Foxp3 expression, but it is not clear whether plasticity is an inherent property of all Foxp3+ T cells or whether it might only be a property or a subpopulation. Foxp3+ T cells induced in vitro are very unstable and may exhibit a “plastic” phenotype, resulting in loss of Foxp3 expression and induction of IL-17 production by a small percentage of the cells (30, 43). Loss of Foxp3 expression is potentiated when Treg cells are cultured with IL-6 (40%), and these former Treg cells are able to produce IL-17. Zhou et al. (44) also showed by genetic lineage tracing that a significant percentage of cells that initially expressed Foxp3 lose expression of Foxp3 in vivo and can develop into pathogenic effector cells. In contrast, Komatsu et al. (45) demonstrated that Foxp3+ T cells are heterogeneous and are composed of a major subset that is committed to the Treg cell lineage, whereas a minor subset (~8%) possesses a “plastic” phenotype and can lose Foxp3 expression. This result is consistent with our observation that a highly enriched population of Foxp3+Helios+ T cells isolated from neonatal thymocytes retains expression of Foxp3 and Helios following transfer to normal recipients. Similarly, our data demonstrating that effector cytokine production is restricted to the human FOXP3+Helios−population is consistent with the possibility that the less committed or “plastic” Treg cells are exclusively found in the Foxp3+Helios− pool.
Lastly, there is a great deal of interest in the analysis of Treg cell function in disease and in the therapeutic use of Treg cells (46). Our results suggest that a significant component of the Foxp3+ T cells population is likely to be composed of induced Treg cells and that this subpopulation is likely to be quite distinct from thymic-derived Treg cells in terms of TCR repertoire expression, stability, and potentially many other characteristics. Furthermore, it is unclear whether defects in Treg cell function will be confined to one or the other of these subpopulations or which subpopulation would be most suited for in vitro expansion and cellular biotherapy. A detailed comparison of the Foxp3+Helios+ and Foxp3+Helios− subpopulations will require generation of a reporter mouse and identification of a cell-surface Ag that will distinguish the sub-populations in humans.
Acknowledgments
This work was supported by funds from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank S. Tanksley and J. Watt for FACS sorting and members of the Shevach and Paul laboratories for helpful discussions.
Abbreviations used in this paper
- DN
double negative
- DP
double positive
- LN
lymph node
- LP
lamina propria
- mLN
mesenteric lymph node
- NIAID
National Institute of Allergy and Infectious Diseases
- qPCR
quantitative PCR
- siRNA
small interfering RNA
- SP
single positive
- Tg
transgenic
- Treg cell
regulatory T cell
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 6.Cobb BS, Smale ST. Ikaros-family proteins: in search of molecular functions during lymphocyte development. Curr Top Microbiol Immunol. 2005;290:29–47. doi: 10.1007/3-540-26363-2_3. [DOI] [PubMed] [Google Scholar]
- 7.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, Morgan BA. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8:508–515. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- 9.Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12:782–796. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 11.Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 12.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA, Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 15.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282:30227–30238. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- 16.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 17.Kang HG, Zhang D, Degauque N, Mariat C, Alexopoulos S, Zheng XX. Effects of cyclosporine on transplant tolerance: the role of IL-2. Am J Transplant. 2007;7:1907–1916. doi: 10.1111/j.1600-6143.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- 18.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 20.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 22.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 23.Rebrikov D, Desai S, Kogan YN, Thornton AM, Diatchenko L. Subtractive cloning: new genes for studying inflammatory disorders. Ann Periodontol. 2002;7:17–28. doi: 10.1902/annals.2002.7.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens GL, Andersson J, Shevach EM. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J Immunol. 2007;178:6901–6911. doi: 10.4049/jimmunol.178.11.6901. [DOI] [PubMed] [Google Scholar]
- 26.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 28.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Q, Dierich A, Oulad-Abdelghani M, Chan S, Kastner P. Helios deficiency has minimal impact on T cell development and function. J Immunol. 2009;183:2303–2311. doi: 10.4049/jimmunol.0901407. [DOI] [PubMed] [Google Scholar]
- 32.Hale JS, Fink PJ. Back to the thymus: peripheral T cells come home. Immunol Cell Biol. 2009;87:58–64. doi: 10.1038/icb.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 34.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daley SR, Ma J, Adams E, Cobbold SP, Waldmann H. A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J Immunol. 2007;179:3648–3654. doi: 10.4049/jimmunol.179.6.3648. [DOI] [PubMed] [Google Scholar]
- 36.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 37.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and non-regulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 42.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD252Foxp32 T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]