Abstract
Women develop certain autoimmune diseases more often than men. It has been hypothesized that this may relate to the development of more robust T-helper (Th)1 responses in women. To test whether women exhibit a Th1 bias, we isolated naïve cluster of differentiation (CD)4+ T cells from peripheral blood of healthy women and men and measured the proliferation and cytokine production by these cells in response to submaximal amounts of anti-CD3 and anti-CD28. We observed that CD4+ T cells from women produced higher levels of IFNγ as well as tended to proliferate more than male CD4+ T cells. Intriguingly, male CD4+ T cells instead had a predilection toward IL-17A production. This sex dichotomy in Th cytokine production was found to be even more striking in the Swiss/Jackson Laboratory (SJL) mouse. Studies in mice and humans indicated that the sexual dimorphism in Th1 and Th17 cytokine production was dependent on the androgen status and the T-cell expression of peroxisome proliferator activated receptor (PPAR)α and PPARγ. Androgens increased PPARα and decreased PPARγ expression by human CD4+ T cells. PPARα siRNA-mediated knockdown had the effect of increasing IFNγ by male CD4+ T cells, while transfection of CD4+ T cells with PPARγ siRNAs increased IL-17A production uniquely by female T cells. Together, our observations indicate that human T cells exhibit a sex difference in the production of IFNγ and IL-17A that may be driven by expressions of PPARα and PPARγ.
Keywords: autoimmunity, cytokines, experimental autoimmune encephalomyelitis, gender, nuclear receptor
For reasons that remain unclear, the incidence of multiple sclerosis (MS) is increasing in women (1) and, overall, women are three times more likely to be diagnosed with MS than men (1, 2). The same is true for a number of other autoimmune diseases (3). In MS, the higher female prevalence of disease emerges around puberty (4), implicating a role for sex hormones in disease risk. Results from both genome-wide association and pathological studies in MS suggest that T-helper (Th)1 and Th17 cells play key roles in the development of this disease (5–7). Furthermore, studies in the rodent model of MS, experimental autoimmune encephalomyelitis (EAE), have provided clues that the higher female preponderance of disease is attributable to the development of more robust Th1 responses in females (8–11). Female myelin-specific T cells produce more IFNγ and less Th2 cytokines than male T cells and are more encephalitogenic upon adoptive transfer (9–11). However, despite numerous attempts to explore this issue in humans (12–16), conclusive evidence in support of a Th1 bias in women is lacking.
Castration of male mice worsens EAE (17), whereas ovariectomy does not have a major impact on disease (18, 19), suggesting that the higher androgen levels in males protects this sex from developing autoimmunity. Regarding possible mediators of these androgen effects, we previously identified that that the nuclear receptor peroxisome proliferator activated receptor (PPAR)α is expressed at higher levels by male vs. female T cells and appears to control T-cell proliferation and Th1 cytokine production uniquely in male mice (20). Furthermore, we observed that a PPARα ligand reduced the incidence of EAE in male, but not female mice, indicating a possible link between PPARα activity and protection from autoimmunity (20). The goal of this study was to explore whether a sex difference in Th cytokine production exists in humans and to investigate the potential role for PPARs in this regulation. Here, we report the observation that naïve cluster of differentiation (CD)4+ T cells from women are intrinsically geared to proliferate and produce higher levels of IFNγ and lower levels of IL-17A compared with male T cells. This sex difference in Th biology was also apparent in Swiss/Jackson Laboratory (SJL) mice. Furthermore, we found that the female Th1 bias was associated with a higher expression of PPARα by male activated T cells and higher expression of PPARγ1 by activated female T cells.
Results
Sex Bias in CD4+ T-Cell Expansion and Th Cytokine Production.
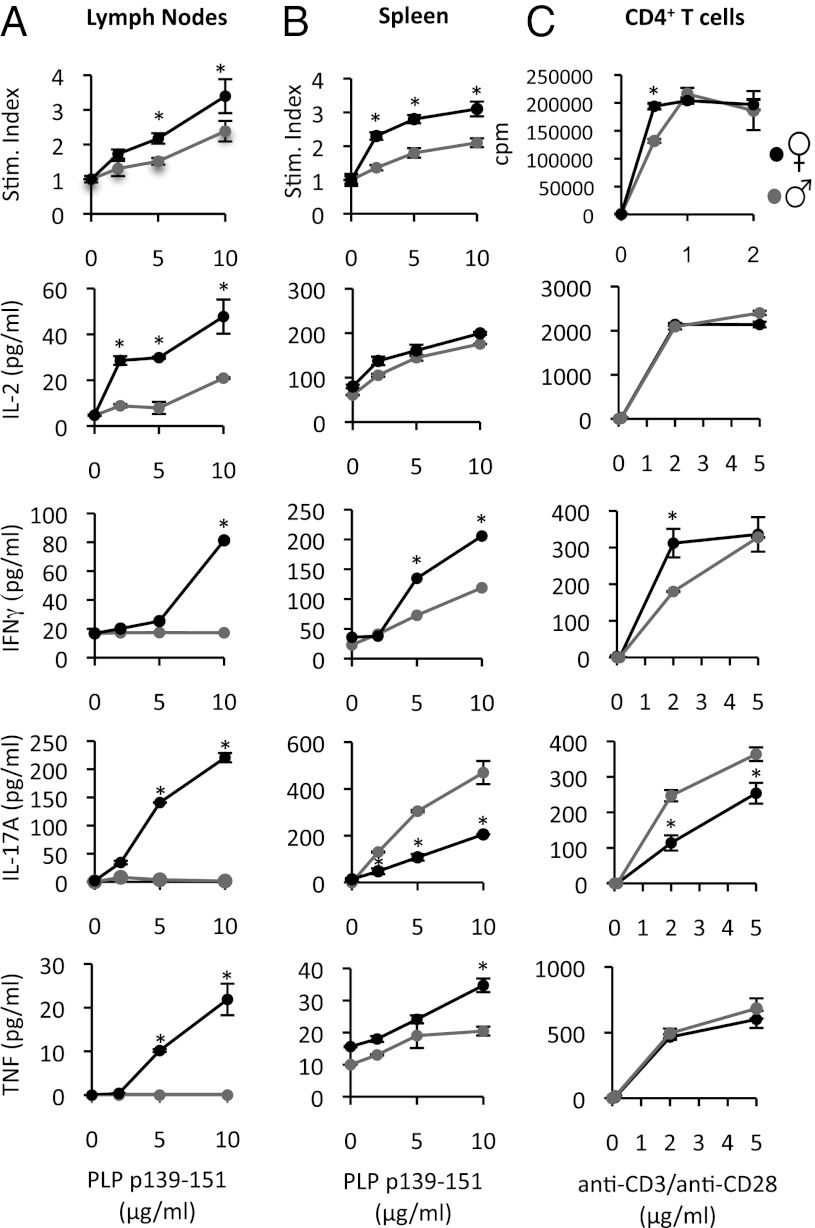
SJL is one of the few mouse strains that exhibits a female bias in EAE development (8, 9). We, thus, used this strain as a model system to explore the basis of the sex differences in Th cytokine production. We immunized male and female mice of this strain with proteolipid protein (PLP) p139-151 in complete Freund’s adjuvant (CFA) and examined the recall proliferation and cytokine responses of splenic and draining lymph node cells to PLP p139-151 ex vivo. We detected PLP p139-151-reactive cells primarily in the spleens of males and in both the spleen and draining lymph nodes of females at 8 d postimmunization, indicating possible sex differences in the trafficking of these cells in vivo (Fig. 1 A and B). However, when considered together, splenocytes and lymph node cells cultured from female mice clearly expanded more than male T cells (Fig. 1 A and B). T regulatory cells (T regs) (CD25+FoxP3+CD4+) also tended to expand more in female mice (Fig. S1A).
Fig. 1.
PLP p139-151-reactive CD4+ from female SJL mice produce more IFNγ and proliferate more than male CD4+ cells. (A and B) Male and female SJL mice (8 wk) were immunized with PLP p139-151 in CFA. Eight days later, cells from draining lymph nodes (A) and spleens (B) were harvested, were pooled within groups, and then cultured with PLP p139-151. (C) CD4+ T cells were isolated from nonimmunized male and female mice and were activated with plate-bound anti-CD3 and anti-CD28. Shown are the proliferation and cytokine responses to the various stimuli. In A and B, proliferation values are expressed as a stimulation (Stim.) index: 3[H]thymidine incorporation in peptide-stimulated wells divided by the media control wells. In C, proliferation values are expressed as cpm. In all cases, values are means ± SEM of triplicate cultures. *Significant difference (P ≤ 0.05) from males, as determined using a t test (two-tailed). Results are representative of three experiments.
Consistent with previous reports (8, 9), we observed that female SJL PLP p139-151-reactive T cells produced higher levels of IFNγ compared with male cells (Fig. 1 A and B). However, instead of exhibiting a Th2 bias (no IL-4 was detected), male splenic PLP p139-151-reactive T cells tended to produce more IL-17A than female cells (Fig. 1B). We also followed several male and female mice to the acute stage of EAE and examined the cytokine production by spinal cord-infiltrating CD4+ cells. We observed a higher frequency CD4+ cells that produced IFNγ (mainly IFNγ- and IL-17A-coproducing CD4+ cells) in the central nervous system of female as compared with male mice (Fig. S1C). Thus, these results indicate that Th cells from female SJL mice have a higher propensity to expand in general and produce more IFNγ and less IL-17A than male T cells. The discrepancy between our results and past studies that indicated a Th2 bias in male SJL mice may relate to the fact that: (i) past studies were conducted before the discovery of Th17 cells; and (ii) the levels of IL-4 reported in these studies were measured in cultures of whole lymph nodes or spleens (8) or by IL-2 expanded T-cell lines (9).
Androgen Dependence of Sex Difference in Th Cytokine Production.
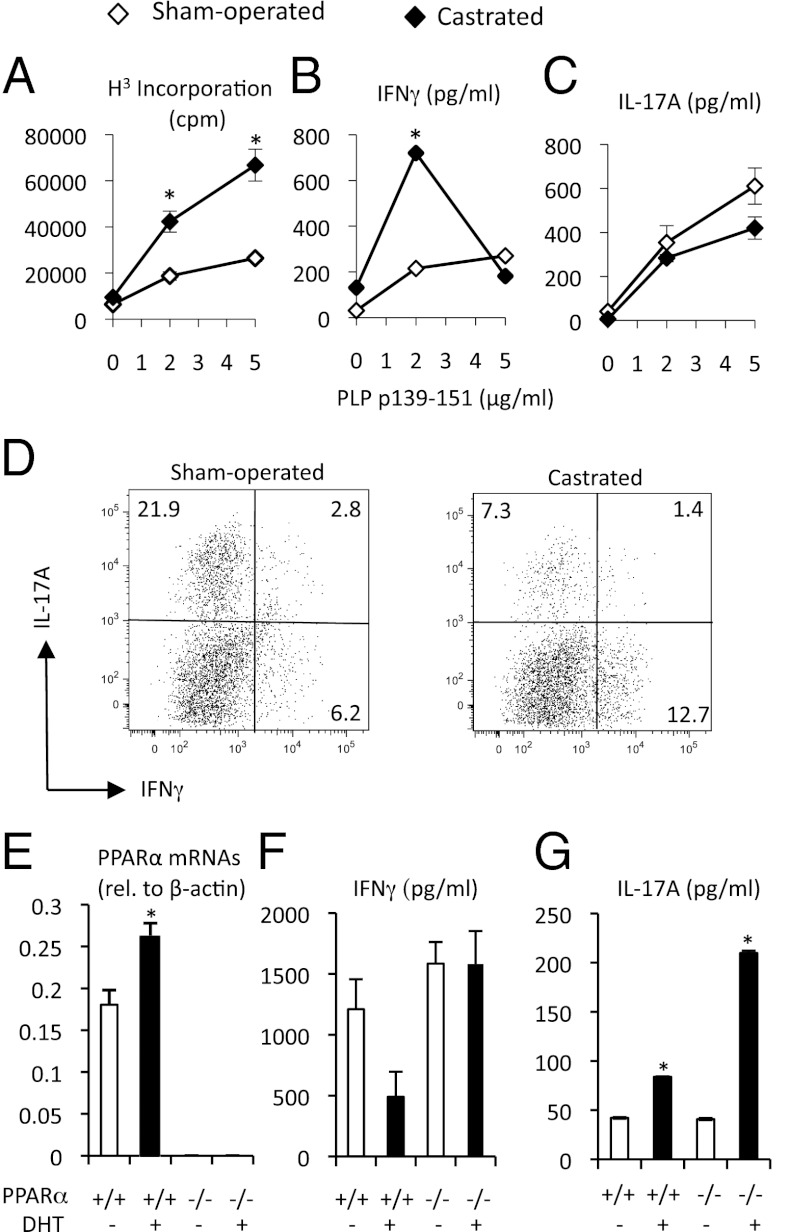
Castration of male mice worsens EAE in males (17), whereas ovariectomy does not have a major impact on this disease in female mice (18, 19). Such observations suggest that it is the higher level of androgens in males that is protecting this sex from EAE development. We, thus, explored the effect of castration on Th proliferation and cytokine production. Male SJL mice (4–5 wk of age) were castrated or provided a sham surgery. When these mice reached adulthood, they were immunized with PLP p139-151 and CFA, and, as before, the recall responses to antigen were measured. We found that castration was associated with a “feminization” of the Th profile in male mice, accompanied by an enhanced expansion of PLP p139-151-reactive T cells over sham-operated counterparts (Fig. 2A) and a selective outgrowth of IFNγ- vs. IL-17A-producing cells (Fig. 2 B vs. C). Castrated mice that were followed to the development of EAE symptoms were also found to exhibit a higher ratio of IFNγ- to IL-17-producing T cells in the central nervous system as compared with sham mice (Fig. 2D). These observations suggest that androgens shift Th cytokines from Th1 to Th17.
Fig. 2.
Sex difference in cytokine production associates with hormone status in male mice. (A–D) Male SJL/J mice (5 wk old; n = 5/group) were subjected to castration or sham surgery. Three weeks later, mice were immunized with PLP p139-151 in CFA. (A–C) Eight days later, the spleens were collected from mice and were pooled, and cells were stimulated with PLP p139-151. *Significantly different (P ≤ 0.05) from sham using a t test (two-tailed). (D) Mononuclear cells were isolated from the spinal cords of male sham and castrated mice during the acute phase of EAE. Mice were all at score 3 and were sick for an equivalent period. (E–G) WT or PPARα−/− SV.129 female mice (5 wk of age; n = 3/group) were implanted with pellet containing DHT (5 mg) or carrier-binder (placebo). Three weeks later, CD4+ T cells were pooled and then cultured with anti-CD3 and anti-CD28 in RPMI containing with 1% autologous serum. (E) Relative abundance of PPARα mRNA (relative to β-actin) in freshly isolated CD4+ T cells. (F and G) Production of IFNγ (F) and IL-17A (G) in T-cell cultures. *Significantly different (P ≤ 0.05) from placebo counterpart, as determined using a one-way ANOVA and Tukey post hoc test. Values are means ± SEM of measurements in triplicate cultures. Results are representative of two to three experiments.
Cellular Basis of T-Cell Expansion and Cytokine Production.
To investigate the cellular basis of the sex difference in Th proliferation and cytokine production, we took advantage of the fact that female SJL T cells do not react against the male Y-linked histocompatibility (HY) antigen (10) and conducted coculture experiments of male or female antigen-presenting cells (APCs) with naïve male or female PLP p139-151-specific T-cell receptor transgenic (TCR Tg) T cells. We found that cultures that contained female APCs and female naïve CD4+ T cells exhibited the highest proliferation to PLP p139-151 (Fig. S2A). On the other hand, the production of Th cytokines more closely mapped with the sex of the T cells in these assays (Fig. S2A). Cultures that contained female T cells produced higher levels of IFNγ and IL-12p40, whereas the cultures that contained male T cells instead exhibited higher levels of IL-17A at the lowest PLP p139-151 concentration tested (Fig. S2A). Again, the levels of IL-4 and IL-10 were low and not found to be different between groups (Fig. S2A). Similar results were observed when we cultured purified CD4+ T cells in isolation with anti-CD3 and anti-CD28 (Fig. 1C). Furthermore, the Th17 bias by male CD4+ T cells was evident regardless of the tissue source of cells (Fig. S2B). Collectively, these results suggest that two mechanisms are operating to regulate the sex differences in Th proliferation and cytokine production: (i) a mechanism that regulates the expansion of T cells that that is determined by the sex of the APCs and the T cell; and (ii) a mechanism that regulates Th cytokine production that is determined primarily by the sex of the CD4+ T cell. Herein, we focused on the T-cell-intrinsic mechanisms that regulate sex differences in the production of IFNγ and IL-17A.
PPARα Is an Androgen-Sensitive Regulator of Th Cytokine Production.
We found previously that PPARα has a male-specific role in dampening Th1 cytokine production in mice (20). To address whether PPARα is an intermediary of androgen effects in shifting the Th cytokine production from Th1 → Th17, we “re-programmed” female WT or PPARα−/− mouse T cells in vivo with the androgen receptor agonist 5α-dihydrotestosterone (DHT). Three weeks later, we isolated splenic CD4+ T cells from mice and, as before, stimulated them in vitro with anti-CD3 and anti-CD28. We observed that DHT-treated WT T cells displayed higher expression of PPARα mRNAs compared with placebo control cells (Fig. 2E). This was associated with lowered IFNγ and higher IL-17A production by WT T cells (Fig. 2 F and G). Interestingly, the decrease in the potential to make IFNγ in response to androgen treatment did not occur in PPARα−/− T cells, whereas the increased potential to make IL-17A did (Fig. 2 F and G). These results indicate that PPARα may be a molecular intermediary of androgen effects on IFNγ, but not IL-17A.
Human Female CD4+ T Cells Are Th1-Prone.
We next set out to investigate whether human CD4+ T cells exhibit a sex difference in Th cytokine production. The variability associated with past reports of sex differences in human Th cytokine production (12–16) may be attributed to the fact that cytokine production was assayed in whole blood or in cultures of peripheral blood mononuclear cells (PBMCs), which are comprised of mixtures of cells that are present at varying frequencies in individuals. We, thus, investigated cytokine production by naive CD4+CD45RA+ T cells in response to anti-CD3 and anti-CD28 stimulation. This stimulus was titrated to produce cytokine levels and proliferative rates that were within the dynamic range of these assays (Fig. S3A). Healthy men and women were recruited in a pair-wise fashion with one woman and one man donating a blood sample each day (n = 25 pairs) to control for the day-to-day variability. Furthermore, to further reduce noise in the system, we controlled for the time of the menstrual cycle in women (follicular phase) (Fig. S3 B and C).
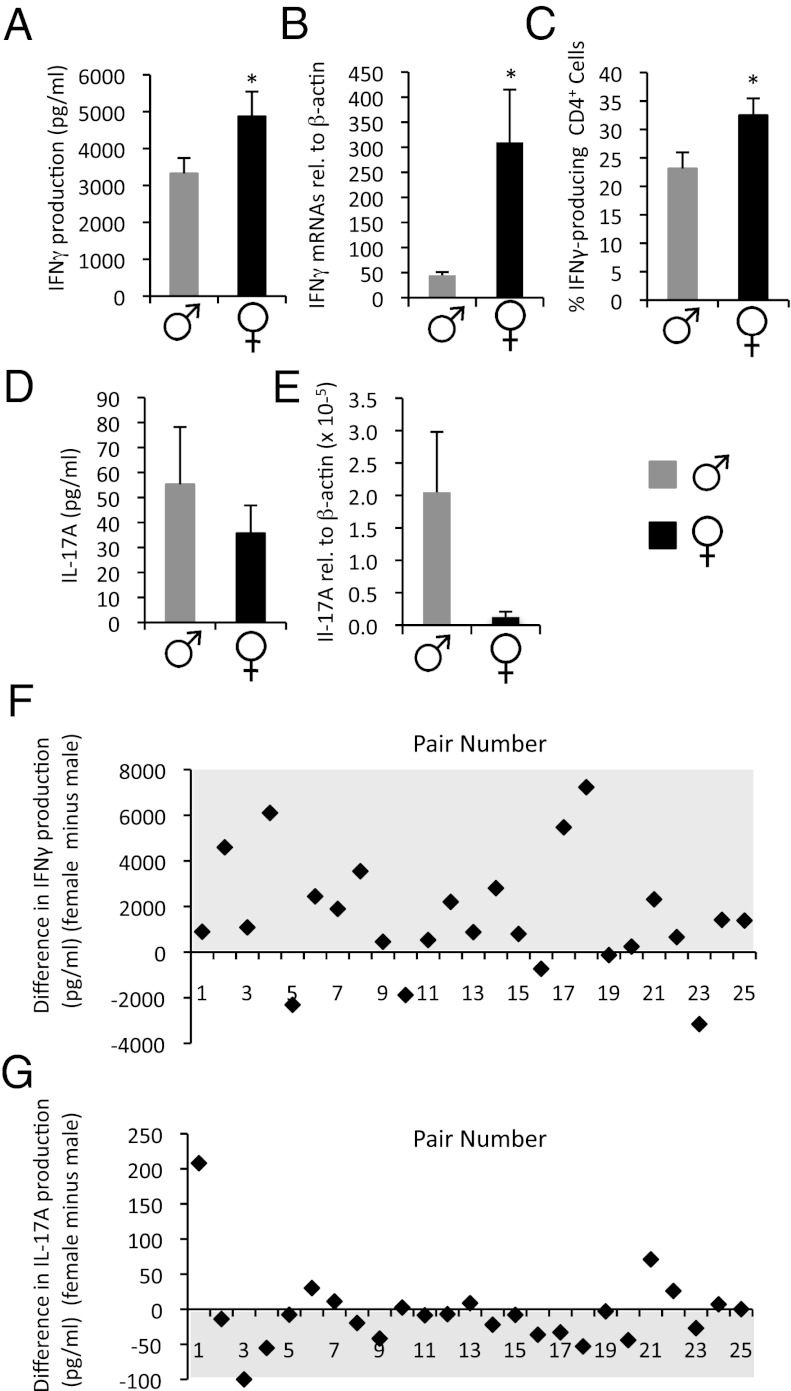
Consistent with our findings in SJL mice, we observed that naïve CD4+ T cells of women secreted significantly higher levels of IFNγ compared with men in response to anti-CD3 and anti-CD28 stimulation (Fig. 3A). In fact, 20 of the 25 women sampled exhibited higher production of IFNγ than their male pair (Fig. 3F). In females, we also detected a higher frequency of IFNγ-producing T cells and significantly higher T-cell expression of IFNγ mRNAs than male counterparts (Fig. 3 B and C and Table S1). A trend for higher proliferative rates by female T cells was also observed (Table S1). Although the level of IL-17A detected in these cultures under Th0 conditions was low and not different between the sexes (Fig. 3D), when IL-17A production was examined in individual pairs, we observed a tendency for higher production of this cytokine in cultures of male vs. female T cells (Fig. 3G). A trend for higher IL-17A mRNA expression by male T cells was also observed (Fig. 3E). As in the SJL mouse, the productions of Th2 cytokines were low and not found to be different between the sexes (Table S1).
Fig. 3.
CD4+ cells from women are more prone to produce IFNγ and less IL-17A than male T cells. Naïve (CD45RA+) CD4+ T cells were isolated from peripheral blood of healthy men and women (n = 25/group) and were cultured in X-VIVO-15 serum-free media in the presence of anti-CD3- and anti-CD28-coated beads. Supernatants were collected at 72 h poststimulation. T cells were expanded in IL-2 containing X-VIVO-15 media for an additional 7 d before stimulation phorbol myristate acetate (PMA)/ionomycin. (A and D) Absolute IFNγ and IL-17A levels detected in culture supernatants. (B and E) IFNγ and IL-17A mRNAs (expressed relative to β-actin). (C) Frequency of IFNγ-producing T cells. Values are means ± SEM measurements from individual human subjects. (F and G) Difference in IFNγ and IL-17A production (pg/mL) in individual pairs (female minus male value). *Significantly different (P ≤ 0.05) from male, as determined using a paired t test (two-tailed).
To further investigate the male Th17 bias, we explored the production of IL-17A in two T culture systems associated with enhanced production of this cytokine: (i) naïve CD4+ T cells activated with anti-CD3 plus peptidoglycan-matured autologous monocytes (21); and (ii) memory CD4+ T cells that were cultured with anti-CD3 plus autologous monocytes before expansion with IL-23 (6). In both of these systems, we observed a higher propensity for IL-17A production when donor cells were derived from men compared with women (Fig. S4). Thus, the presence of male monocytes amplified the sex difference in IL-17A that was specified by TCR- and CD28-elicited signals. Altogether, these findings in human T cells align with our results in the murine system and suggest a Th1 bias in females and a Th17 bias in males.
Regulation of Human PPARα by Androgens and TCR Stimulation.
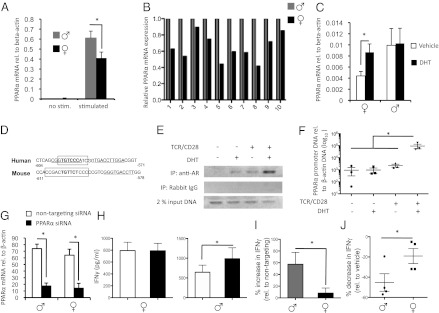
Next, we focused on a possible role for PPARα in the Th1 bias in women. First, we investigated whether PPARα exhibits sexual dimorphic expression in human CD4+ T cells and then followed with experiments to address whether this gene is sensitive to androgen levels. We found that this gene was expressed at low levels in naïve CD4+ T cells of both sexes. However, PPARα mRNAs were strikingly up-regulated in T cells upon TCR and CD28 stimulation, particularly in males (Fig. 4 A and B). Next, we stimulated CD4+ T cells from men and women in the presence of either 100 nM DHT or vehicle, as well as examined the relationship of PPARα mRNAs with circulating testosterone and 17-β-estradiol levels in men and women. We found that in vitro DHT treatment enhanced PPARα expression in T cells of women but did not alter the expression of this gene in T cells of men (Fig. 4C). In addition, we observed that PPARα mRNA expression in freshly isolated male T cells tended to associate with circulating levels of testosterone (P = 0.07) but not 17-β-estradiol levels (Fig. S5). The lack of sensitivity of male T cells to in vitro DHT treatment may relate to the fact that these cells were taken from an environment that already contained high levels of androgens.
Fig. 4.
PPARα has a sex-specific role in inhibiting Th1 cytokine production by human T cells. (A) CD4+ T cells were isolated from peripheral blood of healthy men and women (n = 10/group) and were either frozen down or stimulated with anti-CD3 and anti-CD28 for 31 h. PPARα mRNAs were measured using real-time PCR and were normalized to β-actin mRNAs. (B) Relative expression of PPARα mRNAs in stimulated male and female T cells. (C) PPARα mRNA expression by CD4+ T cells of men or women (n = 4/group) after stimulation for 72 h with 100 nM DHT or vehicle. (D) Location of probable AR (boxed) and estrogen receptor (underlined) binding sites in the human and mouse PPARα promoters. (E and F) PBMCs were isolated from women and were cultured in the presence (+) or absence (−) of anti-CD3/anti-CD28, plus (+) 100 nM DHT or vehicle (−). DNA was isolated from cells for ChIP of the PPARα promoter using an anti-AR or isotype control antibody. Representative gels (one donor) (E) and relative abundance of the PCR-amplified product of the PPARα promoter in three female donors post-ChIP (F). (G–I) CD4+ from men and women (n = 10/group) were transfected with a GFP construct, along with either PPARα or nontargeting control siRNAs, before stimulation with anti-CD3 and anti-CD28 for 48 h. (G) Extent of PPARα knockdown. (H) Absolute IFNγ levels detected in CD4+ T-cell cultures after stimulation. (I) Mean percentage increase in IFNγ over nontargeting control siRNA for male (gray bar) and female (black bar) donors. (J) Percentage decrease in IFNγ in cultures after stimulation of T cells of men and women (n = 4 each) with 2.5 µM fenofibrate relative to vehicle (DMSO) control. In all cases, values are means ± SEM of individual human donors. *Significantly different (P ≤ 0.05), as determined using a t test (two-tailed) or a one-way ANOVA.
To address the molecular basis of androgen-sensitivity human PPARα, we used Alibaba2 prediction software (www.gene-regulation.com/pub/programs.html) to search for hormone-responsive elements in promoter region of this gene. This search revealed the presence of an androgen receptor (AR)/glucocorticoid receptor-binding site in close proximity to an estrogen-binding site in the human and mouse promoters (Fig. 4D). Chromatin immunoprecipitation (ChIP) experiments revealed that DHT and anti-CD3 and -CD28 signals act in synergy to induce the recruitment of the AR to the human PPARα promoter in primary T cells (Fig. 4 E and F). These studies suggest a direct effect of androgens in increasing PPARα mRNAs.
PPARα Represses IFNγ Production by Human T Cells.
To address whether PPARα functions in human T cells to repress IFNγ, we transfected primary T cells of men and women (n = 10/group) with PPARα-specific siRNAs before activating these cells with anti-CD3 and anti-CD28. Approximately half of T cells (52.5 ± 3.0% in men and 56.1 ± 3.8% in women) were transfected using our approach, resulting in a ∼70–75% reduction of T-cell PPARα mRNAs in T cells of both sexes (Fig. 4G). When stimulated with anti-CD3 and anti-CD28, male T cells transfected with PPARα siRNAs produced higher levels of IFNγ compared with those transfected with the nontargeting siRNAs (Fig. 4 H and I). This effect was not observed in women (Fig. 4H), and appeared to be specific to IFNγ (Table S2). Similar results were obtained when siRNA transfection studies were conducted in SJL mice (Fig. S6).
We also investigated whether the PPARα ligand fenofibrate would have sex-specific effects in limiting IFNγ production. We found that fenofibrate reduced IFNγ levels by WT but not by PPARα−/− T cells when administered at concentrations from 1 to 5 μM (Fig. S7A). This same drug also had a sex-specific effect in decreasing IFNγ production by male but not female T cells both in mice (Fig. S7B) and humans (Fig. 4J) when provided at a 5 μM dose. Together with siRNA findings, these results validate that PPARα has a sex-specific role in the negative regulation of T-cell IFNγ production.
Mechanism of Repression of Human IFNγ by PPARα.
The induction of expression of the IFNγ gene is regulated by both TCR- and cytokine-dependent signals acting at multiple genomic elements, located far upstream and downstream of this gene (22, 23). These genomic elements comprise a number of highly conserved noncoding sequences (CNSs) that contain binding sites for transcription factors including T-box expressed in T cells (T-bet) and signal transducer and activator of transcription (STAT)4 (22, 23). Under Th1 conditions, T-bet and STAT4 play key roles in the establishment of histone marks at these regions, thus permitting chromatin opening, transcription factor binding, and recruitment of the transcriptional activation machinery (22, 23). To gain insight into how PPARα may factor into this process, we used ChIP to assess the abundance of the activating histone mark acetylated-histone H4 at two well-characterized CNS regions, CNS-22 and CNS-6, in male T cells that were transfected with PPARα or nontargeting siRNAs (Fig. S8A). We found that T cells transfected with PPARα siRNAs displayed an enhanced abundance of acetylated-histone H4 at both of these CNS regions after anti-CD3 and anti-CD28 stimulation (Fig. S8 B and C). Thus, one way that PPARα may operate to repress IFNγ production is through epigenetic changes in the IFNγ locus that make it less poised for IFNγ transcription.
In light of our previous findings of a higher abundance of RelA (reticuloendotheliosis viral oncogene homolog A) in the nucleus of PPARα−/− T cells (20), we further investigated whether this transcription factor is recruited in increased amounts to these CNS regions. Using ChIP, we detected an enhanced abundance of RelA at these sites in T cells that were transfected with the PPARα siRNAs relative to those transfected with the nontargeting siRNAs (Fig. S8D). This occurred even in quiescent T cells (Fig. S8D). Taken together, our findings suggest that PPARα actions on the IFNγ gene are very complex and involve effects on upstream TCR-signaling intermediates in addition to epigenetic changes at the IFNG locus.
PPARγ Is a Potential Sex-Dependent Regulator of Th17.
Our findings in mice indicated that PPARα expression is not responsible for the higher IL-17A production by male T cells. Indeed, recent reports have implicated roles for a number of other nuclear receptors in the control of IL-17A production [i.e., retinoic acid-related orphan receptor (ROR)γT, vitamin D receptor, retinoic acid receptor, liver X receptor, and PPARγ1] (24). With the knowledge that PPARγ exhibits a female preponderance in certain adipose stores (25, 26), we investigated whether the immune-expressed PPARγ variant, PPARγ1 is expressed by human CD4+ T cells in sex-specific way or is sensitive to androgen levels. In contrast to our findings for PPARα, we observed that PPARγ1 mRNAs were down-regulated more strikingly in T cells of men upon anti-CD3 and anti-CD28 stimulation (Fig. S9A). Furthermore, in vitro DHT treatment inhibited the T-cell expression of this gene in mice and humans, suggesting that PPARγ1 is tuned down in T cells by antigen and androgen signals (Fig. S9 B and G).
Using the approach of siRNA transfection and ligand activation, we next explored whether the control of T-cell IL-17A production by PPARγ1 is sex-dependent. As for our studies for PPARα, we isolated T cells from males and females of both species, transfected these cells with either PPARγ or nontargeting siRNAs and then stimulated cells with anti-CD3 and anti-CD28, this time in the presence of Th17-skewing cytokines. Although we did not detect a sex difference in IL-17A production by the human CD4+ T cells transfected with nontargeting siRNAs (Fig. S9E), we did observe this effect in mice (Fig. S9H). Furthermore, in both the murine and human systems, knockdown of PPARγ1 mRNAs had a more profound effect in increasing IL-17A production by T cells of females vs. males (Fig. S9 E and H). Finally, we observed that the synthetic PPARγ ligand rosiglitazone reduced IL-17A production by CD4+ T cells of women but not men (Fig. S9F). Altogether, these data suggest that PPARγ1 may be one molecule that may have sex-specific effects in limiting the production of IL-17A by female T cells.
Discussion
Based upon work in mice, it has been hypothesized that the reason why women are more prone than men to develop T-cell autoimmune diseases is because they exhibit more robust Th1 adaptive immunity (27). Our finding that naïve CD4+ T cells of women produced higher levels of IFNγ and tended to proliferate more than male T cells provides direct support for this notion. An unexpected feature of our study was the finding that male T cells produced higher levels of IL-17A, which goes against the current dogma that males have a Th2-biased immune system.
It is not yet clear why male SJL mice, in having more Th17 cells (considered by some to be pathogenic), were protected from EAE (8, 9). It is possible that the male PLP p139-151-reactive Th17 cells that we detected were highly encephalitogenic but were unable to cause severe EAE because of a limited potential for expansion or because additional regulatory mechanisms are in place in male mice that counter the pathogenicity of these cells. Although we did not observe higher IL-10 production or higher T reg frequencies by males during EAE, it has been previously reported that male SJL T regs have a greater capacity than female T regs to suppress T effector cells (28). An alternative possibility is that the Th17 cells that we detected in male SJL mice were not highly pathogenic. Indeed, not all Th17 cells are created equally (reviewed in ref. 29). For instance, myelin-reactive Th17 cell lines are highly encephalitogenic if generated with IL-23 but are not when generated in the presence of TGF-β. Certainly, further work is necessary to define what feature(s) of male T cells (i.e., lower IFNγ production, higher threshold of activation, reduced expansion, increased T reg capacity, or lesser propensity for dual IFNγ/IL-17A production) is responsible for protection from EAE.
Our findings of a Th17 bias in males is not surprising when considered in the context of what is known about the cross-regulation between Th1 and Th17 pathways (30). Indeed, IL-2 produced by Th1 cells activates STAT5, which competes with STAT3 for binding at the IL-17A promoter (31). Moreover, the Th1 lineage-determining factor T-bet inhibits Th17 differentiation by preventing the transactivation of the RORγT gene (32). Thus, the higher T-cell production of IL-17A that we observed in males may, in part, be a consequence of lowered IFNγ production by these cells. Notwithstanding, our functional studies indicate that the lower ratio of PPARγ1 to PPARα in male T cells during T-cell activation is an additional T-cell-intrinsic factor that drives this sex bias in cytokine production. We observed that TCR and CD28 signals coupled with androgen signals had a direct effect in up-regulating PPARα mRNAs in T cells of men, whereas these stimuli together extinguished PPARγ1 mRNA expression in female T cells.
In addition to defining the underlying reasons for the sexual dimorphism in PPAR expression, our experiments provided a number of molecular insights into how PPARα may exert its control over IFNγ production in T cells. We found that knockdown of PPARα mRNAs resulted in an increased abundance of an activating histone mark, acetylated histone H4, at CNS-22 and CNS-6 enhancer regions, suggesting a role for PPARα in modulating accessibility at the IFNG locus. Normally, acetylated histone H4 marks chromatin when T-bet or IL-12-STAT-4 activity is high (22, 23). Although we were unable to address T-bet or STAT4 activity in these T cells because of limited sample availability, it has been reported that T-bet is expressed at higher levels in PPARα−/− vs. WT T cells (33). Thus, it is possible that these epigenetic changes that we observed are attributable to increased T-bet activity. In addition to this effect, we observed an enhanced recruitment of RelA to the CNS-22 enhancer region, thus coinciding with our previous finding of a higher abundance of this protein in the nucleus of male PPARα−/− vs. WT T cells (20). Although our study did not address the mechanism of PPARγ repression of IL-17A production by human CD4+ T cells, previous elegant work in mice has indicated that ligand activation of PPARγ prevents the clearance of silencing mediator of retinoid acid and thyroid hormone receptor (SRMT)-containing complexes from the RORγT promoter, resulting in reduced expression of this master regulator of Th17 differentiation (34). Taken together, these results suggest that PPARα and PPARγ, respectively, control key regulatory elements in Th1 and Th17 pathways.
Although we did observe a sexual dimorphism in IFNγ production at the site of inflammation in the early stage of EAE, our study did not address whether women and men develop Th1- or Th17-biased forms of MS. In this regard, there is some precedent in the literature that female MS patients may be more Th1-prone. For instance, Pelfrey et al. reported that female patients with relapsing-remitting MS have a higher frequency of myelin-reactive IFNγ-producing cells in peripheral blood compared with male MS patients (13). Moreover, the frequency of IFNγ-producing CD3+ cells in peripheral blood of MS patients is reported to correlate with the severity of disease in women but not men (16). Finally, analysis of MRI data from a large randomized trial of secondary progressive MS has indicated that women may be more responsive than men to IFNβ therapy (35), a treatment that may preferentially mitigate Th1 inflammation (36). If, indeed, these sex differences in Th cytokine production persist during autoimmune inflammation, it raises the prospect that women and men with MS should be treated differently.
Materials and Methods
To probe for sex differences in Th proliferation and cytokine production, CD4+ T cells were isolated from spleens of female or male SJL/J mice (pooled; n = 5/group) or from peripheral blood of healthy men and women (aged 20–30 y; n = 25/group). Proliferation was measured using [3H]thymidine incorporation assays, and cytokines were measured by ELISA. The hormone status of SJL mice was modulated in some experiments by castrating male mice or implanting female mice with DHT-containing pellets. The role of PPARα and PPARγ in T-cell functioning was probed by transfecting cells with siRNAs specific for these nuclear receptors and by treating cells in vitro with synthetic PPAR-ligand activators. Expressions of PPAR and other molecules were measured using real-time PCR (primer sequences listed in Table S3). Detailed methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Edward Engleman and Paula Colmenero for help with preliminary human experiments; and Drs. Robert Axtell, Christopher Lock, and May Han and the Canadian and Stanford Blood Services staff for help with blood draws and assays. This work was supported by grants from the Canadian Institute of Health Research (CIHR) and the Multiple Sclerosis Society of Canada (MSSC) and Donald Paty Career Development Awards (MSSC) (to S.E.D. and A.P.). M.A.Z. and H.K. are supported by studentships from MSSC and CIHR.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118458109/-/DCSupplemental.
References
- 1.Orton SM, et al. Canadian Collaborative Study Group Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 2.Debouverie M, Pittion-Vouyovitch S, Louis S, Roederer T, Guillemin F. Increasing incidence of multiple sclerosis among women in Lorraine, Eastern France. Mult Scler. 2007;13:962–967. doi: 10.1177/1352458507077938. [DOI] [PubMed] [Google Scholar]
- 3.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96:457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 4.Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6:887–902. doi: 10.1016/S1474-4422(07)70242-9. [DOI] [PubMed] [Google Scholar]
- 5.International Multiple Sclerosis Genetics Consortium. Wellcome Trust Case Control Consortium 2 Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in Multiple sclerosis. Nature. 2011. pp. 214–219. [DOI] [PMC free article] [PubMed]
- 6.Kebir H, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 7.Stadelmann C, Wegner C, Brück W. Inflammation, demyelination, and degeneration - recent insights from MS pathology. Biochim Biophys Acta. 2011;1812:275–282. doi: 10.1016/j.bbadis.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th1 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. J Immunol. 1995;155:4052–4059. [PubMed] [Google Scholar]
- 9.Bebo BF, Jr, Vandenbark AA, Offner H. Male SJL mice do not relapse after induction of EAE with PLP 139-151. J Neurosci Res. 1996;45:680–689. doi: 10.1002/(SICI)1097-4547(19960915)45:6<680::AID-JNR4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Voskuhl RR, Pitchekian-Halabi H, MacKenzie-Graham A, McFarland HF, Raine CS. Gender differences in autoimmune demyelination in the mouse: Implications for multiple sclerosis. Ann Neurol. 1996;39:724–733. doi: 10.1002/ana.410390608. [DOI] [PubMed] [Google Scholar]
- 11.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 12.Aulock SV, et al. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J Interferon Cytokine Res. 2006;26:887–892. doi: 10.1089/jir.2006.26.887. [DOI] [PubMed] [Google Scholar]
- 13.Pelfrey CM, Cotleur AC, Lee J-C, Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J Neuroimmunol. 2002;130:211–223. doi: 10.1016/s0165-5728(02)00224-2. [DOI] [PubMed] [Google Scholar]
- 14.Bouman AM, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol. 2004;52:19–26. doi: 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- 15.Girón-González JA, et al. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen LT, et al. Sex differences in in vitro pro-inflammatory cytokine production from peripheral blood of multiple sclerosis patients. J Neurol Sci. 2003;209:93–99. doi: 10.1016/s0022-510x(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 17.Bebo BF, Jr, et al. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 18.Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- 19.Matejuk A, et al. Estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with EAE. J Neurosci Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 20.Dunn SE, et al. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 22.Aune TM, Collins PL, Chang S. Epigenetics and T helper 1 differentiation. Immunology. 2009;126:299–305. doi: 10.1111/j.1365-2567.2008.03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasubramani A, Mukasa R, Hatton RD, Weaver CT. Regulation of the Ifng locus in the context of T-lineage specification and plasticity. Immunol Rev. 2010;238:216–232. doi: 10.1111/j.1600-065X.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klotz L, Knolle P. Nuclear receptors: TH17 cell control from within. FEBS Lett. 2011;585:3764–3769. doi: 10.1016/j.febslet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki K, Fukino K, Negishi E, Ueno K. Sex differences in PPARgamma expressions in rat adipose tissues. Biol Pharm Bull. 2007;30:818–820. doi: 10.1248/bpb.30.818. [DOI] [PubMed] [Google Scholar]
- 26.Tchoukalova YD, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18:1875–1880. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 28.Reddy J, et al. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2005;175:5591–5595. doi: 10.4049/jimmunol.175.9.5591. [DOI] [PubMed] [Google Scholar]
- 29.Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: Are T cell cytokines relevant in multiple sclerosis? Biochim Biophys Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Paul WE. CD4 T cells: Fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Lazarevic V, et al. Transcription factor T-bet suppresses Th17 differentiation by preventing Runx-1 mediated activation of the RORγt gene. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DC, Ding X, Zhang TY, Daynes RA. Peroxisome proliferator-activated receptor α negatively regulates T-bet transcription through suppression of p38 mitogen-activated protein kinase activation. J Immunol. 2003;171:196–203. doi: 10.4049/jimmunol.171.1.196. [DOI] [PubMed] [Google Scholar]
- 34.Klotz L, et al. The nuclear receptor PPAR γ selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dettke M, Scheidt P, Prange H, Kirchner H. Correlation between interferon production and clinical disease activity in patients with multiple sclerosis. J Clin Immunol. 1997;17:293–300. doi: 10.1023/a:1027374615106. [DOI] [PubMed] [Google Scholar]
- 36.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.