Abstract
This review paper reports the consensus of a technical workshop hosted by the European network, NanoImpactNet (NIN). The workshop aimed to review the collective experience of working at the bench with manufactured nanomaterials (MNMs), and to recommend modifications to existing experimental methods and OECD protocols. Current procedures for cleaning glassware are appropriate for most MNMs, although interference with electrodes may occur. Maintaining exposure is more difficult with MNMs compared to conventional chemicals. A metal salt control is recommended for experiments with metallic MNMs that may release free metal ions. Dispersing agents should be avoided, but if they must be used, then natural or synthetic dispersing agents are possible, and dispersion controls essential. Time constraints and technology gaps indicate that full characterisation of test media during ecotoxicity tests is currently not practical. Details of electron microscopy, dark-field microscopy, a range of spectroscopic methods (EDX, XRD, XANES, EXAFS), light scattering techniques (DLS, SLS) and chromatography are discussed. The development of user-friendly software to predict particle behaviour in test media according to DLVO theory is in progress, and simple optical methods are available to estimate the settling behaviour of suspensions during experiments. However, for soil matrices such simple approaches may not be applicable. Alternatively, a Critical Body Residue approach may be taken in which body concentrations in organisms are related to effects, and toxicity thresholds derived. For microbial assays, the cell wall is a formidable barrier to MNMs and end points that rely on the test substance penetrating the cell may be insensitive. Instead assays based on the cell envelope should be developed for MNMs. In algal growth tests, the abiotic factors that promote particle aggregation in the media (e.g. ionic strength) are also important in providing nutrients, and manipulation of the media to control the dispersion may also inhibit growth. Controls to quantify shading effects, and precise details of lighting regimes, shaking or mixing should be reported in algal tests. Photosynthesis may be more sensitive than traditional growth end points for algae and plants. Tests with invertebrates should consider non-chemical toxicity from particle adherence to the organisms. The use of semi-static exposure methods with fish can reduce the logistical issues of waste water disposal and facilitate aspects of animal husbandry relevant to MMNs. There are concerns that the existing bioaccumulation tests are conceptually flawed for MNMs and that new test(s) are required. In vitro testing strategies, as exemplified by genotoxicity assays, can be modified for MNMs, but the risk of false negatives in some assays is highlighted. In conclusion, most protocols will require some modifications and recommendations are made to aid the researcher at the bench.
Keywords: Nanoparticle characterisation, OECD test method, Gram positive Bacteria, Earthworm, Aquatic tests, Bioaccumulation factor tests
Introduction
The potential environmental hazards from manufactured nanomaterials (MNMs) has been conceptualised (Moore 2006; Owen and Handy 2007), and the experimental evidence of ecotoxicity reviewed (e.g. Handy et al. 2008a; Klaine et al. 2008; Perez et al. 2009; Kahru and Savolainen 2010; Handy et al. 2011). Several key aspects have emerged, including the importance of colloid chemistry in the bioavailability of nanoparticles (NPs), and the demonstration of ecotoxicity to fish and invertebrates at around mg l−1 levels of MNMs in the laboratory. The ecotoxicity of MNMs is likely to be altered by environmental factors that alter the colloid behaviour of particles including: pH, ionic strength, divalent ions such as Ca2+ and the presence of organic matter (e.g. Handy et al. 2008a; Klaine et al. 2008). The studies to date have collected information of direct relevance to risk assessment, such as lethal concentration estimates, as well as fundamental research on possible mechanisms of toxicity, sub-lethal effects and uptake processes. The importance of different methods for preparing stock dispersions of MNMs in the toxicity of MNMs to aquatic species was recognised early on in the research; with shaking, stirring and sonication producing slightly different results (see discussion in Handy et al. 2008a). Such observation, and the difficulty of handling MNMs in aqueous media, has focused attention on the details of test methods and dosing procedures for MNMs (e.g. Crane et al. 2008; Organisation for Economic Cooperation and Development, OECD 2010a).
However, there are many different methodologies being developed, and for a variety of purposes. Those doing fundamental research tend to use more variable, customised methods that are aligned with their specific research objectives, while the regulatory community is more focused on the issue of standardisation. The scientific community is far from reaching international agreement on the precise details and standardisation of ecotoxicity test methods for MNMs. Recently, the OECD made some preliminary recommendations on how to dose toxicity test systems with MNMs (OECD 2010a), and has also started a sponsorship programme with the aim of validating existing regulatory tests for use with a representative set of MNMs over the next few years (OECD 2010b).
The standardisation of ecotoxicity tests is only one aspect of working with MNMs, and in general, the practical details of conducting ecotoxicity experiments with MNMs has been given less attention in the peer reviewed literature. Several scientific networks are currently discussing methodology including the OECD, the International Organisation for Standardization (ISO) and the Society of Environmental Toxicology and Chemistry Nano Advisory Group which has very recently reported (see Handy et al. 2012 on ecotoxicity, and Von der Kammer et al. 2012 on analytical methods). There is also the European network, NIN. This large European network of scientists working on the health and safety of MNMs (http://www.nanoimpactnet.eu/) has already reported on the classification of these materials (Stone et al. 2010), and hosted a technical workshop in Dublin during September 2010 on ecotoxicity test methods. The international workshop gathered together a mixture of researchers from academia, industry, consultancy and government with direct personal experience at the bench in doing experiments with MNMs and/or experience of regulatory procedures. Notably, this group worked independently of other international working parties or advisory groups, with a particular focus on European issues. The group considered several new aspects of chemistry, microbiology and soil organisms not previously debated, as well as the testing strategies for Europe. This paper reports the findings of the workshop and identifies what aspects of the current ecotoxicity testing strategy for new substances may need to be altered for MNMs, as well as practical details of methodology where protocols should be modified, and offers solutions to some of the technical problems at the bench.
Current regulatory toxicity tests and strategies for manufactured manomaterials
Historically, many of the current regulatory ecotoxicity tests (Tables 1, 2), and many of the protocols used in fundamental research, were designed with conventional chemicals in mind. There is a consensus view emerging that the existing methods and framework for hazard assessment (e.g. standard test organisms, mortality, growth and reproduction as routine end points) are generally fit for purpose, but the details within each test, or group of tests may require modification/validation to work well with MNMs (Crane et al. 2008). The regulatory testing strategy has been historically designed so that it is fit for purpose for many different types of chemicals (i.e. one does not have to invent a new protocol every time a new substance emerges). It is critically important that this concept can also work for MNMs. Stone et al. (2010) argues that a substance-based classification system for MNMs is the most pragmatic way forward (e.g. metal NPs, carbon-based NMs, etc.) and that where more than one substance is used in a MNM (composites, functionalised surfaces on materials) that the surface chemistry and physico-chemical properties the surface and shape imparts on material behaviour (lipid solubility, charge, chemical reactivity, etc.) is considered. In essence, this scientific challenge has been met for many different chemical formulations and isomers of traditional chemicals for years. The alternative suggestion of devising MNM-specific protocols for every new MNM (with potentially infinite combinations of surface chemistry and/or shapes) is not a practical proposition for hazard assessment. Instead, NIN advocates a more rational scientific approach, where the properties of MNMs are critically considered with respect to test method execution, and where common properties emerge for different MNMs, that they also share a common solution in term of test method modification. This approach enables the practical aspects of groups of tests with similar methodologies and sample matrices to be considered together for more than one MNM; but at the same time can identify “exceptions to the rule” where a different sub-set of modifications to the test or a different pathway through the overall testing strategy is needed. This approach would also enable the regulator to target resources at modifying tests (e.g. bioaccumulation tests) that need the most modifications. NIN is a European based network, so here we illustrate these issues with reference to the OECD tests.
Table 1.
OECD methods for testing the effects of chemicals on biotic systems
| Compartment | Media | Guideline no. | Guideline title | Principal end point/s | Duration (days) | Recommended species |
|---|---|---|---|---|---|---|
| Aquatic | Water | 209 | Activated sludge, respiration inhibition test | Respiration rate as oxygen consumption | 0.125 | Activated sludge microbial fauna |
| 201 | Alga, growth inhibition test | Growth inhibition (based on biomass measurements) | 3 | Pseudokirchneriella subcapitata, Desmodesmus subspicatus, Navicula pelliculosa, Anabaena flos-aquae, Synechococcus leopoliensis | ||
| 221 | Lemna sp. growth inhibition test | Growth rate based on frond number/biomass | 7 | Lemna minor, Lemna gibba | ||
| 202 | Daphnia sp. acute immobilisation test | Immobilisation | 2 | Daphnia magna, Daphnia pulex | ||
| 211 | Daphnia magna reproduction test | Reproduction | 21 | D. magna | ||
| 203 | Fish, acute toxicity test | Survival | 4 | Brachydanio rerio, Pimephales promelas, Cyprinus carpio, Oryzias latipes, Oncorhynchus mykiss, Poecilia reticulata, Lepomis.macrochirus | ||
| 204 | Fish, prolonged toxicity test: 14-day study | Survival | 14 | B. rerio, P. promelas, C. carpio, O. latipes, O. mykiss, P. reticulata, L.macrochirus | ||
| 210 | Fish, early-life stage toxicity test | Hatching, survival, growth (length/weight) | 32–95 | O. mykiss, P.promelas, B.rerio O. latipes, Cyprinodon variegatus (sw) | ||
| 212 | Fish, short-term toxicity test on embryo and sac-fry stages | Hatching, survival, growth (length/weight) | 8–55 | O. mykiss, B.rerio, C. carpio, P. promelas | ||
| 215 | Fish, juvenile growth test | Growth rate | 28 | O. mykiss, B. rerio, O. latipes | ||
| 229 | Fish short term reproduction assay | Egg production, vitellogenin and 2o sexual characteristics | 21 | P. promelas | ||
| 230 | 21-Day fish assay: a short-term screen for oestrogenic and androgenic activity and aromatase inhibition | Vitellogenin and 2o sexual characteristics | 21 | P. promelas, O. latipes, B. rerio | ||
| Sediment | 218/219 | Sediment–water chironomid toxicity using spiked sediment/water | Larval survival and weight, emergence rate | 28–65 | Chironomus riparius, Chironomus dilutus, Chironomus yoshimatsui | |
| 223 | Sediment–water chironomid life-cycle toxicity test using spiked water or spiked sediment | 1st and 2nd generation larval emergence, sex ratio, egg rope production and fertility | 44–100 | C. riparius, C. dilutus, C. yoshimatsui | ||
| 225 | Sediment–water Lumbriculus toxicity test using spiked sediment | Reproduction and biomass | 28 | Lumbriculus variegatus | ||
| Terrestrial | Soil | 216 | Soil microorganisms: nitrogen transformation test | Inhibition of nitrogen transformation | 28–100 | Endemic natural soil microbial fauna |
| 217 | Soil microorganisms: carbon transformation test | Inhibition of respiration | 28–100 | Endemic natural soil microbial fauna | ||
| 208 | Terrestrial plant test: seedling emergence and growth test | Seedling emergence, biomass and shoot height | 14–21 | Various crop and non-crop species (cotyledon and dicotyledon) | ||
| 227 | Terrestrial plant test: vegetative vigour test | Shoot weight, shoot height and mortality | 21–28 | Various crop and non-crop species (cotyledon and dicotyledon) | ||
| 207 | Earthworm, acute toxicity tests | Survival | 14 | Eisenia fetida, Eisenia andrei | ||
| 220 | Enchytraeid reproduction test | Juvenile production and parent survival | 42 | Enchytraeus albidus | ||
| 222 | Earthworm reproduction test (Eisenia fetida/Eisenia andrei) | Juvenile production and parent survival/growth | 56 | E. fetida, E.andrei | ||
| 232 | Collembolan reproduction test in soil | Adult mortality and reproductive output | 14–21 | Folsomia candida, Folsomia fimetaria | ||
| 226 | Predatory mite (Hypoaspis (Geolaelaps) aculeifer) reproduction test in soil | Female survival, reproductive output | 14 | Hypoaspis (Geolaelaps) aculeifer | ||
| Food | 223 | Avian acute oral toxicity test | Survival | 14 | Colinus virginianus, Coturnix japonica, Anas platyrhynchos, Columba livia, Poephila guttata, Melopsittacus undulatus | |
| 223 | Avian reproduction test | Survival, egg production and viability | 140 | C. virginianus, C. japonica, A. platyrhynchos | ||
| Faeces | 228 | Determination of developmental toxicity of a test chemical to dipteran dung flies | Emergence | 13–18 | Scathophaga stercoraria, Musca autumnalis | |
| Sewage sludge | 224 | Determination of the inhibition of the activity of anaerobic bacteria | Inhibition of gas production | 3 | Sewage sludge microbial fauna |
Table 2.
OECD methods for testing the (bio)degradation and bioaccumulation of chemicals
| Media | Guideline no. | Guideline title | Principal end point/s | Duration (days) | Recommended species |
|---|---|---|---|---|---|
| Sediment | 315 | Bioaccumulation in sediment-dwelling benthic oligochaetes | Uptake rate constant, the elimination rate constant, kinetic bioaccumulation factor (BAFK) | 38 | Lumbriculus variegatus, Tubifex tubifex, Branchiura sowerbyi |
| Soil | 317 | Bioaccumulation in terrestrial oligochaetes | Uptake rate constant, the elimination rate constant, kinetic bioaccumulation factor (BAFK) | 35–42 | Eisenia fetida, Eisenia andrei, Enchytraeus albidus, Enchytraeus crypticus, Enchytraeus luxuriosus |
| Water | 305 | Bioconcentration: flow-through fish test | Uptake rate constant, the elimination rate constant, kinetic bioaccumulation factor (BAFK) | 28–60 | Brachydanio rerio, Pimephales promelas, Cyprinus carpio, Oryzias latipes, Oncorhynchus mykiss, Poecilia reticulata, Lepomis macrochirus, Gesterosteus aculeatus |
| Water | 301 (A–F)/310 | Ready biodegradability | Dissolved organic carbon (DOC), CO2 (inorganic carbon) production, oxygen uptake | 28 | Activated sludge microbial fauna |
| Water | 306 | Biodegradability in seawater | Shake flask: DOC closed bottle: oxygen uptake | 60 | Endemic microbial fauna in natural seawater |
| Water | 308 | Aerobic and anaerobic transformation in aquatic sediment systems | C14 activity or concentration of test substance or transformation products of test substance | <100 | Endemic microbial fauna in natural aquatic sediment |
| Water | 309 | Aerobic mineralisation in surface water—simulation biodegradation test | C14 activity or concentration of test substance or transformation products of test substance | <60 | Endemic microbial fauna in natural aquatic sediment |
| Soil | 307 | Aerobic and anaerobic transformation in soil | C14 activity or concentration of test substance or transformation products of test substance | <120 | Endemic microbial fauna in natural soil |
| Activated sludge | 303 | Simulation test—aerobic sewage treatment—A: activated sludge units; B: biofilms | Elimination of the test substance | 42 | Endemic microbial fauna in activated sewage sludge |
| Sewage sludge | 311 | Anaerobic biodegradability of organic compounds in digested sludge: by measurement of gas production | Biodegradation of test substance (as determined by production of inorganic carbon and methane) | 60 | Endemic microbial fauna in digested sewage sludge |
The OECD testing strategy
The OECD test guidelines for testing chemicals have been used widely for regulatory purposes since the establishment of the MAD principle (Mutual Acceptance of Data) in 1981. This ensures that, if a chemical or substance is tested under the Good Laboratory Practice (GLP) conditions accordingly to an OECD test guideline, the data should be accepted in all OECD countries. The MAD principle depends on member states having confidence in the test protocols, and while surveys suggest that the available regulatory test methods are generally applicable for testing MNMs in terms of their over arching purpose albeit with modifications for MNMs (Crane et al. 2008), the OECD is also proactive in examining the robustness of its protocols for MNMs. The existing OECD test guidelines have been reviewed in the light of their applicability for testing MNMs under the OECD Working Party of Manufactured Nanomaterials (WPMN) (OECD 2009). For these review tasks four subgroups were formed to evaluate the guidelines for: (i) physico-chemical characterisation, (ii) effects on environmental biota, (iii) environmental fate and (iv) health effects with dosimetry. The tasks of the subgroups included identification of the potential problems with each suite of test methods, and also to offer preliminary guidance on testing MNMs in each test, along with any proposed modifications to the existing test guidelines, or to identify needs for new methods. The WPNM quickly identified that the size of the data set on MNMs was not large enough (i.e. not enough experiments done to date) to reach conclusions that could be sufficiently robust to form the basis of any mandatory change in protocols, but nonetheless guidance has now been offered in some areas on what could be done or should be done. For example, there are some common approaches to dispersing MNMs so that known doses are added to test systems (OECD 2010a). Thus currently the guidance on dosimetry for biological effects studies and bioaccumulation tests are similar. This also highlights, that a rationale scientific approach can solve methodological problems for seemingly different MNMs.
The overall testing strategy and what tests to prioritise in the testing regime are also being considered, particularly in the context of the fate and behaviour of MNMs. There are concerns that MNMs will aggregate or agglomerate in natural systems (e.g. in seawater, Klaine et al. 2008), leading to deposition of MNMs on sediment surfaces. In 2007, the OECD adopted a new method for testing the bioaccumulation of chemicals into sediment-dwelling worms using Lumbriculus variegatus (OECD 2008). Clearly, a benthic test of this kind may be more relevant to the behaviour of MNMs, and perhaps should be earlier on in the testing strategy, although it is a longer test. For example by including a benthic test within the base-set of acute toxicity tests (algal growth test, Daphnia immobilisation test, 96 h fish test).
Concerns have been raised that some OECD tests may be inappropriate or even flawed, or at best require very substantial modifications to work with MNMs. This includes, for example, tests designed to measure bioconcentration factors (BCF), such as the OECD BCF test with fish (OECD 305, OECD 1996). Apart from concerns regarding the ability of the experimenter to maintain consistent, if not well characterised exposures over tests that last weeks or months, it is likely that in most cases the relatively large size (1–100 nm) of MNMs compared to molecules (angstroms, <1 nm) may limit their uptake by fish (see Handy et al. 2008b for detailed discussion of uptake). The standard BCF test where the test substance is added to the water until steady-state is achieved with the organism may therefore not be suitable. However, the OECD is looking at alternative ways to achieve dosing, and a dietary bioaccumulation factor (BAF) test with fish is one possibility being considered for organic chemicals (Fisk et al. 1998; Stapleton et al. 2004). This spiked food method is suitable for the testing of poorly soluble large molecules, and might therefore have some utility with some MNMs with similar properties.
The OECD is currently testing a suite of 14 “representative” MNMs (the OECD sponsorship programme; OECD 2010b). The aim of this programme is to identify hazards from a well defined/characterised set of MNMs with different shapes/surface chemistries, but also to evaluate the applicability of the existing OECD test guidelines for testing MNMs. The sponsorship programme is expected to take a few years, but at the end of the process, the OECD should be able to offer better guidance on dosimetry and test designs, as well as having a better understanding of how different the testing of MNMs is compared to their nearest bulk material counterpart, or equivalent conventional chemical as appropriate. Of course, ultimately each test method and any allowable deviations in the test conditions must be validated before the MAD principle can be applied to MNMs. The OECD is therefore only at the start of this process for MNMs.
Generic issues for experiments
Cleaning and preparing apparatus
Research papers on nano ecotoxicology often do not report laboratory procedures for cleaning the ecotoxicity test system, so a systematic review of this aspect in the literature is currently not possible. However, the consensus view from the bench is that normal cleaning procedures, such as acid washing glassware with nitric acid or aqua regia, appear to work for most MNMs. Similar to the situation with traditional chemicals, pilot experiments to determine the adsorption and desorption of MNMs from the test vessels should be performed, especially when there is a need to maintain low concentration during experiments. Many pristine MNMs are hydrophobic and will form a film on the surface of test vessels (e.g. SWCNTs on glass fish tanks, Smith et al. 2007), but this problem is not nano-specific and is known for other hydrophobic substances. In the case of SWCNT, cleaning glass with house hold detergents, rinsing in water, followed by normal acid washing procedures is sufficient. Repeated abrasive cleaning of glass or plastic will scratch the surfaces, and provide points of nucleation for the aggregation of the test MNM; but this can be resolved by using disposable plastic or glass ware.
Nanomaterial interference with electrodes
There are concerns that MNMs may interfere with chemical and biological assays, and this has been discussed for colorimetry (e.g. Monteiro-Riviere et al. 2009). However, the interference of MNMs with electrodes has not been documented and ecotoxicologists may need to measure water pH, conductivity/salinity, dissolved oxygen and sometimes free metal ion concentrations in the test media using potentiometry and related methods. The problems with electrodes can be rationalised into several areas for MNMs: (i) the coating or adsorption of the MNM onto the working parts of the probe, (ii) interference with the electrochemical properties of the solutions or gels inside the probes, (iii) the creation of spurious voltages by MNMs.
Adsorption of MNM to the glass or polymer surfaces of probes has been observed with the less soluble metal oxides including TiO2, and with hydrophobic substances such as carbon black, unfunctionalised C60 and SWCNT (Handy, unpublished observations). For combination glass electrodes like pH probes the MNM can coat the sensitive glass bulb (preventing the analyte reaching the detection surface), or block the sintered plug, with both problems reducing the speed of response and sensitivity of the electrode. The adsorption of hydrophobic chemicals to electrode surfaces is not a new problem, but the MNM-specific issue is that the glass bulb is much harder to clean, and any exposed sintered surface, tape or resin is almost impossible to clean. Washing the electrode using the manufacturer’s recommended cleaning procedure will often be insufficient and only partly restore function, and etching the glass surface of the probe with strong nitric acid for a few seconds may, as a last resort, restore the response. The electrical responsiveness of combination pH electrodes should follow that expected from the Nernst equation (typically 59.16 mV/pH unit at 25°C for a combination pH probe), and the responsiveness of other combination electrodes can be checked using the voltage function on the metre in a similar way.
A second concern is for MNM interference with the filling solutions inside electrodes. Commercially available glass combination ion-selective electrodes (ISEs) are usually filled with high ionic strength media (e.g. 3 M KCl) and often have an internal Ag/AgCl2 reference. However, the glass bulbs of most ion-selective electrodes (including pH probes) are ion-exchange surfaces that create voltage (Durst 1967), they are not porous to the ion being detected per se, and would not be permeable to much large MNMs. Therefore worries such as the precipitation of silver chloride from silver NPs inside the electrode are unfounded for glass combination probes. However, there are now many varieties of “solid state” ISE on the market, and these probes often have a porous membrane covering a graphite or plastic tube filled with an ion-exchange gel or ion-sensitive resin. The external surface of these probes are easier to clean (there is no thin glass bulk to break), but the membrane covering the tip of the probe is a simple mechanical barrier with a mesh size of hundreds of microns, and will be freely permeable to ions and MNMs. The matrix inside the probe is often a fixed polyanion (negatively charged polymer) to detect the cation of interest. The permeability of MNMs through the matrix of solid state electrodes has not been measured experimentally, but MNMs may get tangled with the polymers (steric hindrance). For cationic solid state ISEs specifically, any positively charged MNM trapped in the matrix will theoretically lead to a spurious potential inside the probe (analogous to a junction potential, see below), or repel the dissolved cation of interest (loss of sensitivity). Changing the pH of the test media to the point of zero charge will not resolve this problem, as it is the environment inside the probe that matters. The experimenter has no capacity to replace the resin in the probe (designed to be disposable), and so replacing the electrode is often the only way forward. There are similar concerns of MNM penetration inside the probe for gas-sensing electrodes (e.g. oxygen electrodes, carbon dioxide gas measurements), where the pore sizes in the membrane covering the tip of the probe is usually in the μm range, depending on the gas to be detected (e.g. Horn et al. 2010). However, even if MNMs penetrated inside such a probe, the high ionic strength would likely precipitate the MNM, directly, or in the case of Ag released from the surface of Ag NPs precipitate as AgCl2. Any precipitation of the chloride inside the probe will change the response time of the electrode. The experience at Plymouth with Clarke-type oxygen electrodes is that the probes will work with TiO2 NPs, Ag NPs, Cu NPs, Silica NPs (citrate or alumina-coated), C60, carbon black and SWCNT. However, the variability in reading samples around 100% saturation of oxygen is greater than in clean solutions (i.e. be more careful and take triplicate readings). Replacing the membrane and the filling solution fully restores the function of the oxygen electrode if the calibration criteria are not met.
The problem of spurious voltages is especially important where separate half-cells are used (i.e. separate positive and negative electrodes). This is the case for some commercially available ion-selective electrodes for metals, and also for the electrodes used in physiological experiments to measure membrane potential on single cells, transepithelial potentials across tissue, or compound action potentials in nerves. Some MNM will deposit on the tip of the probes in physiological salines (observed at Plymouth for TiO2 bulk and NPs, Ag NPs, Cu NPs, C60, carbon black and SWCNT, every material examined so far). This creates junction potentials of the order of a few mV, and given that transepithelial potentials on live tissue (e.g. gut, Handy et al. 2000) may be of similar magnitude; it becomes absolutely essential to correct for junction potentials. This problem is well known for traditional dissolved metals, and an experienced physiologist would routinely check for junction potentials in any experiment. This can be done, by checking the short circuit on the half-cells with a salt bridge in the presence/absence of the MNM each time the electrode is calibrated. In addition, if there are concerns about spurious junction potentials from MNMs, then a mixed calibration procedure can be used for ISEs (e.g. see Handy 1989) where the calibrating solutions for the electrode is made with a range of dilutions of the interfering substance. The mixed calibration approach was originally devised for solutes, but will work for MNM providing (most important) that the stirring of the calibration solutions are kept constant as this also alters the size of the junction potentials from particles or dissolved ions.
Experimental design, reference materials and particle size controls
Aspects of experimental design including replication, the types of controls to use and the availability of reference materials. Experimental design and example decision trees on what characterisation could be done for different types of MNMs have been discussed at length elsewhere for ecotoxicity studies (Crane et al. 2008; Hassellöv et al. 2008; Stone et al. 2010). Some key points include characterising the starting material or stock dispersions using more than one technique so that a weight of evidence can confirm the primary particle size, the distribution of sizes in the dispersion and the presence of impurities that might also be toxic to the test organisms. The use of particle size controls should be included in experiments where the aim is to infer a nano scale effect, and metal salt controls where the objective is to understand how nano metal toxicity compares to the traditional dissolved metal paradigms used in metals risk assessments (Handy et al. 2011). Importantly, there is no need for bespoke or totally unique experimental designs for every new type of MNM in the future, and instead the application of the principles outlined here should enable good experimental design with new MNMs as they emerge.
Researchers have attempted to compare ordinary bulk powders with nano scale material of the same chemical to infer particle size-effects (e.g. TiO2 NPs vs. ordinary TiO2 powder, or C60 vs. graphite or carbon black particles). However, in order to truly test a particle-size effect, the bulk material (a conventional material with a size above 100 nm) must be exactly the same as the MNM in every respect, except size. This is often impossible to achieve. The experimenter has the challenge of finding a substance of different sizes with exactly the same crystal structure, surface topography, surface charge, porosity, chemical composition and levels of impurities (see discussion in Ramsden et al. 2009 for TiO2). The use of characterised materials from the OECD sponsorship programme, available via the Joint Research Centre (JRC, http://irmm.jrc.ec.europa.eu/) in Europe, or well characterised materials from other agencies such as the National Institute and Science and Technology (NIST) in the USA will not resolve these problems, as some of the changes in the properties of the material are inherent in making the materials at different sizes. However, with characterised materials becoming available, at least there is an opportunity to select test materials that have minimal differences with respect to particle-size controls. Even for researchers attempting to custom-synthesise particles of different sizes using the same starting chemicals there remains the inherent problem that the particle dispersion characteristics are also a function of size (see Handy et al. 2008a on DLVO theory) and it is inevitable that the experimenter will not be able to maintain exactly the relative size distributions with each material. At best, one might obtain a particle size with reasonably defined limits (e.g. nominal size ±10 nm range) and design the experiment so that the selected mean particle sizes do not overlap.
The question arises as to whether more replication is needed in experiments with MNMs compared to conventional chemicals. This does not seem to be the case, with authors reporting standard deviations or standard error on measurements from MNM treatments with a similar magnitude to those on bulk powder or dissolved metal salt treatments (e.g. TiO2, Galloway et al. 2010; silver, Gaiser et al. 2011). Statistical methods used to estimate the level of replication required (e.g. power analysis) are valid for MNMs, with the caveat for all chemicals that power analysis is only appropriate for normally distributed (parametric) data.
Finally, the use of solvent controls, or more accurately termed, “dispersion controls” in the case of MNMs, should be considered. The environmental relevance, and advantages of different dispersion methods (dispersing agents, sonication, stirring) are discussed at length elsewhere (Crane et al. 2008; Handy et al. 2012), but if dispersing agents are used, then a dispersion control must be included in the experimental design. Notably some dispersing agents that are good at dispersing MNMs from the view point of chemistry (e.g. tetrahydrofuran, THF), are not biocompatible and cause toxicity (e.g. Henry et al. 2007). Inevitably, compromises may be achieved where a reasonable dispersion can be achieved with limited side effects on the test organisms (see Smith et al. 2007 on sodium dodecyl sulphate (SDS) with SWCNT). Dispersants fall into two broad groups from the biological perspective: (i) natural materials such as fulvic acids, humic acids, peptides/proteins and natural gums like gum arabic, or (ii) synthetic substances that are purposefully designed as dispersing agents or surfactants, such as pluronic solutions and SDS. Natural dispersants, such as the humic and fulvic acids from decaying leaf litter in freshwaters may be less toxic and more environmentally relevant, but they are often not well characterised with wide variations of dissolved organic matter occurring in nature. Synthetic dispersing agents at least have a well-defined chemical structure and composition (purity), but can be more toxic than natural materials.
Are current end points adequate for manufactured nanomaterials?
The standard biological end points used in regulatory hazard assessment (e.g. mortality, growth rate, reproduction) remain appropriate for MNMs in the context of supporting data for environmental risk assessment (see discussions in Crane et al. 2008). However, researchers studying the fundamental mechanisms of MNM toxicity are using a wide variety of end points (physiological/behavioural assays, histology, biochemical and molecular methods) that have been used for many years for conventional chemicals (review, Handy et al. 2002). Nanomaterials do show toxic responses (oxidative stress, genotoxicity, organ pathologies, etc., Federici et al. 2007; Smith et al. 2007; Vevers and Jha 2008) that are well known for traditional chemicals. It would therefore seem that these existing approaches (albeit with validation for interference from the particular MNM being tested) are likely to be useful end points for MNMs. So far, unique nano-specific biological responses, or mode of action, have not been sufficiently identified to enable the construction of a bioassay or biomarker for MNM exposure or effect (see Handy et al. 2012). It may also be illogical to seek a single nano-specific diagnostic assay, given the diverse chemistries and surface structures of MNMs.
Methods and practical considerations for determining MNM distribution and size in complex environmental and biological matrices
Fundamentally, the central problem in the measurement of MNMs during ecotoxicity tests is that one is trying to measure a solid-phase material (the MNM being tested) in a matrix of other solid-phase materials (e.g. the components of soil or sediments), an aqueous-phase (seawater, freshwater), or indeed a matrix that may have a combination of solid and aqueous phase properties (e.g. agars and bacterial culture media). In order to measure the MNM techniques must be used that distinguish the MNM from the surrounding matrix. However, most of the colloid behaviours and many other properties of the MNM will be inextricably and unavoidably linked to the properties of the test system. Here, the techniques for finding MNMs in complex solid and solution matrices and determining their properties in situ, are discussed. Perhaps the most fundamental measure of NPs is particle size. The combination of particle size and particle size distribution gives a sense of absolute dimensions and the distribution of the MNM across the whole suspension (the extent of polydispersivity). But determining these characteristics are complicated by the presence of other solids in the matrix. This section briefly describes some of the more robust techniques for locating and characterising MNMs in complex matrices.
Electron microscopy
Direct observation of the MNMs in question is the preferable option for verifying the presence, size and interaction of MNM in the test matrix, and here microscopy methods offer this advantage. However, MNM distribution can be very heterogeneous in an environmental sample and so considerable effort may be expended simply locating MNM in each sample. Microscopy of all kinds can therefore be laborious, but then, this equally applies to traditional methods of detecting the effects of chemicals in organism by microscopy; and this expectation is not new to the ecotoxicologist. Some of the better microscopy techniques for locating MNMs in complex media are discussed below.
Electron microscopy represents an important technique for directing viewing NPs at their original domain sizes (e.g. primary particle size). Transmission electron microscopy (TEM) allows for the highest magnification of nano-sized structures but solely from a two-dimensional perspective, and is best applied to electron dense material (i.e. metal and metal oxide NPs, quantum dots) that can absorb the electron beam and be effectively visualised against the bright field background. However structures that diffract electrons in the beam can also be visualised from the pattern of electron back scattering (Bragg scattering), and this applies to crystalline materials like silica and to carbon nanotubes. Scanning electron microscopy (SEM) also allows for imaging of nano-size domains but has roughly about one to two orders of magnitude less magnification capability, and thus is difficult to view primary MNMs. However, SEM is particularly useful for studying MNMs in complex environmental and biological matrices (freshwater, seawater, pore water extracts from soils, cells/tissues), and for its ability to create three-dimensional images. This is done in SEM by slightly tilting the sample stage between images as to create a stereoscopic image of the particles. Significant improvements in SEM instrumentation have come with the incorporation of field emission technologies which allows scanning electron microscopes to attain magnifications comparable to TEM.
Particle size, shape and size distribution can be determined directly from SEM and TEM images using digital processing, or by scoring images manually. This can be done especially well for metal NPs provided that a suitable dilution is made so that individual particles can be seen (e.g. 10 mg l−1 TiO2 and counting about 100 particles, Federici et al. 2007) for statistical analysis of the mean primary particle size. The counting of carbon nanotubes is often more problematic as the tubes will tangle together during TEM preparation and scoring individual tubes becomes difficult. However, sophisticated topographical analysis can be conducted for complex domains structures, such as characterising particles using such as fractal parameters. For example, Kennedy et al. (2009) showed significant difference in the fractal dimension of multi-walled carbon nanotubes (MWCNTs) (as shown in TEM images) resulting from stirring versus sonicating suspensions.
In addition to high magnification, most electron microscopy systems contain X-ray detection capabilities, such as energy dispersive X-ray (EDX) spectroscopy or X-ray diffraction (XRD). These capabilities are important for identifying and spatially resolving elemental domains within samples in the electron images. This application is particularly important as co-location of elemental domains in particles can provide clues to possible complexation between the MNM surface and constituents and the matrix of interest (Fig. 1). For example, Choi et al. (2009) attributed the reduction of nanosilver toxicity in nitrifying bacteria to the complexation of dissolved silver by biological thiol groups. This conclusion was reached from SEM-EDX images showing immediate co-location of sulphur and dissolved Ag on the cell surface. TEM instruments are increasingly equipped with XRD capabilities. This allows the identification of particles in the image by its X-ray diffraction pattern, assuming the particle is sufficiently crystalline for database matching. EDX and related methods rely on excited electrons falling back into an inner electron orbital within the atom (with subsequent release of the energy as electromagnetic radiations), and therefore only works with atomic numbers greater than four; but in practise anything much smaller than a sodium atom gives a weak signal, with the technique working best for the heavier metals that MNMs also tend to be made from (e.g. Cd, Ti, Ag, Cu, Zn, Fe).
Fig. 1.
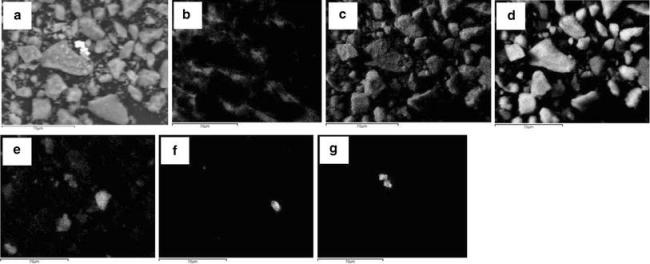
SEM images of silver NPs mixed in soil. a Sample image with the instrument detector switched to backscattering mode, followed by selected X-ray maps showing distribution of different elemental domains; b carbon, c oxygen, d silicon, e potassium, f titanium and g silver (Chappell et al., unpublished). Scale bar 70 μm
Most of the drawbacks from the use of electron microscopes arise from the creation of artefacts due to the fact that samples must be analysed under ultra-high vacuum. This condition requires the sample to be completely desiccated before analysis. Removing the MNM from solution creates a number of different artefacts including aggregation, distortion of particles and potential salting-out of matrix components. These factors can represent substantial complicating factors for interpreting images, particularly for MNMs with highly hydrated or gel-like coatings. New electron microscopes are now available, such as environmental scanning electron microscopy (ESEM) that allow for imaging of samples in liquid and in the presence of a gas phase under non-evacuated conditions. However, the ESEM setup is currently not compatible with field emission sources, necessary for imaging primary particles in SEM.
Electron microscopy equipment sensitive enough to image nanoscale particles can be prohibitively expensive, although new, high capacity, economic microscopes appear to be on the horizon. Like all microscopy techniques, electron microscopy is labour intensive and time consuming, with limited throughput for a large number of samples. Also, EDX detection is limited to percent levels of elements when under high magnifications because of the low amount of incident radiation. Completing a typical EDX map with sufficient spectral resolution at ×9600 magnification can take on the order of 4–6 h per region of interest. Also, particle shape and surface morphology can distort how the particles are represented in the processed image, often making it difficult to identify particles in complex matrices. One can spend an inordinate amount of time “hunting” for MNMs and their distinguishing features by X-ray profiling alone. Fortunately, much of the time spent hunting MNMs in complex matrices can be reduced by switching the detector to backscattering mode, where elements of high electron density or high atomic weight (Z) appear as “bright” images in the microscope. Under this regime, backscattering analysis is not sufficiently sensitive to discriminate between the different high Z elements and so suspected domains must still be mapped by EDX to verify the particle composition in the image.
Dark-field microscopy
One technique that is gaining popularity involves dark-field detection of MNMs. This technology is well suited for high Z NPs that give off distinguishing plasmon resonance signatures. This is important for example with nano silver as plasmon resonance changes both with size and the presence of dissolved silver through particle surface oxidation and dissolution. Coupling this microscopy with a hyperspectral detector expands the range of analysis from those merely observable at visible wavelengths to near infrared (NIR) regions. This is important as plasmon resonance shifts to higher wavelengths beyond the visible spectrum with increasing MNM size. The coupled dark-field, hyperspectral techniques are ideal for MNMs in complex matrices, where other colloidal materials may exist. However, this method is mainly limited by the slow sample throughput when a large number of samples are required. Also, this technology is more limited toward lower Z elements, particularly carbonaceous MNMs, in biological matrices.
X-ray spectroscopies using synchrotron radiation
Synchrotron light is generated at specialised facilities where samples are analysed with high-energy, and monochromatic X-ray electrons are generated and used for high-resolution mapping and chemical speciation of solids. The mapping technique, known as micro-X-ray fluorescence (μ-XRF), works by hitting the sample with an incident X-ray beam and measuring fluorescence to elemental excitation; similar to EDX. However, the flux or “brightness” of the Synchrotron-generated X-ray energy is such that the detection limits can be as much as six-orders of magnitude more sensitive than the combined SEM-EDX technique. Where EDX will mostly be able to detect aggregates representing domains containing percent-levels of NPs, μ-XRF can map much more diffuse distributions in samples.
A distinct advantage of μ-XRF is the ability to couple this technique with X-ray absorption spectroscopy (XAS). This technique is based on the excitation of core electrons in the atomic shell of elements by scanning a sample with incident X-ray electrons tuned at specific energies for an element of interest, resulting in a particular X-ray absorption behaviour. Thus, this technique provides direct structural information regarding NPs and their interaction with their environment. The two forms of XAS are X-ray absorption near-edge spectroscopy (XANES) and extended X-ray absorption fine structure (EXAFS). XANES refers to data collected near the element’s absorption edge while EXAFS refers to data collected at extended energies well above the absorption edge. With appropriate background correction, spectral normalization and Fourier-transformation, EXAFS data can be fitted to quantum mechanical models of X-ray scattering to describe the important details with respect to the type and number of coordinating atoms and their corresponding bond distances. For example, Fig. 2 shows the extended region for Pd NPs. Fitting of the data shows that Pd EXAFS spectra typical of what is seen as purely metallic Pd, with second-neighbouring Pd atoms occurring between 2.7 and 3.9 Å. The NPs show no evidence of oxygen formation (e.g. typically observed between 1 and 2 Å), such as due to sorption of surface oxygens, confirming the particles are metallic. This data demonstrates the powerful capabilities of XAS as many elemental and mineral domains within soils, etc. are quasi-crystalline at best, and thus, not distinguishable by common X-ray diffraction techniques.
Fig. 2.
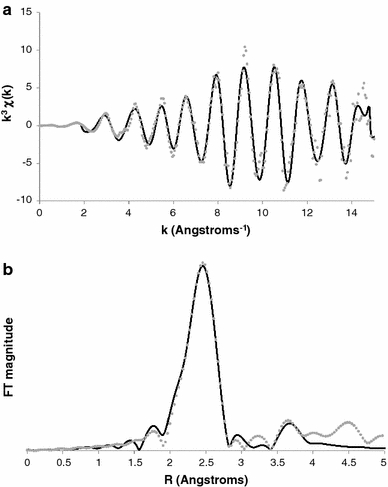
Extended X-ray absorption fine structure (EXAFS) spectra (a) and corresponding Fourier transformation (b) of Pd nanoparticles imbedded in a cotton textile (Chappell et al., unpublished)
The quality of both XANES and EXAFS data is highly concentration dependent. In general, EXAFS requires higher concentrations of elements to collect adequate signal for modelling than XANES. In cases where elemental concentrations are too low for EXAFS analysis, some structural information can be extracted from a statistical fit of XANES region data based on sets of known standard salts or reference sorbents. The most common involves a two-fold analysis: first, a principal component analysis to determine the maximum number of standards that are relevant for describing the sample, followed by a linear combination analysis to calculate the proportion of standard that is represented within the sample. These fits are evaluated diagnostically using both a reduced χ2 value, which refers to goodness of fit of the model proposed, and a R-factor, which represents the fractional misfit of the data.
XAS can be combined with μ-XRF to speciate mapped out MNM domains. Typically, μ-XANES is possible on μ-XRF maps, but under special circumstances, μ-EXAFS can be performed as well. Although the substantial loss of incident energy, due to focusing the X-ray beam into a small spot size, reduces the signal to noise ratio of analysis.
An important advantage of these X-ray techniques is that they require little or no sample preparation for analysis. Samples can be brought to the beamline and analysed “as is” without requiring extraction, drying, or any other common preparatory techniques. Analyses are regularly conducted in a variety of complex matrices, including highly heterogeneous geologic material and biological tissues. For example, Scheckel et al. (2004) conducted a combined μ-XRF/XANES study on potted plants by hanging a leaf on the sample stage in the X-ray beam path to determine the distribution and speciation of As. Chappell and co-workers regularly combines both liquid speciation determinations sample extracts with XAS and μ-XRF studies to demonstrate the difference between the soluble and non soluble phases on contaminants in soil.
There are perhaps two main limitations of utilising XAS: One involves beamline access to these specialised facilities. Currently, there are only a handful of Synchrotron devices throughout the world. Access is typically granted on a competitive proposal basis. Another limitation involves the fact that considerable expertise is necessary to adequately apply this technology for analysis. In particular, there are numerous pitfalls in experimental setup, data collection and data processing that only those who are very experienced with the technology can avoid. In short, there is no such thing as a casual user of XAS. Fortunately, most research utilizing μ-XRF and XAS involves highly collaborative projects, including partners with varying expertise in these techniques.
Light scattering techniques
This method represents the most common forms of particle size analysis—light scattering and X-ray scattering. Light scattering is especially popular given its ready adaptability to most solid–liquid system and high sample throughput. These measurements are based on the relationship between a particle’s diffusion coefficient in solution and its size-dependent Brownian motion. Particle motion is calculated by time-integrated measurements of incidences of light scattering within a sample. Two major forms of light scattering are commercially available—dynamic light scattering (DLS)—a strictly particle sizing technique, measuring light scattering from a sample at one detection angle. Outfitting a DLS instrument with a special cell that allows for an applied voltage provides measures of particle charge or zeta potential—a measure designed to quantify the particle response to the applied electric field. DLS therefore works for spherical solid particles (i.e. almost all metal NPs), but is poor for hollow structures like C60 and does not work for high aspect ratio materials like rigid carbon nanotubes which are far from spherical. The other common form of light scattering is called static light scattering (SLS). Instrumentation for SLS employs multiple detectors placed at different scattering angles so that differential intensities of the detection can be attributed to a particle’s shape size. In SLS, a particle’s scattering “pattern” is modelled based on expected scattering of geometric shapes for the calculation of a radius of gyration (RG) value. In simple terms, the RG value provides the average distance of motion around a particle’s geometric center. For dissolved polymers, SLS is useful for estimating a polymer’s molecular weight from its RG value. For colloids, RG values can be used for determining a particle’s hydrodynamic dimensions or conformation in solution if the particle structure is well characterised (Cantor and Schimmel 1998).
It is important for the user to be aware of the limitations of light scattering instrumentation. First, and foremost, light scattering measurements are purely estimates of particle size and shape. For DLS, particle size calculations are based on the assumption of spherical geometry. These equations can be modified for non-spherical objects, but become very complex and are impractical for the ecotoxicologist. Fortunately, spheres represent a lower energy state for solid surfaces, and even flexible nanotubes will prefer to take on a spherical structure. Chappell and co-workers have observed CNT “hairballs” in solution.
Light scattering instrumentation optimised for the nano scale may also be limited with respect to the size of particles and suspension concentration. Light scattering intensity is generally related to the R6 (where R = particle radius) of the particle. This means that there is an eventual particle size that will saturate the detector and essentially “blind” the instrument. One can attempt to circumvent this problem by allowing samples to settle on the bench top for approx. 24 h, or for example, the DLS instrument can be configured for an upper-particle limit of approximately 3000 nm. The instrument also needs an adequate number of particles to scatter and in practise dispersions in tens of mg l−1 range work well, but samples too concentrated (hundreds of mg l−1) will give poor scattering. Too few particles will also prevent detection and in practise the DLS detection limit for particle dispersions is about 1 mg l−1, and its use for ecotoxicology is therefore limited to acute toxicity studies or measurements in stock dispersions used for dosing. Light scattering methods are also poor at distinguish between particles of varying composition. Most natural waters (freshwater, seawater, pore water from soils/sediments) and the body fluids of organisms contain quantities of natural colloids where light scattering will not discriminate the MNMs in the sample.
A variant of SLS is known as SAXS or small-angle X-ray scattering (SAXS). Similar to SLS, SAXS measures the scattering of photons due to a colloidal shape. However, the shorter wavelength of X-ray radiation allows for much greater resolution and finer detail of particle shape and size than visible light scattering. This analysis is proving to be particularly useful for characterising high-aspect ratio MNMs such as CNTs. Figure 3 shows an example of MWCNTs sterically stabilised in an aqueous solution containing nonionic surfactant, Brij 35. Modelling the SAXS data using a potential distribution function calculated the CNT dispersion possessing a RG of 15.2 nm. The size distribution of the CNT suspension was modelled as having a mean particle diameter of 27.7 nm, consisting of a trimodal particle size distribution centred on populations of 9.3, 27 and 51 nm. The size distribution of CNTs best conformed to a spheroidal geometric shape with an aspect ratio = 1.0.
Fig. 3.
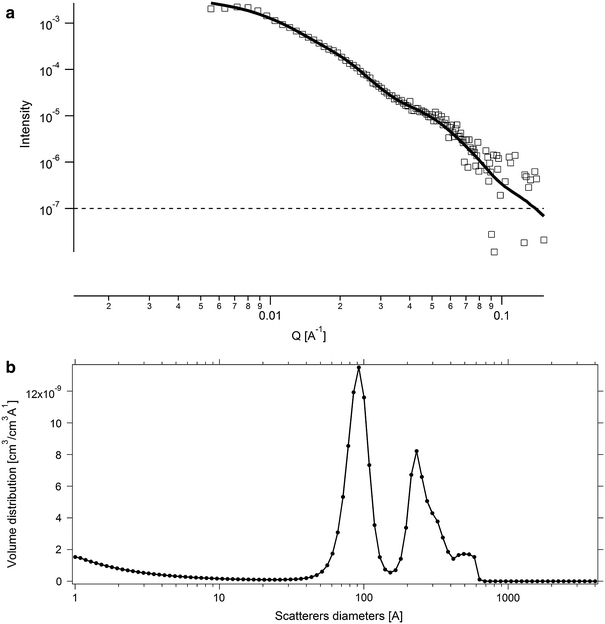
a SAXS data for CNT particles dispersed in an aqueous solution of Brij 35, modelled by fitting to a spheroidal geometry (aspect ratio, AR = 1). b Calculated particle size distribution of the CNT dispersion (Chappell et al., unpublished). See text for details
Chromatographic techniques
Size exclusion chromatography can be used to separate particles. In principle, MNMs are injected into a very long column containing a porous, chemically inert, but well-characterised stationary phase. The particle plume spreads out based on individual particle sizes with the larger particle sizes traversing the column faster than the smaller particles. Once the particles elute from the column, they can be detected by any number of analyte or particle detection techniques (e.g. UV absorption, in-line DLS, fluorescence spectroscopy etc.). Substantial improvements to this technique include optimising the separation for nano-sized particles by introducing re-circulating flow through the original column or additional columns to enhance the separation resolution, especially for NPs in the range of 1–10 nm (Al-Somali et al. 2004). In addition to DLS, a SLS and capillary viscometer can be added in series for measuring particle RG and suspension viscosity, respectively.
The advantage of adding a chromatography step is that it provides improved resolution of the particle size distribution when compared to simple batch light scattering measurements. However, disadvantages include the potential for micron scale material to lodge into pores and clog the column, making a preliminary size separation sometimes necessary (such as 24 h settling to remove the larger particles). Chromatography methods are also sensitive to the presence of other colloids and polymers in the system. However, the addition of particle or elemental specific detectors, such as ICP-MS, can alleviate some of these issues. For example, Bednar et al. (2007), use SEC-UV-ICP-MS to characterise organouranium complexes extracted from plant material.
Field flow fractionation (FFF) is similar to gel permeation chromatography except that NPs are separated through a combination of longitudinal and lateral flow gradients that separate particles by differential movement related to hydrodynamic size. This technique has proven especially useful for separating metallic NPs in natural freshwater containing organic matter where the dispersion may be reasonably stable. The advantages to FFF include customisable control of flow conditions enabling a high resolution of particle size distribution, non-destructive sampling so that the sample can be used for other measurements (bioassays, analytical chemistry). For example, connecting an ICP-MS in series with FFF allows for one to distinguish between dissolved and particulate forms of silver (Kennedy et al. 2010). Additionally, the ICP-MS elemental specific detector is extremely sensitive, allowing detection of NPs at the parts per billion range (Poda et al. 2011).
An important disadvantage of FFF is that it requires very stable suspensions of NPs in order to conduct the separation, as particle agglomerations will be separated based on the size of the agglomeration. For the ecotoxicologist, typical “unstable” samples that are less suited to FFF are unfunctionalised MNMs in seawater (high ionic strength media), crude homogenates of organisms, soil or sediment samples. A surfactant can be added to the mobile phase to sterically stabilise suspensions, but this creates its own artefacts including increasing hydrodynamic size, falsely shifting the particle size distribution to smaller values, as well as altering the ecotoxicity of any fractions subsequently tested in bioassays.
Predicting dispersion and dissolution properties through theoretical calculations
An alternative approach, when direct measurement is either technically not possible, or difficult for practical/logistics reasons in an ecotoxicity test, is to predict MNM behaviour from theoretical calculations. Currently, methods for measuring particle size distributions are ineffective below about 1 mg l−1, and with an urgent need to collect ecotoxicological data at low μg l−1 or ng l−1 concentrations to reflect likely environmental scenarios, having at least a mathematical estimate of what may be happening in the dispersion would greatly aid data interpretation. Computational methods have imperfections, but here, they are at least derived from some 80+ years of research on natural colloids, and therefore have an established scientific foundation (i.e. applying established math to MNMs). This approach is mainly applicable to ecotoxicity tests using aqueous media (freshwater, seawater, defined media like hydroponic solutions used in plant tests or the salines for nematode tests). For dispersion stability, some degree of surface charge is likely on the NP. Thus, application of Derjaguin-Landau-Verwey-Overbeak theory (DLVO theory, Derjaguin and Landau 1941; Verwey and Overbeek 1948) which describes the theoretical interaction between two charged particles is useful. In this theory, the behaviour of spherical particles represents the sum of both the inter-particle attractive forces (i.e. van der Waals) and electrostatic repulsive forces that control whether the particles in a suspension have sufficient energy to remain dispersed, or will aggregate upon contact with each other (see Handy et al. 2008a for discussion of the theory from an ecotoxicology perspective). DLVO theory is useful because it allows the input of multiple particle and media properties, providing a basis by which to “comprehend” how the various measured properties come together to define particle behaviour. Of course, it is not expected that a typical ecotoxicologist at the bench could do these theoretical calculations manually, but ecotoxicologists do routinely use chemical speciation software, for example, and it is simply a matter of developing a user-friendly software that could be of practical value at the bench for understanding dispersion in experimental media.
On the Wolfram web site (http://www.wolfram.com/), Chappell and co-workers have published a demonstration programme for DLVO written in Mathematica 7.0 (Blaustein and Chappell 2011) that can be used to approximate whether a particular NP dispersion is stable under given experimental conditions. In the current version, sliders allow for the input of particle-specific parameters, such as particle size, zeta potential, and matrix-dependent parameters, such as background electrolyte type and concentration. The software is therefore very easy for the ecotoxicologist to use. Future versions will expand on this programme to include effects of pH and coating type on dispersion properties.
The output includes two plots—an interaction potential plot (Fig. 4) and a plot showing what is called the CCC, or critical coagulation concentration. The latter parameter determines what solution conditions will cause the defined NP to aggregate out of solution, where the stability ratio (W) of the suspension equals 1, as calculated from the DLVO equation. The W parameter is directly indicative of the stability of the NP dispersion. When W > 1, the dispersion is predicted to be stable (W is reported on a sliding scale). The calculation of W is straight forward and enables the ecotoxicologist to understand whether or not their test conditions will promote flocculation, and what parameters might be adjusted before the experiments start to avoid this (if desired). For data interpretation, the software would also enable some appreciation of bioavailability to the organism in the test system, for example in a salinity experiment where one could rapidly estimate flocculation with different NaCl concentrations.
Fig. 4.
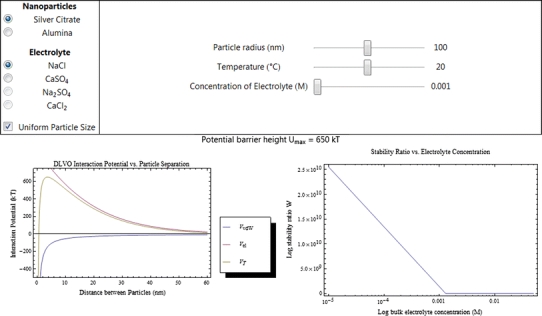
Screenshot of Wolfram demonstration project created by Blaustein and Chappell (2011) where particle stability for two types of NPs are calculated using classical DLVO theory
W can also be estimated using a simplified equation (Morrison and Ross 2002),
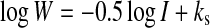 |
1 |
where I is the ionic strength (in units of molarity or M). If we assume ks (solubility constant) is negligible, then W is calculated only from the ionic strength as
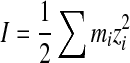 |
2 |
where, m and z represent the concentration (in units of M) and valence of ith ion species. For a simple monovalent, 1:1 electrolyte (e.g. NaCl = Na+ and Cl− ions of both the same charge), I can be estimated from electrical conductivity measurements (Evangelou 1998).
DLVO theory does have some important limitations and weaknesses, including inaccuracies with respect to NP size, shape, surface coating and polymer components in the matrix (Holtze et al. 2010, and references therein). For the ecotoxicologist, classical DLVO theory at least gives a qualitative estimate of dispersion on which practical decisions about the experiment, such as water changes, can be made in the laboratory. For example, even though the prediction of W above assumes full thermodynamic equilibrium, one can still gain a qualitative sense for the dispersion half-life (t1/2), which is proportional to the magnitude of W.
The dissolution potential of NPs can be estimated in a variety of ways, from complex geochemical speciation modelling to simplistic calculations involving a material’s solubility product (Ksp). For example, we can conservatively approximate the dissolution of Ag+ from nanosilver (nAg) particles through the following steps. Since metallic Ag has virtually no solubility, it can be assumed that the metallic Ag atoms remain on the surface of the particle, and are gradually oxidized to form a surface oxide coating. This coating contains a variety of Ag to O stoichiometries (Roy et al. 2007), but the solubility of surface oxides can be approximated from the dissolution of AgOH. Here, the equilibrium concentration of Ag+ can be estimated by the K1/2sp for AgOH. It is important to bear in mind that this calculation does not reflect time-dependent release of Ag+, only what the expected total concentration will be if the reaction is allowed to reach full equilibrium. Thus, if first-order kinetics of dissolution is assumed (i.e. the dissolution is not rate limited by continues to equilibrium) then the proportion of maximum Ag solubility with time (t) can be calculated from the integrated rate equation as:
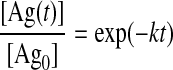 |
[3] |
where [Ag0] and [Ag(t)] represent the concentration of Ag in solution at the initial concentration and with respect to time. By referring to the literature for the rate constant k, the proportion of maximum dissolution can be calculated with given periods of values for time. Clearly, this approach is a first approximation as it does not account for effects from pH, ionic strength, redox potential and polymeric components such as surfactants, chelators and humic substances. Nonetheless, it enables some appreciation of whether dissolution is likely to occur, and if so, the possible magnitude.
Practical approaches to estimating MNM dispersion and dissolution using simple measurements during experiments
The detailed measurement techniques above require the test media samples to be taken to a specialist instrument that may also need additional expert technical support. Then, with each sample taking minutes or much longer to read, there is little prospect of the ecotoxicologist being able to monitor the exposure, or correct dosing problems, during the early stages of an experiment. However, some simple direct approaches are available to provide some information on the behaviour of the MNM in the test media while experiments are still in progress. For example, tracking the settling of MNMs in aqueous test media can be done using simple optical methods. Settling can be followed using a typical UV–vis spectrometer at wavelengths where the NPs do not absorb (typically around 550–650 nm). The spectrophotometer therefore simply measures particle absorbance (optical density) based on the notion that particles in the suspension will prevent photons reaching the detector. Repeated measurements over time will at least tell the ecotoxicologist if the test material is settling out of the experimental media, and if careful time courses are done using defined media, then it may be possible to calculate a particle settling rates (Chappell et al. 2009). Absorbance measurements can also be used to track the settling of fine MNM suspensions, where very slow settling rates could be measured using micro-cuvettes. Optical density measurements should be corrected for the turbidity of the test media by blanking the spectrophotometer with the test media (no particles added), but this should also include time-matched sampling in cases where naturally turbid test media has some settling of its own. Particle mass concentration could also be calculated from a calibration curve constructed from absorbance measurements of the MNM in a serial dilution of the test media.
Measuring the dissolution of NPs during an experiment is more difficult, but is possible for some metals using ISEs (see above on electrodes). The main advantage of ISEs is that they should only detect free ion activity, and sometimes, this can be specific to one chemical species. For example, ISEs for dissolved Ag often are designed to detect only Ag+ ions, and cannot detect other dissolved Ag complexes (AgOH2−, AgOH0, etc.). The general problems of using electrodes (see above) also apply to ISEs, and measurements may be tedious with temporal drift in the electrode response, but this approach does provide a direct measure of metal ion activity, without any modification of the test system or media. Other approaches include high-speed centrifugation of aliquots of the test media to provide a supernatant that contains the dissolved fraction of the metal. These can then be analysed by traditional methods (e.g. ICP-MS, ICP-OES or F-AAA), or indeed the free ion activity in the supernatant measured with ISEs. However, centrifugation approaches require validation of the assumption that all of the particulate matter has been removed from the supernatant. One should also remember that dissolution will still be occurring while the samples are in the centrifuge, so centrifugation steps should be as short as possible (e.g. a few minutes at high speed using small volumes). An alternative approach is to verify dissolution in separate dialysis experiments, where the MNM can be dialysed with the test media, and samples of the test media analysed for the dissolved fraction. However, this latter approach requires pilot studies in advance of the main ecotoxicology experiment. If such pilots are done without the test organism present (missing the ligands secreted by the organism), or with different volumes of media compared to the main ecotoxicity test protocol, then dissolution data from dialysis may be difficult to interpret.
Minimum characterisation during ecotoxicology tests
Clearly, a range of methods are available for MNM characterisation, and a few of the simple methods described above enable some understanding of dispersions during experiments. However, some further thought is required on what is the minimum practical characterisation expected of the ecotoxicologist while experiments are running. Samples should be collected for measurement of the mass concentration of the MNM, as is done for ecotoxicology tests with conventional chemicals. The simple optical methods above may enable the experimenter to obtain relative measures of the amount of settling of the test material, and inform when the test media should be replaced. For many regulatory aquatic toxicity tests, validation criteria state that at least 80% of the nominal test concentration should be achieved during the exposure. The simple optical measure will at least give the experimenter an idea if this is being achieved while the experiment is in progress.
The minimum characterisation requirements for stock dispersions of MNMs have been discussed (e.g. Crane et al. 2008; Stone et al. 2010), but technology gaps and a lack of rapid particle characterisation methods are limiting what the ecotoxicologist can do while the experiment is in progress. The worker at the bench cannot simply store samples for latter analysis because the MNM may aggregate, agglomerate, or even dissolve, during storage. Particle size distribution measurements, or particle number, would allow data to be expressed using a dose metric other than mass concentration, but there is a big technology gap in achieving this from samples collected during experiments. Most DLS instruments, and nanoparticle tracking analysis (NTA) instruments only have reliable quantification down to about 1 mg l−1, and environmentally relevant concentrations are predicted at μg l−1 levels or less (e.g. Gottschalk et al. 2009). In addition, the methods are slow, with triplicate DLS measurements on a single natural water sample taking up to 20 min. Currently, there is no real prospect of detailed characterisation of the test media while toxicity tests are running, and the next best pragmatic solution is to follow settling or dissolution with simple methods, and to do pilot experiments with test media that focus just on the characterisation issue.
Soil tests using invertebrates and terrestrial plants
For testing hazards of chemicals to soil invertebrates, several standard tests are available (e.g. OECD tests 207, 216, 217, 220, 222 and 232; see Crane et al. 2008 for a summary of OECD ecotoxicity tests). Protocols are also available to test invertebrates in sediments (e.g. OECD 225). The soil tests include different species (earthworm, collembolan and enchytraeid species, various microorganisms), different end points (e.g. growth, mortality, reproduction), and exposure scenarios (from a few days to several weeks/months). Clearly, there is an extensive battery of existing soil and sediment tests that could be used, or modified, to work with MNMs. However, there are many practical issues to resolve including how to prepare and dose the test media, characterisation of MNMs in complex matrices like soil, the selection of species, choice of end points and inclusion of controls for MNMs in the test design.
Test medium preparation
There are essentially two main approaches to adding MNMs to test soils. The MNM may be added as a dry powder and mixed into the dry soil, or the MNM can be added (or sprayed) onto the soil as a liquid suspension. Both methods have been applied in soil tests with MNMs (powder: e.g. Hu et al. 2010a; suspension: e.g. Scott-Fordsmand et al. 2008; Johansen et al. 2008; Roh et al. 2009), and each approach has advantages and disadvantages (Table 4). For example dry mixing may enable the MNM to spread throughout the soil sample, but addition as liquid to the soil surface may be more environmentally relevant. Current regulatory tests do not prescribe exactly how the test substance should be mixed with the soil. It may be prudent to tighten up this aspect of methodology with a standard mixing protocol (for example using a mechanical food mixer where the settings are known), rather than just allowing the experimenter to do this arbitrarily. Effective mixing of the soil could be monitored using an inert marker (e.g. chromic oxide that is widely used in the blending of experimental animal feeds), even if the original test material cannot be tracked.
Table 4.
Advantages and disadvantages of different methods for spiking soils with MNMs, identified at the NIN workshop
| Adding as powder | Adding in suspension without a dispersing agent | Add in suspension with a dispersing agent | |
|---|---|---|---|
| Yield | High concentrations possible (no limit) | Low concentrations (μg l−1 to mg l−1 range) | High concentrations possible (g l−1 range) |
| Ease of preparation | Potential occupational hazards from dusts. Short preparation (hours) | Easy to apply, but potentially long preparation time for the stock dispersion (for stirring methods, up to months) | Easy to apply, and short preparation time (hours) |
| Control of the dosing | If the soil is relatively dry and mixed with dry powder then a reasonable spread of the test material in the soil occurs | Poor reproducibility of the stock dispersion could produce variable dosing. Depending on the hydroscopic nature and viscosity of the solution, and properties of the MNM, the material may not evenly spread in the soil sample | Improved reproducibility of the stock dispersion, and more chance that the test material will spread evenly in the soil sample. However, dispersing agents controls are needed in the test design |
| Characterisation | Possible in the stock dispersion, but not in the soil matrix | Possible in the stock dispersion, but not in the soil matrix | Possible in the stock dispersion, but not in the soil matrix |
| Surface modification of the test material | Weathering effects less likely with dry mixing | Long preparation times of stock dispersions may lead to oxidation, hydroxylation or other chemical/physical modifications of the surface. Soil effect relative to the stock preparation effect on surface modifications are mostly unknown | Short preparation times imply less likely to produce spontaneous changes in the particle surface, but dispersing agents will coat/modify the surface. Interaction of dispersing agent with the soil and particle surface will depend on soil type and the stability of any surface coating in the soil matrix |
| Dosing for chronic tests | Suitable dosing method, but MNM may age, particle ageing control should be included in the experimental design | Suitable dosing method, but MNM may age, particle ageing control should be included in the experimental design | Suitable dosing method, particle ageing may be different with dispersing agent present. Degradation of the dispersing agent is likely |
The dosing method may influence bioavailability of the MNM and therefore the results of the test. For instance, the use of synthetic dispersing agents can alter MNM availability or subsequent toxicity in soil tests (Zhu et al. 2006). Natural dispersing agents are also available for soil matrices. Recently, organic material extracted from test soil was used as a dispersing agent to prepare a stock suspension of fullerene particles (Van der Ploeg et al. 2011). In this way a suspension with high concentrations of MNMs could be established, without adding interferences or toxicity of the synthetic dispersing agents (outlined above). “Dispersing agent controls” with only exposure to the dispersing agent should be included in the test design, as even additions of extra natural organic matter could change the soil properties.
Given the diversity of soil types, and MNMs, it is important to include a “bench mark” with a standard (artificial) soil in the test design, so that results of tests can be compared between studies. Indeed, many regulatory protocols for soils specify that an artificial soil of known composition must be used in the test, and then give details of how to make it. For example, in the OECD 222 earthworm reproduction test the soil is specified to be comprised of 10 per cent sphagnum peat (as close to pH 5.5–6.0 as possible), 20% kaolin clay, 0.3–1.0% calcium carbonate (to ensure the initial pH is 6.0 ± 0.5), and 70% air-dried quartz sand with more than 50% of the particles between 50 and 200 microns. This is typical of the recipes for artificial soils in regulatory protocols, which have a high sand content and only one type of organic matter or clay added. Nonetheless, while artificial soils may be criticised for being over-simplified and not being representative of the diversity of natural soils, the original purpose of using a defined artificial soil for bench marking and inter-laboratory comparisons is equally useful for tests with MNMs, especially while data are being collected to generate a consensus view on exactly how soil tests should be done.
Meanwhile, researchers are encouraged to provide more information on the exposure method than currently indicated in OECD test methods for soils, which were originally devised with conventional chemicals in mind. Specifically, for MNMs, this should include exact details of the method of dosing; such as the amounts of MNM added, volumes of MNM to soil, moisture content at the time of dosing, the speed/duration and method of any mixing, and information on attempts to verify that the mixing has achieved a homogenous spread of the MNM in the soil sample (or otherwise). Details of the types, concentrations, volumes and batch numbers, of dispersing agents should be documented. This would be in addition to the usual reporting of the measured details of the soil matrix (proportions of sand, organic matter, clay, pH, etc.) and robust characterisation of any starting material or stock dispersions (using the techniques above), including details of how stock dispersions were prepared (e.g. sonication times and intensities, stirring times/speeds, mass concentration, measured particle size distribution). In this respect, the current OECD guidelines are insufficient and collecting this extra information will allow cross-study comparisons (meta-analysis) and better interpretation of test results and understanding of test soil reproducibility with MNMs. At this stage, it is too early to give the experimenter a precise universal protocol for dosing and mixing soils with MNMs, because the particle-soil interactions are not understood. In the future however, it may at least be possible to arrive at guidelines for dosing particular types of soil (e.g. sandy soil, clay soil, etc.) with particular generic types of MNMs (e.g. hydrophilic or hydrophobic).
Characterisation issues specific to soil matrices
In addition to the generic discussions on characterisation (above), there are some specific issues that relate to tests using soil as the matrix. It is possible to characterise the MNM stock at the start of the experiment, i.e. the MNM as a powder (Scott-Fordsmand et al. 2008; Hu et al. 2010a), or in suspension (Johansen et al. 2008; Roh et al. 2009; Van der Ploeg et al. 2011), prior to adding it to the soil. However, no studies are available in which MNMs have been characterised in the test soil itself. Methods to characterise MNMs, like TEM and DLS for size distribution, or methods to assess zeta-potential are currently not applicable directly in soil samples. Furthermore, characteristics of the MNMs in the stock dispersion are unlikely to be informative or representative of the behaviour of the MNM in the soil, because the soil properties (e.g. pH, organic matter, clay content, cation exchange capacity, concentrations of major nutrients) may alter the aggregation state, surface chemistry and other properties of MNM (Hu et al. 2010b). Hence, characterisation of MNMs in the test soil remains an essential step in order to epitomise the exposure conditions. Techniques like energy dispersive spectroscopy or Fourier transform infrared spectroscopy have been applied to characterise NPs in plant tissues (e.g. Lee et al. 2008). Another approach, combining a modified application of SEM (WetSEM™) with energy dispersive X-ray spectroscopy has been applied to image Au-NPs in soil suspensions (Tiede et al. 2009). Ag NPs could be visualised in activated sludge by hydrodynamic chromatography-ICP-MS (Tiede et al. 2010). Field-flow fractionation, coupled with specific detection methods like ICP-MS may also be used to analyse MNMs in soil or sediments (Dubascoux et al. 2010). Application of such techniques in soil medium may be a valuable approach for further development, although most of them need some sort of sample preparation in order to extract the MNM from the soil, which is likely to change their properties. This may be overcome by the application of for instance Raman spectroscopy, which has been used to analyse minerals in ore samples, although not at the nano-scale but at the micron-scale (Stefaniak et al. 2009).
Additionally, net exposure may be assessed by analysing MNMs concentrations in the test animals (Roh et al. 2009; Unrine et al. 2010a), although this will not give insight into the underlying MNM properties leading to such exposure levels. Based on this, a Critical Body Residue (CBR) approach may be developed (Ma 2005), in which CBR is defined as the lowest total body concentration of a compound in an organism, associated with adverse toxic effects. This approach may circumnavigate practical problems related to the characterisation of MNMs in soil, and may be useful in hazard assessment.
Selection of test species and end points for soil tests with invertebrates
In the OECD guidelines, several standard test species are described including, earthworm, collembolan and enchytraeid species. Other species have also been used in studies on conventional soil contaminants e.g. Caenorhabditis elegans (Höss et al. 2009), isopods, mites and other micro-arthropods (Graff et al. 1997; Dallinger et al. 1992; Vijver et al. 2006). However, the published data set on individual species are currently insufficient to form a consensus view on what type of MNM is most toxic to each species. For example, a Web of Knowledge search reveals only 22 papers on earthworms and NPs up until September 2011; and of these only 12 reports are on ecotoxicity, and even fewer included metal salt controls or detailed data on particle chemistry to enable data interpretation. Heckmann et al. (2011) performed a limit test with metallic NPs (1000 mg kg−1 of the test materials) using earthworms (Eisenia fetida), and of a bank of metal salts, bulk and nano scale materials tested, only Ag NPs, Cu NPs and TiO2 NPs were toxic at the high dose used in the limit test. This might suggest that earthworms are an insensitive species to metallic NPs, but a more likely explanation is that the OECD limit test using a soil matrix is simply not allowing the exposure to occur (low bioavailability). Longer experiments do report effects of carbon-based materials on earthworm species, isopods and C. elegans (Jemec et al. 2008; Roh et al. 2009; Scott-Fordsmand et al. 2008; van der Ploeg et al. 2011). For example, van der Ploeg et al. (2011) found that 15.4 and 154 mg C60 kg−1 soil caused some mortality, reduced juvenile growth rate and altered cocoon production by Lumbricus rubellus. This provides evidence that C60 in soils does alter parameters in individuals that are critical to population level survival in this species, but with only a handful of such papers, one is far from concluding that carbon-based MNMs are a risk to soil invertebrate populations generally. Researchers are also beginning to unravel the complex of issue of exposure dose versus bioavailable metal for metallic MNMs (e.g. Unrine et al. 2010a, b; Shoults-Wilson et al. 2011). For example, Unrine et al. (2010a) showed that there was metal accumulation from Ag NP exposures in the earthworm E. fetida, and that on a particle number basis 20 nm Ag NPs were more bioavailable than 55 nm Ag NPs. Shoults-Wilson et al. (2011) also showed that Ag NPs with a hydrophilic (polyvinylpyrrolidone) or amphiphilic (oleic acid) coat caused some reproductive toxicity in E. fetida that was associated with tissue accumulation of Ag from the Ag NPs. However, overall there are not enough data to arrive at a consensus view on the most sensitive invertebrate species to use in soil tests with MNMs, or to construct species sensitivity distributions towards specific MNMs.
Species may be selected for logistics reasons given the difficulty of maintaining exposures with MNMs. For example, reproduction tests with earthworms can be relatively long-term (e.g. OECD 222 has 4 weeks exposure, then a further 4 weeks exploring reproductive end points), in comparison to reproduction experiments with C. elegans that can be conducted in about 21 days. The idea of changing the regulatory testing strategy for soil to a different organism so that a shorter test can be used is attractive from the view point of logistics and cost. The C. elegans reproduction test uses a liquid media containing essential salts, and this at least offers a media where some particle characterisation may be possible compared to soils at present. However, ease of experimentation is not the only consideration. In addition to the standard regulatory end points of mortality, growth and reproduction, species may be selected because they have features or utility in relation to specific modes of actions of chemicals. For example, one might select the earthworm to investigate oxidative stress or immunotoxicity because their coelomocyte responses are reasonably well described (e.g. Burch et al. 1999). However, the mode of action of most MNMs, in individual species, is still unknown. Where species selection on the basis of mode of action, or species sensitivity to the MNM cannot be made, it may be helpful to use omic-techniques to screen for effects. In this respect, the genomes of the earthworm Lumbricus rubellus, and the nemtatode C. elegans, have been sequenced (for Lumbricus genome, see http://xyala.cap.ed.ac.uk/Lumbribase/index.shtml; for C. elegans see www.wormbase,org). After such screening, it may then be possible to rationally select more specific end points or model organisms for soil testing.
Use of control observations in soil tests with MNMs
One concern in soil tests, especially the chronic tests involving reproduction that can last for weeks, is that the MNM may be chemically modified, resulting in changes in the surface properties, or physically modified (polymerised, or even dissolved in the case of some metal NPs). For instance, in a study on the accumulation of zinc oxide NPs in isopods, it was shown that the primary route of accumulation was through uptake of dissolved zinc that was generated by the dissolution of the particles (Pipan-Tkalec et al. 2010). In order to account for potential effects of dissolved metals, the test design can include metal salt controls. This control would represent the worst case scenario (complete dissolution of the MNM). For other materials, the effect of weathering or ageing may change the surface properties of the MNM (for example, auto oxidation and the generation of hydroxyl groups on the surface). In this case, the test design should include time-matched sampling of the controls, but also an aged soils containing aged MNMs. This approach essentially involved an ageing control where the soil is spiked with MNMs months before adding the test organisms. This would be in addition to the no-added MNM control (negative control), and dispersing agent controls (discussed above). Hence, a full design of a soil test will include controls of non-spiked soil (always), controls for ionic metal exposure in the case of metal MNMs, dispersing agent controls, aged soil/MNM control, as well as an artificial soil for bench marking.
Tests using terrestrial plants
For terrestrial plants, some standard tests are available that have been widely used for conventional chemicals (e.g. OECD test 208, 227). Many of the issues relating to soil invertebrate testing also apply to plant testing. Exposure via the soil is commonly used in research (Shah and Belozerova 2009), and in the OECD 208 seedling emergence and growth test there is a choice of different types of artificial sandy soils containing up to 1.5% organic carbon (notably, not the same recipe as the soils used for earthworm tests) as well as the choice to use natural soils. In the OECD 208 test there is a caveat relating to the use of clays in the soil that may inadvertently adsorb the test substance. For MNMs, research is needed to identify which soil recipes reduce the bioavailability of MNMs, and then a similar caveat can be inserted in the guidance documents. The OECD 227 vegetative vigour test involves spray applications of the test substance onto the plant. It has been shown that small MNMs may be accumulated through the stomata of leaves (Eichert et al. 2008). This process is thought to be very variable, and unpredictable for MNMs. For very small particles (<1.5 nm) this may demand specific tests, in which MNMs are deposited directly on the leaves of plant, after which accumulation or toxicity end points (e.g. inhibition of photosynthesis) could be measured.
In most regulatory tests the end points include emergence and/or growth of the plant. The latter is measured as plant biomass, and this may be recorded for different parts of the plant (e.g. root elongation) or whole seedlings biomass. This approach works for some MNMs when the seeds are grown in a MNM suspension and germination is allowed to occur on a filtration paper where the structure of the seedling can be easily observed (e.g. Lin and Xing 2007). Germination experiments have also been reported, using soil exposure (Au and Cu NPs) with lettuce (Shah and Belozerova 2009). Respiration and/or photosynthesis may also be useful end points for MNMs. Ma et al. (2010) reviewed the phytotoxicity of MNMs, describing effects on seedlings and plants. Toxicity to plants depended on the properties of the MNMs, and results differed between species. Like the soil tests with invertebrates, there is insufficient data to construct species sensitivity distributions or make recommendations on which species listed in the OECD tests should be used. At this stage of the research, the traditional end points should still be used (germination, growth, etc.), but biochemical end points relating to photo-inhibition by MNMs should also be explored.
Plant tests with MNMs could also use media other than soil. These include agar made of a simple polysaccharide medium containing minerals (e.g. Lee et al. 2008; Lee et al. 2010), or hydroponic media containing essential minerals and nutrients in solution (e.g. Seeger et al. 2009). Hydroponic exposure, or the use of agar as growing medium, enables characterisation of the MNM, and can result in a well defined exposure scenario. For instance, Lee et al. (2008) used agar as exposure medium to test effects of Cu-NPs on two plant species. They applied scanning-electron microscopy-cathodololuminescence to visualise NPs in the agar, although they used Zn-NPs as a substitute for the Cu-NPs because Zn-NPs have cathodololuminescence activity, and Cu-NPs do not. Similarly, Lee et al. (2010) used scanning electron microscopy to illustrate the dispersion of metal oxide NPs in agar. However, other properties like particle size, hydrodynamic diameter and zeta potential of the different NPs were only assessed in the aqueous growth medium, and not in the agar medium. Clearly, measurements of particle size that are based on the diffusion of particle (like hydrodynamic diameter), are easier to perform in media that is less viscous than agar. Anything that can be done to simplify the composition of the test media is likely to aid MNM characterisation. However, the choice of growth medium also depends on the scientific question being asked. The fact that growth media differ in ionic strength, viscosity, pH, etc. is an opportunity to explore the effects of environmental chemistry on MNM aggregation and bioavailability, and should not be seen just as negative issue relating to the challenges of MNM characterisation.
Similar to soil invertebrates, an alternative strategy for confirming exposure is to measure MNM on/in the plants themselves. For example, energy dispersive spectroscopy has been used to characterise internal Cu-NPs in roots of mung bean (Phaseolus radiatus). Lin et al. (2009) use light field microscopy, combined with FTIR-spectroscopy to assess C70 fullerenes in different plant tissues (seed, root, leaf and stem). However, these measurements can be laborious, and in order to detect MNMs in tissue numerous images would need to be examined to form an opinion on how much or where the MNM is locating in the tissue. These approaches are therefore unlikely to be of routine use in regulatory tests. Nevertheless, for validation of specific routes of accumulation, or of the fate of MNMs in biological samples, these are very useful tools.
Testing microbes in soil
There are many hazard assessment protocols that involve the use of microorganisms (OECD tests in Table 2; Crane et al. 2008). These include tests relating to biodegradation including the biological oxygen demand (BOD) assay, ready or inherently biodegradability tests, aerobic/anaerobic biodegradation tests (e.g. activated sludge tests), mutagen tests (e.g. Ames test), and tests that relate to metabolism of microbes in soil (enzyme tests, soil respiration, nitrogen or carbon transformation tests). However, all these tests were designed with conventional chemicals in mind and assume that the test substance has access to the inside of the cell, but this has not been proven for MNMs.
The cell wall of microbes as a barrier to MNMs
The prokaryotic cell is protected by its cell wall around the cytoplasmic membrane, and some of the nano issues for each layer of the bacterial envelope are summarised (Table 3). The structure of microbial cell walls is diverse, and they therefore present very different types of barriers to MNM uptake. In gram-negative Bacteria, there is an extra layer (the outer membrane) not present in Gram-positive Bacteria, but then this may be compensated by the thicker murein layer in the latter (Table 3). Ultimately the structure and ligand chemistry of the cell wall will have a strong influence on how MNMs interact with each type of microbes, with nano-specific consequences (Table 3). For example, the murein protects against hydrophobic molecules due to the fixed polyanionic residues (e.g. charged amino acids, amino sugars) of the gram-positive peptidoglycan. This charge effect should repel MNMs with fixed negative charge on their surfaces (or with a net negative charge) as well as hydrophobic materials. The same argument applies to the additional outer membrane found in Gram-negative Bacteria with negatively charged molecules such as the bacteria-specific lipopolysaccharide (Neidhardt et al. 1990), but in addition the outer membrane pore size is far too small to allow diffusive entry of MNMs (Table 3). Emerging toxicological data suggest these differences in cell wall morphology are important. For example, carboxyfullerenes inhibit Gram-positive but not Gram-negative Bacteria (Tsao et al. 2002; Lyon and Alvarez 2008). However, there are also significant knowledge gaps.
Table 3.
The prokaryote envelope as a barrier to NPs
| Structure | Archaea | Gram positive bacteria | Gram negative bacteria | Nano issue |
|---|---|---|---|---|
| Cytoplasmic membrane | Lipid bilayer of mainly glycerol-ether lipids. Contains membrane spanning proteins | Lipid bilayer of mainly glycerol-ester lipids. Contains membrane spanning proteins | Lipid bilayer of mainly glycerol-ester lipids. Contains membrane spanning proteins | Hydrophobic layers, pore sizes in proteins <1 nm. Only lipid dispersible, or lipid coated MNMs may associate with latter |
| Murein layer | Absent | Relatively thick layer, 10–50 nm wide. Peptidoglycan, teichoic acids and polysaccharides. Contains fixed polyanions and hydrophilic | Relatively thin layer, 2–3 nm wide. Mostly peptidoglycan. Contains fixed polyanions and hydrophilic | Interactions of MNMs with peptidoglycans unknown. Hydrophobic MNMs less likely to penetrate this layer |
| Outer membrane | Absent | Absent | A thin peptidoglycan layer, 7–8 nm thick. Contains lipopolysaccharides. Membrane spanning porins. Contains fixed polyanions and hydrophilic | Hydrophilic MNMS likely to associate with the outer membrane. Porins too small (<1 nm pore) for NPs |
| S-layer | Glycoprotein coat forming the outer-most cell envelope layer | Glycoprotein layer covalently linked to the murein layer. Lattice structure with a pore size 2–8 nm | Glycoprotein layer covalently linked to the outer membrane. Lattice structure with a pore size 2–8 nm | S-layer interactions with MNMs not investigated. MNMs < 8 nm may theoretically penetrate the (large pore size) lattice |
Note. For clarity, the cyanobacterial cell wall is excluded, but consists in essence of a Gram positive-like murein layer with an outer layer
Archaea have so far been neglected in any culture-based ecotoxicological tests with MNMs, and are different from Bacteria in that they lack the murein layer and outer membrane (Table 3), but instead, rely on the S-layer (see below) or a reinforcement of the cytoplasmic membrane (Kandler and König 1993; Howland 2000). The lipids in the cytoplasmic membrane of the Archaea (mainly glycerol-ether lipids, De Rosa et al. 1986; Koga and Morii 2005) are different to those of the Bacteria (mainly glycerol-ester lipids, see Kandler and König 1993; Howland 2000), providing different permeability in each type of membrane. These structural differences have yet to be addressed for MNM uptake, and in relation to assay validity.
Many Bacteria and Archaea have a crystalline surface layer (S-layer), which is the outermost layer of the microbial cell and therefore will be critical for MNM interactions with the surface of the organisms. The S-layers is composed of glycoprotein molecules which non-covalently interact with each other, but are usually covalently linked to the appropriate underlying layer in Bacteria (Table 3). On average, the S-layer lattice has pores of 2–8 nm and is 5–10 nm thick (Sleytr and Messner 1988; Debabov 2006), with sugar moiety of the glycoproteins imparting a fixed negative charge to the S-layer. The S-layer could be regarded as a size-fractionating sieve for traditional chemicals and some macromolecules, but MNM interactions with this layer have not been investigated. This is a concern because the S-layer appears to be a non-conserved structure, which can even be influenced by environmental factors, and different strains of a particular species may be able to synthesise different S-layer proteins (Sleytr and Beveridge 1999). For toxicity tests, this could mean that MNM effects on microbes could be related to the variation of the structure and charge of the S-layer, requiring a greater number of microbial species to be incorporated into the testing strategy.
In contrast to the lack of knowledge on the function of the S-layer, it is known that passive entry of small hydrophilic molecules and ions into the cell is restricted in Gram-negative Bacteria and Cyanobacteria to small channels (porins) in the outer membrane. The sizes of the porins are generally too small to permit passive diffusion of MNMs. For example, the porin proteins OmpU and OmpT of Vibrio cholerae have an effective radius of about 0.55 nM and 0.43 nm, respectively (Duret and Delcour 2010). Secondly, the inner surface of the porin channel often exhibit charged amino acids (Neidhardt et al. 1990), and thus would also be a selective barrier to metal ions released by dissolution of particles at the cell surface.
The current model for the antimicrobial action of MNMs
From this short summary of the prokaryotic cell wall and cell surface it becomes apparent that single NPs as primary particles, and aggregates of NPs, are unlikely to penetrate an intact cell wall. Effects may also be material specific. Fullerenes and CNTs may not penetrate into the charged surface layers or peptidoglycan layer of the cell wall due to their hydrophobicity. Metal-NPs that may associate with the hydrophilic cell surface, will not penetrate the cell membrane because the porins/channels available are < 1 nm (see above). Therefore, it appears that the initial steps in MNM toxicity involves insult to the cell wall, which may then lead to damage of the mechanical cell defenses, and eventually, to bacterial death. Membrane damage via oxidative stress has been shown in microbes. Metal-NPs are known to lead to the photocatalytic production of ROS (e.g. Ling et al. 2004). Fullerenes also result in oxidative damage to the prokaryotic cell, despite the fact that fullerenes seem not to produce these ROS, with the targets likely being proteins rather than lipid membranes (Lyon et al. 2008a, b). Other, possible direct biophysical or chemical interactions at the NP-bio interface (e.g. hydrodynamic, electrodynamic, steric and polymer-bridging interactions) are less well known (see review; Nel et al. 2009).
Damage to the cell wall components may be prevented or delayed when the cell surface is coated by exopolymers like excreted polysaccharides (Neal 2008). These exopolymers represent a mechanical barrier to MNMs. However, once the cell wall components are damaged, the mechanical defenses would be largely lost, and MNM movement across the inner cell membrane is possible. Whether endocytotic uptake of MNMs into the cell represents a real alternative pathway is unknown. However, the recent report that endocytosis, a widely distributed process among eukaryotes, also occurs in the Planctomycete species Gemmata obscuriglobus (Lonhienne et al. 2010) may indicate that it is possible. Moreover, if so, then this path of MNM uptake may also occur in other deeply branching phyla (Forterre and Gribaldo 2010; Santarella-Mellwig et al. 2010). Once inside the bacterial cell, the NPs would have the opportunity to interact with cytoplasmic molecules (e.g. lipids, proteins, DNA), such as those evidenced by the formation of a NP-protein corona on the surface of particles (Cedervall et al. 2007), ultimately leading to the loss of structural and functional integrity of the cytoplasmic biomolecules (Nel et al. 2009), which represent the final stages of prokaryotic cell death. Clearly, oxidative damage or other biochemical changes in the cell envelope of microbes could form the basis of new ecotoxicity assay for MNMs.
An alternative mode of antimicrobial action is provided by the release of ions from metal-containing MNMs, in particular nano-Ag. Ag+ is likely to penetrate the cell wall via ion channels due to its small size and hydrophilic character, without damaging cell membranes (Yamanaka et al. 2005). Silver ions have been shown to bind to the thiol groups of enzymes (and possibly to DNA) leading to defective bacterial metabolism. In particular, the expression of enzymes required for ATP production are altered, resulting eventually in cell death (Uchida et al. 2003; Yamanaka et al. 2005). The toxicity of oxidised silver ions (i.e. Ag+ ions) has been experimentally demonstrated by comparison of oxidised Ag NPs to those that were synthesised under reducing (H2-atmosphere) conditions (Fang et al. 2007). Thus the antimicrobial action of MNMs identified so far include (Lyon and Alvarez 2008), (i) ROS generation from metal oxides, (ii) ROS-independent protein oxidation (mediated by fullerenes) followed by loss of membrane potential (only detected in Gram-positive bacteria) and cellular respiration and (iii) the leaching of metal ions into the cell via dissolution of metals from NPs at the surface of the organism.
Modifying current OECD guidelines involve microorganisms for MNMs
The validity of the current tests (Table 2; Crane et al. 2008) will depend on whether there is a logical mechanistic reason for the MNM interacting with the microbe in a way that will generate a change in the measured end point in the test. For some tests, this is clearly not the case. For example, similar to conventional dissolved metals, the biodegradability tests are not likely to be relevant to elemental MNMs, but it is also unclear on how (or if) carbon-based MNMs can be degraded by microbes. Furthermore, if the main modes of bacterial toxicity of MNMs involve attack of the cell wall components (see above), then some tests that involve intracellular mechanisms (e.g. Ames test) may be less sensitive than expected for MNMs. Except perhaps in the case of metal ion release from metal-NPs, where mutagenic effects may occur via direct association of the metal cation with the negatively charged DNA, or indirectly through inhibition of enzymatic functions involved in (for example) DNA repair. However, such an effect has not yet been reported.
In contrast, soil or sediment tests that involve some aspects of bacterial metabolism/measurements of enzyme activity in soil may be more versatile. For example, net changes in soil enzyme activity could be mediated through alterations in cellular activity or in the total number of cells in the sample. Therefore these tests should also include a component that measures microbial biomass (e.g. ISO 1997a; ISO 1997b), but even this will not account for environmentally relevant changes in the biodiversity of microbial communities (see below). Metabolic and physiological aspects of microbes are under utilized in the current testing regime, and given the potential interaction of MNMs with cell walls and membranes, a test based on changes in bacterial membrane potential would be a valuable contribution to current tests. Oxidative stress end points could also be utilized to measure the oxidation of lipids and proteins, either via ROS-mediated or ROS-independent pathways, or the direct action of metal ions on the thiol groups of proteins inside the cell (e.g. Ag+). Such assays are already applied in fundamental research with microbial cultures in the laboratory (e.g. Lyon and Alvarez 2008; Lok et al. 2006), and it would be a small step to modify these assays to work with soil samples, or indeed to extract the microbes from the soil using physical and chemical approaches (e.g. Kowalchuk et al. 2004) prior to applying the assays.
New approaches to testing microbial responses to MNMs
In the long-term, new, additional tests that utilise novel information on the cellular response of microorganisms specific to their contact with MNM may become feasible. A possible approach, for example, could include assessment of quantitative changes in the expression of genes associated with the cell wall, or profiles of genes representative of the cellular response of the whole microbial community in a sample. First examples of potential cellular responses at the molecular level stimulated by the treatment with Ag NPs are provided by Lok et al. (2006), while Yamanaka et al. (2005) present results from similar proteomic studies, except that cells were treated with Ag+ instead of NPs (see above). The findings from the proteomic analyses of the response to Ag NPs which indicate an increased expression of, among others, genes coding for outer membrane protein precursors was further corroborated by physiological assays that revealed destabilisation of the outer membrane, collapsing of the plasma membrane potential and depletion of the levels of intracellular ATP (Lok et al. 2006).
Changes in microbial community structure may also be environmentally relevant, but the vast diversity of prokaryotes cannot be cultured in the laboratory, despite sophisticated isolation techniques (e.g. Joint et al. 2010). Many important groups of microbes are not represented in the suite of current regulatory tests, but fundamental molecular research has provided a way forward. Shifts in the microbial composition in natural samples may occur during exposure to MNMs, and this can only be evaluated using molecular techniques involving DNA extractions from the soil/sediment sample (e.g. Bradford et al. 2009). These investigations are labour-intensive and are usually only undertaken in the course of academic research, but nevertheless, reveal important insights into microbial biodiversity that cannot be provided by any of the other tests. For example, a simple enzymatic test may show similar activities in test and control samples, although the composition in the microbial community may have changed. Such a scenario is feasible with chronic exposure to antimicrobial MNMs where long-term exposure ultimately leads to the selection of resistant cells. If those resistant cells then replace cells that have been inhibited by MNM exposure, then no overall effect in the enzymatic test may be detected.
In addition, measures of resistance that reflect the co-localisation or expression of more than one gene may be especially useful. For example, it is now clear that metal-contaminated sediments from marine harbours contain microbes with increased antibiotic resistance (e.g. Baker-Austin et al. 2006). This occurs in the absence of any appreciable antibiotic pollution at these sites, and arises because the genes for resistance to antibiotics are often incidentally co-selected with those for metal resistance. Both sets of genes are often encoded on the same plasmid (e.g. Hernández et al. 1998). Metal contaminated soils have been found to contain more plasmids than uncontaminated soils (Rasmussen and Sorensen 1998). Furthermore, the linkage of these genes on plasmids allows them to be transferred to other cells via horizontal gene transfer, thus facilitating the spreading of both resistance patterns despite the presence of only one selective pressure. Therefore, tests of co-selection for antibiotic resistance (Mühling et al. 2009) may also be useful for metal-containing MNMs.
Up to now, all of the studies into potential changes in microbial diversity caused by the exposure to MNMs have used genetic fingerprint techniques (e.g. denaturing gradient gel electrophoresis, DGGE: Tong et al. 2007; Bradford et al. 2009) that provide only a limited genetic resolution. However, recent advances in modern high-throughput sequencing techniques (e.g. 16S-tag pyrosequencing: Gilbert et al. 2010) and the use of group-specific PCR primers (e.g. Mühling et al. 2008) now allow targeted and in-depth analyses of shifts in the composition of the microbial communities upon MNM exposure and should be applied to future investigations. However in the scramble to use new molecular technology, one must not lose sight of the most important prerequisite for the development of standardised tests involving molecular techniques; that is the reproducible isolation of total environmental nucleic acids (DNA, RNA) from the soil sample. Fortunately, details of appropriate methods are available in laboratory manuals (e.g. Kowalchuk et al. 2004), and those have been shown to work for the extraction of high quality environmental DNA from soil and sediments (e.g. marine sediments, Bradford et al. 2009).
Environment conditions during experiments and taxa specific issues with MNMs
The question of whether or not aged soils or MNMs (discussed above) will interfere or alter the results of microbial tests is currently unclear. In the case of the assays involving environmental samples consideration must be given to details describing the status of the environment in which the MNM pollution is likely to occur. For example, Ag NPs proved to be antimicrobial under toxic conditions, while the same size of Ag NPs that were produced and tested against microorganisms under a reducing atmosphere (N2 atmosphere) resulted in no detectable toxicity (Lok et al. 2007). Apart from the reducing effects on metal-NPs the exclusion of oxygen should also result in a lower likelihood of damaging cells through ROS. Such environments that are characterised by anoxic reducing conditions represent likely sinks in which NPs may well accumulate (e.g. in the anoxic sediments of aquatic environments including water reservoirs, lakes, rivers and estuaries; see Klaine et al. 2008).
Moreover, the OECD guidance manual for the testing of MNMs indicates that if the substance is expected to partition into the soil, then microbial toxicity testing relating to nitrogen fixation or carbon transformation may be necessary. It also indicates that the impact of MNMs to anaerobic microorganisms should be considered (OECD 2000). Therefore, standardised tests of representative microbial activities under anoxic conditions (e.g. denitrification, dissimilatory sulphate reduction, methane production, dissimilatory metal reduction) need to be developed; and if tests are to be carried out on representative isolates, these need to be defined. Currently, the only available OECD test that involves anaerobic microorganisms evaluates inhibition of their metabolic activity by measuring the reduction of gas production from anaerobically digesting (sewage) sludge (OECD Test No. 224). In this context it should be noted that methane production is performed by microorganisms that are not specifically included in any of the regulatory tests: the Archaea. These organisms are responsible for all of the methane production and also contribute to other globally important metabolic services. As outlined above, Archaea may be affected in a different way by MNMs as compared to Bacteria due to differences in the composition of their cell wall. Therefore, future research should include the analysis of the impact of MNMs on Archaea and the development of standardised test(s) for anaerobic microorganisms, as well as aerobic and anaerobic strains of the Archaea.
Representative soils for microbial tests
In addition to the experimental design issues discussed above for soil tests with invertebrates and plants, natural variation in the soil composition and even seasonal changes in the same soil, can have a dramatic effect on microbial activity and/or diversity. The use of reference soils are therefore also recommended for microbial tests. These can be sterilised and then inoculated with a defined test microorganism(s), so at least the microbial composition of the soil is bench marked at the start of an experiment. The soil parameters that are particularly crucial to control or monitor for microbial tests (in addition to organic nutrients, clays etc., above) are the water and oxygen content, the redox potential and the pH. Nanomaterials that generate ROS are likely to alter the latter three parameters. Interestingly, the natural diversity and richness of soil bacterial communities differ by ecosystem type and these differences appear to be largely explained by soil pH (Fierer and Jackson 2006). Storage of sterile soil samples can be conducted at +4°C for up to 4 weeks, or longer if performed at −20°C (see ISO 1993). The replication issues are also similar to above on soil invertebrates. Notably, despite the much higher microbial diversity in soils (e.g. Curtis et al. 2002), a triplicated design also seems to work for sets of individual natural marine sediment samples (i.e. not from a defined artificial test matrix, e.g. Bradford et al. 2009).
Tests with aquatic primary producers
Algae are included in many hazard assessment schemes as representatives of the aquatic primary producers. Standardised protocols have been developed for regulatory testing (Table 1 for the OECD tests, also ISO 1989; ASTM 2003; US EPA 1996a; see Janssen and Heijerick 2003 for a review). Less standardised protocols have been developed for testing marine microalgae (US EPA 1995; US EPA 1996a) but guidelines for marine algae tests are available (Thursby et al. 1993; Walsh 1988). Most protocols for marine algae use artificial sea water recipes (Berges et al. 2001). All standardised tests on algae are based on growth inhibition. The guidelines do give recommendations on experimental conditions for algal tests including duration (usually 72–96 h), the chemical composition of the media, light conditions (quality, intensity and photoperiod), shaking and salinity (for marine algae). However, the researcher is given considerable choice in the parameter for lighting and shaking of the cultures, and the latter especially will alter particle settling. The test guidelines may therefore require further standardisation of these parameters for MNM exposures. The protocols often include a choice of several test species of algae (see Table 1), and with insufficient data on MNMs in these tests, it is not yet clear if one species is more sensitive than another. Macrophytes as representatives of higher aquatic plants are also important in risk assessment schemes. Currently, standardised growth inhibition tests have been published only for the duckweed Lemna sp. (e.g. protocol numbers DIN EN ISO 20079, ISO 20079, OECD 221, see US EPA 1996b). Lemna is a floating fresh water plant, with roots suspended in the water column. The exposure if therefore via the water, and this at least lends the media to some of the particle characterisation methods discussed above. Data on tests using macrophytes are currently lacking for MNMs.
Abiotic factors and MNMs in algal tests
The composition of the test media is standardised with respect to ionic composition, but there is a conflict for MNMs. The salts in the media ensures that the algae are not nutritionally limited for growth (the main end point), but the same salts will promote NP aggregation and therefore removal of the test substance from the water column. The OECD 201 algal growth test growth includes 18 mg l−1 CaCl2 and 15 mg l−1 MgSO4, these cations will promote aggregation by charge screening on the electric double layer (for example) but without these nutrients the algae will not grow well. Critically, unlike toxicity tests with animals, the algae obtain all their minerals from the water and for MNM exposures there is no option of removing these minerals; although Navarro et al. (2008a) have suggested reducing the mineral content. Algae also produce exudates containing macromolecules and mucous-like proteins (Soldo et al. 2005) which may promote agglomeration of the test material. Alternatively, small organic macromolecules could coat the NPs to provide more stable dispersions than expected. The key point is that the release of exudates is likely a much bigger problem for MNM experiments compared to traditional chemicals, and the experimenter has little control over this for maintaining the exposure. For metal-based MNMs, the precise composition of the algal media will influence dissolution rates, and the bioavailability of dissolved metal ions in the solution. Dynamic changes in particle number and particle size distribution are therefore expected, and pragmatically one can attempt to measure MNM behaviour in the test media frequently during the experiment. These issue are well documented in a recent study by Hartmann et al. (2009) using three different sizes of TiO2 NPs. Establishing a concentration–response relationship for the three particle sizes was difficult, and the reproducibility of the exposures was influenced by the concentration-dependent aggregation of the NPs, and their sedimentation and sorption to test vessels, as well as the effects of exopolymeric exudates.
The presence of MNM aggregates in algal experiments deserves particular attention because this will influence light penetration into the media, photosynthesis and therefore growth. Lighting regimes are well known to alter algal growth tests (Cleuvers and Weyers 2003), but the specific problem for MNM experiments is to include a control for light scattering or shading effects. Researchers have used two-compartment vessels to physically separate the MNMs from the algae, and illuminated the cultures from the side containing the MNM suspension. This method has been used to assess growth inhibition of TiO2 NPs and shading effects were limited or absent even with larger sized aggregates (Aruoja et al. 2009; Hartmann et al. 2009; Hund-Rinke and Simon 2006). However, the possibility that MNMs could precipitate and cover the surface of the algae, or shade the entire dispersion cannot be excluded (Aruoja et al. 2009). In addition, MNMs on the surface of the algae or in the suspension could alter nutrient availability or induce other physical effects on algal growth (Hartmann et al. 2009). One possible amendment to protocols could be to increase the light intensity, and is already recommended in tests to assess the toxicity of coloured substances to algae (Cleuvers and Weyers 2003). Effects of MNM aggregates on algal assays have also been associated with other indirect effects. Sorption of TiO2 NPs to algae resulted in a 2.3-fold increase of cellular weight (Huang et al. 2005), potentially confounding biomass measurements. Similarly, aggregates of carbon black adsorbed to sperm cells reduced fertilization success of the marine seaweed Fucus serratus, mainly due to physical effects (Nielsen et al. 2008).
End points in studies with primary producers and MNMs
Most studies examining the effects of MNMs on primary producers, so far, have reported concentrations that inhibit growth and/or photosynthesis in mainly freshwater algae (see review; Kahru and Dubourguier 2010) and in some marine algae (Miao et al. 2009; Wei et al. 2010). The current state of knowledge does not allow any firm conclusions on the mechanisms leading to growth inhibition for any type of MNM and there is a clear need to explore a variety of additional variables including molecular, biochemical and physiological end points, as well as structural/cytological end points. For instance, measurements of glutathione in a study with functionalised multiwalled carbon nanotubes (f-MWCN) suggested oxidative stress occurred in the marine algae, Dunaliella tertiolecta (Wei et al. 2010). In the case of TiO2 NPs, toxicity to algal growth has been suggested to result from particle adhesion to algal cells, with disruption of the cell wall and/or the formation of ROS (Hartmann et al. 2009), but these hypothetical mechanisms remain to be examined. The use of fluorescence techniques, including staining with fluorochromes specific for cellular structures combined with detection by flow cytometry, or exploring specific cellular constituents (e.g. intracellular Ca spikes measured with FURA-2) by fluorescence microscopy, are promising approaches to provide mechanistic information. Such experiments would need to include blanks to assess the interference of the MNMs with each fluorescent probe, and dye calibrations spiked with the appropriate MNM.
Morphological observations using light microscopy should also be performed in algal experiments with MNMs to examine effects on cell shape, cytology and the integrity of the cell wall. These observations can simply be qualitative to indicate the potential effects or type of exposure to the MNM. For instance, microscopic observations have indicated cellular structural changes in the green algae Chlamydomonas reinhardtii exposed to silver NPs that are distinct from those elicited by dissolved silver ions, suggesting nano-specific effects (Behra, unpublished results). Morphological observations on cells can also be quantitative to enable statistical analysis of the data.
Currently, it is unclear if MNMs can be internalised by algae and plant cells. Similar to the situation described above for microbes, plant and algal cells also have a polyanionic cell wall (consisting of cellulose, glycoproteins and polysaccharides) that constitutes a barrier to the internalization of MNMs. It is also a primary site of interaction with particles (Navarro et al. 2008b). The diameter of pores across algal cell walls have a size ranging from 5 to 20 nm, and determines their selective properties; allowing the passage of small molecules while limiting the passage of larger ones (Fleischer et al. 1999; Zemke-White et al. 2000). Consequently, only primary particles or agglomerates with a size smaller than the pores are expected to pass through the cell wall and reach the plasma membrane (Navarro et al. 2008b). Permeability of the cell wall might change during reproduction with the newly synthesised cell wall being more permeable to MNMs, suggesting that growth (involving cell divisions) should be a sensitive end point for these materials. Like microbes, damage to the cell wall will facilitate particle internalisation, and one might therefore expect threshold effects on biochemical end points that rely on the MNM being inside the cell.
Aquatic invertebrate tests
Ecotoxicity tests with aquatic invertebrates can be conducted in water (e.g. artificial water, natural freshwater or seawater) or using aquatic sediment where organisms are exposed to a sediment with some overlying water. Many of the exposure issues described above for protocols using freshwater algae and soils apply to tests with invertebrates in water or sediment systems respectively, but issues specific to aquatic invertebrates are described here.
Tests with invertebrates in aquatic media
Aqueous tests can be conducted without renewal (static tests), or with daily renewal (semi-static) of the test media. Using current OECD protocols, exposure vessels for aquatic invertebrate tests are not stirred or shaken (unlike the algal tests). It is therefore inevitable that some settling of the MNM will occur with the current protocols. It is possible to gently stir beakers of invertebrates, or to create some mixing of the media with aeration. This may be appropriate to some marine and river invertebrates where they might normally experience significant water movement in their natural habitats. However, for organisms that live in still waters like the water flea, Daphnia magna, or the larval stages of chronomids, there is a risk of mechanical damage to the delicate structures (chaetae, sense organs, etc.) on the surface of the animals. Careful experiments remain to be done to define what level of mixing/aeration can be achieved in beakers of invertebrates to improve dispersion of the MNM without causing stress to the test organisms.
The Daphnia immobilisation test is a widely used acute toxicity test (OECD test 202, Table 1), but there are some issues with using immobilisation as an end point with MNMs. It is possible that the physical effect of MNMs sticking to the carapace and appendages of the Daphnia could result in restricting the movements of the animals (false positive on the immobility end point; Rosenkranz et al. 2009; Gaiser et al. 2011) when MNM concentrations are very high (e.g. mg l−1 levels). However, there is also a toxicological feature to these effects in Daphnia species. They ventilate by movement of the appendages to create a flow of water over the respiratory surface. If this movement is stopped, then toxicity by suffocation is possible. Roberts et al. (2007) demonstrated that mg l−1 concentrations of lysophophatidylcholine coated single-walled carbon nanotubes could adhere to Daphnia magna and cause abnormal swimming where the animals would sink to the bottom of the test containers. One interpretation of this phenomenon is to simply allow it occur during the test, and regard it as a “non-chemical method” of producing the immobilisation end point. A “particle control” for the mechanical effects of MNMs might be needed, but exactly how this could be done is unclear at present.
Another concern is whether or not to feed invertebrates during ecotoxicity experiments with MNMs. Many invertebrate cultures are feed on unicellular algae, or other particulate food material. For short tests lasting only a few days, it is simply a matter of not feeding the test organisms. This is, for example, the standard procedure for acute Daphnia test (48 h duration; OECD 202). This is not the case, in longer tests such as the Daphnia reproduction test (e.g. OECD 211) which lasts 21 days. In such experiments, it is an essential husbandry requirement to feed the test organisms. Clearly, any food particles added to the media will interfere with attempts to measure the size distribution of the MNM being tested. A simple modification of protocols can avoid this problem, for example, by feeding the animals for a few minutes before a water change, and renewal of the test media immediately after the animals have fed. There are also some scientific concerns about the exposure in the presence of food particles. At least one report confirms that the uptake of MNMs by Daphnids can be quite different in presence of food particles, comparing to exposures taking place in the absence of food (TiO2 NPs, Zhu et al. 2010). This is perhaps not surprising, since aquatic invertebrates will often increase the processing of water over their body surface to extract any food when it is present, and the food particles will also act as a surface for agglomeration of the test material. Both of these processes might increase exposure of the test organism, but how this might alter the outcome of a reproduction test (for example) is unclear. Critically, invertebrate studies are reporting apparent MNM accumulation in the organism, when in fact it is simply ingested material present in the gut lumen. The correct use of terminology is required. For example, for kinetic flux studies the net uptake is usually defined as the net transfer of the substance from the external environment to the systemic circulation of the animal. Accumulation occurs when the steady state unidirectional influx exceeds the efflux, and thus refers to the internal compartments of the animal only. The gut lumen contents represent the ingested dose not the accumulated body burden. Experimentally, even if animals are depurated of gut contents or rinsed in clean water, the measured concentration for the whole body may include a surface-bound component (adsorption rather than true uptake). If the methodology is carefully reported, one can at least correctly interpret whole body measurements.
Tests with invertebrates in aquatic sediments
Most of the issues described above for measuring MNMs in soils, and the inability to routinely track MNMs in the exposure media, equally applies to sediment tests with invertebrates. Consider the OECD sediment tests using Chironomus species, non-biting midges (OECD 223), and Lumbriculus variegatus, a freshwater oligochaete (OCED 225, see Table 1). In the chironomid tests and the Lumbriculus test, the current protocols allow directing spiking of the sediment; with all the issues or how to achieve representative mixing as described for soils (Table 4). The aim of spiking the sediment with the test material is the same as that in setting up a soil test; to evenly mix the test MNM throughout the sediment sample, and do this in a way that minimises variability between replicates of each concentration of MNM. However, in the chironomid tests it is also possible to spike the overlying water. The approach of spiking the water may be more environmentally relevant, and such spiking methods can give a consistent coating of the MNM being tested on the surface layers of the sediment, especially if the test material rapidly settles out of the water phase (e.g. Bradford et al. 2009). In all these sediment tests it is permissible to use natural sediments and/or waters “for specific testing purposes”. Similar to the soil tests and some of the microbial tests, there is a vast range of natural sediments (marine to freshwater) with very different compositions (percent of sand, clay, organic matter etc.). An artificial sediment might therefore be used for “bench marking” while a consensus view on the effects of sediment type on nanotoxicity is emerging, and further standardisation of the mixing/dosing of sediments with MNMs is evaluated.
Dosing of the sediment, or overlying water, will be subject to all the experimental design issues discussed above for soils with no-added MNM controls, dispersion controls where dispersing agents are used, bulk material controls and/or metal salt controls as appropriate to the material being tested. The Lumbriculus test runs over 28 days, and the chironomid tests can be up to 100 days. The issue of MNM ageing or modification identified above for soil tests also applies to sediment tests. Similar to soil tests, it remains impractical to renew the test sediment to prevent MNM modification, or to maintain exposure during the test, although it is possible to refresh the overlying water in a sediment test. If food is added to the overlying water, similar to aquatic tests, this could be done just prior to water changes, although it is inevitable that material will settle onto the surface of the sediment. In the Lumbriculus test, it is common practice to mix food into the sediment, when the test chambers are initially prepared. This method is also practical for MNMs. The end points of the Lumbriculus test are biomass and the total number of worms per replicate. Optionally, reproduction (as increase of worm numbers) and growth (as increase of dry biomass) can also be evaluated, in relation to the initial values. Mortality may also be recorded. There is currently no evidence that these current end points are inappropriate for tests with MNMs. the mixing protocol for MNMs in both sediment and soil tests is needed for regulatory tests.
Fish toxicity tests
Animal husbandry in experiments with MNMs
There are some particular animal husbandry problems with setting up fish tests that are specific to MNMs. Trout, for example, are especially sensitive to ammonia or low oxygen levels, and for this reason, researchers might prefer to use flow-through methods from the view point of animal husbandry. However, this approach would also generate lots of waste water (e.g. hazardous waste in the case of CNTs in the UK), and so semi-static exposures are preferred for MNMs (Federici et al. 2007; Smith et al. 2007). Animal husbandry also requires that the animals can be easily observed every day, and the semi-static methods also allows close observation of the animals in situations where the MNM may also discolour the water (e.g. very dark water from high concentrations of C60). Increases in aggressive behaviours have also been noted in trout during MNM exposures after about 5 or 6 days (TiO2 Federici et al. 2007; SWCNT, Smith et al. 2007). This problem is not likely to be an animal husbandry issue for acute tests lasting only a few days, but can be in 14 day or longer experiments. Feeding can reduce aggression and current regulatory protocols that exclude feeding (i.e. experiments with unfed fish) should be reconsidered to address this problem for MNMs. A single short feed with a maintenance ration (2% of body mass) immediately after the water change can reduce aggression without compromising water quality (Smith et al. 2007). Aggression can result in mortalities, and this is therefore an important husbandry issue, especially for CNTs (Smith et al. 2007). Also, exclude apparatus from the tank that can become the focus of aggressive behaviours, such as air stones that can be bitten by the fish (also releasing particles into the water in the process).
Ethical considerations require experimental replication (i.e. the use of animals) to be carefully considered. Traditionally, regulatory tests have used pseudo-replication (one tank/treatment), but this must be balanced against the particular problem of maintaining MNM dispersions, and the possibility that between-tank variability with some MNMs will inevitably be greater than conventional soluble chemicals. Measurements from very carefully controlled triplicate tanks in semi-static tests with trout show that the replication was good, enabling (in theory) the pooling of the data within triplicates for statistical analysis where the individual fish are also can be identified (e.g. Federici et al. 2007). However, it would be prudent to judge this on a case by case basis with each MNM until a weight of evidence is available to confirm whether or not pseudo-replication is “adequate” for a regulatory test, and whether this will remain ethically acceptable.
Maintaining exposure in fish tests with MNMs
Unlike many of the algal and invertebrate tests, fish can produce large quantities of mucus when irritated by chemicals in the water column (see Handy and Maunder 2009 for a review of fish mucus). MNMs readily form aggregates/agglomerates with fish mucus components (e.g. SWCNT, Smith et al. 2007), which inevitably remove the test material from the water column, and deposits clumps of MNM-contaminated mucus on the bottom of the tank (which the fish may then ingest). The semi-static exposure method can ensure these clumps are siphoned off with regular water changes, and the test water renewed. This semi-static renewal of test water involves some labour, but is achievable over experiments lasting a couple of weeks. The prospect of doing at least twice daily water changes for longer (e.g. a month) for a chronic fish test is not ideal from a logistics view point, but it is possible to use this method to maintain exposures via the water for many weeks. For experiments with fish larvae and embryos, and small fish such as zebrafish, experience has shown that the problems of mucus production and loss of the test material are less important; and it may be possible to limit water changes to once a day.
Bioaccumulation tests and dietary tests for MNMs
Bioaccumulation tests were originally designed for conventional chemicals, to test the notion that organisms can take up substances from the water column faster than they can excrete them, leading to a net bioaccumulation in the long term. When the organism is at steady state (in equilibrium) with the substance in the water, BCF may be calculated (Veith et al. 1979). However, the idea of steady state equilibria is at best theoretically difficult, or even inappropriate for colloid chemistry. For colloids, the concept is one of a dynamic system that creates the dispersion; one which is not at a steady equilibrium state (Handy et al. 2008a). It would therefore seem that one of the founding assumptions of “steady-state” in the BCF measurement does not apply to the chemistry of MNMs. At present, there are also some technical barriers in our ability to measure MNMs in tissues and the water accurately, and so for example, measuring the unidirectional flux of a MNM across the gills is difficult or impossible for many materials. In addition, even if such a flux could be measured, until the mechanism(s) of uptake are established it would be difficult to assign curve fitting or kinetic theory to any data obtained (e.g. the Michaelis-Menton kinetic plots usually used for solute flux may not apply). Moreover, if MNMs are being moved across the epithelial tissue like gills by endocytosis (See Handy et al. 2008b), then simple diffusion models like the Fick equations will also not apply. These are substantial conceptual problems that undermine the validity of bioaccumulation tests. It would be illogical and unhelpful to simply do the current bioaccumulation tests, and then fit the data with no underlying rationale for the kinetics or shape of the curves produced.
Given the practical difficulties of maintaining MNM exposures in the water column, one possible alternative suggestion is to dose via the food for chronic studies. While the approach of developing a dietary bioaccumulation factor (BAF test) for organic chemicals may be appropriate to overcome practical problems associated with aqueous exposures, the fundamental conceptual problem that the chemistry is not “steady state” and the kinetics not based on solute transporters remains for the gut mucosa. In addition, the technical problems of doing dietary exposures are equally as challenging as doing exposures with dispersions in water. For example, there are considerable problems with the matrix of fish diets (see discussion in Ramsden et al. 2009). Essentially, the diet will contain a myriad of natural nanoscale particles, making it technically difficult (currently impossible) to verify the particle distribution in the food (i.e. a similar problem to soil above). In addition, some naturally occurring micron scale materials are also already present in many fish formulations (e.g. natural minerals in the ingredients, TiO2, silicates, iron, etc.) making the notion of a particle-size control in such experiments very difficult to apply. If one attempts to remove such natural mineral particles from the food, or to “dilute” them with other dry ingredients (e.g. by adding extra protein or carbohydrate) then the nutritional value of the food may be compromised, or at least abnormal, and could lead to false positives in the testing regimes (i.e. nutritional deficiency not MNM toxicity). For example, it is well known that aquafeeds containing oxidising metals can strip antioxidant vitamins from the food (e.g. Cu salts depleting vitamin E levels in fish food, Baker et al. 1998); resulting in the food being nutritionally deficient. These problems can be overcome by adjusting feed formulations, but the necessary information to do this is lacking for MNMs. Furthermore, one cannot simply change the feed formulation without first understanding the effects on the digestibility of the food, and subsequent bioavailability of the MNM. The current understanding of how MNM behave in a food matrix is very rudimentary, and there is a high risk of misinterpreting results from any dietary bioaccumulation factor test with MNMs at present.
In vitro testing methods
In vitro testing strategies using cell culture systems or isolated cells to screen toxic substances or to test for particular toxic mechanisms (e.g. immunotoxicity, genotoxicity) have been discussed at length for conventional chemicals (e.g. Schirmer 2006; Galloway and Handy 2003; Jha 2008); and are now being applied to MNM (review, Handy and Shaw 2007; Stone et al. 2009). Similar to the testing of other substances, there is also an ethical driver to find non-animal alternatives for the hazard assessment of MNMs. However, like the in vivo tests above there are methodological concerns about dosing, maintaining exposure, the effects of MNMs on the cells or other biological material in the test, and whether or not the current measurements in each test are valid for MNMs. There are a wide variety of in vitro methods used in traditional ecotoxicology including studies with perfused organs (e.g. Handy et al. 2000), cell culture systems (Schirmer 2006), membrane vesicle preparations (Glover and Wood 2008); and now omics with “systems toxicology” (review, Handy 2008). Rather than discuss individual details for all the possible in vitro methods, the approach here is to use genotoxicity as a case study to highlight some of the key issues for in vitro testing with MNMs.
Currently, assessment of the genotoxicity of substances can be performed using a variety of end points, such as single- and double-strand breaks, point mutations, cytogenetic assays (e.g. induction of chromosomal aberrations, micronuclei), DNA repair and cell-cycle measurements (Ng et al. 2010). These end points apply to both human-related toxicity in vitro (human and mammalian cells), as well for ecogenotoxicity evaluation (Dixon et al. 2002; Jha 2004; Raisuddin and Jha 2004; Jha 2008; Papis et al. 2011). For MNMs it is worth keeping this broad perspective in testing strategies so that relative risk to both wildlife and humans can be assessed. Additionally, the use of different cell types from different organs allows a more flexible approach while data on the most appropriate target organs or tissues is emerging from in vivo studies.
The current tiered approach for the genotoxicity testing of chemicals involves an initial in vitro screen for genotoxic potential using the Ames test (see below) and this is usually followed by an appropriate in vitro cytogenetic assay. If both tests are negative, then the substance is regarded as “non-genotoxic”. However, if either of the in vitro tests provide a positive result, then the second tier would involve in vivo testing (often the micronucleus (Mn) assay, below and DNA repair assays using liver samples). The tiered approach and the associated technical difficulties for each method have been recently reviewed for MNMs (Singh et al. 2009; Landsiedel et al. 2009; Doak et al. 2009). For both the human health and ecotoxicology, an additional tier (either in parallel or series) near the start of the testing strategy is needed to incorporate information on particle characterisation, and to determine whether or not the nano form can be demonstrated to have a different hazard to the nearest equivalent bulk chemical. Like the in vivo studies above, the use of dispersion controls and an appropriate bulk powder or mineral salt control should also be incorporated in the test methods.
For ecogenotoxicity a range of species that represent organisms from different habitat and/or trophic level should be used. This is especially important at this early stage in fate and behaviour studies, where there remains uncertainty over exactly where MNMs will accumulate in ecosystems. The current organisms used for ecogenotoxicity include collecting cells from a variety of marine and freshwater fish, bivalves (e.g. Mytilus species) and sea urchins, as well as the more traditional organisms used in genetic studies like Drosophila (Jha 2004, 2008). Similar, to the soil tests above, there is not enough information on species sensitivity to MNMs to establish which is the most appropriate species or cell model to use for a particular type of MNM in the in vitro testing strategy. In the absence of such information for MNMs, researchers should continue to consider the ecosystem or habitat being protected, trophic level, appropriate models for the geographical region (e.g. cells from temperate or tropical species), and ethical considerations for non-destructive sampling (e.g. blood samples) of vertebrate animals or other protected species.
Several genotoxicity assays have been proposed for MNMs which together, determines both intrinsic and expressed genotoxicity, and at different levels of genetic organisation (i.e. base pairs, genes, DNA strands and chromosomes). The most commonly applied methods for detecting genotoxicity in wildlife and mammals include the bacterial Ames test (plasmid nicking assay), DNA strand break measurements in cells (e.g. the comet assay), and the cytogenetic assays (Mn and chromosomal aberration assays, including the use of fluorescence in situ hybridization and chromosome paintings). The application of these tests for the ecogenotoxicity testing of MNMs is discussed below.
Ames test
In regulatory toxicology the most commonly used genotoxicity assay is the prokaryotic point mutation Ames test, a bacterial reversion mutation test (Mortelmans and Zeiger 2000). The test involves using bacterial strains with a gene mutation for histidine synthesis. The bacteria cannot grow in histidine minimal (i.e. histidine deficient) agar media. After treatment of the bacteria with mutagenic compounds, reverse mutations in the histidine gene can occur enabling the bacteria to synthesise histidine and thus grow and form colonies in the minimal histidine media. The Salmonella/microsome Ames test has been used to assess the genotoxicity of several MNMs, including C60 fullerenes (Mori et al. 2006; Shinohara et al. 2009), MWCNT (Szendi and Varga 2008; Di Sotto et al. 2009; Wirnitzer et al. 2009), aluminium oxide NMs (Balasubramanyam et al. 2010), iron-platinum nano composites (Maenosono et al. 2007, 2009), ZnO NPs (Yoshida et al. 2009), and various NPs generated as combustion by-products (Miraglia et al. 2005). However, there are a number of concerns as to why the Ames test may not be appropriate for MNMs. First, the basis of the test is that the genetic material inside the organism is exposed to the test substance. For the reasons outlined above on microbes, many MNMs may not cross the cell wall, and therefore will produce false negatives in the test. In addition, the agar media (polyanionic) may prevent the migration of positively charged MNMs, also producing false negatives in the test. It is also unknown how MNMs will alter amino acid metabolism in bacteria, and one should not assume that histidine synthesis will occur normally (down regulation, false negative; up regulation, false positive). It is therefore perhaps not surprising that the Ames test has, so far, produced largely negative results (no effect) for MNMs (Landsiedel et al. 2009), despite the fact that in principle, MNMs could interact with bacterial DNA (An et al. 2010). Given the risk of false negatives, the Ames test should not be used alone as a critical tool in the decision making on hazard, but should always be considered in context with the results of other genotoxicity assays.
Single cell gel electrophoresis (Comet assay) and other techniques to measure DNA strand breaks
Single cell gel electrophoresis, or the comet assay, has been used extensively to evaluate direct as well as oxidative DNA damage (Collins et al. 1996; Dušinská and Collins 1996; Reeves et al. 2008; Vevers and Jha 2008). The assay detects DNA damage at the level of a single cell. Under alkaline conditions, DNA breaks are relaxed from super coiling; and following electrophoresis ‘comet tails’ are formed from the DNA fragments. The assay can measure the general magnitude of the strand breaks (size/shape of the comet tail), as well as specific DNA lesions. The latter is determined by incubating the nuclei embedded in agarose with (for example) lesion specific bacterial endonucleases III (endo III), or formamidopyrimidine DNA glycosylase (Fpg). Fpg recognises oxidatively modified purine bases, and thus can be used to detect oxidative DNA damage (Collins et al. 1996; Dušinská and Collins 1996). The issue of MNMs interfering with the properties of the agarose gel during electrophoresis is being investigated (Dušinská, personal communication), because of a theoretical possibility of false positives if the MNM causes the DNA fragments to aggregate, or in some other way slows the migration of fragments in the agarose gel. However, this concern is only theoretical, and researchers at the bench are not reporting significant additional problems with the electrophoresis in MNM experiments so far. Interference of MNMs with any enzymes used for incubations should also be tested before starting the assay, by measuring enzyme activity in MNM-spiked solutions.
Some MNMs are photo-reactive, and the generation of ROS resulting ultimately in oxidative DNA damage, can be measured using the comet assay. Several authors have measured ROS generation (with/without UV activation) in cell culture systems that lend themselves to the comet assay (Reeves et al. 2008; Vevers and Jha 2008; Dodd and Jha 2009, 2011). The comet assay is currently being validated by ECVAM/JaCVAM and a draft guideline protocol for mammalian regulatory purposes is being developed for conventional chemicals, and within the OECD sponsorship programme for MNMs. Some of the advantages of this technique for conventional chemicals (rapid, sensitive, inexpensive, ability to study DNA repair and cell death in different cell types, see review, Jha 2008), are likely to apply to MNMs. However, the sensitivity and reliability of the assay needs establishing for MNMs incorporating some of the precautions suggested above.
Another assay to detect DNA strand breaks is DNA unwinding and hydroxylapatite chromatography (Dušinská and Slamenová 1992). This assay has been used for detection of DNA damage in mammalian cells, and in ecotoxicology (e.g. in pyloric caeca of the sea star Asterias rubens L., in fish RTG-2 cell line derived from rainbow trout (Oncorhynchus mykiss) gonadal tissue; Hansen et al. 2009). The technique is based on the time-dependent partial alkaline unwinding of DNA and determination of single and double stranded DNA using chromotography (Everaarts et al. 1998). Similarly, pulsed-field gel electrophoresis (PFGE) has been used for detection of double strand breaks in fish RTG-2 cells (Hansen et al. 2009). However, these DNA unwinding assays have not yet been applied to MNMs, although the issues relating to false negatives in electrophoresis gels and solid phases in chromatography will apply. Where chromatograph is used, the effects of the MNM on the solvent front, and capacity/binding/elution characteristics of the column should be tested using assay blanks (the MNM and no biological sample), and also reference material (e.g. DNA standards) with/without the MNM to ensure the chromatography column is working correctly.
Micronucleus assay
The Mn assay, and related chromosomal techniques, were originally developed for mammalian systems, and the more varied karyotypes of non-mammalian cells present an inherent technical difficulty for scoring chromosomes from the organisms used in ecotoxicology (Dixon et al. 2002; Jha 2004; Raisuddin and Jha 2004; Papis et al. 2011). For example, metaphase techniques such as sister chromatid exchanges and chromosomal aberrations are not practical methods for many aquatic species. However, it is possible to score cells from a few fish species for chromosomal aberrations in the laboratory. Recently, Wise et al. (2010) applied these methods to a cell line from the medaka (Oryzias latipes), and found that exposure to Ag NPs (30 nm primary particle size) could induce chromosomal aberrations and aneuploidy in the cells. Unfortunately, the fish and invertebrate species that have found some utility in the laboratory for karyotyping, have proven less useful in field situations (Dixon et al. 2002; Hooftman and de Raat 1982; Al-Sabti and Metcalfe 1995; Grisolia and Cordeiro 2000). As a consequence, the Mn assay, which only requires identification of DNA fragments outside the main nucleus of the cell (i.e. the micronuclei) and is easier to score, has found greater utility than more complicated karotyping. The Mn assay is widely used to assess genotoxic hazard and has shown potential for the in situ monitoring of water quality (Bolognesi and Hayashi 2011). Mn are formed during the anaphase of mitotic cell divisions from chromosomal fragments, or whole chromosomes, that are “left behind” when the nucleus divides. After the telophase, these fragments may not be included in the nuclei of daughter cells, and form single or multiple micronuclei in the cytoplasm (OECD 1997). The Mn test detects both clastogenic and aneugenic effects; and therefore allows the detection of genotoxicity for a wide range of compounds including MNMs. The nucleoplasmic bridge and binucleated cells formed during cell divisions in the assay provide an additional and complementary measure of chromosome rearrangement. The assay has been further developed with cytochalasin-B, a cytokinesis blocking agent that inhibits cell-division, giving an increased occurrence of binucleated cells in the preparation, and thus increasing the sensitivity of the assay in terms of the number of potential Mn that may subsequently form, and the ability to visualise chromosomes more easily in the dividing nucleus. Differential staining methods (e.g. use of fluorescent acridine orange) have also been employed in an attempt to increase sensitivity of the Mn assay, as well as to differentiate between young and mature erythrocytes in fish species (see Jha 2008). Such methods can now be routinely used for measuring chromosome breakage, impairment in DNA repair, chromosome loss, nondisjunction, necrosis, apoptosis and cytostasis in cells (Fenech 2007). The Mn assay can be applied in different target tissues such as erythrocyte, gills, kidneys and livers of fish under laboratory and field conditions (Williams and Metcalfe 1992; Hayashi et al. 1998; De Flora et al. 1993; Klingerman 1982; Mersch and Beauvais 1997), and therefore offers flexibility in the testing regime while the target organs for different MNMs are being established. Scoring the Mn present in blood cells from aquatic species requires some care with MNMs in order to avoid false positives. For example, the presence of virus particles, or damage to the nucleus associated with infection that could be mistaken for Mn from MNM exposure; such as erythrocytic necrosis in fish cells and similar conditions in the haemocytes of bivalves (Dixon et al. 2002). The observer should also be familiar with the morphology of the MNM being tested, and the types of aggregates or agglomerates that may form, in order to reduce the risk of falsely identifying MNM aggregates as Mn fragments. The latter can be avoided by using histological staining to positively identify nucleic acids.
Overall, the most promising assays for nano ecogenotoxicology testing in vitro seems to be Mn and comet assays, but identifying the most sensitive species and cell type for these assays remain to be established for most MNMs. In addition, the relative risk of nano genotoxicity in human/mammalian systems compared to wildlife is unclear, and cross-species comparisons of mammalian, fish and invertebrate cells in each assay are required. A range of cell types from different organs should also be used while the target organs for MNM toxicity are being established. In vitro assays using cells or cell cultures of MNMs should incorporate similar controls to in vivo experiments (dispersion controls, positive controls, nearest equivalent conventional chemical for bench marking), but current experiments have often used high μg or mg concentrations of MNMs and concentrations need to be reduced to reflect the likely low μg or ng circulating levels of MNMs inside organisms.
Conclusions
In conclusion, the experience of researchers at the NIN workshop and evidence from the literature indicate that most of the experimental approaches used for fundamental research and regulatory testing can be applied to MNMs. However, most standardised protocols intended for hazard assessment require modifications, and careful consideration should be given to even the very basics of measurement methods (cleaning apparatus, interferences with assays and electrodes) in all experimental work. The key practical recommendations for methodology are indicated below.
Experimental designs for MNM studies should include controls relevant to the scientific question being asked. For the hazard assessment of metallic MNMs, a metal salt control is recommended to allow comparison with the existing metals literature. Bulk powders should be used with caution on the data interpretation as they are rarely a true “particle size” only control and the ecotoxicity of many bulk powders has not been established with respect to identifying materials as positive or negative controls for ecotoxicity tests. Dispersing agents should be avoided, but if they must be used to facilitate the exposure, then a dispersion control must be included in the experimental design. The interpretation of the results should reflect the likelihood that the MNM will be coated with the dispersant, and that this may not be an environmentally relevant exposure.
It is possible to reliably dose ecotoxicity test systems with MNMs. For soil tests, dosing can be achieved by dry mixing of the MNM or by adding the MNM as a dispersion. For aquatic tests, dosing with freshly characterised stock dispersions, and the use of stirring/mixing of the water, or water changes (semi-static exposure), are practical approaches for maintaining the exposure. Precise details of the dosing method, volumes, stirring rates, etc., should be reported. Several techniques are available for characterising MNMs, and while it is expected that researchers will perform detailed characterisation of stock dispersions, time constraints and technology gaps indicate that full characterisation of test media during ecotoxicity tests is not a practical proposition. Detecting MNMs in complex matrices like soil, sediment and animal food is currently not possible, against the background of natural nanomaterials already present in these matrices. For soil tests an artificial soil should be included in the design to allow bench marking between studies. In tests that use liquid media (water, physiological saline, dilute agars) it may be possible to track exposures using simple optical methods during experiments, and user-friendly predictive software should be developed to enable the researcher at the bench to estimate the likely behaviour or settling time of the MNM, so that logistical decisions about media changes for maintaining the exposure can be made.
The validation of existing and new end points is needed in several regulatory tests. For microbial assays, the cell wall is a formidable barrier to MNMs and end points that rely on the test substance penetrating the cell (e.g. microbial respiration assays) may not be sensitive for MNMs. Instead methods based on the cell envelope may be more appropriate. In algal and plant tests, growth rate is an important end point, but the effects of MNMs in the media may indirectly inhibit growth by removing essential minerals/nutrients from the test media during particle aggregation, or by shading effects that prevent photosynthesis. For algal growth tests especially, it is therefore vital to give precise details and actual measurements of lighting regimes (intensity, duration, types of lamps, etc.) and any shaking or mixing of the media. New more sensitive end points based on the biochemistry of photosynthesis are suggested for algae and plants. Measurements of mechanical interference of MNM with Daphnia mobility, and the use of omics in soil organism tests should also be considered.
There are some extra animal husbandry considerations for fish tests that are specific to MNMs, but the use of in vitro methods as alternatives to animal testing are encouraged for MNMs. In vitro methods for MNMs should continue to be validated with a range of different primary cells from different organs, and cell lines, while the target organs for MNMs are being established. A weight of evidence approach with a suite of in vitro assays is advocated while the utility of individual tests is being established for MNMs. Theoretical risks of false negatives or positives of some in vitro assays need to be verified by experimentation.
Bioaccumulation studies with MNMs are problematic, and fundamentally flawed in that the tests were originally designed for measuring solute concentrations in steady-state in the water and the test organism. This idea of steady state equilibrium does not apply to the colloid chemistry of MNMs. Modifying the exposure route from water to dietary will not resolve the fundamental conceptual problems with bioaccumulation tests. It is recommended that new bioaccumulation tests are developed for MNMs.
Research needs
The most urgent research need relates to being able to confirm MNM exposure during experiments. There is a technology gap. Rapid methods for measuring particle size distribution in a range of liquid media, and in soils/sediments containing large quantities of natural nano-scale materials are not available. The sensitivity of such instruments needs to increase by at least two orders of magnitude to detect environmentally relevant concentrations of MNMs. Rapid and reliable measurement methods for MNMs in the tissues of organisms are also needed to understand uptake and bioavailability, but also to ensure correct interpretation of ecotoxicity test results for risk assessments. Research with ecologically relevant test species (not just the standard OECD organisms, or standard cell lines) and in real environmental scenarios is needed. For microbes, studies on complex communities are needed, and data on some groups such as anaerobic bacteria and on Archaea are particularly lacking. The role of the S-layer in the defense against MNM-induced damage, and the co-incidence of antibiotic resistance genes and metal response genes in microbes, are priority knowledge gaps within microbial studies. Several core laboratory techniques are confounded by the presence of MNMs. For cell culture work, tests with unicellular algae, or with microbes, high throughput methods of cell counting by flow cytometry are confounded by particle aggregation. Polymerase chain reaction (PCR) and DNA extraction methods for complex natural samples (soils, sediments and whole organisms) containing MNMs should be validated to ensure confidence in the use of ‘omics techniques in nano ecotoxicology. Finally, new tests that are equivalent in purpose to the bioaccumulation tests for conventional solutes are needed, and with the ability to verify both exposure and particle uptake during the test.
Acknowledgments
The authors would like to thank all those who participated in the discussions at the NanoImpactNet workshop held in Dublin during September 2010. Funding from the European Union’s 7th Framework Programme Coordination Action NanoImpactNet (NMP4-CA-2008-218539) to the workshop is acknowledged. In addition, R. Handy was supported by the Natural Environment Research Council, UK (NE/G001812/1).
References
- Al-Sabti K, Metcalfe CD. Fish micronuclei for assessing genotoxicity in water. Mutat Res. 1995;343:121–135. doi: 10.1016/0165-1218(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Al-Somali AM, Krueger KM, Falkner JC, Colvin VL. Recycling size exclusion chromatography for the analysis and separation of nanocrystalline gold. Anal Chem. 2004;76:5903–5910. doi: 10.1021/ac049355h. [DOI] [PubMed] [Google Scholar]
- An H, Liu Q, Ji Q, Jin B. DNA binding and aggregation by carbon nanoparticles. Biochem Biophys Res Comm. 2010;393:571–576. doi: 10.1016/j.bbrc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- ASTM (2003) Standard guide for conducting 96 h toxicity tests with microalgae, ASTM E 1218-97a. In: Standards on biological effects and environmental fate (2nd ed) Vol. 11.05, American Society for Testing and Materials, West Conshohocken, PA
- Baker RTM, Handy RD, Davies SJ, Snook JC. Chronic dietary exposure to copper affects growth, tissue lipid peroxidation, and metal composition of the grey mullet, Chelon labrosus. Mar Environ Res. 1998;45:357–365. [Google Scholar]
- Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam A, Sailaja N, Mahboob M, Rahman MF, Hussain SM, Grover P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol In Vitro. 2010;24:1871–1876. doi: 10.1016/j.tiv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Bednar AJ, Medina VF, Ulmer-Scholle DS, Frey BA, Johnson BL, Brostoff W, Larson SL. Effects of organic matter on the speciation of uranium in soil and plant matrices. Chemosphere. 2007;70:237–247. doi: 10.1016/j.chemosphere.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Berges JA, Franklin DJ, Harrison PJ. Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol. 2001;37:1138–1145. [Google Scholar]
- Blaustein GS, Chappell MA (2011) DLVO theory approach to colloidal behavior of nanoparticles (In Review) [Online]. Available by Wolfram Demonstrations Project http://demonstrations.wolfram.com
- Bolognesi C, Hayashi M. Micronucleus assay in aquatic animals. Mutagenesis. 2011;26:205–213. doi: 10.1093/mutage/geq073. [DOI] [PubMed] [Google Scholar]
- Bradford A, Handy RD, Readman WJ, Atfield A, Mühling M. Impact of silver nanoparticle contamination on the genetic diversity of natural bacterial assemblages in estuarine sediments. Environ Sci Technol. 2009;43:4530–4536. doi: 10.1021/es9001949. [DOI] [PubMed] [Google Scholar]
- Burch SW, Fitzpatrick LC, Goven AJ, Venables BJ, Giggleman MA. In vitro earthworm Lumbricus terrestris coelomocyte assay for use in terrestrial toxicity identification evaluation. Bull Environ Contam Toxicol. 1999;62:547–554. doi: 10.1007/s001289900910. [DOI] [PubMed] [Google Scholar]
- Cantor CR, Schimmel PR. Biophysical chemistry. Part II: Techniques for the study of biological structure and function. W.H. Freeman and Company, New York; 1998. [Google Scholar]
- Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, George AJ, Porter BE, Price CL, Dontsova KM, Kennedy AJ, Steevens JA. Surfactive properties of dissolved soil humic substances for stabilizing multi-walled carbon nanotubes dispersions. Environ Pollut. 2009;157:1081–1087. doi: 10.1016/j.envpol.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Choi O, Clevenger TE, Deng B, Surampalli RY, Ross L, Hu Z. Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res. 2009;43:1879–1886. doi: 10.1016/j.watres.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Cleuvers M, Weyers A. Algal growth inhibition test: does shading of coloured substances really matter? Water Res. 2003;37:2718–2722. doi: 10.1016/S0043-1354(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Collins AR, Dušinská M, Gedik C, Štetina R. Oxidative damage to DNA; do we have a reliable biomarker? Environ Health Perspect. 1996;104:465–469. doi: 10.1289/ehp.96104s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane M, Handy R, Garrod J, Owen R. Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology. 2008;17:421–437. doi: 10.1007/s10646-008-0215-z. [DOI] [PubMed] [Google Scholar]
- Curtis TP, Sloan WT, Scannell JW. Estimating prokaryotic diversity and its limits. Proc Natl Acad Sci USA. 2002;99:10494–10499. doi: 10.1073/pnas.142680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallinger R, Berger B, Birkel S. Terrestrial isopods: useful biological indicators of urban metal pollution. Oecologia. 1992;89:32–41. doi: 10.1007/BF00319012. [DOI] [PubMed] [Google Scholar]
- Flora S, Vigano L, Dagostini F, Camoirana A, Bagnasco M, Bennecelli C, Melodia F, Arillo A. Multiple genotoxicity biomarkers in fish exposed in situ to polluted river water. Mutat Res. 1993;319:167–177. doi: 10.1016/0165-1218(93)90076-p. [DOI] [PubMed] [Google Scholar]
- Rosa M, Gambacorta A, Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debabov VG. Bacterial and archaeal S-layers as a subject of nanobiotechnology. Mol Biol. 2006;38:482–493. [Google Scholar]
- Derjaguin BV, Landau L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys Chim URSS. 1941;14:633–662. [Google Scholar]
- Sotto A, Chiaretti M, Carru GA, Bellucci S, Mazzanti G. Multi-walled carbon nanotubes: lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol Lett. 2009;184:192–197. doi: 10.1016/j.toxlet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Dixon DR, Pruski AM, Dixon LRJ, Jha AN. Marine invertebrate eco-genotoxicology: a methodological overview. Mutagenesis. 2002;17:495–507. doi: 10.1093/mutage/17.6.495. [DOI] [PubMed] [Google Scholar]
- Doak SH, Griffiths SM, Manshian B, Singh N, Willaims PM, Brown AP, Jenkins GJS. Confounding experimental consideration in nanogenotoxicology. Mutagenesis. 2009;24:285–293. doi: 10.1093/mutage/gep010. [DOI] [PubMed] [Google Scholar]
- Dodd NFJ, Jha AN. Titanium dioxide induced cell damage: a proposed role of the carboxyl radical. Mutat Res. 2009;660:79–82. doi: 10.1016/j.mrfmmm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Dodd NFJ, Jha AN. Photoexcitation of aqueous suspensions of titanium dioxide nanoparticles: an electron spin resonance spin trapping study of potentially oxidative reactions. Photochem Photobiol. 2011;87:632–640. doi: 10.1111/j.1751-1097.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- Dubascoux S, Hecho I, Hassellöv M, Kammer F, Gautier MP, Lespes G. Field-flow fractionation and inductively coupled plasma mass spectrometer coupling: History, development and applications. J Anal Atomic Spectrom. 2010;25:613–623. [Google Scholar]
- Duret G, Delcour AH. Size and dynamics of the vibrio cholerae porins ompu and ompt probed by polymer exclusion. Biophys J. 2010;98:1820–1829. doi: 10.1016/j.bpj.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst RA. Mechanism of the glass electrode response. J Chem Educ. 1967;44:175–176. [Google Scholar]
- Dušinská M, Collins AR. Detection of oxidised purines and UV-induced photoproducts in DNA, by inclusion of lesion-specific enzymes in the comet assay (single cell gel electrophoresis) ATLA. 1996;24:405–411. [Google Scholar]
- Dušinská M, Slameňová D. Application of alkaline unwinding assay for detection of mutagen-induced DNA strand breaks. Cell Biol Toxicol. 1992;8:207–216. doi: 10.1007/BF00156731. [DOI] [PubMed] [Google Scholar]
- Eichert T, Kurtz A, Steiner U, Goldbach HE. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol Plantarum. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Evangelou VP (1998) Environmental soil and water chemistry: Principles and applications John Wiley & Sons, Inc., New York
- Everaarts JM, Besten PJ, Hillebrand MTJ, Halbrook RS, Shugart LR. DNA strand breaks, cytochrome P-450-dependent monooxygenase system activity and levels of chlorinated biphenyl congeners in the pyloric caeca of the seastar (Asterias rubens) from the North Sea. Ecotoxicology. 1998;7:69–79. [Google Scholar]
- Fang J, Lyon DY, Wiesner MR, Dong J, Alvarez PJJ. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ Sci Technol. 2007;41:2636–2642. doi: 10.1021/es062181w. [DOI] [PubMed] [Google Scholar]
- Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84:415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk AT, Norstrom RJ, Cymbalisty CD, Muir DCG. Dietary accumulation and depuration of hydrophobic organochlorines: bioaccumulation parameters and their relationship with the octanol/water partition coefficient. Environ Toxicol Chem. 1998;17:951–961. [Google Scholar]
- Fleischer A, O’Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre P, Gribaldo S. Bacteria with a eukaryotic touch: a glimpse of ancient evolution? Proc Natl Acad Sci USA. 2010;107:12739–12740. doi: 10.1073/pnas.1007720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiser BK, Biswas A, Rosenkranz P, Jepson M, Lead JR, Stone V, Tyler CR, Fernandes TF. Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J Environ Monit. 2011;13:1227–1235. doi: 10.1039/c1em10060b. [DOI] [PubMed] [Google Scholar]
- Galloway TS, Handy RD. Immunotoxicity of organophosphorus pesticides. Ecotoxicology. 2003;12:345–363. doi: 10.1023/a:1022579416322. [DOI] [PubMed] [Google Scholar]
- Galloway T, Lewis C, Dolciotti I, Johnston BD, Moger J, Regoli F. Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ Pollut. 2010;158:1748–1755. doi: 10.1016/j.envpol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Field D, Swift P, Thomas S, Cummings D, Temperton B, Weynberg K, Huse S, Hughes M, Joint I, Somerfield PJ, Mühling M. The taxonomic and functional diversity of microbes at a temperate coastal site: a ‘multi-omic’ study of seasonal and diel temporal variation. PLoS ONE. 2010;5:e15545. doi: 10.1371/journal.pone.0015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover CN, Wood CM. Histidine absorption across apical surfaces of freshwater rainbow trout intestine: mechanistic characterization and the influence of copper. J Membrane Biol. 2008;221:87–95. doi: 10.1007/s00232-007-9088-y. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modelled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Graff S, Berkus M, Alberti G, Kohler HR. Metal accumulation strategies in saprophagous and phytophagous soil invertebrates: a quantitative comparison. Biometals. 1997;10:45–53. [Google Scholar]
- Grisolia CK, Cordeiro CMT. Variability in micronucleus induction with different mutagens applied to several species of fishes. Genet Mol Biol. 2000;23:235–239. [Google Scholar]
- Handy RD. The ionic composition of rainbow trout body mucus. Comp Biochem Physiol A. 1989;93:571–575. [Google Scholar]
- Handy RD. Systems toxicology: Using the systems biology approach to assess chemical pollutants in the environment. In: Hogstrand C, Kille P, editors. Toxicogenomics. Advances in experimental biology. London: Elsevier; 2008. pp. 249–281. [Google Scholar]
- Handy RD, Maunder RJ. The biological roles of mucus: Importance for osmoregulation and osmoregulatory disorders of fish health. In: Handy RD, Bury N, Flik G, editors. Osmoregulation and ion transport: Integrating physiological, molecular and environmental aspects, Essential Reviews in Experimental Biology. London: Society for Experimental Biology Press; 2009. pp. 203–235. [Google Scholar]
- Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007;9:125–144. [Google Scholar]
- Handy RD, Musonda MM, Phillips C, Falla SJ. Mechanisms of gastro-intestinal copper absorption in the African Walking Catfish: copper dose-effects and a novel anion-dependent pathway in the intestine. J Exp Biol. 2000;203:2365–2377. doi: 10.1242/jeb.203.15.2365. [DOI] [PubMed] [Google Scholar]
- Handy RD, Jha AN, Depledge MH (2002) Biomarker approaches for ecotoxicological biomonitoring at different levels of biological organisation. In: Burden F, McKelvie I, Förstner U, Guenther A (eds) Handbook of environmental monitoring, McGraw Hill, New York. Chapter 9, pp. 9.1-9.32
- Handy RD, Henry TB, Scown TM, Johnstone BD, Tyler CR. Manufactured nanoparticles: their uptake and effects on fish—a mechanistic analysis. Ecotoxicology. 2008;17:396–409. doi: 10.1007/s10646-008-0205-1. [DOI] [PubMed] [Google Scholar]
- Handy RD, Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- Handy RD, Al-Bairuty G, Al-Jubory A, Ramsden CS, Boyle D, Shaw BJ, Henry TB. Effects of manufactured nanomaterials on fishes: a target organ and body systems physiology approach. J Fish Biol. 2011;79:821–853. doi: 10.1111/j.1095-8649.2011.03080.x. [DOI] [PubMed] [Google Scholar]
- Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, Metcalfe C, Steevens JA, Klaine SJ, Koelmans AA, Horne N. Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem. 2012;31:15–31. doi: 10.1002/etc.706. [DOI] [PubMed] [Google Scholar]
- Hansen PD, Wittekindt E, Sherry J, Unruh E, Dizer H, Tüg H, Rosenthal H, Dethlefsen V, von Westernhagen H (2009) Genotoxicity in the environment (eco-genotoxicity). In: Barceló D, Hansen P-D (eds) Biosensors for environmental monitoring of aquatic systems. The handbook of environmental chemistry series, volume 5 J, Springer, Dordrecht, pp. 203-226
- Hartmann NB, Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A. Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2009;269:190–197. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Hassellöv M, Readman JW, Ranville JF, Tiede K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology. 2008;17:344–361. doi: 10.1007/s10646-008-0225-x. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ueda T, Uyeno L, Wada K, Kinae N, Saotome K, Tanaka N, Takai A, Sasaki YF, Asano N, Sifuni T, Ojima Y. Development of genotoxicity assay systems that use aquatic organisms. Mutat Res. 1998;399:125–133. doi: 10.1016/s0027-5107(97)00251-0. [DOI] [PubMed] [Google Scholar]
- Heckmann LH, Hovgaard MB, Sutherland DS, Autrup H, Besenbacher F, Scott-Fordsmand JJ. Limit-test toxicity screening of selected inorganic nanoparticles to the earthworm Eisenia fetida. Ecotoxicology. 2011;20:226–233. doi: 10.1007/s10646-010-0574-0. [DOI] [PubMed] [Google Scholar]
- Henry TB, Menn F, Fleming JT, Wilgus J, Compton RN, Sayler GS. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ Health Perspect. 2007;115:1059–1065. doi: 10.1289/ehp.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A, Mellado RP, Martínez JL. Metal accumulation and vanadium-induced multidrug resistance by environmental isolates of Escherichia hermannii and Enterobacter cloacae. Appl Environ Microbiol. 1998;64:4317–4320. doi: 10.1128/aem.64.11.4317-4320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze EM, Phenrat T, Lowry GV. Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual. 2010;39:1909–1924. doi: 10.2134/jeq2009.0462. [DOI] [PubMed] [Google Scholar]
- Hooftman RN, Raat WK. Induction of nuclear anomalies (micronuclei) in the peripheral blood erythrocytes of the eastern mud minnow Umbra pygmea by EMS. Mutat Res. 1982;104:147–152. doi: 10.1016/0165-7992(82)90136-1. [DOI] [PubMed] [Google Scholar]
- Horn JJ, Mccreedy T, Wadhawan J. Amperometric measurement of gaseous hydrogen sulfide via a Clark-type approach. Anal Methods. 2010;2:1346–1354. [Google Scholar]
- Höss S, Jänsch S, Moser T, Junker T, Römbke J. Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol Environ Saf. 2009;72:1811–1818. doi: 10.1016/j.ecoenv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Howland JL. The surprising Archaea: discovering another domain of life. Oxford. pp: Oxford University Press; 2000. [Google Scholar]
- Hu CW, Li M, Cui YB, Li DS, Chen J, Yang LY. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem. 2010;42:586–591. [Google Scholar]
- Hu JD, Zevi Y, Kou XM, Xiao J, Wang XJ, Jin Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci Total Environ. 2010;408:3477–3489. doi: 10.1016/j.scitotenv.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Huang CP, Cha DK, Ismat SS (2005) Progress report: short-term chronic toxicity of photocatalytic nanoparticles to bacteria, algae, and zooplankton. University of Delaware, EPA Grant Number: R831721. Available at: http://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/7384/report/2005
- Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ Sci Pollut Res. 2006;13:225–232. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- ISO (1989) Water quality—Fresh water algal growth. Inhibition test with Scenedesmus subspicatus and Pseudokirchneriella subcapitata. ISO 8692, International Organization for Standardization, Geneva, Switzerland
- ISO (1993) Soil quality—sampling. Guidance on the collection, handling and storage of soil for the assessment of aerobic microbial processes in the laboratory. ISO 10381-6:1993. Available at: http://www.iso.org/iso/catalogue_detail.htm?csnumber=19248
- ISO (1997a) Soil quality—determination of soil microbial biomass—part 1: Substrate-induced respiration method. ISO 14240-1:1997. Available at: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=21530
- ISO (1997b) Soil quality—determination of soil microbial biomass—part 2: Fumigation-extraction method. ISO 14240-2:1997. Available at: http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=23951
- Janssen CR, Heijerick DG. Algal toxicity tests for environmental risk assessments of metals. Rev Environ Contam Toxicol. 2003;178:23–52. doi: 10.1007/0-387-21728-2_2. [DOI] [PubMed] [Google Scholar]
- Jemec A, Drobne D, Remskar M, Sepcic K, Tisler T. Effects of ingested nano-sized titanium dioxide on terrestrial isopods (Porcellio scaber) Environ Toxicol Chem. 2008;27:1904–1914. doi: 10.1897/08-036.1. [DOI] [PubMed] [Google Scholar]
- Jha AN. Genotoxicological studies in aquatic organisms: an overview. Mutat Res Fund Mol Mech. 2004;552:1–17. doi: 10.1016/j.mrfmmm.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Jha AN. Ecotoxicological applications and significance of the comet assay. Mutagenesis. 2008;23:207–221. doi: 10.1093/mutage/gen014. [DOI] [PubMed] [Google Scholar]
- Johansen A, Pedersen A, Jensen KA, Karlson U, Hansen BM, Scott-Fordsmand JJ, Winding A. Effects of C-60 fullerene nanoparticles on soil bacteria and protozoans. Environ Toxicol Chem. 2008;27:1895–1903. doi: 10.1897/07-375.1. [DOI] [PubMed] [Google Scholar]
- Joint I, Mühling M, Querellou J. Culturing marine bacteria—an essential prerequisite for biodiscovery. Microb Biotechnol. 2010;3:564–575. doi: 10.1111/j.1751-7915.2010.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru A, Dubourguier HC. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269:105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Kahru A, Savolainen K. Potential hazard of nanoparticles: from properties to biological and environmental effects. Toxicology. 2010;269:89–91. doi: 10.1016/j.tox.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Kandler O, König H (1993) Cell envelopes of archaea: structure and chemistry. In: The Biochemistry of Archaea (Archaebacteria); Kates M, Kushner DJ, Matheson AT (eds) Elsevier, Amsterdam, pp. 223–259
- Kennedy AJ, Gunter JC, Chappell MA, Goss JD, Hull MS, Kirgan RA, Steevens JA. Influence of nanotube preparation in aquatic bioassays. Environ Toxicol Chem. 2009;28:1930–1938. doi: 10.1897/09-024.1. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Hull MS, Bednar AJ, Goss JD, Gunter JC, Bouldin JL, Vikesland PJ, Steevens JA. Fractionating nanosilver: importance for determining toxicity of aquatic test organisms. Environ Sci Technol. 2010;24:9571–9577. doi: 10.1021/es1025382. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Klingerman A. Fishes as a biological detector of the effect of genotoxic agents. In: Heddle JA, editor. Mutagenicity, new horizons in genetic toxicology. New York: Acadmic Press; 1982. pp. 435–453. [Google Scholar]
- Koga Y, Morii H. Recent advances in structural research on ether lipids from archaea including comparative and physiological aspects. Biosci Biotechnol Biochem. 2005;69:2019–2034. doi: 10.1271/bbb.69.2019. [DOI] [PubMed] [Google Scholar]
- Kowalchuk GA, Bruijn FJ, Head IM, Akkermans ADL, Elsas JD, editors. Molecular microbial ecology manual. Volumes I & II: Kluwer Academic Publishers, Dordrecht; 2004. [Google Scholar]
- Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: methods, preparation and characterisation of test material, potential artefacts and limitations-many questions, some answers. Mutat Res. 2009;681:241–258. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Lee WM, An YJ, Yoon H, Kweon HS. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water insoluble nanoparticles. Environ Toxicol Chem. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJJ. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem. 2010;29:669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Lin S, Reppert J, Hu Q, Hunson JS, Reid ML, Ratnikova T, Roa AM, Luo H, Ke PC. Uptake, translocation and transmission of carbon nanomaterials in rice plants. Small. 2009;5:1128–1132. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- Ling CM, Mohamed AR, Bhatia S. Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere. 2004;57:547–554. doi: 10.1016/j.chemosphere.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- Lonhienne TGA, Sagulenko E, Webb RI, Lee K-C, Franke J, Devos DP, Nouwens A, Carroll BJ, Fuerst JA. Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA. 2010;107:12883–12888. doi: 10.1073/pnas.1001085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DY, Alvarez PJJ. Fullerene water suspension (nC(60)) exerts antibacterial effects via ROS-independent protein oxidation. Environ Sci Technol. 2008;42:8127–8132. doi: 10.1021/es801869m. [DOI] [PubMed] [Google Scholar]
- Lyon DY, Brown DA, Alvarez PJJ. Implications and potential applications of bactericidal fullerene water suspensions: effect of nC(60) concentration, exposure conditions and shelf life. Water Sci Technol. 2008;57:1533–1538. doi: 10.2166/wst.2008.282. [DOI] [PubMed] [Google Scholar]
- Lyon DY, Brunet L, Hinkal GW, Wiesner MR, Alvarez PJJ. Antibacterial activity of fullerene water suspensions (nC(60)) is not due to ROS-mediated damage. Nano Lett. 2008;8:1539–1543. doi: 10.1021/nl0726398. [DOI] [PubMed] [Google Scholar]
- Ma WC. Critical body residues (CBRs) for ecotoxicological soil quality assessment: copper in earthworms. Soil Biol Biochem. 2005;37:561–568. [Google Scholar]
- Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Maenosono S, Suzuki T, Saita S. Mutagenicity of water-soluble FePt nanoparticles in Ames test. J Toxicol Sci. 2007;32:575–579. doi: 10.2131/jts.32.575. [DOI] [PubMed] [Google Scholar]
- Maenosono S, Yoshida R, Saita S. Evaluation of genotoxicity of amine-terminated water-dispersible FePt nanoparticles in the Ames test and in vitro chromosomal aberration test. J Toxicol Sci. 2009;34:349–354. doi: 10.2131/jts.34.349. [DOI] [PubMed] [Google Scholar]
- Mersch J, Beauvais MN. The micronucleus assay in fresh water mussel, Dreissena polymorpha, to in situ monitor genotoxicity in fresh water environments. Mutat Res. 1997;393:141–149. doi: 10.1016/s1383-5718(97)00099-5. [DOI] [PubMed] [Google Scholar]
- Miao AJ, Schwehr KA, Xu C, Zhang SJ, Luo ZP, Quigg A, Santschi PH. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ Pollut. 2009;157:3034–3041. doi: 10.1016/j.envpol.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Miraglia N, Simonelli A, Acampora A, Pascarella L, D’Alessio A, Sgro LA, Sannolo N. Combustion generated nanoparticles: mutagenicity and chemical reactivity. G Ital Med Lav Ergon. 2005;27:326–328. [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharm. 2009;234:222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Mori T, Takada H, Ito S, Matsubayashi K, Miwa N, Sawaguchi T. Preclinical studies on safety of fullerene upon acute oral administration and evaluation for no mutagenesis. Toxicology. 2006;225:48–54. doi: 10.1016/j.tox.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Morrison ID, Ross S. Colloidal dispersions: suspension, emulsions, and foams. New York: Wiley; 2002. [Google Scholar]
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Mühling M, Woolven-Allen JA, Murrel JC, Joint I. Improved group-specific PCR primers for DGGE analysis of the genetic diversity of complex microbial communities. ISME J. 2008;2:379–392. doi: 10.1038/ismej.2007.97. [DOI] [PubMed] [Google Scholar]
- Mühling M, Bradford A, Somerfield PJ, Readman J, Handy RD. An investigation into the effect of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar Environ Res. 2009;68:278–283. doi: 10.1016/j.marenvres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17:372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- Neal AL. What can be inferred from bacterium–nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology. 2008;17:362–371. doi: 10.1007/s10646-008-0217-x. [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Ingraham JL, Schaechter M. Physiology of the bacterial cell—a molecular approach. Sunderland: Sinauer Associates Inc.; 1990. [Google Scholar]
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Ng C-T, Li JJ, Bay B-H, Yung L-Y L (2010) Current studies into the genotoxic effects of nanomaterials. J Nucleic Acids 2010:Article ID 947859. doi:10.4061/2010/947859 [DOI] [PMC free article] [PubMed]
- Nielsen HD, Berry LS, Stone V, Burridge TR, Fernandes TF. Interactions between carbon black nanoparticles and the brown algae Fucus serratus: inhibition of fertilization and zygotic development. Nanotoxicology. 2008;2:88–97. [Google Scholar]
- OECD Test guideline 305. Paris: Bioconcentration: Flow-through fish test. Organization for Economic Cooperation and Development; 1996. [Google Scholar]
- OECD (1997) Guidelines for testing of chemicals. Mammalian erythrocyte micronucleus test. Guideline 474. Organization for Economic Cooperation and Development, Paris
- OECD (2008) OECD Test Guideline 225. Sediment-water Lumbriculus toxicity test using spiked sediment. Organization for Economic Cooperation and Development, Paris
- Preliminary review of OECD test guidelines for their applicability to manufactured nanomaterials. Paris: Organization for Economic Cooperation and Development; 2009. [Google Scholar]
- OECD (2010a) Preliminary guidance notes on sample preparation and dosimetry for the safety testing of manufactured nanomaterials. OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials, No. 24, ENV/JM/MONO(2010)25, 31 May 2010, Organisation for Economic Co-operation and Development, Paris. http://www.oecd.org/officialdocuments/displaydocumentpdf/?cote=ENV/JM/MONO(2010)25&doclanguage=en
- OECD (2010b) Current Developments/Activities on the safety of manufactured nanomaterials. OECD Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials, No. 26, ENV/JM/MONO(2010)42, 22nd September 2010, Organisation for Economic Co-operation and Development, Paris. http://www.oecd.org/officialdocuments/displaydocumentpdf/?cote=env/jm/mono(2010)42&doclanguage=en
- Owen R, Handy RD. Formulating the problems for environmental risk assessment of nanomaterials. Environ Sci Technol. 2007;41:5582–5588. doi: 10.1021/es072598h. [DOI] [PubMed] [Google Scholar]
- Papis E, Davies SJ, Jha AN. Relative sensitivity of fish and mammalian cells to the antibiotic, trimethoprim: cytotoxic and genotoxic responses as determined by neutral red retention, Comet and micronucleus assays. Ecotoxicology. 2011;20:208–217. doi: 10.1007/s10646-010-0572-2. [DOI] [PubMed] [Google Scholar]
- Perez S, Farre M, Barcelo D. Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. Trends Anal Chem. 2009;28:820–832. [Google Scholar]
- Pipan-Tkalec Z, Drobne D, Jemec A, Romih T, Zidar P, Bele M. Zinc bioaccumulation in a terrestrial invertebrate fed a diet treated with particulate ZnO or ZnCl2 solution. Toxicology. 2010;269:198–203. doi: 10.1016/j.tox.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Poda AR, Bednar AJ, Kennedy AJ, Harmon A, Hull M, Mitrano DM, Ranville JF, Steevens JA. Characterization of silver nanoparticles using field-flow fractionation interfaced to inductively coupled plasma mass spectrometry. J Chromatogr A. 2011;27:4149–4159. doi: 10.1016/j.chroma.2010.12.076. [DOI] [PubMed] [Google Scholar]
- Raisuddin S, Jha AN. Relative sensitivity of fish and mammalian cells to sodium arsenate and arsenite as determined by alkaline single-cell gel electrophoresis and cytokinesis block micronucleus assay. Environ Mol Mutagen. 2004;44:83–89. doi: 10.1002/em.20027. [DOI] [PubMed] [Google Scholar]
- Ramsden CS, Smith TJ, Shaw BJ, Handy RD. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology. 2009;18:939–951. doi: 10.1007/s10646-009-0357-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen LD, Sorensen SJ. The effect of long term exposure to mercury on the bacterial community in marine sediment. Curr Microbiol. 1998;36:291–297. doi: 10.1007/s002849900312. [DOI] [PubMed] [Google Scholar]
- Reeves JF, Davies SJ, Dodd NJ, Jha AN. Hydroxyl radicals (•OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 2008;640:113–122. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin SJ, Ke PC, Rao AM, Klaine SJ. In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol. 2007;41:3025–3029. doi: 10.1021/es062572a. [DOI] [PubMed] [Google Scholar]
- Roh J, Sim SJ, Yi J, Park K, Chung KH, Ryu D, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Rosenkranz P, Chowdry Q, Stone V, Fernandes TF. A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ Toxicol Chem. 2009;28:2142–2149. doi: 10.1897/08-559.1. [DOI] [PubMed] [Google Scholar]
- Roy R, Hoover MR, Bhalla AS, Slawecki T, Dey S, Cao W, Li J, Bhaskar S. Ultradilute Ag-aquasols with extraordinary bactericidal properties: role of the system Ag-O-H2O. Mater Res Innov. 2007;11:3–18. [Google Scholar]
- Santarella-Mellwig R, Franke J, Jaedicke A, Gorjanacz M, Bauer U, Budd A, Mattaj IW, Devos DP. The compartmentalized bacteria of the Planctomycetes-Verrucomicrobia-Chlamydiae superphylum have membrane coat-like proteins. PLoS Biol. 2010;8:e1000281. doi: 10.1371/journal.pbio.1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheckel KG, Lombi E, Rock SA, McLaughlin MJ. In vivo synchrotron study of thallium speciation and compartmentation in Iberis intermedia. Environ Sci Technol. 2004;38:5095–5100. doi: 10.1021/es049569g. [DOI] [PubMed] [Google Scholar]
- Schirmer K. Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology. 2006;224:163–183. doi: 10.1016/j.tox.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scott-Fordsmand JJ, Krogh PH, Schaefer M, Johansen A. The toxicity testing of double-walled nanotubes-contaminated food to Eisenia veneta earthworms. Ecotoxicol Environ Saf. 2008;71:616–619. doi: 10.1016/j.ecoenv.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Seeger E, Baun A, Kastner M, Trapp S. Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J Soil Sediment. 2009;9:46–53. [Google Scholar]
- Shah V, Belozerova I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009;197:143–148. [Google Scholar]
- Shinohara N, Matsumoto K, Endoh S, Maru J, Nakanishi J. In vitro and in vivo genotoxicity tests on fullerene C60 nanoparticles. Toxicol Lett. 2009;191:289–296. doi: 10.1016/j.toxlet.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Shoults-Wilson WA, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV, Unrine JM. Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida) Nanotoxicology. 2011;5:432–444. doi: 10.3109/17435390.2010.537382. [DOI] [PubMed] [Google Scholar]
- Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ, Doak SH. Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Sleytr UB, Beveridge TJ. Bacterial S-layers. Trends Microbiol. 1999;7:253–260. doi: 10.1016/s0966-842x(99)01513-9. [DOI] [PubMed] [Google Scholar]
- Sleytr UB, Messner P. Crystalline surface layers in prokaryotes. J Bacteriol. 1988;170:2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Shaw BJ, Handy RD. Toxicity of single walled carbon nanotubes on rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol. 2007;82:94–109. doi: 10.1016/j.aquatox.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Soldo D, Hari R, Sigg L, Behra R. Tolerance of Oocystis nephrocytioides to copper: intracellular distribution and extracellular complexation of copper. Aquat Toxicol. 2005;71:307–317. doi: 10.1016/j.aquatox.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Letcher RJ, Li J, Baker JE. Dietary accumulation and metabolism of polybrominated diphenyl ethers by juvenile carp (Cyprinus carpio) Environ Toxicol Chem. 2004;23:1939–1946. doi: 10.1897/03-462. [DOI] [PubMed] [Google Scholar]
- Stefaniak EA, Alsecz A, Frost R, Mathe Z, Sajo IE, Torok S, Worobiec A, Grieken R. Combined SEM/EDX and micro-Raman spectroscopy analysis of uranium minerals from a former uranium mine. J Hazard Mater. 2009;168:416–423. doi: 10.1016/j.jhazmat.2009.02.057. [DOI] [PubMed] [Google Scholar]
- Stone V, Johnston H, Schins RPF. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39:613–626. doi: 10.1080/10408440903120975. [DOI] [PubMed] [Google Scholar]
- Stone V, Nowack B, Baun A, Brink N, Kammer F, Dušinská M, Handy R, Hankin S, Hassellöv M, Joner E, Fernandes TF. Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterisation. Sci Total Environ. 2010;408:1745–1754. doi: 10.1016/j.scitotenv.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Szendi K, Varga C. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 2008;28:349–352. [PubMed] [Google Scholar]
- Thursby GB, Anderson BS, Walsh GE, Steele RL (1993) A review of the current status of marine algal toxicity testing in the United States. In: Landis WG, Hughes JS, Lewis MA (eds) Environmental toxicology and risk assessment. American Society for Testing and Materials (ASTM), Special Technical Publication, STP 1179. ASTM, Philadelphia, pp 362–377
- Tiede K, Tear SP, David H, Boxall ABA. Imaging of engineered nanoparticles and their aggregates under fully liquid conditions in environmental matrices. Water Res. 2009;43:3335–3343. doi: 10.1016/j.watres.2009.04.045. [DOI] [PubMed] [Google Scholar]
- Tiede K, Boxall ABA, Wang X, Gore D, Tiede D, Baxter M, David H, Tear SP, Lewis J. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J Anal Atomic Spectrom. 2010;25:1149–1154. [Google Scholar]
- Tong Z, Bischoff M, Nies L, Applegate B, Turco RF. Impact of fullerene (C60) on a soil microbial community. Environ Sci Technol. 2007;41:2985–2991. doi: 10.1021/es061953l. [DOI] [PubMed] [Google Scholar]
- Tsao N, Luh T-Y, Chou C-K, Chang T-Y, Wu J-J, Liu C-C, Lei H-Y. In vitro action of carboxyfullerene. J Antimicrob Chemotherapy. 2002;49:641–649. doi: 10.1093/jac/49.4.641. [DOI] [PubMed] [Google Scholar]
- Uchida M, Yamamoto T, Taniguchi A, Nakata S, Nakagawa Z. Reaction of silver ions and some amino acids. J Antibact Antifung Agents. 2003;31:695–704. [Google Scholar]
- Unrine JM, Tsyusko OV, Hunyadi S, Judy J, Bertsch P. Effects of particle size on chemical speciation and bioavailability of Cu to earthworms (Eisenia foetida) exposed to Cu nanoparticles. J Environ Qual Sci. 2010;39:1942–1953. doi: 10.2134/jeq2009.0387. [DOI] [PubMed] [Google Scholar]
- Unrine JM, Hunyadi SE, Tsyusko OV, Rao W, Shoults-Wilson WA, Bertsch PM. Evidence for bioavailability of Au nanoparticles from soil and biodistribution within earthworms (Eisenia fetida) Environ Sci Technol. 2010;44:8308–8313. doi: 10.1021/es101885w. [DOI] [PubMed] [Google Scholar]
- US EPA (1996a) Ecological effects test guidelines. OPPTS 850.5400. Algal toxicity, tiers I and II. EPA 712-C-96-164. US Environmental Protection Agency, Washington
- US EPA (1996b) Ecological effects test guidelines. OPPTS 850.4400. Aquatic plant toxicity test using Lemnasp., tiers I and II. EPA 712-C-96-156. US Environmental Protection Agency, Washington
- Ploeg MJ, Baveco JM, Hout A, Bakker R, Rietjens IM, Brink NW. Effects of C60 nanoparticle exposure on earthworms (Lumbricus rubellus) and implications for population dynamics. Environ Pollut. 2011;159:198–203. doi: 10.1016/j.envpol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Veith GD, DeFoe DL, Bergstedt BV. Measuring and estimating the bioconcentration factor of chemicals in fish. J Fish Res Board Can. 1979;36:1040–1048. [Google Scholar]
- Verwey E, Overbeek JThG. The theory of the stability of lyophobic colloids: the interaction of sol particles having an electric double layer. Amsterdam: Elsevier; 1948. [Google Scholar]
- Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–420. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- Vijver MG, Vink JPM, Jager T, Straalen NM, Wolterbeek HT, Gestel CAM. Kinetics of Zn and Cd accumulation in the isopod Porcellio scaber exposed to contaminated soil and/or food. Soil Biol Biochem. 2006;38:1554–1563. [Google Scholar]
- Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem. 2012;31:32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- Walsh GE. Principles of toxicity testing with marine unicellular algae. Environ Toxicol Chem. 1988;7:979–987. [Google Scholar]
- Wei LP, Thakkar M, Chen YH, Ntim SA, Mitra S, Zhang XY. Cytotoxicity effects of water dispersible oxidized multiwalled carbon nanotubes on marine alga, Dunaliella tertiolecta. Aquat Toxicol. 2010;100:194–201. doi: 10.1016/j.aquatox.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Williams RC, Metcalfe CD. Development of an in vivo hepatic micronucleus assay with rainbow trout. Aquat Toxicol. 1992;23:193–202. [Google Scholar]
- Wirnitzer U, Herbold B, Voetz M, Ragot J. Studies on the in vitro genotoxicity of baytubes, agglomerates of engineered multi-walled carbon-nanotubes (MWCNT) Toxicol Lett. 2009;186:160–165. doi: 10.1016/j.toxlet.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Wise JP, Goodale BC, Wise SS, Craig GA, Pongan AF, Walter RB, Thompson WD, Ng AK, Aboueissa AM, Mitani H, Spalding MJ, Mason MD. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat Toxicol. 2010;97:34–41. doi: 10.1016/j.aquatox.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Hara K, Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Kitamura D, Maenosono S. Mutagenicity of water-soluble ZnO nanoparticles in Ames test. J Toxicol Sci. 2009;34:119–122. doi: 10.2131/jts.34.119. [DOI] [PubMed] [Google Scholar]
- Zemke-White WL, Clements KD, Harris PJ. Acid lysis of macroalgae by marine herbivorous fishes: effects of acid pH on cell wall porosity. J Exp Mar Biol Ecol. 2000;245:57–68. [Google Scholar]
- Zhu S, Oberdörster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C-60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res. 2006;62(Suppl):S5–S9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chang Y, Chen Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere. 2010;78:209–215. doi: 10.1016/j.chemosphere.2009.11.013. [DOI] [PubMed] [Google Scholar]


