Abstract
Background
Impulsivity is a multifaceted personality construct associated with numerous psychiatric disorders. Recent research has characterized four facets of impulsivity: ‘urgency’ (the tendency to act rashly especially in the context of distress or cravings); ‘lack of premeditation’ (not envisaging the consequences of actions); ‘lack of perseverance’ (not staying focused on a task); ‘sensation seeking’ (engaging in exciting activities). Urgency is particularly associated with clinical populations and problematic disinhibited behaviour.
Methods
We used magnetic resonance spectroscopy (MRS) to measure concentration of the inhibitory neurotransmitter γ-amino butyric acid (GABA) in the dorso-lateral prefrontal cortex (dlPFC) in two cohorts of 12 and 13 participants.
Results
We find that variation in trait urgency in healthy men correlates with GABA concentration in the dlPFC. The result was replicated in an independent cohort. More GABA predicted lower urgency scores, consistent with a role in self-control for GABA-mediated inhibitory mechanisms in dlPFC.
Conclusions
These findings help account for individual differences in self-control, and thus clarify the relationship between GABA and a wide range of psychiatric disorders associated with impaired self-control.
Keywords: Self-control, inhibition, urgency, GABRA2, externalizing, neurochemistry, personality, Stop-signal
Introduction
The ability to flexibly regulate one’s behaviour in a changing environment is key to adaptive social life. Failure in this regulation is salient in psychiatric disorders, where impulsivity is the second commonest symptom in the Diagnostic and Statistical Manual of mental disease (1, see 2). However, impulsivity is an umbrella term comprising several facets, which have been clarified in the UPPS model as: Urgency (or ‘rash impulsivity’), the tendency to act rashly in response to distress or other strong emotions and urges; Premeditation (lack of), the tendency to envisage the consequences of an act before engaging in it; Perseverance (lack of), the ability to remain focused on a difficult task; Sensation Seeking, the tendency to engage in exciting activities (3).
Of these four traits, urgency has been most strongly linked to problematic levels of alcohol consumption, smoking, gambling, binge eating, illicit drug use, aggression and risky sexual behaviour (4,5). High levels of urgency represent one extreme of wide natural variation in this trait, and the UPPS model is an example of how it is fruitful to understand human behaviour and its maladaptive variants along a continuum (6). Thus identifying the neurobiological bases of individual variation in urgency may be of key importance for understanding the pathophysiology of psychiatric disorders.
Building from previous focus on serotonin and dopamine (2,7,8), there is now increasing interest in the role of GABA-ergic neurotransmission in impulsivity. Variation in GABA receptor genes has been associated with problematic levels of alcohol use, obesity and drug use, as well as bipolar spectrum disorders, conduct problems and distress regulation (9-16). Notably, Dick et al. (16) found that the GABRA2 gene is involved in the predisposition to alcohol dependence through a generalized ‘externalizing’ pathway, a concept that captures a range of disinhibited behaviours that commonly co-occur, including aggression, aspects of impulsivity, antisocial behavior and drug abuse (17), forming a coherent spectrum of personality and psychopathology. Research connecting externalizing psychopathology and personality traits (18) has shown that externalizing liability is a normally distributed dimension of risk, with urgency having the largest loading on the general externalizing factor (5,19). Thus, given that genetic variation in GABA transmission has been linked to general externalizing, and urgency is the best personality trait marker of the general externalizing liability, we predict that variation in GABA levels would be related to the urgency facet of impulsivity.
Any relationship between GABA and urgency may be specific to particular brain regions. Several studies have related general impulsivity to activity or grey matter thickness in the lateral prefrontal cortex (20-23). Increased delay discounting of rewards may reflect a general vulnerability to externalizing (24) and TMS disruption of dlPFC leads to such enhanced discounting (25). Increased externalizing traits in undergraduate subjects are related to reduced amplitude of the error-related negativity (ERN, 26) brain response, a signal that is abolished following lesions of lateral PFC (27). Variation in urgency is linked to the ability to adaptively regulate emotions and cravings (5), and recent research links dlPFC to this aspect of control (e.g.,28). Moreover, individual variation in emotion regulation abilities has also been linked to dlPFC function (29). In addition, MRS and post-mortem studies show alterations in GABA in dlPFC for psychiatric disorders linked with heightened emotional dysregulation and poor self-control, including cocaine addiction, depression and schizophrenia (30-32). Hence, dlPFC would be a strong candidate area in which a relationship between urgency and GABA levels might be manifest.
There are also reasons to propose the inferior frontal gyrus (IFG) or the anterior cingulate cortex (ACC) as candidate areas for a GABA-urgency relationship. IFG, for example, has been strongly linked with response inhibition in the stop-signal task (33), and variation in response inhibition has been suggested to be an important mechanism underlying externalizing vulnerability (34). ACC, together with dlPFC, is thought to be a neural generator of the ERN (35). Further, Matsuo et al. (36) found that gray matter volume in rostral ACC was correlated with impulsivity (as measured by the Barrat Impulsiveness Scale 37).
Advances in MRS (38-41) allow us to investigate individual differences in GABA concentration in specific regions of the human brain. This approach has proved successful in discovering group differences in regional GABA concentration between psychiatric and control cohorts (e.g., 32,42), and more recently in relating specific behavioural and physiological measures in healthy participants to individual differences in regional GABA levels, even in the small cohort size of typical MR studies (43-48). In frontal cortex in particular, higher GABA concentration measured in the region of the frontal eye fields was correlated with lower eye movement distractibility (i.e. better control, 49), leading us to predict that the likely direction of any association between frontal GABA and impulsivity would be higher GABA concentration, lower impulsivity (better control).
In this study we take a two-step approach. First, we explored whether resting GABA levels in any of the three candidate brain regions (dlPFC, IFG and ACC) were related to urgency, or indeed any of the other three impulsivity facets. Second, having identified a correlation between urgency and GABA in dlPFC, we aimed to test whether this would replicate in a second cohort. We also include data from two control regions, the parietal cortex and supplementary motor area (SMA), which were measured in the same participants for the purpose of a different study (46).
Materials and Methods
Overview
In the first experiment, using edited MR spectroscopy (38,40), we measured GABA concentration from a 3cm□3cm□3cm region of the right dorsal lateral prefrontal cortex (dlPFC) including, but not limited to, a sub-region of the middle frontal gyrus (we refer to this as the dlPFC region, Figure 1a,b). The voxel size is dictated by signal-to-noise ratio; smaller voxels have been used previously for group averages, but not for individual differences (e.g.,42,50,51). We also acquired (over two MR sessions per participant) MRS measurements from voxels in the inferior frontal gyrus (IFG), the anterior cingulate cortex (ACC), the parietal lobe, and the supplementary motor area (SMA), as well as an anatomical MRI scan. The SMA and parietal voxels are included here as control voxels, though the primary purpose for their measurement was for a different study (investigating the relationship between masked priming and GABA in SMA, for which the prefrontal voxels served as control (46). Note that in the present GABA-edited MRS protocol we acquire an average spectrum from a single predefined volume, and thus measurements for each volume were taken separately (12min each). On a separate occasion, each participant completed the UPPS questionnaire and was then tested on the stop-signal task (participants also performed other tasks for the purpose of the priming study; neither the impulsivity data nor stop-signal relationships have been published before).
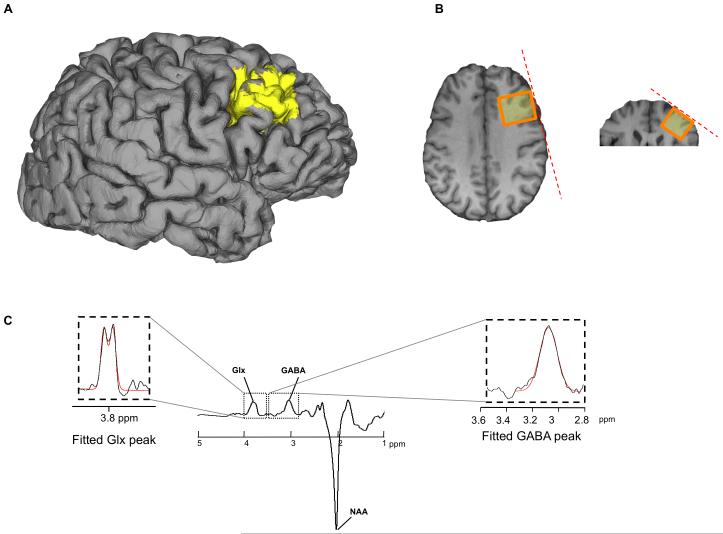
Figure 1.
Methodology for spectroscopy. (a, b) the MRS voxel (yellow, 3 cm × 3cm × 3cm voxel) was placed over the anatomical location of the dlPFC. Edited MR spectra (c) allow the quantification of GABA concentration by extracting the area under the GABA peak (39-41, 68). In a similar way, we extracted a measure of glutamate+glutamine, Glx, (also marked is the N-acetyl-aspartate peak, NAA). The GABA peak will contain signal from co-edited macromolecules, but although we do not know the exact contribution, we do not expect any individual differences in macromolecules to drive any correlation with our behavioural measures.
The aim of the second experiment was to test in an independent cohort the robustness of the relationship found between GABA and urgency scores in the first experiment. There was one MR session per participant consisting of an anatomical MRI scan followed by MRS measurement from the dlPFC voxel. Parietal and SMA MRS voxels were again included in this session for the purpose of the priming study (46), and are presented here as control regions. On a separate occasion, participants completed the UPPS questionnaire and were tested in the Stop-signal task (as well as the priming study tasks).
Participants
For the first experiment, twelve volunteers (aged 21-32, mainly Caucasian) were recruited within the School of Psychology, Cardiff University. For the second experiment, thirteen volunteers were similarly recruited (aged 19-35, mainly Caucasian). Since menstrual cycle may affect GABA levels (52,53), and since there are gender differences in impulsivity (54), all the participants were male in order to remove these potential sources of variance. All had normal or corrected-to-normal vision, no neurological or psychiatric history and were right-handed. They received payment for their time. Participants were aware that the purpose of the scan was spectroscopy, and that they would complete a personality questionnaire and performance tasks, but no further specification was given as to the nature of the hypotheses. The local Research Ethics Committee approved all procedures.
Structural MRI
A 1-mm3 isotropic resolution, T1-weighted anatomical MRI scan (FSPGR) was carried out to allow MRS voxel placement, and subsequent reconstruction of the cortical surface and segmentation of the MRS voxel. To segment the volume we used both FAST (http://www.fmrib.ox.ac.uk/fsl/) and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), and these methods showed a high degree of correlation for grey matter volume (r>0.95). In the reported results, grey matter estimates came from FreeSurfer.
MRS
GABA-edited MEGA-PRESS spectra (38,40) were acquired on a General Electric 3T-HDx MRI scanner from voxels positioned according to anatomical landmarks. For each participant, the dlPFC voxel was placed in the right middle frontal gyrus (i.e. between the superior and inferior frontal sulci, with the posterior surface of the voxel aligned with the precentral sulcus), using oblique localizing slices to match edges of the voxel to the subject’s cortical surface (see Figure 1-a). Directly inferior to this voxel, we placed a voxel of similar dimensions in the IFG (between the inferior frontal sulcus and the horizontal ramus), again matching the edge of the voxel to the subject’s cortical surface. The anterior cingulate voxel was placed over the midline, and between the cingulate and the pericallosal sulci; this voxel was 3cm□2cm□4cm – reducing the inferior-superior dimension helped avoid including part of the insula and corpus callosum. The parietal voxel was positioned in the right superior parietal lobule aligned with the cortical surface, while the SMA voxel was placed on the midline with its posterior surface aligned with the central sulci, (for more information see 46).
The following experimental parameters were used: TE=68-ms; TR=1800-ms; 400 transients of 4096 data points were acquired in 12-min; 16-ms Gaussian editing pulses were applied either to the GABA spins (1.9ppm), or symmetrically about the water peak (7.5ppm) in an interleaved manner. A further eight transients were acquired, without water suppression, as an internal concentration reference. Phased-array coil data (8 channels) were combined (using the first point of the unsuppressed water free induction decay signal) and spectra were processed by locally written software. Three-Hertz exponential line broadening and a high-pass water filter were applied, and the MEGA-PRESS difference spectrum was produced.
The edited GABA signal at 3ppm and the unsuppressed water signal were integrated: the integral of the GABA peak was calculated automatically using a linear fit of the baseline and a Gaussian fit to the peak itself. The water signal was fitted using a Lorentzian-Gaussian lineshape (55). A Gaussian model was chosen for the GABA peak, rather than a doublet, because empirically we rarely find a clear doublet with this protocol (45,46,56), but the integral derived is very consistent whichever fitting model is used (r>0.95). The reason a doublet does not appear is complex. The pseudo-doublet has a small center-peak (Figure 3 in 57), the size of which will depend on sequence parameters such as refocusing bandwidth and refocusing slice profile. There is also likely to be a contribution from macromolecules, which is unknown and will depend on sequence parameters. The extent to which any pseudo-doublet splitting is filled in will therefore depend on these factors and the underlying linewidth of the signals (~12Hz in this study). In other studies using a Siemens MEGA-PRESS implementation, a doublet appears in, at most, half of edited spectra (Figure 5 in 40,48).
The GABA integral was scaled to account for the fraction of cerebrospinal fluid (CSF) within the voxel, and the water signal was scaled to account for the different water content in CSF, grey and white matter (58). A concentration measurement in institutional units was derived from the ratio of the GABA and water signals by using a single scalar to adjust for the editing efficiency and the T1 and T2 relaxation times of water (59) and GABA. Since T1 and T2 values for GABA are not available in the literature, they were estimated from the range for other metabolites (60,61,62). Note that this scalar adjustment changes only the absolute concentration values reported, not the relative values between individuals. We also obtained a combined measure of glutamate and glutamine levels, by fitting the “Glx” peak (Figure 1-c) with a Gaussian doublet centered on 3.75.
Measuring impulsivity
After completing behavioural tasks of cognitive and automatic motor control (masked-priming, stop-signal, Simon tasks – 46), participants were administered the UPPS impulsive behaviour scale. This scale was derived by Whiteside and Lynam (3) from a combined analysis of the five-factor model of personality (63) and a total of 21 subscales across 10 impulsivity measures (e.g.,the Barratt Impulsiveness Scale-11, 37). This resulted in a fourfold division of impulsivity into facets of: Urgency, Premeditation (lack of), Perseverance (lack of) and Sensation Seeking (hence “UPPS”). Example questions for urgency include: ‘It is hard for me to resist acting on my feelings’; ‘When I am upset I often act without thinking’. Representative questions for the other facets include: ‘I usually think carefully before doing anything’ (Premeditation); ‘I finish what I start’ (Perseverance); ‘I would enjoy water skiing’ (Sensation seeking). The UPPS scales have excellent reliability (Cronbach alpha ~ 0.9, see 3), and have been validated, for example, against interview-based impulsivity assessments (4). UPPS items are assessed on a scale ranging from 1 (Agree strongly) to 4 (disagree strongly), and the UPPS is scored such that higher scores represent higher levels of impulsivity.
Results
The mean scores and their standard deviations in each subscale are given in Table 1. These were similar across the two cohorts (all p>.07), and comparable to those reported in similar populations of university staff and students.
Table 1.
Mean scores (and standard deviation) obtained on the 4 subscales of the UPPS impulsivity measurement. Values are total scores from each of the UPPS scales. Number of items per scale: urgency – 12 items, Premeditation – 11 items, Perseverance – 10 items, sensation seeking – 12 items
| (Feeling of) Urgency |
(Lack of) Premeditation |
(Lack of) Perseverance |
Sensation (seeking) |
Age (year) | |
|---|---|---|---|---|---|
| Cohort 1 | 24 (4.5) | 20.7 (4.1) | 17.8 (3.7) | 32.9 (4) | 27 (1.3) |
| Cohort 2 | 23.7 (5.6) | 20.9 (3.2) | 19.8 (6.1) | 33.3 (5) | 22.7 (1.4) |
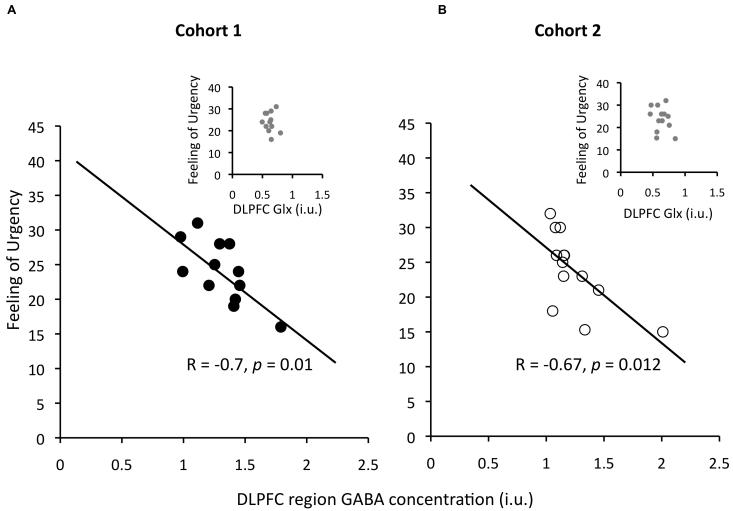
The main result for the first cohort was a correlation between GABA concentration in dlPFC and urgency, such that participants with higher GABA concentration scored lower on this factor (Pearson’s r=−0.70, Spearman’s ρ=−0.77; Figure 2-left). There was also a correlation of dlPFC GABA with perseverance (r=−0.58). However, with our sample size, these correlations are not significant after correcting for multiple comparisons (5 areas □ 4 impulsivity facets). Therefore we tested for replication in a second independent cohort, using the results of the first cohort to specify planned comparisons. The correlation between dlPFC GABA and urgency was replicated (Pearson’s r=−0.67, p=.013, 1-tailed, corrected for 2 comparisons, Spearman’s ρ=−0.78, p=.0015; Figure 2-right). We also performed bootstrapping to check whether just one or two points drove the correlations. This involved taking N random samples from within our data, with replacement (where N is the number of participants), computing the correlation coefficient r, and repeating 10000 times to find the 95th percentile value of r (i.e. the value for which 95% of the values produced in bootstrapping were greater or equal to this value). If a correlation is driven by just one or two points, since in many of the iterations these would be left out, near zero r values would occur more than 5% of the time. Thus if the 95th percentile r-value were near zero or crossed it, it would indicate the correlation is not robust. The Bootstrap 95th percentile values for R for the Pearson’s r for cohorts 1 and 2 were −0.31 and −0.33, respectively, indicating they are robust to subsampling.
Figure 2.
Urgency correlates with GABA in the dlPFC region. Higher dlPFC GABA predicted lower urgency score across individuals (a). This result was replicated in a second cohort (b). GABA concentration measurements are stated in institutional units (i.u.). The p-value for cohort 2 is given 1-tailed, and corrected for two comparisons, since there were two planned tests of replication that arose from cohort 1. Inserts show that the relation observed for GABA levels did not hold for glutamate+glutamine (Glx).
This relationship did not generalize to the Glx peak in the spectrum (glutamate+glutamine, Figure 2-left insert; 1st cohort: r=−0.15, p>.05, Figure 2-right insert; 2nd cohort: r=−0.3, p>.05). Neither did Glx correlate with GABA (1st cohort: r=0.39, p>.20; 2nd cohort: r=0.37, p > .23) or with any impulsivity subscale (1st cohort: all ∣r∣<0.39, all p > .2; 2nd cohort: all ∣r∣<0.22, all p>.45). However, note that the Glx peak is not a pure measure of glutamate, because glutamate cannot be unambiguously separated from glutamine using this technique at a 3T field strength, therefore this absence of correlation cannot be taken to mean that individual differences in glutamate play no role.
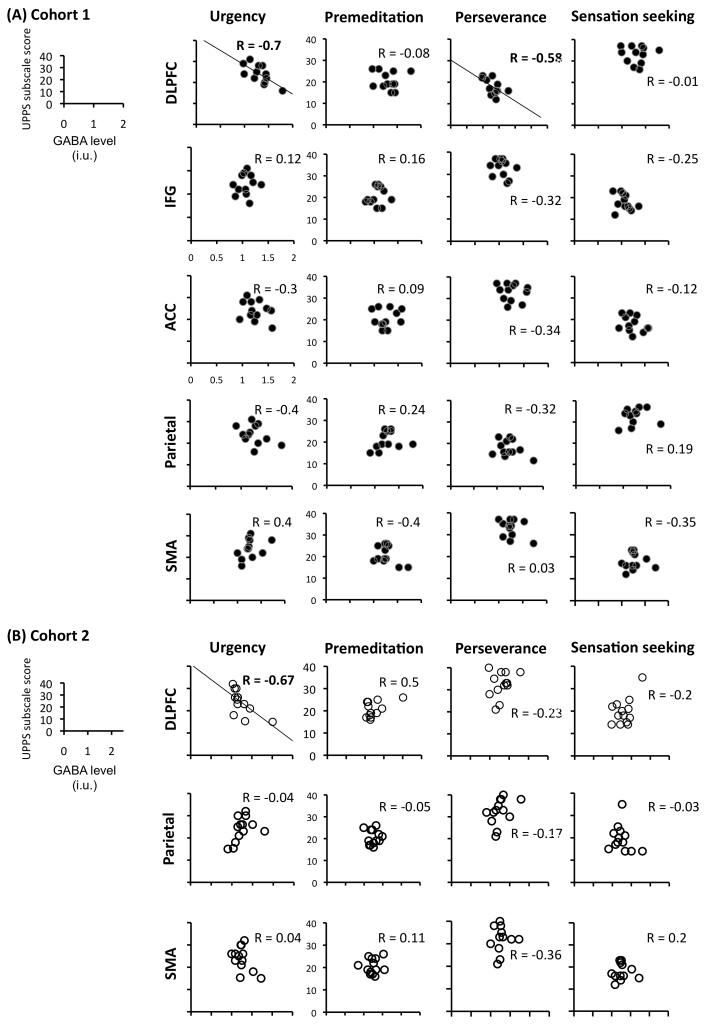
GABA concentration in the dlPFC did not correlate with Premeditation or Sensation-seeking, and the correlation with Perseverance found at first did not replicate in the second cohort (Figure 3-a,b, top rows). This stresses the importance of an independent replication when evaluating correlations obtained with small samples and multiple possible comparisons. In terms of regional specificity, we found no evidence that any of the impulsivity traits correlated with GABA measures taken from voxels in the other two candidate regions (IFG and ACC) or with the other two regions for which we obtained measurements with these cohorts (SMA and parietal).
Figure 3.
Reliable correlation between GABA and impulsivity was specific to the dlPFC region and the urgency component. GABA concentration measurements are stated in institutional units (i.u.).
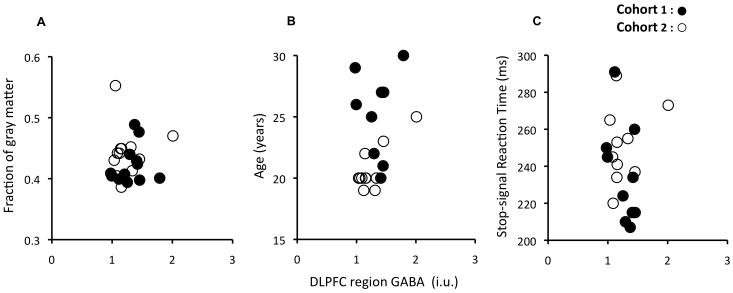
We tested whether the correlation between urgency and GABA in the dlPFC region might arise due to other individual factors such as age or grey matter fraction in the voxel. It did not; there were no significant correlations of either the urgency scores or dlPFC GABA with these factors (Figure 4-a,b), and when controlling for them, correlations between dlPFC GABA and urgency remained strong or even improved (Controlling for the Gray matter fraction: 1st cohort: rpartial=−0.77, p<.005; 2nd cohort: rpartial=−0.69, p<.012; Controlling for age: 1st cohort: rpartial=−0.70, p<.016; 2nd cohort: rpartial=−0.68, p<.014). We also tested whether the correlation might be related to irregular sleep patterns, tobacco, alcohol, drug use, or weight in our participants (for the 15 participants who provided this information). There was no relationship with any of these factors. None of the participants was taking any medication at the time of the study.
Figure 4.
The fraction of gray matter in the dlPFC MRS voxel, the participants’ age and Stop-signal reaction time do not correlate with GABA in the dlPFC region. (a) The fraction of gray matter in the dlPFC MRS voxel was assessed using Freesurfer (Martinos center for biomedical imaging, Cambridge, Mass, USA) and showed no correlation with dlPFC GABA – first cohort, r = 0.2, p < .5; second cohort, r= 0.07, p < .8. (b) Age in our sample was not related to GABA in the dlPFC region – first cohort, r = −0.1, p < .75; second cohort, r = 0.07, p < .8. (c) The experimental measure of response inhibition is not predicted by our neurochemical measurement. Both correlations were not significant – first cohort, r = −0.49, p < .1 (bootstrap 90% conf. int.: [−0.8, 0.0]); second cohort, r= 0.35, p < .3 (bootstrap 90% conf. int.: [−0.2, 0.7])– and even in opposite directions. The Stop-signal task (86), can be conceived as an enriched version of a Go/No-go task (e.g.,87), bringing detailed information about the timing of inhibitory motor processes by estimating a Stop-signal reaction time. In our case, participants made speeded button presses to a shape cue, but on a subset of trials a second stimulus was presented that instructed them to withhold their response. The interval between go and stop signals is modulated to find the interval at which participants successfully stop on 50% of the stop trials.
Because externalizing vulnerability has been linked to impaired response inhibition (34), we also tested whether results of the Stop-signal task, an experimental measure of response inhibition, correlated with dlPFC GABA (and with Urgency). We did not find any consistent relationship between GABA and either stopping ability (Figure 4-c), or overall response speed (1st & 2nd cohorts: all ∣r∣<0.08, p>.8). It is worth noting that performance in the stop task itself has previously been more strongly related to IFG and pre-SMA than to DLPFC (64-67), but we found no significant correlation between stopping and IFG GABA (1st cohort: r=0.24, p>.4) or between stopping and GABA in our voxel centered on the SMA, which contained part of the pre-SMA (1st cohort: r =0.21, p>.5). We also did not find a relationship between stopping ability and any of the components of impulsivity.
Discussion
The neurochemical balance in different brain regions has long been thought instrumental in the regulation of personality traits and psychiatric disorders, but it is not straightforward to link genetic or pharmacological data implicating particular neurotransmitter systems with particular brain regions (e.g.,8). Employing magnetic resonance spectroscopy (MRS) offers a way to fill this gap, enabling us to report here that individual differences in GABA concentration in a specific region (dlPFC) were reliably related to a specific personality trait (urgency). It is worth emphasizing that the correlation did not arise from general GABA concentration over the whole brain – there was no correlation with the other frontal and parietal areas we measured, although low signal-to-noise in edited MRS techniques may have contributed to absence of detection of correlations in these regions. Previous reports have found no evidence for correlation of GABA concentration between brain regions (46,49,68). Thus on current data, it seems that having high GABA concentration in dlPFC does not necessarily mean that a person will have high GABA concentration in other brain regions, underlining the importance of targeting specific brain regions in clinical GABA MRS studies (32,69,70).
Recently, a series of genetic studies have linked variation in GABAergic neurotransmission with a variety of co-occurring disorders characterized by emotional dysregulation and poor self-control (9-11,14-16), thought to reflect a coherent externalizing spectrum (18). Our results support the utility of a dimensional approach to personality-psychopathology relations (e.g.,6). The results also link GABAergic neurotransmission with the role of dlPFC in self-control (e.g.,28,29,71). The exact mechanism by which differences in dlPFC GABA level would influence self-control ability is unknown. Our findings are consistent with the view that dlPFC neurons adapt their properties to robustly maintain information that is relevant to current goals (72). In the context of such ‘adaptive coding’ in the control of working memory, GABAergic inhibition has been posited as the mechanism for adjusting the focus of dlPFC neurons to the task at hand, leading to increased robustness of goal-representations in the face of distraction (e.g. from rewards or stressors, 73). Similarly, Sawaguchi et al. (74) found that GABA continuously and preferentially suppresses neuronal activity via GABA-A receptors to limit the population of prefrontal neurons activated by a particular behaviour, consistent with adaptive selectivity. This process may have parallels with the role of GABAergic inhibition in tuning the response properties of visual cortex neurons, sharpening orientation selectivity, for example (56,75). Thus it is plausible that higher GABA concentration can lead to more robust adaptive coding of goals in dlPFC, providing more effective integration of salient motivational information with current task goals, thus promoting adaptive behaviour (76).
The details of the relationship between GABAergic neurotransmission in dlPFC and functional inhibition of behaviour are likely to be complex. The dlPFC is part of a network involving loops through striatum and thalamus as well as numerous connections to other cortical and subcortical areas relevant for controlling behaviour. Similarly, the roles of dopamine and serotonin are yet to be clarified (7,8,23), and are likely to interact with that of GABA (77,78). Moreover, the reasons why people may differ in MRS measures of regional GABA concentration, and how this relates to variation in receptor genes, remain unknown. Also unknown is the contribution that macromolecules make to the GABA measurement: They are likely to make a significant contribution to the absolute measures, but are currently thought unlikely to drive the differences between individuals, which correlate with neurophysiological and behavioural differences that are readily explained by GABA variation, but would be difficult to explain by macromolecule variation (43-45,47,48,56,79). Most likely, differences in our measure of GABA concentration will be related to the number of GABA interneurons or the number and type of synapses, and we do not yet know what implications this could have for the wiring of GABAergic and glutamatergic excitation-inhibition circuits across the different cell layers of the neocortex (80,81). Importantly, however, such regional individual differences should not be expected to mimic the effects of agonist/antagonist pharmacological agents, which disturb neurotransmission over larger parts of the brain, and probably do not change the number of interneurons present. Even when regionally specific, pharmacological agents may not mimic natural variation: for example, GABAA receptor agonists such as muscimol can be used to temporarily ‘deactivate’ regions of cortex (e.g.,82), but deactivating dlPFC would be expected to reduce impulse control (25) and thus be associated with higher urgency. Our finding that higher GABA predicts lower urgency is opposite to this prediction, and shows that natural variation in GABA should not simply be considered analogous to more or less cortical deactivation.
Our findings also imply that neither GABA nor impulsivity have a simple relationship with response inhibition as measured by the Stop-Signal reaction time alone. We found no correlation between either GABA or impulsivity and the stop-task in our samples. Although correlation between impulsivity and the stop-task has been reported previously (83,84), other studies have not replicated this (e.g.,85). Young et al.’s findings (34) are informative in this respect. They found that the relationship between externalizing disinhibitory traits and response inhibition is better captured at the level of a latent response inhibition variable, measured across several ‘inhibition’ tasks, than by any single task. Overall, these results suggest that dlPFC GABA is more related to a higher-level trait indexed by urgency, than specific to the performance in the Stop-signal task, although such conclusion based on absent correlations must be only tentative.
In sum, we have found that that one facet of impulsivity, urgency, a quantitative trait linked at high levels with several clinically problematic externalizing, disinhibitory conditions, correlates with the concentration of GABA measured in dlPFC, a region implicated in self-control. We hope that these results, as well as future research using regionally specific MRS in humans, will help to bridge the gap between the regional specificity provided by imaging studies and the biochemical data from genomic and pharmacological investigations.
Acknowledgements
This work was supported by the Wellcome Trust, and the Wales Institute of Cognitive Neuroscience (WICN). CUBRIC was established with support from the UK Department of Trade and Industry, Cardiff University and the Welsh Assembly Government.
Footnotes
Financial disclosure The authors report no direct or indirect biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev ed Washington, DC: 2000. [Google Scholar]
- 2.DeYoung C. Impulsivity as a personality trait. In: Vohs KD, Baumeister RF, editors. Handbook of Self-Regulation: Research, Theory, and Applications. Second ed Guilford Press; New York: 2010. pp. 485–502. [Google Scholar]
- 3.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and human differences. 2001;30:669–689. [Google Scholar]
- 4.Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- 5.Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 7.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, et al. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci U S A. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer L, Dick D, Bierut L, Bucholz K, Edenberg H, Kuperman S, et al. Obesity, smoking, and frontal brain dysfunction. Am J Addict. 2010;19:391–400. doi: 10.1111/j.1521-0391.2010.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enoch MA, Hodgkinson CA, Yuan Q, Shen PH, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67:20–27. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, et al. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Molecular Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green EK, Grozeva D, Moskvina V, Hamshere ML, Jones IR, Jones L, et al. Variation at the GABAA receptor gene, Rho 1 (GABRR1) associated with susceptibility to bipolar schizoaffective disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1347–1349. doi: 10.1002/ajmg.b.31108. [DOI] [PubMed] [Google Scholar]
- 14.Dick DM, Bierut L, Hinrichs A, Fox L, Bucholz KK, Kramer J, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 15.Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009;66:649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markon KE, Krueger RF. Categorical and continuous models of liability to externalizing disorders: a direct comparison in NESARC. Arch Gen Psychiatry. 2005;62:1352–1359. doi: 10.1001/archpsyc.62.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer M. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyoung C, Peterson J, Seguin J, Tremblay R. Externalizing behavior and the higher order factors of the Big Five. Journal of abnormal psychology. 2008;117:947–953. doi: 10.1037/a0013742. [DOI] [PubMed] [Google Scholar]
- 20.Valdes IH, Steinberg JL, Narayana PA, Kramer LA, Dougherty DM, Swann AC, et al. Impulsivity and BOLD fMRI activation in MDMA users and healthy control subjects. Psychiatry Res. 2006;147:239–242. doi: 10.1016/j.pscychresns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Asahi S, Okamato Y, Akado G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:245–2512004. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- 22.Brown SM, Manuck SB, Flory JD, Hariri AR. Contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- 23.DeYoung C, Hirsh J, Shane M, Papademetris X, Rajeevan N, Gray J. Testing predictions from personality neuroscience: brain structure and the Big Five. Psych Sci. 2010:1–34. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 26.Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullsperger M, von Cramon DY, Muller NG. Interactions of focal cortical lesions with error processing: evidence from event-related brain potentials. Neuropsychology. 2002;16:548–561. doi: 10.1037//0894-4105.16.4.548. [DOI] [PubMed] [Google Scholar]
- 28.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drabant EM, Mcrae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65:367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. International Journal of Neuropsychopharmacology. 2010;13:411–420. doi: 10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, et al. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci U S A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young S, Friedman N, Miyake A, Willcutt E, Corely R, Haberstick B, et al. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo K, Nicoletti MA, Peluso MA, Hatch JP, Nemoto K, Watanabe Y, et al. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar disorder. Bipolar Disord. 2009;11:628–636. doi: 10.1111/j.1399-5618.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 37.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 40.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magnetic Resonance in Medicine. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR in Biomedicine. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 42.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 43.Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. Journal of Neurophysiology. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual Differences in Subconscious Motor Control Predicted by GABA Concentration in SMA. Curr Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. Journal of Neuroscience. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.07.017. DOI:10.1016/j.neuroimage.2010.1007.1017. [DOI] [PubMed] [Google Scholar]
- 49.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nature Neuroscience. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 50.Jensen J, Blaise de BF, Renshaw P. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR in Biomedicine. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 51.Choi IY, Lee SP, Merkle H, Shen J. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage. 2006;33:85–93. doi: 10.1016/j.neuroimage.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 53.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 54.Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21 doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, et al. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magnetic Resonance in Medicine. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 56.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. Journal of Neuroscience. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waddell K, Avison M, Joers J, Gore J. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magnetic Resonance Imaging. 2007;25:1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain .1. Compartments and Water. Journal of Magnetic Resonance Series B. 1993;102:1–8. [Google Scholar]
- 59.Wansapura J, Holland S, Dunn R, Ball W. NMR relaxation times in the human brain at 3.0 tesla. Journal of magnetic resonance imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 60.Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W, et al. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1.5 and 3 Tesla. Magn Reson Med. 2003;50:1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- 61.Traber F, Block W, Lamerichs R, Gieseke J, Schilld HH. 1H Metabolite Relaxation Times at 3.0 Tesla: Measurements of T1 and T2 Values in Normal Brain and Determination of Regional Differences in Transverse Relaxation. Journal of Magnetic Resonance Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 62.Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human Brain-Structure Resolved T2 Relaxation Times of Proton Metabolites at 3 Tesla. Magnetic Resonance in Medicine. 2007;57:983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]
- 63.McCrae R, Costa P. Validation of the five-factor model across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- 64.Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, et al. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: Evidence from functional MRI and Huntington’s disease. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 67.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 68.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. Journal of Magnetic Resonance Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 69.Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biological Psychiatry. 2005;58:969–973. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, Mattews PM, et al. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. International Journal of Neuropsychopharmacology. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 71.Kim S, Lee D. Prefrontal Cortex and Impulsive Decision Making. Biol Psychiatry. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 73.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sawaguchi T. Unmasking the silent ‘task-related’ neuronal activity in the monkey prefrontal cortex by a GABA-A antagonist. Neuroscience Research. 2001;39:123–131. doi: 10.1016/s0168-0102(00)00204-2. [DOI] [PubMed] [Google Scholar]
- 75.Li G, Yang Y, Liang Z, Xia J, Yang Y, Zhou Y. GABA-mediated inhibition correlates with orientation selectivity in primary visual cortex of cat. Neuroscience. 2008;155:914–922. doi: 10.1016/j.neuroscience.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 76.Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–9193. doi: 10.1523/JNEUROSCI.22-21-09185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Almeida J, Mengod G. Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem. 2008;107:488–496. doi: 10.1111/j.1471-4159.2008.05649.x. [DOI] [PubMed] [Google Scholar]
- 79.Stagg CJ, Bachtiar V, Johansen-Berg H. The Role of GABA in Human Motor Learning. Curr Biol. 2011 doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 81.Douglas RJ, Martin KAC, Whitteridge D. A canonical microcircuit for neocortex. Neural computation. 1989;1:480–488. [Google Scholar]
- 82.Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- 83.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- 84.Vigil-Colet A, Codorniu-Raga M. Aggression and inhibition deficits, the role of functional and dysfunctional impulsivity. Personality and individual differences. 2004;37:1431–1440. [Google Scholar]
- 85.Reynolds B, Ortengren A, Richard JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and individual differences. 2006;40:305–315. [Google Scholar]
- 86.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 87.Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, et al. Monoamine oxidase-a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]