Abstract
Objective
To estimate all cause mortality from untreated familial hypercholesterolaemia free from selection for coronary artery disease.
Design
Family tree mortality study.
Setting
Large pedigree in Netherlands traced back to a single pair of ancestors in the 19th century.
Subjects
All members of pedigree aged over 20 years with 0.5 probability of carrying a mutation for familial hypercholesterolaemia.
Main outcome measure
All cause mortality.
Results
A total of 70 deaths took place among 250 people analysed for 6950 person years. Mortality was not increased in carriers of the mutation during the 19th and early 20th century; it rose after 1915, reached its maximum between 1935 and 1964 (standardised mortality ratio 1.78, 95% confidence interval 1.13 to 2.76; P=0.003), and fell thereafter. Mortality differed significantly between two branches of the pedigree (relative risk 3.26, 95% confidence interval 1.74 to 6.11; P=0.001).
Conclusions
Risk of death varies significantly among patients with familial hypercholesterolaemia. This large variability over time and between branches of the pedigree points to a strong interaction with environmental factors. Future research is required to identify patients with familial hypercholesterolaemia who are at extreme risk and need early and vigorous preventive measures.
What is already known on this topic
Familial hypercholesterolaemia is associated with excess mortality in families of patients who present with cardiovascular disease
Population data are lacking
What this study adds
Many untreated patients with familial hypercholesterolaemia (about 40%) reach a normal life span
Standardised mortality ratio was normal in the 19th century and rose to a peak in the 1930s to 1960s
The variation in mortality suggests an interaction between genetic and environmental factors
Introduction
Familial hypercholesterolaemia is associated with premature cardiovascular disease1,2 and decreased life expectancy.3–5 These associations, however, have been described in families that were investigated because the probands—or even multiple family members—had presented with cardiovascular disease at young age.1–6 Because susceptibility to cardiovascular disease is likely to be affected by additional genetic and environmental factors,7–9 mortality from familial hypercholesterolaemia may have been preferentially studied in patients and families with multiple risk factors for cardiovascular disease.5 The natural course of the disorder has not been studied without selection for cardiovascular disease, and estimates for carriers in the general population are therefore lacking.
The recent introduction of molecular diagnostic tools allows the disorder to be diagnosed with certainty,10 and large scale family screening has been shown to be highly effective.11 We assessed mortality risk in a large pedigree of carriers of the V408M mutation or Afrikaner-2 mutation in exon 9 of the low density lipoprotein receptor gene.12
Subjects and methods
Hypercholesterolaemia was detected in probands A and B during routine screening. In proband C, hypercholesterolaemia was detected after myocardial infarction at the age of 51 years. The probands had mean fasting total serum cholesterol concentrations of 10.24, 9.20, and 12.78 mmol/l, respectively. The three probands were carriers of the V408M mutation on an identical haplotype, and this suggested that they were (distantly) related. We performed genealogical searches using official records of births, marriages, and deaths. Dutch official records for previous centuries are virtually complete because they were stored at several places. The authorities performed these registrations irrespective of socioeconomic status.
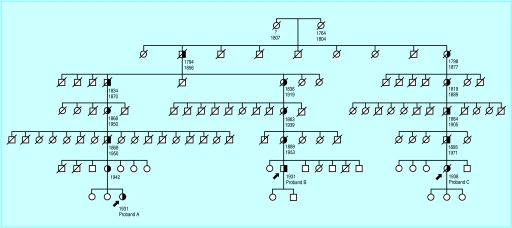
The genealogical searches had two phases. Firstly, we traced all maternal and paternal ancestors of the three carriers of the mutation throughout as many generations as possible. We found only one pair of ancestors shared by and connecting the three probands. Secondly, we traced all descendants of this pair and screened all living descendants for the V408M mutation. Figure 1 shows a small part of the pedigree. All first degree relatives of people on the transmission lines of the mutation had a mendelian probability of 0.5 of being affected. As a result of the increasing size of the pedigree in recent generations, the information about ancestors on transmission lines is confirmed many times. Our analyses could have been influenced by consanguinity or non-paternity if the genuine fathers were also carriers of the V408M mutation. Deceased parents and their ancestors could then be interpreted incorrectly as affected. We therefore did genealogical searches of the spouses of the pedigree and tested for the V408M mutation in the living descendants of their siblings. We did not detect consanguinity and we did not find any indication for non-paternity.
Figure 1.
Ascertainment of complete sibships by genealogical searches in a small part of the pedigree. Three probands were heterozygous for the V408M mutation. The lines of transmission (half solid symbols) connect the three probands with the shared pair of distant ancestors. All family members in the sibships along the lines of transmission had 0.5 mendelian probability of carrying the mutation
The molecular diagnosis of familial hypercholesterolaemia was based on the presence of the V408M mutation. This molecular method has been described elsewhere.12 The molecular and genealogical studies were approved by the hospitals' review boards, and all family members studied gave informed consent.
Statistical methods
The mortality in the pedigree was compared with the mortality in the Dutch population standardised for age, sex, and calendar period as described previously.13–15 In brief, the standardised mortality ratio is the ratio of observed to expected number of deaths. We calculated the expected mortality by multiplying the total number of years lived by the people in the pedigree in each calendar period for each age and sex category by the age and sex specific mortality rates of the Dutch population for each calendar period. The probands and the parental years before birth of the proband were excluded from the analyses. We also ignored the first two decades of life for all people in the pedigree because the registration of juvenile mortality in the 19th century may have been incomplete. Moreover, ancestors who were certainly affected may have been missed because premature death could have decreased the chance of passing on their mutation to present generations. This would lead to underestimation of risk of death. Therefore, we did the primary analyses in relatives of complete sibships (all siblings available for analysis), who had 0.5 probability of being affected. As a result the observed standardised mortality ratios exhibit 50% of the excess mortality from the mutation causing familial hypercholesterolaemia. Secondary analyses were done on people who were definitely affected. We ended all analyses in December 1989, when statins became available to our patients.
We compared mortality between subgroups with Poisson regression (relative risk). Cumulative survival was analysed with Cox's regression (relative risk) and the Kaplan-Meier method. We calculated the 95% confidence interval of the standardised mortality ratio assuming a Poisson distribution of the observed number of deaths and using exact limits. We calculated the 95% confidence interval of the relative risk with Poisson or Cox's regression as the exponent of the regression coefficient and its standard error. Significance was assessed at the 5% level of probability.
Results
Genealogical searches
We traced a total of 412 descendants in eight generations along the lines of transmission of the V408M mutation in the pedigree. Four sibships with incomplete data due to emigration were excluded from the analyses. The complete sibships contained 387 (94%) relatives, of whom 250 survived for 20 years or more.
Standardised mortality: variance over time
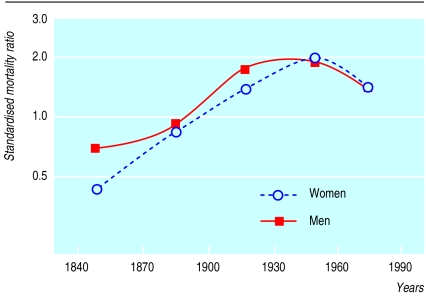
Between 1830 and 1989, a total of 70 deaths took place among 250 people with a 0.5 probability of carrying the mutation who were analysed over 6950 person years (table). The overall standardised mortality ratio of these people was 1.32 (95% confidence interval 1.03 to 1.67; P=0.02). Thirty of the 118 people who were definitely affected died, giving a standardised mortality ratio of 1.59 (1.07 to 2.26; P=0.02). In the 20th century, mortality from familial hypercholesterolaemia rose, peaking between 1935 and 1964 (standardised mortality ratio 1.78, 1.13 to 2.76; P=0.003) and then falling. The standardised mortality ratios of relatives who were definitely affected followed a similar trend, with a maximum of 2.29 (1.14 to 4.09; P=0.005) between 1935 and 1964.
Figure 2 shows the point estimates of the standardised mortality ratios for men and women over time. The period had a significant effect on risk of death after differences in the distribution of age and sex were adjusted for (P<0.001).
Figure 2.
Mortality from familial hypercholesterolaemia according to sex and time. Mortality was estimated among 250 persons with 0.5 probability of carrying the V408M. Probands and the first 20 years of life were ignored
Standardised mortality: variance within generations
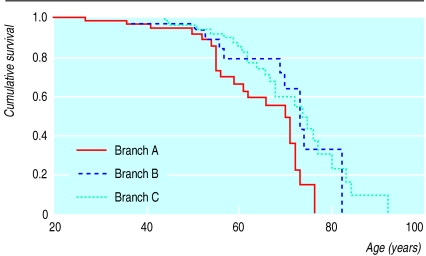
Analysis by Poisson regression showed that the risk of death in the family members of probands B and C relative to those of proband A was 1.74 (95% confidence interval 0.82 to 3.67; P=0.1) and 3.26 (95% confidence interval 1.74 to 6.11; P=0.001) respectively. Analysing the data with a Cox's proportional-hazards model did not materially alter the results (data not shown). Figure 3 shows the difference in survival between the branches. The standardised mortality ratio of branch A was 1.04 (95% confidence interval 0.66 to 1.43; P=0.9).
Figure 3.
Survival in the pedigree with V408M mutation. Data represent Kaplan-Meier estimates of family members with 0.5 probability of being affected. Branches A and C were ascertained through probands A and C, respectively. Life expectancy was significantly better in branch A than branch C (Logrank test P=0.001). Branch B differed from the other two with respect to the distribution of sex and time. Probands and first 20 years of life were ignored
The Poisson regression analysis allowed synchronous estimation of the variance of risk of death in two directions: through the centuries and within generations. Time had a significant effect on risk of death (P=0.002) after adjustment for age, sex, and position in the pedigree. The deaths took place in seven generations of the pedigree, and consequently the deceased relatives shared only a small fraction of their genome. Statistical adjustment for the exact family ties did not change the results (data not shown).
Discussion
We found that the excess mortality from familial hypercholesterolaemia varied over time. In the 19th century, mortality seemed lower than in the general population. It rose after 1915, reached a maximum during the 1950s, and decreased thereafter. During the decades with excess mortality, survival in the branches of the pedigree differed significantly, ranging from normal life expectancy to severe excess mortality. This large variation of risk suggests that previous studies, with families based on selected patients, may have overestimated mortality. Moreover, such large variation in mortality in two directions (over time and within generations) in a pedigree indicates that the disorder has strong interactions with environmental factors.
Strengths and weaknesses
The strength of our study is that the natural course of the disorder was assessed free from selection for cardiovascular disease. We started with three probands and traced the pair of distant ancestors from whom the disorder originated. All descendants of these distant ancestors were subsequently identified, the only factor affecting selection being the completeness of the Dutch official records. We then identified the transmission lines of the mutation through the pedigree so that all carriers of the mutation could be analysed. We studied all cause mortality rather than cardiovascular mortality because cause of death was often poorly defined in earlier records.
Our findings are based on a large pedigree of carriers of the V408M mutation in the low density lipoprotein receptor gene. This mutation is consistently associated with greatly raised plasma cholesterol concentrations and may be indicative of familial hypercholesterolaemia in general.12 Moreover, the mortality from different types of mutations may be similar.5 However, we cannot exclude the existence of rare mutations that cause a higher risk of death from familial hypercholesterolaemia.
Our analyses are unlikely to have been affected by competing risk. In the past, mortality from other causes, mainly infectious disease, could have been so high that people died from these other causes before they could develop cardiovascular disease. However, this will not have affected our calculations because the standardised mortality ratio and the Poisson model were based on age specific rates, which are not influenced by competing risk.16
Implications
People in the first generations of our pedigree reached old age. Such higher survival of ancestors with familial hypercholesterolaemia has been reported previously in Utah pedigrees,3 and hypercholesterolaemia may have conferred a survival advantage when infectious disease was prevalent. This hypothesis is supported by the observation that genetically modified mice with high cholesterol concentrations were protected against severe Gram negative infections.17
Harlan et al described normal survival in a large pedigree with familial hypercholesterolaemia in 1966. They suggested that “the precocious onset of cardiovascular disease and the bad prognosis of familial hypercholesterolemia have been overemphasized because many of the early studies were of the relatives of patients who had sought medical attention.”18 These findings were largely ignored, and since then many studies have been done in probands (and relatives) who presented with symptoms of cardiovascular disease in lipid clinics. We found many affected people who had normal lifespans. Nevertheless, increased mortality does exist in some affected families. These families are probably characterised by clustering of risk factors. Lifestyle factors such as high fat diet, cigarette smoking, and physical activity3,8,19,20 and factors that influence lipid metabolism8,21 have been associated with a higher prevalence of cardiovascular disease in patients with familial hypercholesterolaemia. Because of the large time span of our study, we do not have information on specific environmental factors.
Future research should be directed at determining which patients with familial hypercholesterolaemia are at particularly high risk of premature cardiovascular disease and which environmental factors are effective in modulating this risk. Individual regimens can then be developed to prevent premature death in patients with familial hypercholesterolaemia who show different combinations of risk factors.
Table.
Mortality from familial hypercholesterolaemia in pedigree compared with Dutch population
| Years | 50% affected (n=250)
|
100% affected (n=118)
|
|||||
|---|---|---|---|---|---|---|---|
| Person years* | No of deaths | Standardised mortality ratio (95% CI) | Person years* | No of deaths | Standardised mortality ratio (95% CI) | ||
| 1830-1869 | 306 | 3 | 0.58 (0.12 to 1.71) | 127 | 1 | 0.45 (0.01 to 2.51) | |
| 1870-1904 | 466 | 7 | 0.87 (0.35 to 1.79) | 162 | 3 | 1.20 (0.25 to 3.52) | |
| 1905-1934 | 892 | 11 | 1.49 (0.74 to 2.67) | 349 | 4 | 1.21 (0.33 to 3.09) | |
| 1935-1964 | 2008 | 23 | 1.78 (1.13 to 2.67) | 892 | 11 | 2.29 (1.14 to 4.09) | |
| 1965-1989 | 3278 | 26 | 1.33 (0.87 to 1.95) | 1657 | 11 | 1.81 (0.90 to 3.24) | |
| All years | 6950 | 70 | 1.32 (1.03 to 1.67) | 3186 | 30 | 1.59 (1.07 to 2.26) | |
The probands and the first 20 years of life were ignored.
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Müller C. Angina pectoris in hereditary xanthomatosis. Arch Intern Med. 1939;64:675–700. [Google Scholar]
- 2.Stone NJ, Levy RI, Fredrickson DS, Verter J. Coronary artery disease in 116 kindred with familial type II hyperlipoproteinemia. Circulation. 1974;49:476–488. doi: 10.1161/01.cir.49.3.476. [DOI] [PubMed] [Google Scholar]
- 3.Williams RR, Hasstedt SJ, Wilson DE, Ash KO, Yanowitz FF, Reiber GE, et al. Evidence that men with familial hypercholesterolemia can avoid early coronary death. JAMA. 1986;255:219–224. [PubMed] [Google Scholar]
- 4.Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893–896. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sijbrands EJG, Westendorp RGJ, Lombardi MP, Havekes LM, Frants RR, Kastelein JJP, et al. Additional risk factors influence mortality in heterozygous familial hypercholesterolaemia. Atherosclerosis. 2000;149:421–425. doi: 10.1016/s0021-9150(99)00336-6. [DOI] [PubMed] [Google Scholar]
- 6.Hazzard WR, Goldstein JL, Schrott HG, Motulsky AG, Bierman EL. Hyperlipidemia in coronary heart disease. III. Evaluation of lipoprotein phenotypes of 156 genetically defined survivors of myocardial infarction. J Clin Invest. 1973;52:1569–1577. doi: 10.1172/JCI107333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seed M, Hoppichler F, Reaveley D, McCarthy S, Thompson GR, Boerwinkle E, et al. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990;322:1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 8.Hill JS, Hayden MR, Frohlich J, Pritchard PH. Genetic and environmental factors affecting the incidence of coronary artery disease in heterozygous familial hypercholesterolemia. Arterioscler Thromb. 1991;11:290–297. doi: 10.1161/01.atv.11.2.290. [DOI] [PubMed] [Google Scholar]
- 9.Williams RR, Hunt SC, Hopkins PN, Wu LL, Schumacher MC, Stults BM, et al. Evidence for gene-environmental interactions in Utah families with hypertension, dyslipidaemia and early coronary heart disease. Clin Exp Pharmacol Physiol. 1992;19 (Suppl 20):1–6. [PubMed] [Google Scholar]
- 10.Lombardi P, Sijbrands EJG, Van de Giessen K, Smelt AHM, Kastelein JJP, Frants RR, et al. Mutations in the low density lipoprotein receptor gene of familial hypercholesterolemic patients detected by denaturing gradient gel electrophoresis and direct sequencing. J Lipid Res. 1995;36:860–867. [PubMed] [Google Scholar]
- 11.Umans-Eckenhausen MAW, Defesche JC, Sijbrands EJG, Scheerder RLJM, Kastelein JJP. Screening for familial hypercholesterolemia in the Netherlands: the first five years of experience. Lancet. 2001;357:165–168. doi: 10.1016/S0140-6736(00)03587-X. [DOI] [PubMed] [Google Scholar]
- 12.Defesche JC, Van Diermen DE, Lansberg PJ, Lamping RJ, Reymer PW, Hayden MR, et al. South African founder mutations in the low-density lipoprotein receptor gene causing familial hypercholesterolemia in the Dutch population. Hum Genet. 1993;92:567–570. doi: 10.1007/BF00420940. [DOI] [PubMed] [Google Scholar]
- 13.Coleman MP, Herman C, Douglas A. Person-years (PYRS): a further program for cohort study analysis. Lyons: WHO; 1989. [Google Scholar]
- 14.Rosendaal FR, Heijboer H, Briet E, Buller HR, Brandjes DPM, De Bruin K, et al. Mortality in hereditary antithrombin-III deficiency - 1830 to 1989. Lancet. 1991;337:260–262. doi: 10.1016/0140-6736(91)90867-o. [DOI] [PubMed] [Google Scholar]
- 15.Hille ETM, Westendorp RGJ, Vandenbroucke JP, Rosendaal FR. Mortality and causes of death in families with the factor V Leiden mutation (resistance to activated protein C) Blood. 1997;89:1963–1967. [PubMed] [Google Scholar]
- 16.Rothenberg RB. Competing mortality and progress against cancer. Epidemiology. 1994;5:197–203. doi: 10.1097/00001648-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Netea MG, Demacker PNM, Kullberg BJ, Boerman OC, Verschueren I, Stalenhoef AFH, et al. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe gram-negative infections. J Clin Invest. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlan WR, Graham JB, Estes EH. Familial hypercholesterolemia: a genetic and metabolic study. Medicine. 1966;45:77–110. [Google Scholar]
- 19.Beaumont V, Jacotot B, Beaumont J-L. Ischaemic disease in men and women with familial hypercholesterolaemia and xanthomatosis. A comparative study of genetic and environmental factors in 274 heterozygous cases. Atherosclerosis. 1976;24:441–450. doi: 10.1016/0021-9150(76)90136-2. [DOI] [PubMed] [Google Scholar]
- 20.Nobili A, D'Avanzo B, Santoro L, Ventura G, Todesco P, La Vecchia C. Serum cholesterol and acute myocardial infarction: a case control study from the GISSI-2 trial. Br Heart J. 1994;71:468–473. doi: 10.1136/hrt.71.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotze MJ, Davis HJ, Bissbort S, Langenhoven E, Brusnicky J, Oosthuizen CJ. Intrafamilial variability in the clinical expression of familial hypercholesterolemia: importance of risk factor determination for genetic counselling. Clin Genet. 1993;43:295–299. doi: 10.1111/j.1399-0004.1993.tb03821.x. [DOI] [PubMed] [Google Scholar]