Abstract
Metabolomics studies hold promise for discovery of pathways linked to disease processes. Cardiovascular disease (CVD) represents the leading cause of death and morbidity worldwide. A metabolomics approach was used to generate unbiased small molecule metabolic profiles in plasma that predict risk for CVD. Three metabolites of the dietary lipid phosphatidylcholine, namely choline, trimethylamine N-oxide (TMAO), and betaine, were identified and then shown to predict risk for CVD in an independent large clinical cohort. Dietary supplementation of mice with choline, TMAO or betaine promoted up-regulation of multiple macrophage scavenger receptors linked to atherosclerosis, and supplementation with choline or TMAO promoted atherosclerosis. Studies using germ-free mice confirmed a critical role for dietary choline and gut flora in TMAO production, augmented macrophage cholesterol accumulation and foam cell formation. Suppression of intestinal microflora in atherosclerosis-prone mice inhibited dietary choline-enhanced atherosclerosis. Genetic variations controlling expression of flavin monooxygenases (FMOs), an enzymatic source of TMAO, segregated with atherosclerosis in hyperlipidemic mice. Discovery of a relationship between gut flora-dependent metabolism of dietary phosphatidylcholine and CVD pathogenesis provides opportunities for development of both novel diagnostic tests and therapeutic approaches for atherosclerotic heart disease.
Keywords: metabolomics, atherosclerosis, intestinal microbiota, diet, phospholipid, choline
The pathogenesis of cardiovascular disease (CVD) includes genetic and environmental factors. A known environmental risk factor for the development of CVD is a diet rich in lipids. A relationship between blood cholesterol and triglyceride levels and cardiovascular risk is well established. However, less is known about the role of the third major category of lipids, phospholipids, in atherosclerotic heart disease pathogenesis.
Another potential yet controversial environmental factor in the development or progression of atherosclerotic heart disease is inflammation due to infectious agents. Studies have suggested associations between coronary disease and pathogens such as Cytomegalovirus (CMV), Helicobactor, Chlamydia, or C pneumoniae1–4. However, prospective randomized trials with antibiotics in humans have thus far failed to demonstrate cardiovascular benefit5–7 and studies with germ-free hyperlipidemic mice confirm that infectious agents are not necessary for murine atherosclerotic plaque development8. While a definite cause-and-effect relationship between bacterial or viral pathogen and atherosclerosis in humans has not yet been established, the prospect of a role for microbes and atherosclerosis susceptability remains enticing.
Intestinal microbiota (“gut flora”), comprised of trillions of typically non-pathogenic commensal organisms, serve as a filter for our greatest environmental exposure - what we eat. Gut flora play an essential role, aiding in the digestion and absorption of many nutrients9. Animal studies have recently shown that intestinal microbial communities can influence the efficiency of harvesting energy from diet, and consequently influence susceptibility for obesity10. Metabolomics studies of inbred mouse strains have also recently shown that gut microbiota may play an active role in the development of complex metabolic abnormalities, such as susceptibility to insulin resistance and non-alcoholic fatty liver disease11. A link between gut flora dependent phospholipid metabolism and atherosclerosis risk through generation of pro-atherosclerotic metabolites has not yet been reported.
RESULTS
Metabolomics studies identify elevated plasma levels of specific analytes that are associated with increased risk for CVD
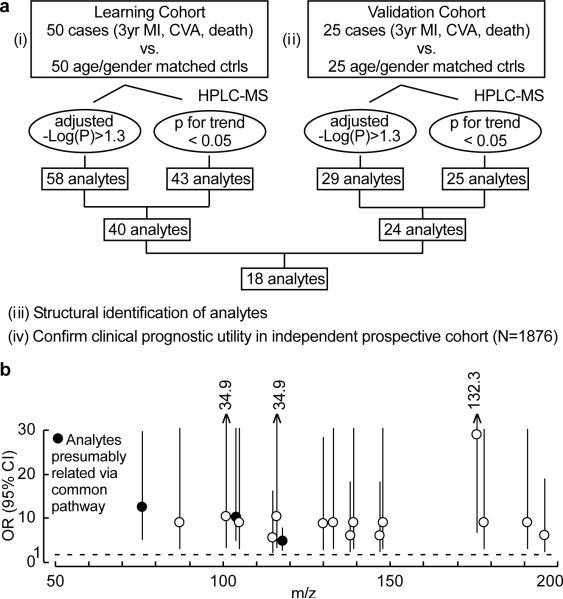
In initial studies we sought to discover unbiased small molecule metabolic profiles in plasma that predict increased risk for cardiovascular disease (CVD). An initial “Learning Cohort” was used comprised of plasma from stable patients undergoing elective cardiac evaluation who subsequently experienced a heart attack (myocardial infarction, MI), stroke or death over the ensuing three year period versus age- and gender-matched subjects who did not. Liquid chromatography with on-line mass spectrometry (LC/MS) analysis of plasma was performed to define analytes associated with cardiac risk as described in Methods. Of an initial 2000+ analytes monitored, 40 met all acceptability criteria within the Learning Cohort. Subsequent studies within an independent “Validation Cohort” led to identification of 18 analytes that met acceptability criteria in both Learning and Validation Cohorts (Fig. 1a,b, Supplementary Fig. 1a, Supplementary Table 1).
Figure 1. Strategy for metabolomics studies to identify plasma analytes associated with cardiovascular risk.
a, Overall schematic to identify plasma analytes associated with cardiac risk. b, Odds ratio (OR) and 95% confidential intervals (CI) of prospective (3 year) risk for myocardial infarction (MI), stroke (CVA) or death of the 18 plasma analytes that met all selection criteria in the Learning and Validation Cohorts, OR and CI shown are for the highest vs. lowest quartile for each analyte. Filled circles represent the analytes (m/z=76, 104, 118) focused on in this study. m/z, mass to charge ratio of an analyte monitored in positive MS1 mode.
The structural identity of the 18 small molecules in plasma whose levels track with cardiac risks was not known since the compounds were screened based upon their retention time and mass-to-charge ratio (m/z) when analyzed by LC/MS. Among the 18 analytes, those with m/z 76, 104 and 118 demonstrated significant (p<0.001) correlations amongst one another, suggesting their potential relationship via a common biochemical pathway (Supplementary Fig. 1b). We therefore initially sought to structurally define these three analytes.
Identification of dietary phosphatidylcholine metabolites as markers for increased CVD risk
The candidate compound in plasma with m/z of 76 associated with CVD risks was isolated and unambiguosly identified as trimethylamine N-oxide (TMAO) using multinuclear NMR, MSn, LC/MS/MS, and GC/MS/MS following multiple derivitization strategies (see Methods, Supplementary Figs. 2a–d, and Supplementary Table 2). TMAO, an oxidation product of trimethylamine (TMA), is a relatively common metabolite of choline in animals12–13. Foods rich in the lipid phosphatidylcholine (PC, also called lecithin), which predominantly includes eggs, milk, liver, red meat, poultry, shell fish and fish, are believed to be the major dietary sources for choline, and hence TMAO production14. Briefly, initial catabolism of choline and other trimethylamine containing species (e.g. betaine) by intestinal microbes forms the gas TMA13, which is efficiently absorbed and rapidly metabolized by at least one member of the hepatic flavin monooxygenase (FMO) family of enzymes, FMO3, to form TMAO15–16. Identification of the plasma analyte associated with CVD risk with m/z 76 as TMAO therefore suggested that the plasma analyte with m/z 104 might be choline. Further, these results also suggested that the plasma analyte with m/z 118 associated with CVD might be related to PC (choline) metabolism.
To test the hypothesis that the plasma analytes with m/z 76 (TMAO), 104 and 118 might all be derived from the major dietary lipid PC, mice were fed egg yolk PC (via oral gavage) and plasma levels of analytes over time were monitored. In both male and female mice, analytes with the same m/z (76, 104 and 118) and the same retention times as the corresponding analytes of interest observed in human plasma all increased following oral PC feeding (Supplementary Figs. 3a and b), strongly suggesting the m/z 104 analyte was choline, and the analyte at m/z 118 was derived from PC. Confirmation that the plasma analyte (m/z 104) associated with CVD risk was choline was achieved by MSn, LC/MS/MS, and GC/MS/MS following multiple derivitization strategies (Supplementary Fig. 4a–d; Supplementary Table 3).
We next studied the plasma analyte with m/z 118. We hypothesized that the analyte was either betaine or one of several potential methyltransferase metabolites of choline (see Supplementary Fig. 5a for structures and strategy for discrimination amongst these isomers). To both distinguish amongst these species, and explore a role for intestinal generation of the various metabolites, different isotopically labeled choline precursors were administered to mice either via oral (gavage) vs. parenteral (intraperitoneal, i.p.) route. The observed m/z of new isotopically labeled analytes at the appropriate retention times identified in plasma following these isotope tracer studies are sumarized in Fig. 2a. Oral administration of non-labeled choline resulted in time-dependent increases in plasma levels of analytes with m/z 76, 104 and 118, consistent with TMAO, choline and either betaine or a methylated choline species (Supplementary Fig. 6a). Use of selectively deuterated choline species at either the trimethylamine moiety (d9-isotopomer) or the ethyl moiety (d4-isotopomer) unambigously confirmed the m/z 118 analyte as betaine (Figs. 2a, Supplementary Fig. 6b). Further confirmation was acquired by observing the same retention time in LC/MS and an identical CID mass spectrum (Supplementary Fig. 5b). Moreover, supplementation of PC or choline isotopomers via gavage or i.p. injection showed an absolute requirement for oral route in TMAO production, whereas betaine production from PC or choline was formed via both oral and i.p. routes (Fig. 2, Supplementary Fig. 7a).
Figure 2. Identification of metabolites of dietary PC and an obligatory role for gut flora in generation of plasma analytes associated with CVD risks.
a, Summary schematic indicating structure of metabolites and routes (oral or intraperitoneal, i.p.) of formation observed in choline challenge studies in mice using the indicated isotope labeled choline. The m/z in plasma observed for the isotopmers of the choline metabolites are shown. b, Plasma levels of d9-metabolites following i.p. challenge with d9(trimethyl)-dipalmitoylphosphatidylcholine (d9-DPPC). c, d9-TMAO production following oral d9-DPPC in mice, following suppression of gut flora with antibiotics (3 weeks), and then following placement (4 weeks) into conventional cages with non-sterile mice (i.e. – “conventionalized”). Data are presented as mean ± SE from 4 independent replicates.
Demonstration of an obligate role for gut flora in TMAO formation from dietary PC
Intestinal microflora play a role in TMAO formation from dietary free choline13. We therefore hypothesized that commensal organisms (i.e. gut flora) might also have an obligate role in TMAO formation from dietary PC. To test this, deuterated PC was synthesized whereby the choline-methyl groups were deuterium labelled (i.e. d9-PC) and used as isotope tracer for feeding studies. When mice were fed via oral gavage with d9-PC, time-dependent appearance of the anticipated d9-isotopomer of TMAO was observed in plasma (Fig. 2c). Interestingly, pre-treatment of mice with a three-week course of broad spectrum antibiotics to suppress intestinal flora completely suppressed appearance of d9-TMAO in plasma following oral d9-PC (Fig. 2c). A similar pattern was observed following oral administration of d9-choline to mice, with d9-TMAO produced in untreated mice, but not in the same mice following a three-week course of broad spectrum antibiotics (Supplementary Fig. 7b), or in germ free mice born sterilely by Caesarian section (Supplementary Fig. 7c). In a final series of studies, mice with suppressed intestinal microflora following antibiotics were placed in conventional cages with normal (i.e. non-germ-free) mice to permit intestinal colonization with microbes. After four weeks, repeat oral d9-PC challenge of the now “conventionalized” mice resulted in readily detectable plasma levels of d9-TMAO (Fig. 2c). Similar results were observed following conventionalization of germ-free mice and oral d9-choline (Supplementary Fig. 7c). Collectively, these results show an obligate role for intestinal microbiota in generation of TMAO from the dietary lipid PC. They also reveal the following metabolic pathway for dietary PC producing TMAO: PC → choline → TMA → TMAO.
Confirmation of elevated plasma levels of the dietary PC metabolites TMAO, choline and betaine as predictors of cardiovascular risk in a large independent clinical cohort
We next sought to independently confirm the prognostic value of monitoring fasting plasma levels of TMAO, choline and betaine in a large independent clinical cohort distinct from subjects examined in both the Learning and Validation Cohorts. Stable subjects (N=1876) undergoing elective cardiac evaluations were enrolled. Clinical, demographic and laboratory characteristics of the cohort are provided in Supplementary Table 4a. Fasting plasma levels of TMAO, choline and betaine were quantified by LC/MS/MS using methods specific for each analyte (Supplementary Fig. 8). Elevated levels of choline, TMAO and betaine were all observed to show dose-dependent associations with the presence of CVD (Fig. 3a–c) and multiple individual CVD phenotypes including peripheral artery disease (PAD), coronary artery disease (CAD), and history of myocardial infarction (MI) (Supplementary Tables 5a–d for multilogistic regression models, and Supplementary Table 5e for Somers' Dxy correlation). The associations between increased risk of all CVD phenotypes monitored and elevated systemic levels of the three PC metabolites held true following adjustments for traditional cardiac risk factors and medications usage (Figs. 3a–c, Supplementary Tables 5a–e).
Figure 3. Choline, TMAO and betaine are associated with atherosclerosis risks in humans and promote atherosclerosis in mice.
a–c, Spline models of the logistic regression analyses reflecting risk of cardiovascular disease (CVD) (with 95% CI) according to plasma levels of choline, TMAO and betaine in the entire cohort (n = 1876 subjects). d, Comparison in aortic lesion area among 20 week old female C57BL/6J.Apoe−/− mice fed with chow diet supplemented with the indicated amounts (wt/wt) of choline or TMAO from time of weaning (4 weeks). e, Relationship between plasma TMAO levels and aortic lesion area. f, Relationship between fasting plasma levels of TMAO versus CAD burden among subjects (N=1020). Boxes represent 25th, 50th and 75th percentile, and whiskers 5th and 95th percentile plasma levels. Single, double and triple coronary vessel disease refers to number of major coronary vessels demonstrating ≥ 50% stenosis on diagnostic coronary angiography.
Dietary choline or TMAO supplementation enhances atherosclerotic lesion development in mice
We next investigated whether the strong associations noted between plasma levels of the dietary PC metabolites and CVD risk reflected some hidden underlying pro-atherosclerotic mechanism. Atherosclerosis-prone mice (C57BL/6J.Apoe−/−) at time of weaning were placed on either normal chow diet (contains 0.08–0.09% total choline, wt/wt) or normal chow diet supplemented with intermediate (0.5%) or high amounts of additional choline (1.0 %) or TMAO (0.12%). At 20 weeks of age enhanced aortic atherosclerotic plaque was noted in both male and female mice on diets supplemented with either choline or TMAO (Fig. 3d, Supplementary Fig. 9a). Analysis of plasma levels of choline and TMAO in each of the dietary arms showed nominal changes in plasma levels of choline, but significant increases of TMAO in mice receiving either choline or TMAO supplementation (Supplementary Fig. 10). Parallel examination of plasma cholesterol, triglycerides, lipoproteins, glucose levels, and hepatic triglyceride content in the mice failed to show significant increases that could account for the enhanced atherosclerosis (Supplementary Table 6, Supplementary Fig. 11). Interestingly, all dietary groups of mice revealed a significant positive correlation between plasma levels of TMAO and atherosclerotic plaque size (Fig. 3e, Supplementary Fig. 9b). Of note, plasma TMAO levels observed within the female mice (which get enhanced atherosclerosis relative to their male counterparts), even on normal chow diet, were significantly higher than those observed among male mice (Supplementary Fig. 10). No significant gender differences in plasma levels of TMAO were observed in humans (P=0.47); however, a clear dose-response relationship was observed between TMAO levels and clinical atherosclerotic plaque burden in subjects undergoing coronary angiography (Fig. 3f).
Genetic and biochemical studies implicate hepatic flavin monooxygenases (FMOs) in TMAO production and atherosclerosis susceptability
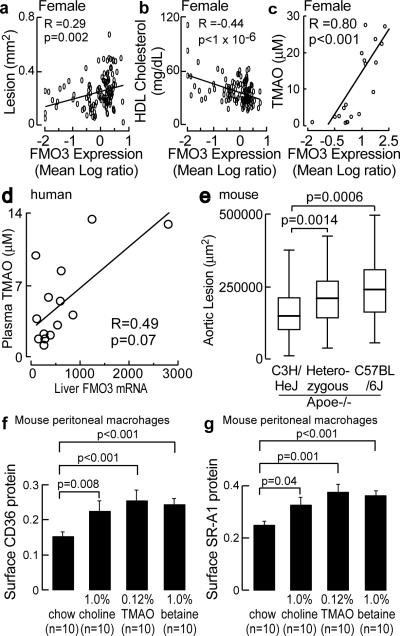
Hepatic FMO3 is a known enzymatic source for TMAO in humans, based on the recent recognition of the etiology of an uncommon genetic disorder called trimethylaminuria (also known as fish malodor syndrome)15,17. Subjects with this metabolic condition have impaired capacity to convert TMA, which smells like rotting fish, into TMAO, an odorless stable oxidation product17. We therefore sought to identify possible sources of genetic regulation and role of FMO3 in atherosclerosis using integrative genetics in mice18. Expression levels of FMO3 were determined by microarray analysis in the livers of mice from an F2 intercross between atherosclerosis-prone C57BL/6J.Apoe−/− mice and atherosclerosis-resistant C3H/HeJ.Apoe−/− mice and compared with quantitative measures of atherosclerosis. The expression level of FMO3 showed marked differences between genders (females > 1,000 fold higher than in males). Significant positive correlations were readily found between hepatic FMO3 expression and atherosclerotic lesions (Fig. 4a; Supplementary Fig. 12 top row; Supplementary Fig. 13). Interestingly, a highly significant negative correlation with plasma high density lipoprotein (HDL) cholesterol levels was noted in both male and female mice (Fig. 4b; Supplementary Fig. 12, middle row). Further, plasma levels of the PC metabolite, TMAO, showed a significant positive correlation with hepatic FMO3 expression level in mice (Fig. 4c; Supplementary Fig. 12 bottom row).
Figure 4. Hepatic FMOs are linked to atherosclerosis and dietary PC metabolites enhance macrophage scavenger receptor expression.
a–c, Correlation between hepatic FMO3 expression and aortic lesion, plasma HDL cholesterol and TMAO in female mice from the F2 intercross between atherosclerosis prone C57BL/6J.Apoe−/− and atherosclerosis resistant C3H/HeJ Apoe−/− mice. d, Correlation between human hepatic FMO3 expression and plasma TMAO. e, Effect of FMO3 genotype (SNP rs3689151) on aortic sinus atherosclerosis in male mice from the C57BL6/J Apoe−/− and C3H/HeJ Apoe−/− F2 intercross. f.g, Quantification of scavenger receptor CD36 and SR-A1 in macrophages harvested from C57BL/6J mice (13 week) following three weeks of standard chow verses chow supplemented with the indicated amounts (wt/wt) of choline, TMAO or betaine. Data are presented as mean ± SE from the indicated numbers of mice in each group.
FMO3 is one member of a family of FMO enzymes, the majority of which are physically located as a cluster of genes on chromosome 1 in both humans and mice. The various FMOs share sequence homology and overlapping substrate specificities. Further, while rare mutations in or near the FMO3 gene have been identified in individuals with trimethylaminuria19, the impact of these mutations on other FMO genes remains unknown. Examination of the hepatic expression levels of the various FMOs revealed that many are highly correlated with each other in both mice and humans (Supplementary Table 7). Examination of hepatic expression levels of additional FMOs in mice from the atherosclerosis F2 intercross revealed multiple FMOs are significantly correlated with aortic lesion formation, HDL cholesterol concentrations, and plasma TMAO levels (Supplementary Figs. 14–16), suggesting that multiple members of the FMO family of enzymes may participate in atherosclerosis and the PC → TMAO metabolic pathway. To explore the relationship between hepatic FMOs and plasma TMAO levels in humans, paired samples of liver and plasma from subjects undergoing elective liver biopsy were examined. Amongst all of the human FMO monitored, only a trend toward positive association was noted between hepatic expression of FMO3 and plasma TMAO levels (Fig. 4d; Supplementary Fig. 17).
Next, we focused on the genetic regulation of hepatic FMO3 expression (and other FMOs) using eQTL (expression quantitative trait locus) analyses in the F2 mouse cross. The eQTL plot for FMO3 mRNA levels is shown in Supplementary Fig. 18, and demonstrates a strongly suggestive cis locus (LOD=5.9) on mouse chromosome 1 at 151 Mb. FMO3 (and several other FMOs) is located at 164.8 Mb in a region identified as non-identical by descent between C3H/HeJ and C57BL/6 (http://mouse.perlegen.com). This region is just distal to the 95% confidence interval of a previously reported murine atherosclerosis susceptibility locus20. Examining the effect of the closest SNP to FMO3 (rs3689151) as a function of alleles inherited from either parental strain indicated a strong effect on atherosclerosis in both genders of the F2 mice (Kruskal-Wallis test, p<1.×10−6). Bonferroni corrected pair-wise comparisons indicated a dose-dependent significant increase in atherosclerosis in F2 mice heterozygous or homozygous for the C57BL/6J allele (Fig. 4e). Although the resolution on average for an F2 intercross of this size is in excess of 20 Mb and thus does not provide `gene level' resolution, these data show that the locus encompassing the FMO gene cluster on chromosome 1 is associated with atherosclerotic lesion size. Collectively, these results indicate that: (i) hepatic expression levels of multiple FMOs are linked to plasma TMAO levels in mice; (ii) hepatic expression levels of multiple FMOs are associated with both the extent of aortic atherosclerosis and HDL cholesterol levels in mice; (iii) hepatic expression levels of FMO3 suggest an association with plasma TMAO levels in humans; and (iv) a genetic locus containing the FMO gene cluster on chromosome 1 in mice has a strong effect on atherosclerosis.
Dietary supplementation with PC-Mderived metabolites induces gut flora-dependent in vivo up-regulation of atherogenic macrophage scavenger receptors and foam cell formation
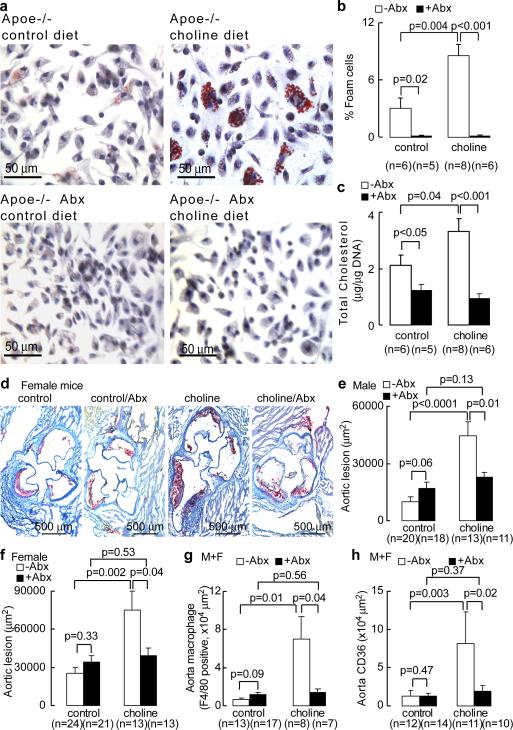
To explore potential mechanisms through which dietary choline and its metabolites might exert their pro-atherosclerotic effects, C57BL/6J.Apoe−/− mice at time of weaning were placed on a normal chow diet supplemented with either choline, TMAO or betaine (for > 3 weeks). Both mRNA levels (Supplementary Fig. 19) and surface protein levels (Figs. 4f, g; Supplementary Fig. 20) of two macrophage scavenger receptors implicated in atherosclerosis, CD36 and SR-A1, were then determined in peritoneal macrophages. Relative to normal chow diet, animals supplemented with either choline, TMAO or betaine all showed enhanced macrophage levels of CD36 and SR-A1. We next examined the impact of dietary choline and gut flora on endogenous formation of cholesterol-laden macrophage foam cells, one of the earliest cellular hallmarks of the atherosclerotic process. Hyperlipidemic C57BL/6J.Apoe−/− mice were fed diets with defined levels of choline as follows: (i) “control” (0.07–0.08%, wt/wt), which is similar to the choline content of normal chow (0.08–0.09%); versus (ii) high “choline”, corresponding to > 10-fold higher level of choline (1.0%, wt/wt) than normal chow. Concomitantly, half of the mice were administered broad spectrum antibiotics for 3 weeks in order to suppress intestinal microflora, which was confirmed by the reduction of plasma TMAO levels by >100-fold (plasma TMAO concentrations in groups receiving antibiotics were <100 nM). While mice on the control diet showed modest evidence of endogenous macrophage foam cell formation, as indicated by oil-red-O staining of peritoneal macrophages, mice on the 1% choline supplemented diet showed markedly enhanced lipid-laden macrophage development (Fig. 5a). In contrast, suppression of intestinal flora significantly inhibited dietary choline-induced macrophage foam cell formation (Fig. 5a,b). These results were confirmed by microscopic quantification of endogenous foam cell levels (Fig. 5b), and analytical quantification of the cholesterol content of recovered macrophages (Fig. 5c). Histopathology and biochemical studies of livers recovered from these mice showed no evidence of steatosis (Supplementary Fig. 21). Parallel analyses of plasma PC metabolites also demonstrated no significant changes in choline or betaine levels between the different dietary groups, and significant increases of plasma TMAO levels only in mice on the high choline diet in the absence of antibiotics (males, control vs. choline diet, 2.5±0.1 μM vs. 28.3±2.4 μM, p<0.001; for females, control vs. choline diet, 4.0±0.5 μM vs. 158.6±32.9 μM, p<0.001).
Figure 5. Obligatory role of gut flora in dietary choline enhanced atherosclerosis.
a, Choline supplementation promotes macrophage foam cell formation in gut flora dependent fashion. C57BL/6J.Apoe−/− mice at time of weaning (4 weeks) were provided drinking water with versus without broad spectrum antibiotics (Abx), and placed on chemically defined diets similar in composition to normal chow (control diet, 0.08 ± 0.01% total choline, wt/wt) or normal chow with high choline (choline diet, 1.00% ± 0.01% total choline, wt/wt). Resident peritoneal macrophages were recovered at 20 weeks of age. Typical images of oil-red-O/hematoxylin stained macrophages in each diet group are shown. b, Foam cell quantification from peritoneal macrophages recovered from mice in studies described in panel a. c, Macrophage cellular cholesterol content. d, Representative oil-red-O/hematoxylin stained aortic root sections from female C57BL/6J.Apoe−/− mice fed control and high choline diets in the presence versus absence of Abx. e,f, Aortic lesion area in 20 week old C57BL/6J.Apoe−/− mice off versus on Abx and fed with control versus choline diet. g, Aortic macrophage quantification with anti-F4/80 antibody staining. h, Quantitation of scavenger receptor CD36 in aorta within the indicated groups. Error bars represent s.e.m. from the indicated numbers of mice.
Demonstration of gut flora involvement in dietary choline-induced atherosclerosis
In additional studies we sought to test whether gut flora is involved in dietary choline-induced atherosclerosis. At the time of weaning (4 week old), atherosclerosis-prone C57BL/6J.Apoe−/− mice were placed on either a control diet (0.08±0.01%, wt/wt, choline) or a diet supplemented with 1% choline (wt/wt, choline diet). Half of the mice were also treated with broad spectrum antibiotics to suppress intestinal microflora. Serial plasma measurements confirmed suppression of TMAO levels to virtually non-detectable levels (<100 nM) throughout the duration of the study. At 20 weeks of age, mice were sacrificed and aortic root lesion development was quantified. In the absence of antibiotics (i.e. preserved intestinal microflora), choline supplementation augmented atherosclerosis in both male and female mice nearly three fold (Figs. 5d,e,f). In contrast, suppression of intestinal flora completely inhibited dietary choline-mediated enhancement in atheroscelerosis (Figs. 5d,e,f). Aortic macrophage content and scavenger receptor CD36 immunoreactive surface area within aortic lesions were markedly increased on the high choline diet, but not when intestinal microflora was suppressed with antibiotic treatment (Fig. 5g,5h; Supplementary Figs. 22,23). Both histopathological and biochemical examination of liver sections from mice showed no evidence of steatosis or altered neutral lipid (triglyceride or cholesterol/cholesterol ester) levels on either diet in the absence or presence of antibiotics (Supplementary Fig. 21 and Supplementary Table 8). Finally, the structural specificity of PC metabolites in promoting a proatherogenic macrophage phenotype was examined. Mice fed diets supplemented with trimethylamine species (choline or TMAO) showed increased peritoneal macrophage cholesterol content and elevated plasma levels of TMAO. In contrast, dietary supplementation with the choline analog 3,3-dimethyl-1-butanol (DMB), where the quaternary amine nitrogen of choline is replaced with a carbon, resulted in no TMAO increase and no increased cholesterol in macrophages (Supplemental Fig. 24).
DISCUSSION
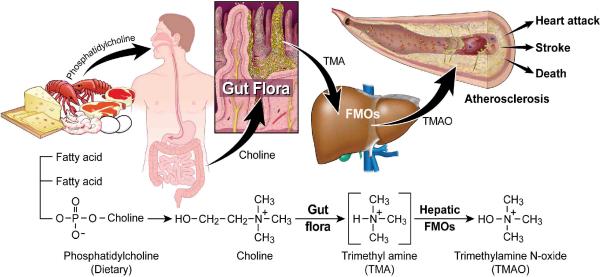
Using a targeted metabolomics approach aimed at identifying plasma metabolites whose levels predict risk of CVD in subjects, we have identified a novel pathway linking dietary lipid intake, intestinal microflora and atherosclerosis (Fig. 6). The pathway identified (dietary PC/choline -> gut flora-formed TMA -> hepatic FMO-formed TMAO) represents a unique additional nutritional contribution to the pathogenesis of CVD that involves choline metabolism, an obligate role for the intestinal microbial community, and regulation of surface expression levels of macrophage scavenger receptors known to participate in the atherosclerotic process. The pro-atherogenic gut flora-generated metabolite, TMAO, is formed in a two-step process initiated by gut flora-dependent cleavage of a trimethylamine species (e.g. PC, choline, betaine) generating the precursor TMA, and subsequent oxidation by FMO3 and possibly other FMOs (Fig. 6). PC is by far the most abundant dietary source of choline in most humans. The present results indicate that both environmental exposure (dietary lipid) and microbial flora participate in TMAO production and the atherogenic macrophage phenotype. While the present genetic studies also suggest a role for hepatic expression levels of one or more FMOs in both enhanced atherosclerotic plaque and decreased HDL levels in mice, the participation of FMOs in human atherosclerosis and HDL cholesterol levels remains to be established. Strong associations between systemic TMAO levels and both angiographic measures of coronary artery atherosclerotic burden and cardiac risks were observed among subjects; however, no correlation was observed between plasma TMAO levels and HDL cholesterol levels in subjects. It remains to be determined whether genetic impairment in FMO3 alone or in combination with other FMOs, is protective for CVD. No phenotype other than the objectionable odor accompanying this disorder is known. In fact, individuals with trimethylaminuria often become vegans, since reducing ingestion of dietary lipids decreases TMA production and the associated noxious odor. Little is also known about the biologic functions of TMAO in humans. TMAO apparently serves as an osmolite in the freeze-avoidance response of some species21. In vitro it can function as a small molecule chaperone, affecting the folding and functioning of some proteins22–23. In addition, TMAO and TMA accumulate in plasma of subjects on maintenance hemodialysis24, suggesting that TMAO might contribute to the well-established enhanced CVD risk noted among subjects with end-stage renal disease.
Figure 6. Gut flora dependent metabolism of dietary PC and atherosclerosis.
Schematic summary illustrating newly discovered pathway for gut flora mediated generation of pro-atherosclerotic metabolite from dietary PC.
Choline is an essential nutrient that is usually grouped within the vitamin B complex. Choline and its metabolite, betaine, are methyl donors, along with folate, and are metabolically linked to transmethylation pathways including synthesis of the CVD risk factor homocysteine. Deficiency in both choline and betaine are suggested to produce epigenetic changes in genes linked to atherosclerosis25–26, and acute choline and methionine deficiency in rodent models causes lipid accumulation in liver (steatohepatitis), heart and arterial tissues27. Alternatively, some studies have reported an association between increased whole blood levels of total choline and cardiovascular disease28–29. Few clinical studies have examined the relationship between choline intake and CVD30, probably because accurate measures of the choline content of most foods has only recently become available14 (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf). The association between dietary choline (and alternative trimethyl amine containing species) and atherosclerosis is complex and will be influenced by the composition of the intestinal microflora.
The human intestinal microbial community is an enormous and diverse ecosystem with known functions in nutrition, gut epithelial cell health, and innate immunity31. Intestinal flora also has recently been implicated in development of some metabolic phenotypes such as obesity and insulin resistance, as well as alterations in immune responses11,32–34. As far as we know, the present studies are the first to identify a direct link between intestinal microflora, dietary PC and CVD risk. These results suggest that an appropriately designed probiotic intervention may serve as a therapeutic strategy for CVD. Interestingly, production of TMAO can be altered by probiotic administration35. Thus, in addition to the current clinical recommendation for general reduction in dietary lipids, manipulation of commensal microbial composition may be a novel therapeutic approach for the prevention and treatment of atherosclerotic heart disease and its complications. Finally, the present studies suggest an additional novel treatment for atherosclerosis - blocking the presumed pathogenic biochemical pathway at the level of the gut flora through use of a non-systemically absorbed inhibitor.
METHODS SUMMARY
Plasma samples and associated clinical study data were identified in patients referred for cardiac evaluation at a tertiary care center. All subjects gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved all study protocols. Unbiased metabolic profiling was performed using liquid chromatography coupled to electrospray ionization mass spectrometry (LC/MS). Target analyte structural identification was achieved using a combination of LC/MS/MS, LC-MSn, multinuclear NMR, gas chromatography-mass spectrometry, and choline isotope tracer feeding studies in mice as outlined in Methods. Statstical analyses were performed using R (version 2.10.1)36. Intestinal microflora was suppressed by supplementation of drinking water with a cocktail of broad spectrum antibiotics37. Germ-free mice were purchased from Taconic SWGF. QTL analyses to identify atherosclerosis related genes were performed on F2 mice generated by crossing atherosclerosis prone C57BL/6J.apoe−/− mice and atherosclerosis resistant C3H/HeJ.apoe−/− mice38. mRNA expression was assayed by Microarray Analysis and Real Time PCR. Aortic root lesion area in mice was quantified by microscopy after staining39. Mouse peritoneal macrophages were collected by lavage for foam cell quantification and cholesterol accumulation assay. Surface protein levels of scavenger receptors, CD36, SR-A1, were determined by flow cytometry.
METHODS
General Procedures
Lipids were extracted by chloroform:methanol (2:1, v/v)40. Cholesterol was quantified by GC/MS41. Triglyceride was quantified by GPO reagent set (Pointe Scientific)42. Cell DNA content was quantified by PicoGreen43. RNA was isolated by TRIZOL® reagent (Invitrogen) and RNeasy Mini Kit (Qiagen). All reagents were purchased from Sigma unless otherwise specified.
Research Subjects
Plasma samples and associated clinical data were collected as part of two studies involving stable non-symptomatic subjects undergoing elective cardiac evaluations at a tertiary care center. The first study, GeneBank, is a large well-characterized tissue repository with longitudinal data from subjects undergoing elective diagnostic left heart catheterization or elective coronary computed tomography angiography44. The second study, BioBank, includes subjects undergoing cardiac risk factor evaluation/modification in a preventive cardiology clinic45. CAD included adjudicated diagnoses of stable or unstable angina, MI or angiographic evidence of ≥ 50% stenosis of one or more epicardial vessels. PAD was defined as any evidence of extra-coronary atherosclerosis. Atherosclerotic CVD was defined as the presence of either CAD or PAD. All subjects gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved all study protocols.
Discovery metabolomics analyses began with an unbiased search for plasma (fasting, EDTA purple top tube) analytes linked to CVD risk using a case:control design (Learning Cohort, N=50 cases and 50 controls). Cases were randomly selected from GeneBank subjects who experienced an MI, stroke or death over the ensuing three year period. An age- gender-matched control group was randomly selected from GeneBank subjects that did not experience a CVD event. An independent non-overlapping Validation Cohort (N=25 cases and 25 controls) was also from GeneBank. A third large (N=1876) independent study comprised of non-overlapping subjects then evaluated clinical associations of identified analytes. Approximately half (N=1020) of the subjects enrolled were from GeneBank and the remaining (N=856) were from BioBank. Similar patient characteristics within each cohort and the combined cohort are observed, as shown in Supplementary Table 4a.b. The association of each PC metabolite and various cardiovascular phenotypes within each cohort (GeneBank and BioBank) are also similar (Supplementary Tables 4c–e). All subjects in the large independent clinical study had similar inclusion and exclusion criterion, negative cardiac enzymes (troponin I < 0.03 ng/ml) and no recent history of MI or CABG. Estimate of glomerular filtration rate was calculated using the MDRD formula46. Fasting blood glucose, C reactive protein, troponin I and lipid profiles were measured on the Abbott ARCHITECT platform (Abbott Diagnostics).
Metabolomics analyses
Plasma proteins were precipitated with 4 volumes of ice cold methanol and small molecule analytes within supernatants were analyzed following injection onto a phenyl column (4.6 × 250 mm, 5 μm Rexchrom Phenyl; Regis, Morton Grove, IL) at a flow rate of 0.8 ml/min using a Cohesive HPLC (Franklin, MA) interfaced with an PE Sciex API 365 triple quadrupole mass spectrometer (Applied Biosystems, Foster, CA) with Ionics (Ontario, Canada) HSID+, EP10+, XT+ redesigned source and collision cell as upgrades in positive MS1 mode. LC gradient (LC1) starting from 10 mM ammonium formate over 0.5 min, then to 5 mM ammonium formate, 25 % methanol and 0.1 % formic acid over 3 min, held for 8 min, followed by 100% methanol and water washing for 3 min at a flow rate of 0.8 ml/min was used to resolve analytes. Spectra were continuously acquired after the initial 4 minutes. Peaks within reconstructed ion chromatograms at 1 amu increments were integrated and both retention times and mass-to-charge ratio (m/z) of analytes were used for statistical analyses.
Selection criteria for determining analytes of interest were based upon the composite of MACE as the clinical phenotype, defined as incident myocardial infarction, stroke or death at 3 years, and included: (i) demonstration of a statistically significant difference between cases vs. controls using a Bonferoni adjusted two sided t-test (P<0.05); (ii) evidence of a significant (P<0.05) dose-response relationship between analyte level and clinical phenotype using Cochran-Armitage trend test; and (iii) a minimal signal-to-noise ratio of 5:1 for a given analyte.
Chemical characterization of unknown metabolites
To chemically define the structures of the plasma analytes selected for further investigation (i.e. analytes with m/z 76, 104 and 118 in positive MS1 mode), multiple approaches were used. Analytes of interest were isolated by HPLC, vacuum dried, re-dissolved in water and injected onto the same phenyl column with a distinct HPLC gradient (LC2, flow rate: 0.8 ml/min) starting from 0.2% formic acid over 2 min, then linearly to 18% acetonitrile containing 0.2 % formic acid over 18 min and further to 100% acetonitrile containing 0.2 % formic acid over 3 min. The targeted analytes were identified by their m/z and the appropriate fractions recovered. After removal of solvent, dry analytes were used for structural identification.
Samples analyzed by GC-MS were derivatized using Sylon HTP kit (HMDS + TMCS + Pyridine (3 : 1 : 9), Supelco). Derivitizations of TMAO and the plasma analyte at m/z 76 also included initial reduction by titanium (III) chloride47 and further derivatized by reaction with 2,2,2-trichloroethylchloroformate48. Analyses were performed on the Agilent Technolgies 6890/5973 GC/MS in positive ion chemical ionization mode. The GC/MS analyses used a J&W Scientific (Folsom) DB-1 column (30 m, 0.25 mm inner diameter, 0.25-μm film thickness) for separations.
Quantitation of TMAO, choline and betaine
Stable isotope dilution LC/MS/MS was used for quantification of TMAO, choline and betaine. TMAO, choline and betaine were monitored in positive MRM MS mode using characteristic precursor - product ion transitions: m/z 76→58, m/z 104→60, and m/z 118→59, respectively. The internal standards TMAO-trimethyl-d9 (d9-TMAO) and choline-trimethyl-d9 (d9-choline), were added to plasma samples prior to protein precipitation, and were similarly monitored in MRM mode at m/z 85→68 and m/z 113→69, respectively. Various concentrations of TMAO, choline and betaine standards and a fixed amount of internal standards were spiked into control plasma to prepare the calibration curves for quantification of plasma analytes. TMA was similarly quantified from acidified plasma by LC/MS/MS using MRM mode.
Aortic root lesion quantification
Apolipoprotein E knockout mice (C57BL/6J.Apoe−/−) were weaned at 4 weeks of age and fed with either standard chow control diet (Teklad 2018) or a custom diet comprised of normal chow supplemented with 0.5% choline (Teklad TD.07863), 1.0% choline (Teklad TD.07864) or 0.12% TMAO (Teklad TD.07865) for 16 weeks. Mice were anesthetized with Ketamine/Xylazine prior to cardiac puncture to collect blood. Hearts were fixed and stored in 4% paraformaldehyde prior to frozen OCT sectioning and staining with Oil-Red-O and hematoxylin. Aortic root lesion area was quantified as the mean value of 6 sections39. Aortic sections were immunostained with rat anti-mouse F4/80 antibody (ab6640, abcam) followed by goat anti-rat IgG-FITC antibody (sc-2011, Santa Cruz) and FITC conjugated CD36 mAb (Cayman Chemical) for F4/80 and CD36, respectively. Sections were mounted in Vectashield DAPI (H-1200, Vectashield) to take pictures under a Leica DMR microscope (W. Nuhsbaum) equipped with a Q Imaging Retiga EX camera (Burnaby, BC, Canada). Use Image-Pro Plus Version 7.0 (MediaCybernetics) to integrate the positive staining area of F4/80 and CD36 in aorta.
Flow cytometry assays on scavenger receptors
Cell surface expression of scavenger receptors, SR-A1 and CD36, were quantified on peritoneal macrophages from female mice by flow cytometry after immunostaining with fluorochrome conjugated antibodies. Fluorescence intensity was quantified on a FACSCalibur flow cytometry instrument with FlowJo software (BD Biosciences). >10,000 total events were acquired to obtain adequate macrophages numbers. The following antibodies were used to stain macrophages: CD36 mAb FITC (Cayman Chemical), anti-mouse SR-AI/MSRA1 (R&D Systems), goat anti-rat IgG-FITC (Santa Cruz Biotechnology), Alexa Fluor® 647 anti-mouse F4/80 (eBioscience), Alexa Fluor® 647 anti-mouse CD11b (eBioscience) and the isotype controls, Alexa Fluor® 647 rat IgG2b (eBioscience), Alexa Fluor® 647 rat IgG2a (eBioscience), normal mouse IgA-FITC (Santa Cruz). Cells were incubated with antibodies for 30 min at 4°C and washed with 0.1% BSA in PBS. Cells with double positive staining for F4/80 and CD11b were gated as macrophage49–51 for the quantification of fluorescence intensity for CD36 and SR-A1 (Supplementary Figure 20), with results normalized to F4/80.
eQTL studies
C57BL/6J.Apoe−/− (B6.Apoe−/−) mice were purchased from the Jackson Laboratory and C3H/HeJ.Apoe−/− (C3H.Apoe−/−) mice were bred by backcrossing B6.Apoe−/− to C3H/HeJ for 10 generations. The F2 mice were generated by crossing B6.Apoe−/− with C3H.Apoe−/− and subsequently intercrossing the F1 mice. Mice were fed Purina Chow containing 4% fat until 8 weeks of age, and then transferred to a Western diet (Teklad 88137) containing 42% fat and 0.15% cholesterol for 16 weeks until euthanasia at 24 weeks of age. Mouse atherosclerotic lesion area was quantified using standard methods39. eQTL analyses were performed as previously described38. Each individual sample was hybridized against the pool of F2 samples. Significantly differentially expressed genes were determined as previously described52. Expression data in the form of mean log ratios (mlratios) were treated as a quantitative trait in eQTL analysis using Rqtl package for the R language and environment for statistical computing (http://cran.r-project.org/).
Antibiotic knockdown of endogenous gut flora, germ-free mice, and conventionalization studies
An antibiotic cocktail (0.5 g/L vancomycin, 1 g/L neomycin sulfate, 1 g/L metronidazole, 1 g/L ampicillin) previously shown to be sufficient to deplete all detectable commensal bacteria37 was administered in drinking water ad lib. In additional studies, 8 week old female Swiss Webster Germ Free mice (Taconic SWGF) underwent an oral (gavage) choline challenge (see below) immediately following their removal from their germ-free microisolator shipper. After the choline or PC challenge, the germ-free mice were placed in conventional cages with non-sterile C57BL/6J female mice to facilitate transfer of commensal organisms. Four weeks later, the conventionalized mice underwent a second choline or PC challenge.
In vivo macrophage studies
C57BL/6J mice or B6.Apoe−/− mice were fed with either standard chow control diet (Teklad 2018) or a custom diet supplemented with 1.0% betaine (Teklad TD.08112), 1.0% choline (Teklad TD.07864), 0.12% TMAO (Teklad TD.07865) or 1.0% dimethylbutanol (DMB) supplemented in drinking water for at least 3 weeks. Elicited mouse peritoneal macrophages (MPMs) were harvested by peritoneal lavage with ice-cold PBS 3 days after intraperitoneal (i.p.) injection of 1 mL 4% thioglycollate. Some studies with mice were performed using a custom diet with low but sufficient choline content (0.07% total; Teklad TD.09040) vs. high choline diet (1.0% total; Teklad TD.09041) in the presence or absence of antibiotics. Choline content of all diets was confirmed by LC/MS/MS.
Foam cell staining
Foam cells were identified by microscopy cultured peritoneal macrophages on glass cover-slips following 6 hours in RPMI 1640 medium supplemented with 3% lipoprotein deficient serum. Cells were fixed with paraformaldehyde and stained with Oil Red O/hematoxylin53. Cells containing >10 lipid droplets were scored as foam cells50. At least 10 fields and 500 cells/condition were counted.
Real time PCR
Real time PCR of CD36, SR-A1 and flavin monooxygenases (FMOs) was performed using Brilliant II SYBR® Green QRT-PCR kit (Strategene). The forward and reverse primers, CD36, GAPDH, SR-A1, FMOs and F4/80, were synthesized by IDT based on sequences reported54–58.
Synthesis of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine-N,N,N-trimethyl-d9 (d9-DPPC) and preparation of lipid vesicles
d9-DPPC was synthesized by reacting 1,2 -dipalmitoyl-sn-glycero-3-phosphoethanolamine (Genzyme Pharmaceuticals) with per-deuteromethyliodide (CD3I, Cambridge Isotope Laboratories)59–60. The product was purified by preparative silica gel TLC and confirmed by both MS and NMR. Egg yolk lecithin (Avanti Polar Lipids) and d9-DPPC liposomes used for gavage feeding and i.p. injection of mice were prepared by the method of extrusion through polycarbonate filters61.
Metabolic challenges in mice
C57BL/6J mice were administered (gavage) unlabeled or the indicated stable isotope labeled choline or PC (egg yolk lecithin or d9-DPPC) using a 1.5 inch 20 gauge intubation needle. Choline challenge: gavage consisted of 150 μl of 150 mM choline-d9. PC challenge: gavage or i.p. injection of 300 μl 5 mg/ml of unlabeled PC or labeled d9-DPPC. Mice were fasted 12 hours prior to PC challenge. Plasma (50 μl) was collected via the saphenous vein from mice at baseline and post gavage or i.p. injection time points.
Statistical analysis
Student's t test and Wilcoxon rank sum test were employed to compare group means62–63. Pearson correlation, Spearman rank correlation and Somers' Dxy correlation were used to investigate the correlation between two variables64–65. Comparison of categorical measures between independent groups was done using χ2 tests66. Odds ratios and 95% confidence intervals (CI) for cardiovascular phenotypes (history of MI, CAD, PAD, CVD and CAD+PAD) were calculated with R, Version 2.10.1 (www.r-project.org), using logistic regression67 with case status as the dependent variable and plasma analyte as independent variable. Trend tests in frequencies across quartiles were done using Cochran-Armitage Trend tests68. Levels of analytes were adjusted for traditional CAD risk factors in a multivariate logistic regression model including individual traditional cardiac risk factors (age, gender, diabetes, smoking, hypertension, lipids, CRP and estimated creatinine clearance) and medication usage (statin or other lipid lowering agents, antihypertensive agents including angiotensin-converting enzyme inhibitor, angiotensin receptor blocking agent, diuretic, calcium channel blocker or beta blocker, and aspirin or other platelet inhibitors).
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank Dr. Effie Sehayek, Cleveland Clinic, for stimulating discussions, Dr. Lawrence W. Castellani, University of California, Los Angeles, for help with lipoprotein profile analysis, and Fallon McNally, Michael Berk, and Mike Pepoy, Cleveland Clinic, for technical assistance. This research was supported by National Institutes of Health grants R01 HL103866, P01 HL098055, P01HL087018-020001, P01 HL28481, and P01 HL30568. BJB was supported by supported by NIH Training grant T32-DK07789. The clinical study GeneBank was supported in part by P01 HL076491-055328, R01 HL103931 and the Cleveland Clinic Foundation General Clinical Research Center of the Cleveland Clinic/Case Western Reserve University CTSA (1UL1RR024989). Some of the laboratory studies (hemaglobin A1C, fasting glucose) in GeneBank were supported by R01 DK080732, and Abbott Diagnostics Inc. provided supplies for performance of some of the fasting lipid profile, glucose, creatinine, troponin I and hsCRP measured in GeneBank.
Footnotes
AUTHOR CONTRIBUTIONS Z.W. performed metabolomics analyses, and biochemical, cellular, animal model, and mass spectrometry studies. He assisted with statistical analyses, and assisted in both drafting and critical review of the manuscript. E.K., B. D. and J.D.S. assisted with performance of animal models and their analyses. B.S.L. synthesized d9-DPPC and assisted in metabolomics / mass spectrometry analyses. B.J.B., H.A. and A.J.L. performed the mouse eQTL experiments and analyses, and assisted in both drafting and critical review of the manuscript. A.J.L. provided funding for the study. R.K., E.B.B. and X.F. performed mass spectrometry analyses of clincal samples. Y.W. performed statistical analysis. A.E.F. and P.S. helped with collection of human liver biopsy material and interpretation of biochemical and pathological examination of animal liver for steatosis. W.H.W.T. assisted in GeneBank study design and enrollment, as well as analyses of clinical studies, and critical review of the manuscript. J.A.D. assisted in clinical laboratory testing for human clinical studies, animal model experimental design, and critical review of the manuscript. S.L.H. conceived of the idea, designed experiments, assisted in data analyses, the drafting and critical review of the manuscript, and provided funding for the study.
REFERENCES
- 1.Epstein SE, et al. The role of infection in restenosis and atherosclerosis: focus on cytomegalovirus. Lancet. 1996;348(Suppl 1):s13–17. doi: 10.1016/s0140-6736(96)98005-8. [DOI] [PubMed] [Google Scholar]
- 2.Patel P, et al. Heart disease and cardiovascular risk factors. BMJ. 1997;311:711. doi: 10.1136/bmj.311.7007.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? The Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 4.Saikku P, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. The Lancet. 1988;332:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor CM, et al. Azithromycin for the Secondary Prevention of Coronary Heart Disease Events: The WIZARD Study: A Randomized Controlled Trial. JAMA. 2003;290:1459–1466. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 7.Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2641–2647. doi: 10.1001/jama.293.21.2641. [DOI] [PubMed] [Google Scholar]
- 8.Wright SD, et al. Infectious agents are not necessary for murine atherogenesis. J. Exp. Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 11.Dumas ME, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashman JR, et al. Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr Drug Metab. 2003;4:151–170. doi: 10.2174/1389200033489505. [DOI] [PubMed] [Google Scholar]
- 13.al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 14.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 15.Lang DH, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes : Selective catalysis by fmo3. Biochemical Pharmacology. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37:515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SC, Smith RL. Trimethylaminuria: The fish malodor syndrome. Drug Metab Dispos. 2001;29:517–521. [PubMed] [Google Scholar]
- 18.Schadt EE, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005;37:710–717. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- 20.Wang SS, et al. Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res. 2007;101:e11–30. doi: 10.1161/CIRCRESAHA.107.152975. [DOI] [PubMed] [Google Scholar]
- 21.Treberg JR, Wilson CE, Richards RC, Ewart KV, Driedzic WR. The freeze-avoidance response of smelt Osmerus mordax: initiation and subsequent suppression of glycerol, trimethylamine oxide and urea accumulation. J Exp Biol. 2002;205:1419–1427. doi: 10.1242/jeb.205.10.1419. [DOI] [PubMed] [Google Scholar]
- 22.Devlin GL, Parfrey H, Tew DJ, Lomas DA, Bottomley SP. Prevention of polymerization of M and Z alpha1-Antitrypsin (alpha1-AT) with trimethylamine N-oxide. Implications for the treatment of alpha1-at deficiency. Am J Respir Cell Mol Biol. 2001;24:727–732. doi: 10.1165/ajrcmb.24.6.4407. [DOI] [PubMed] [Google Scholar]
- 23.Song JL, Chuang DT. Natural osmolyte trimethylamine N-oxide corrects assembly defects of mutant branched-chain alpha-ketoacid decarboxylase in maple syrup urine disease. J Biol Chem. 2001;276:40241–40246. doi: 10.1074/jbc.M107242200. [DOI] [PubMed] [Google Scholar]
- 24.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, Yoon W, Goldschmidt-Clermont PJ. DNA methylation and atherosclerosis. J Nutr. 2002;132:2406S–2409S. doi: 10.1093/jn/132.8.2406S. [DOI] [PubMed] [Google Scholar]
- 26.Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135:5–8. doi: 10.1093/jn/135.1.5. [DOI] [PubMed] [Google Scholar]
- 27.Salmon WD, Newberne PM. Cardiovascular disease in choline-deficient rats. Effects of choline deficiency, nature and level of dietary lipids and proteins, and duration of feeding on plasma and liver lipid values and cardiovascular lesions. Arch Pathol. 1962;73:190–209. [PubMed] [Google Scholar]
- 28.Danne O, Lueders C, Storm C, Frei U, Mockel M. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta. 2007;383:103–109. doi: 10.1016/j.cca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 29.LeLeiko RM, et al. Usefulness of elevations in serum choline and free F2)-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009;104:638–643. doi: 10.1016/j.amjcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 30.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckburg PB, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Li M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reigstad CS, Lunden GO, Felin J, Backhed F. Regulation of serum amyloid A3 (SAA3) in mouse colonic epithelium and adipose tissue by the intestinal microbiota. PLoS One. 2009;4:e5842. doi: 10.1371/journal.pone.0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin FP, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo ML. Statistical computing with R. Chapman & Hall/CRC; 2008. [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, et al. Genetic and genomic analysis of a fat mass trait with complex inheritance reveals marked sex specificity. PLoS Genet. 2006;2:e15. doi: 10.1371/journal.pgen.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baglione J, Smith JD. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol Med. 2006;129:83–95. doi: 10.1385/1-59745-213-0:83. [DOI] [PubMed] [Google Scholar]
- 40.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 41.Robinet P, Wang Z, Hazen SL, Smith JD. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J Lipid Res. 2010;51:3364–3369. doi: 10.1194/jlr.D007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millward CA, et al. Genetic factors for resistance to diet-induced obesity and associated metabolic traits on mouse chromosome 17. Mamm Genome. 2009;20:71–82. doi: 10.1007/s00335-008-9165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn SJ, Costa J, Emanuel JR. PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996;24:2623–2625. doi: 10.1093/nar/24.13.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 45.Nicholls SJ, et al. Lipoprotein (a) levels and long-term cardiovascular risk in the contemporary Era of statin therapy. J Lipid Res. 2010 doi: 10.1194/jlr.M008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoves J, Lindley EJ, Barnfield MC, Burniston MT, Newstead CG. MDRD equation estimates of glomerular filtration rate in potential living kidney donors and renal transplant recipients with impaired graft function. Nephrol Dial Transplant. 2002;17:2036–2037. doi: 10.1093/ndt/17.11.2036. [DOI] [PubMed] [Google Scholar]
- 47.Barham AH, et al. Appropriateness of cholesterol management in primary care by sex and level of cardiovascular risk. Prev Cardiol. 2009;12:95–101. doi: 10.1111/j.1751-7141.2008.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.daCosta KA, Vrbanac JJ, Zeisel SH. The measurement of dimethylamine, trimethylamine, and trimethylamine N-oxide using capillary gas chromatography-mass spectrometry. Anal Biochem. 1990;187:234–239. doi: 10.1016/0003-2697(90)90449-j. [DOI] [PubMed] [Google Scholar]
- 49.Schledzewski K, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 50.Cailhier JF, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 51.Kunjathoor VV, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 54.Miles EA, Wallace FA, Calder PC. Dietary fish oil reduces intercellular adhesion molecule 1 and scavenger receptor expression on murine macrophages. Atherosclerosis. 2000;152:43–50. doi: 10.1016/s0021-9150(99)00446-3. [DOI] [PubMed] [Google Scholar]
- 55.Westendorf T, Graessler J, Kopprasch S. Hypochlorite-oxidized low-density lipoprotein upregulates CD36 and PPARgamma mRNA expression and modulates SR-BI gene expression in murine macrophages. Mol Cell Biochem. 2005;277:143–152. doi: 10.1007/s11010-005-5873-z. [DOI] [PubMed] [Google Scholar]
- 56.Rasooly R, Kelley DS, Greg J, Mackey BE. Dietary trans 10, cis 12-conjugated linoleic acid reduces the expression of fatty acid oxidation and drug detoxification enzymes in mouse liver. Br J Nutr. 2007;97:58–66. doi: 10.1017/S0007114507257745. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Cashman JR. Quantitative analysis of FMO gene mRNA levels in human tissues. Drug Metab Dispos. 2006;34:19–26. doi: 10.1124/dmd.105.006171. [DOI] [PubMed] [Google Scholar]
- 58.de Vries TJ, Schoenmaker T, Hooibrink B, Leenen PJ, Everts V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J Leukoc Biol. 2009;85:919–927. doi: 10.1189/jlb.0708402. [DOI] [PubMed] [Google Scholar]
- 59.Chen FCM, Benoiton LCJC. A new method of quatenizing qmines and its use in amino acid and peptide chemistry. Can. J. Chem. 1976;54:3310–3311. [Google Scholar]
- 60.Morano C, Zhang X, Fricker LD. Multiple isotopic labels for quantitative mass spectrometry. Anal. Chem. 2008;80:9298–9309. doi: 10.1021/ac801654h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenberg ME, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 62.Gauvreau K, Pagano M. Student's t test. Nutrition. 1993;9:386. [PubMed] [Google Scholar]
- 63.Wijnand HP, van de Velde R. Mann-Whitney/Wilcoxon's nonparametric cumulative probability distribution. Comput Methods Programs Biomed. 2000;63:21–28. doi: 10.1016/s0169-2607(00)00058-4. [DOI] [PubMed] [Google Scholar]
- 64.Gaddis ML, Gaddis GM. Introduction to biostatistics: Part 6, Correlation and regression. Ann Emerg Med. 1990;19:1462–1468. doi: 10.1016/s0196-0644(05)82622-8. [DOI] [PubMed] [Google Scholar]
- 65.Deichmann M, et al. S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol. 1999;17:1891–1896. doi: 10.1200/JCO.1999.17.6.1891. [DOI] [PubMed] [Google Scholar]
- 66.Goodall CM, Stephens OB, Moore CM. Comparative sensitivity of survival-adjusted chisquare and normal statistics for the mutagenesis fluctuation assay. J Appl Toxicol. 1986;6:95–100. doi: 10.1002/jat.2550060206. [DOI] [PubMed] [Google Scholar]
- 67.Traissac P, Martin-Prevel Y, Delpeuch F, Maire B. Logistic regression vs other generalized linear models to estimate prevalence rate ratios. Rev Epidemiol Sante Publique. 1999;47:593–604. [PubMed] [Google Scholar]
- 68.Gautam S. Test for linear trend in 2 × K ordered tables with open-ended categories. Biometrics. 1997;53:1163–1169. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.