Abstract
The aryl hydrocarbon receptor (AHR), the receptor for dioxin, has been known to cause immunosuppression after binding dioxin. It has recently been discovered that the receptor may be central to T cell differentiation into FoxP3+ T regulatory cells vs. TH17 cells. In this paper we demonstrate that kynurenine, the first breakdown product in the indoleamine 2,3-dioxygenase(IDO)-dependent tryptophan degradation pathway, activates the AHR. We furthermore show that this activation leads to AHR-dependent T regulatory cell generation. We additionally investigate the dependence of TGF-β on the AHR for optimal Treg generation, which may be secondary to the upregulation of this receptor that is seen in T cells after exposure to TGF-β. These results shed light on the relationship of IDO to the generation of regulatory T cells, in addition to highlighting the central importance of the AHR in T cell differentiation. All tissues and cells were derived from mice.
Introduction
It has long been recognized that the immune system is in a fine balance between immunity and self tolerance. The concept of suppressor T cells playing a role in this balance was first proposed in the 1970s (1). Efforts to identify these cells were generally unsuccessful, and their very existence was brought into question in the early 1980’s by molecular biologists who failed to locate an elusive “suppressor” gene in the mouse MHC class II locus (2). The suppressor T cell concept was dropped and remained out of vogue until it re-emerged as the CD4+CD25+ regulatory T cell (Treg), first described in detail by Sakaguchi in 1995 (3). Since that time, numerous studies have characterized these cells, and the role they play in autoimmunity, control of infection, and transplant rejection. Identification of FoxP3, a transcription factor for Treg development, has led to further characterization of the importance of regulation (4, 5). More recently a new T helper cell lineage, termed TH17, was described (6, 7). These IL-17 secreting cells are thought to play a major role as effectors in autoimmunity and transplant rejection. Interestingly, this new data has led investigators to question previously held beliefs about terminal cell differentiation and stability of Tregs, as the ability of Tregs to “redifferentiate” into TH17 cells in the appropriate inflammatory milieu has now been described (8, 9).
Recent publications implicate the aryl hydrocarbon receptor (AHR) as a central player in T cell differentiation. The AHR is best known as the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (“dioxin”, or TCDD). Activation of the AHR by this environmental pollutant can lead to a range of toxic endpoints, including hepatocellular damage, epithelial changes, cancer, birth defects, thymic involution, and immunosuppression (10, 11). Although the AHR is well known for its role in toxicology, this receptor has also been shown to play a role in vascular and hematopoietic development (12, 13). Moreover, several potential endogenous ligands have been shown to bind to the AHR with variable affinity and potency (14). The fact that this receptor has been conserved in evolution (15), and that invertebrate orthologs of the AHR are well preserved and yet don’t bind to TCDD (16), is consistent with the idea that a physiologically relevant endogenous ligand for this receptor exists (17).
In evaluating the mechanisms for immunosuppression seen after exposure to TCDD, it was discovered that activation of the AHR with TCDD leads to the generation of Tregs in vitro or in vivo (18), and alternatively activation with a different endogenous ligand, 6-formylindolo[3,2-b]carbazole (FICZ), leads to TH17 cell formation (19). Although this unusual pharmacology, where one AHR agonist diverts T cells towards regulators, and another agonist generates effectors is surprising, multiple studies have confirmed the importance of the AHR in the generation of TH17 cells both in vitro and in vivo (20, 21). Regarding Treg generation, the direct relationship of regulatory cells to the AHR has been less clear (22). This has led investigators to question whether the AHR truly has a direct effect on the generation of these cells (23, 24).
Our laboratory has focused on the role of indolylic products as potential endogenous ligands of the AHR (25, 26). Therefore, we began to think about the potential for an interaction between the AHR and the indoleamine 2,3-dioxygenase (IDO) pathway. The IDO enzyme catalyzes the rate-limiting step of tryptophan degradation along the kynurenine pathway (27). IDO is present and activated in subsets of DCs (particularly plasmacytoid, or pDCs,), and thought to be central to Treg generation from T cell precursors by DC-T cell interactions (28, 29). The exact mechanistic pathway by which IDO leads to Tregs has been debated, and both tryptophan starvation and direct effects of tryptophan metabolites (including kynurenine) have been proposed (30–32). In addition to a connection via indole metabolism, the IDO-AHR interaction was particularly interesting in light of the observation that IDO may be upregulated by the AHR (33, 34) and that kynurenine and related metabolites may be AHR agonists (35–37).
In this report, we demonstrate an important role for kynurenine, the first tryptophan metabolite of the IDO pathway, in Treg generation. We provide evidence that kynurenine activates the AHR at a dose clinically relevant in humans and leads to Tregs in vitro. The role for the AHR in this process is supported by two observations. First, kynurenine does not influence Treg generation in AHR-null T cells. Second, kynurenine can be shown to activate the AHR using classical response genes such as Cyp1a1 and Cyp1b1. In our model the AHR on T cells is required for the generation of Tregs by kynurenine. We further define the importance of the AHR for optimal generation of Tregs by TGF-β, and characterize potential mechanisms for this.
Materials and Methods
Mice
C57BL/6J wild type (WT) and BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, Maine). AHR-null (AHR-deficient B6) mice on a C57BL/6J background (13) were bred and maintained under specific pathogen-free conditions. All animal experiments were carried out according to institutional guidelines approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee.
Isolation and Differentiation of Bone Marrow-Derived Dendritic Cells
The method of Bone Marrow Derived Murine Dendritic Cells (BMDCs) was derived as previously described (38). Briefly, bone marrow was obtained from mice femurs. After RBC lysis the cells were plated in 6 well plates with a density of 1×106/ml in complete RPMI media supplemented with 30ng/ml GM-CSF. On day 3, non-adherent cells and 75% of culture media was exchanged for fresh media. On day 6 the cells were either harvested as immature DCs or cultured an additional day to maturity by again exchanging 75% of the media, with the addition of 50 ng/ml of LPS. 80% of the cell population stained positive for CD11c by flow cytometry. For analysis of mRNA expression, cells were treated with or without TCDD (10 nM). As mentioned above, 50 ng/ml of LPS was utilized for maturation of BMDC. There is a previous publication that LPS alone can lead to IDO (39). It should be clarified that this only occurred when higher doses of LPS (5ug/ml) were used. This response is dose dependent, as seen by other investigators (33, 40, 41). As an additional control, an LPS titration was performed with BMDC as further confirmation, the results of which are in the supplemental data (supplemental figure 1). No increase in IDO mRNA was seen until at least 100ng/ml of IDO was used in these assays.
Luciferase Assay
A mouse hepatoma cell line H1L6.1c3, stably carrying a DRE-driven firefly luciferase reporter gene (a gift from Dr. Denison (42)) was maintained with 0.3 mg/ml of G418 in completed DMEM media. Briefly, 0.6×106 cells were seeded in each well of a 6 well plate overnight and were then treated with different concentrations of TCDD, L-tryptophan, L-kynurenine, hydroxykynurenine, hydroxyanthranilic acid, anthranilic acid, nicotinamide, and quinolinic acid for the time specified. All of the kynurenines3 (including kynurenine) were purchased from Sigma-Aldrich, and purity was listed at 98% or better confirmed by HPLC. They were placed in solution in 0.5M HCL, as recommended by the manufacturer for maximum solubility. Cells were lysed by lysis buffer (Promega) and the luciferase assay was performed by using a BD monolight 3010 luminometer. The relative light unit (RLU) is the indicator of luciferase expression level. All experiments were repeated three times and each sample was tested in triplicate each time.
Real time PCR (qPCR)
Total RNA was extracted using the reagents: RNeasy Mini Kit and RNase-Free DNase Set (QIAGEN). 500ng of total RNA in each group was used for RT reaction (iScript cDNA Synthesis Kit, BIO-RAD or High-capacity cDNA Reverse Transcription Kits, Applied Biosystems). The Relative Quantitation PCR for IDO1 (Mm00492586-m1), GAPDH (4352339-E0806018), and IFN-γ (Mm99999071-m1) were performed in the Applied Biosystems 7900HT Fast Real-Time PCR System and TaqMan Universal PCR Master Mix was used as a reaction reagent. The Relative Quantitation PCR for Foxp3, Cyp1a1, Cyp1b1 and GAPDH were processed by the BioRad iCycler and iQ SYBR Green Supermix as the reaction reagent
Isolation of Naïve CD4 T Cells and T Cell Differentiation
Naive CD4 T cells were isolated from spleens of C57BL/6J and AHR-null mice using the CD4 CD62L Isolation Kit (Miltenyi Biotec) and an autoMACS. This kit includes a depletion cocktail, including the addition of a CD25 and an anti-TCRγ/δ+ antibody. CD62L is expressed on naïve T cells, and down-regulated upon activation. A small subset of central memory T cells also express CD62L, and could be included in this separation. These represent a very small proportion of the final separation, and we will refer to separated cells as naïve T cells. Cells were tested for purity after sorting and consistently showed greater than 90% purity for CD4+CD62+CD25− cells. An example of analysis of the separations is included in supplementary data (supplemental figure 2A). Viability at the beginning of culture was typically greater than 98% as seen by trypan blue staining. For qPCR analysis, 2–5 × 105 cells were cultured in each well of a 96 well round bottom plate, coated with 0.5µg/ml anti-CD3 and anti-CD28 overnight, and then washed with PBS twice before seeding the cells. The naïve T cells were maintained in F10 media supplemented with 10% heat-inactivated FBS, 100µg/ml streptomycin, 100 units/ml penicillin, 50µm 2-mercaptoethanol, 25mM HEPES, and 2mM L-glutamine and were treated with 10nM TCDD, 100nM FICZ, 2–10 ng/ml TGF-β (as specified), 50µM kynurenine, or 25µM of each kynurenines (hydroxykynurenine, hydroxyanthranilic acid, anthranilic acid, nicotinamide, quinolinic acid). After 5 days the cultured cells were harvested for RNA assay. Prior to this cells were checked for viability using live-dead staining with flow cytometry. The majority of cells were viable, but dead cells were gated out in flow cytometry analysis. For flow cytometric analysis, purified naïve T cells were stimulated with the CD3/CD28 T cell Activation/Expander Kit (Miltenyi) for 5 days. As indicated, cultures were supplemented with recombinant cytokines and reagents: human TGF-β1 (R&D Systems), mouse IL-6 (20 ng/ml; R&D Systems), kynurenine, FICZ (100 nM), AHR antagonist CH-223191 (Calbiochem).
Intracellular FoxP3 and IL-17 Cytokine Staining
To Stain for Foxp3, T cells were first surface stained with anti-CD4 and anti-CD25 and then fixed and permeabilized with the Fixation/Permeabilization buffer (eBioscience) for 30 minutes at 4°C. Following this, cells were stained with Pacific Blue-conjugated anti-Foxp3. For intracellular IL-17 staining, T cells were first stimulated with 50 ng/ml PMA (Sigma) and 800 ng/ml ionomycin (Sigma) for 4 hours in the presence of GolgiStop (BD PharMingen) for the final 2 hours. Cells were then fixed and permeabilized with the Fixation/Permeabilization buffer and then stained with PE-conjugated anti-IL-17. All antibodies were from eBioscience. Flow cytometric analysis was performed using an LSR-II (BD Biosciences).
pDC/T cell coculture
Naïve CD4+ CD25− T cells were isolated from WT and AHR-null mice and co-cultured with BALB/c pDCs isolated using the Miltenyi mouse pDC isolation kit at a ratio of 20 to 1 or 10 to 1 (example of pDC separation in supplemental figure 2B). CpG, FICZ and kynurenine were added at the start of culture. On day 5, cells were harvested and subjected to flow cytometric analysis.
Results
AHR activation in dendritic cells leads to IDO induction
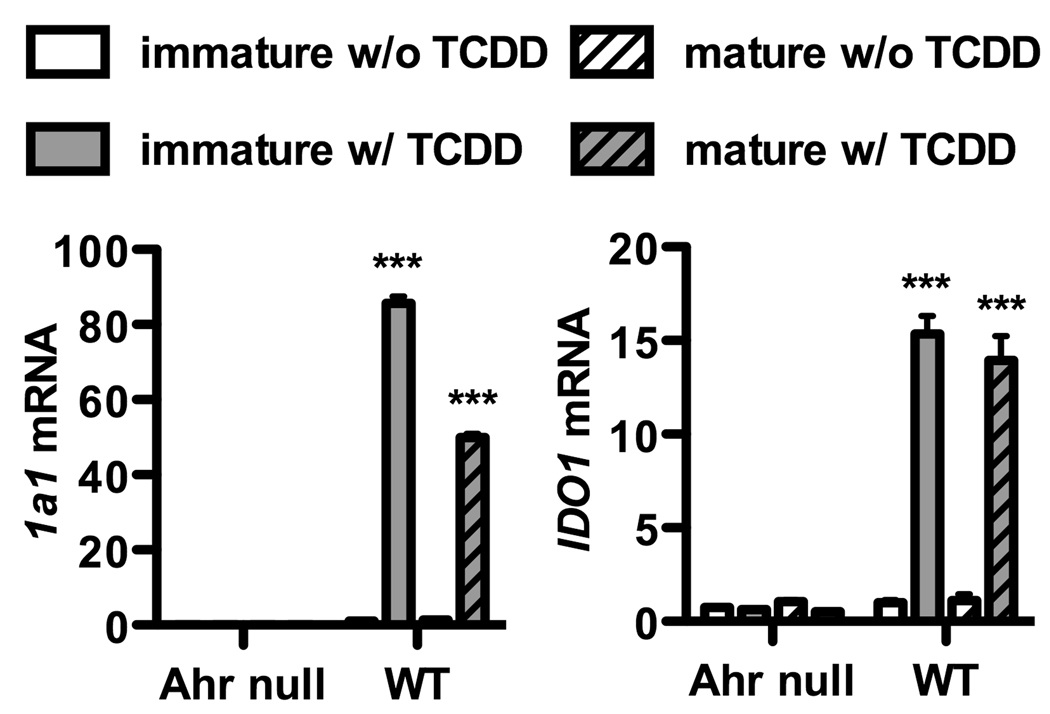
We first examined the role that the AHR in DCs might play in directing T cell differentiation. Based on previously published data (29, 33, 34), we considered the possibility that IDO may be a mediator of the crosstalk between these cells. We initially set out to confirm that IDO could be induced by activation of the AHR. Figure 1 shows that cultured bone marrow derived DCs, when exposed to the AHR agonist TCDD, led to Cyp1a1 induction, confirming activation of the AHR in these cells. Exposure to TCDD also increased IDO mRNA levels. DCs produced Cyp1a1 and IDO in both immature and mature states, indicating they did not have to be activated in order to have this response. AHR-null DCs did not exhibit IDO production after exposure to TCDD. In supplementary figure 3, mRNA for IDO was analyzed at earlier time points than 48 hours used above, to control for any indirect affect that may have led to IDO expression. As early as 7 hours after culture, IDO mRNA was generated.
Figure 1. AHR activation in mouse BMDCs leads to IDO.
BMDCs were generated from the bone marrow of C57BL/6J wild type (WT) and AHR null mice as described in Material and Methods. Cells were harvested on day 6 as immature BMDCs or on day 7 as mature BMDCs following addition on day 6 of LPS at a dose of 50ng/ml, a concentration which itself does not cause IDO expression, confirmed in supplemental figure 1. BMDCs were cultured in the presence or absence of TCDD (10nM) added on day 0 of culture. mRNA was isolated from immature or mature BMDCs and assayed for the expression of Cyp1a1 (left panel), a marker of AHR activation, and IDO1 (right panel). Data was normalized to WT control. Post ANOVA testing comparisons are to cultures without TCDD; ***, p < 0.001. Cyp1a1 mRNA was undetectable in all AHR null PCR reactions. Each graph is representative of three independent experiments.
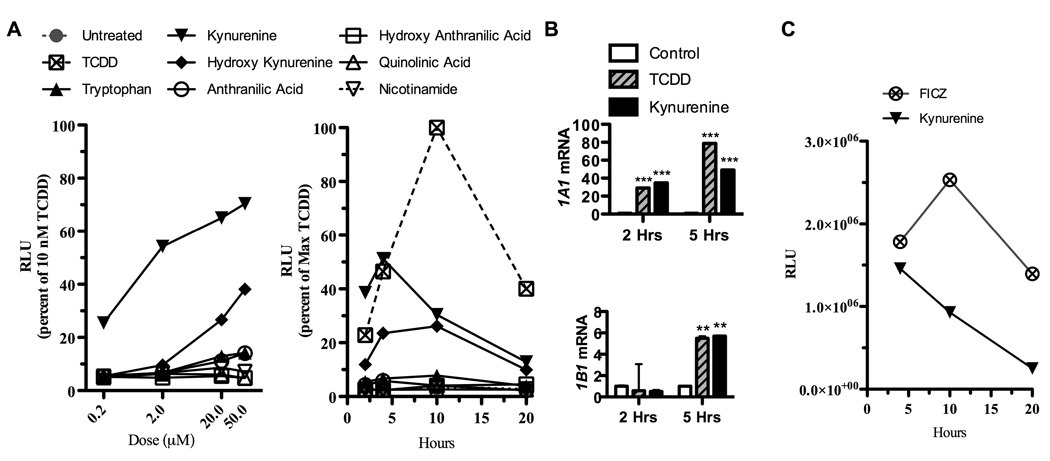
Kynurenine activates the AHR, while other tryptophan breakdown products downstream to kynurenine do not
Given that IDO can be generated by AHR activation in DCs, and that tryptophan breakdown products have been known to generate AHR ligands, we examined all of the tryptophan breakdown products of the IDO pathway for their ability to activate the AHR. We employed a mouse cell line of hepatoma cells, termed Hepa1. These cells have been transfected with a luciferase reporter gene fused to the dioxin-responsive elements (DRE) (43). We tested each of the commercially available substrates in the kynurenine pathway3, and compared their ability to activate the DRE with TCDD. Interestingly, kynurenine itself showed the strongest activity (figure 2A). Peak activity was at 5 hours, with a dose (50 µm) which is comparable to levels encountered clinically in humans in areas of inflammation after activation of IDO (44) (figure 2A). All other breakdown products showed less DRE activity, with decreasing peaks of activity the further down the kynurenine pathway that products were examined. As seen in figure 2B, we further tested this cell line using real time PCR, and found that after exposure to kynurenine in vitro, a substantial increase in Cyp1a1 and Cyp1b1 mRNA was seen, confirming activation of the AHR by this ligand. FICZ was also tested to compare its efficacy as an activator of the DRE, in comparison to kynurenine. It is a potent ligand, with a peak at a later time point (10 hours), seen in figure 2C.
FIGURE 2. Kynurenine, but not other tryptophan breakdown products, directly activates the AHR.
Mouse Hepa1 cells that were transfected with luciferase reporter gene fused to the dioxin-responsive elements (DRE) were seeded at 0.6×106 cells per well. Cells were then exposed to tryptophan breakdown products along the kynurenine pathway (50 µM, except as indicated) for 4 hours except as indicated. Luciferase activity was measured on a luminometer. A, Data was converted as a percent of response to TCDD (10nM) to determine the dose response curve (left) and time-course (right) of luciferase activity. B, mRNA was isolated from Hepa1 cells following 2 and 5 hour exposure to TCDD (10nM) and kynurenine (50 µM). qPCR analysis was performed to determine expression levels of Cyp1a1 (upper) and Cyp1b1 to confirm activation of the AHR. Post ANOVA testing comparisons are to control; ***, p < 0.001; **, p < 0.01. C, Time-course of luciferase activity following exposure of Hepa1 cells to FICZ (200nM) or kynurenine (50 µM). All figures represent 1 of 3 independent experiments.
Presence of the AHR is necessary in T cells for optimal generation of FoxP3+ Tregs
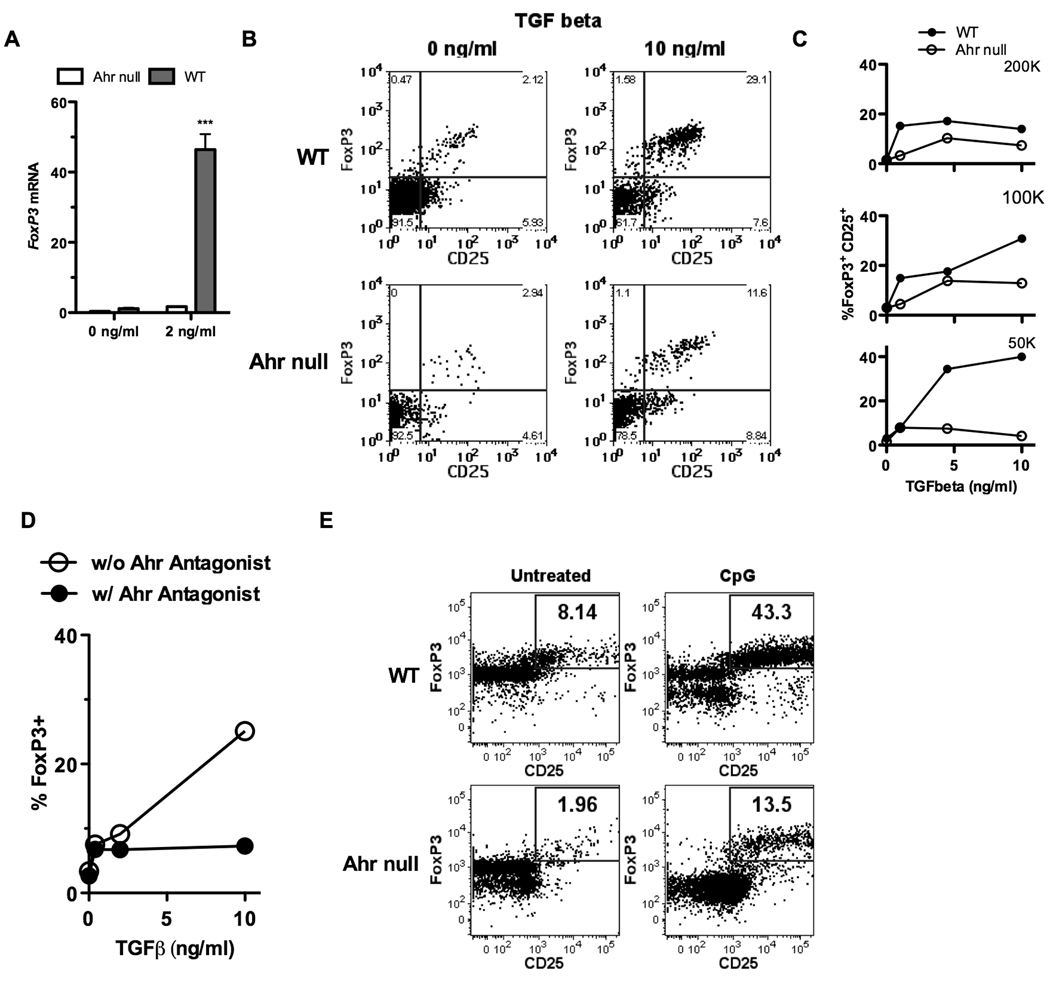
The observation that IDO can be upregulated in DCs in an AHR dependent manner, and kynurenine activates the AHR, led us to consider that kynurenine could generate Tregs through the AHR. Using the well documented technique for Treg generation with TGF-β and antibody stimulation (45), we initially exposed naïve CD4+ T cells from wild-type and AHR-null animals to 2 ng/ml of TGF-β, and analyzed collected mRNA for FoxP3 expression. As seen in figure 3A, wild type cells generated FoxP3, more than 40 times the response in AHR nulls. To further support this finding we performed a similar experiment exposing wild type or AHR-null cells to antibody stimulation and a higher dose of TGF-β, and measured Treg generation by flow cytometry. This is represented in figure 3B, where optimal Treg populations were generated in wild type cells (29.1% in this representative assay), with a muted response from null cells (11.6% in this same assay). By titrating doses of TGF-β, we were able to yield increasing numbers of CD25+FoxP3+ cells seen by flow, represented graphically in figure 3C. When naïve CD4+ T cells were separated from AHR-null animals, increasing doses of TGF-β had little effect on the generation of Tregs (figure 3B, C). To further test the importance of AHR-ligand binding in Treg generation, we repeated antibody stimulation of naïve wild-type T cells with titrating doses of TGF-β, and this time included an AHR antagonist (CH-223191), known to competitively bind to the receptor. As seen in figure 3D, the addition of antagonist blocked the increase of Tregs seen by flow cytometry after exposure to TGF-β in vitro.
Figure 3. Presence of the AHR is necessary in T cells for optimal generation of FoxP3+ Tregs in Treg-polarizing conditions with and without cell-cell contact.
A, Naïve CD4 T cells (CD4+CD62L+ T cells) were generated by magnetic bead separation. 5×105 cells/well were cultured for 5 days with anti CD3/CD28 beads in the presence of no or 2ng/ml of TGF-β. qPCR was used to test the generation of FoxP3. B, Naïve CD4 T cells (CD4+CD62L+ T cells) were generated by magnetic bead separation. 5×105 cells/well were cultured for 5 days with anti CD3/CD28 beads in the presence of titrating doses of TGF-β. Flow cytometry was used to analyze for CD25 and intracellular FoxP3. C, Graphical representation of a similar experiment as B, utilizing titrating doses of TGF-β and titrating numbers of T cells per well. D, Similar to C, except some naïve cells were exposed to a soluble AHR antagonist. E, Naïve CD4+ CD25− T cells were isolated from B6 WT and AHR-null mice and co-cultured with pDCs isolated from BALB/c mice using the Miltenyi mouse pDC isolation kit at a ratio of 20 to 1. CpG was added at the start of culture in some experiments. On day 5, cells were harvested and subjected to flow cytometric analysis. Percentages are the fraction of gated live CD4+ cells that were FoxP3/CD25 double positive. The figures are representative of 3 independent experiments.
The above experiments all demonstrate the importance of the AHR in antibody-stimulated Treg generation via TGF-β. Given our suspicion that cell-cell contact is important in AHR-dependent Treg generation, we employed an in vitro system separating plasmacytoid DCs (pDC) and exposing them to allogeneic naïve CD4+ T cells (pDC were derived from BALB/c mice, and naïve T cells from C57BL/6J mice). This system was previously shown to be dependent on IDO for successful generation of Tregs (29). We were able to repeat the findings that pDCs exposed to CPG led to significant generation of FoxP3+ Tregs in wild-type allogeneic naïve T cells (figure 3E). When naïve T cells were isolated from AHR-null mice, a low percentage of Tregs were identified prior to manipulation. Addition of CpG did increase Treg generation, but the expression was dramatically less robust than in the wild-type cells (figure 3E).
Kynurenine induces generation of FoxP3+ Tregs in an AHR-dependent manner
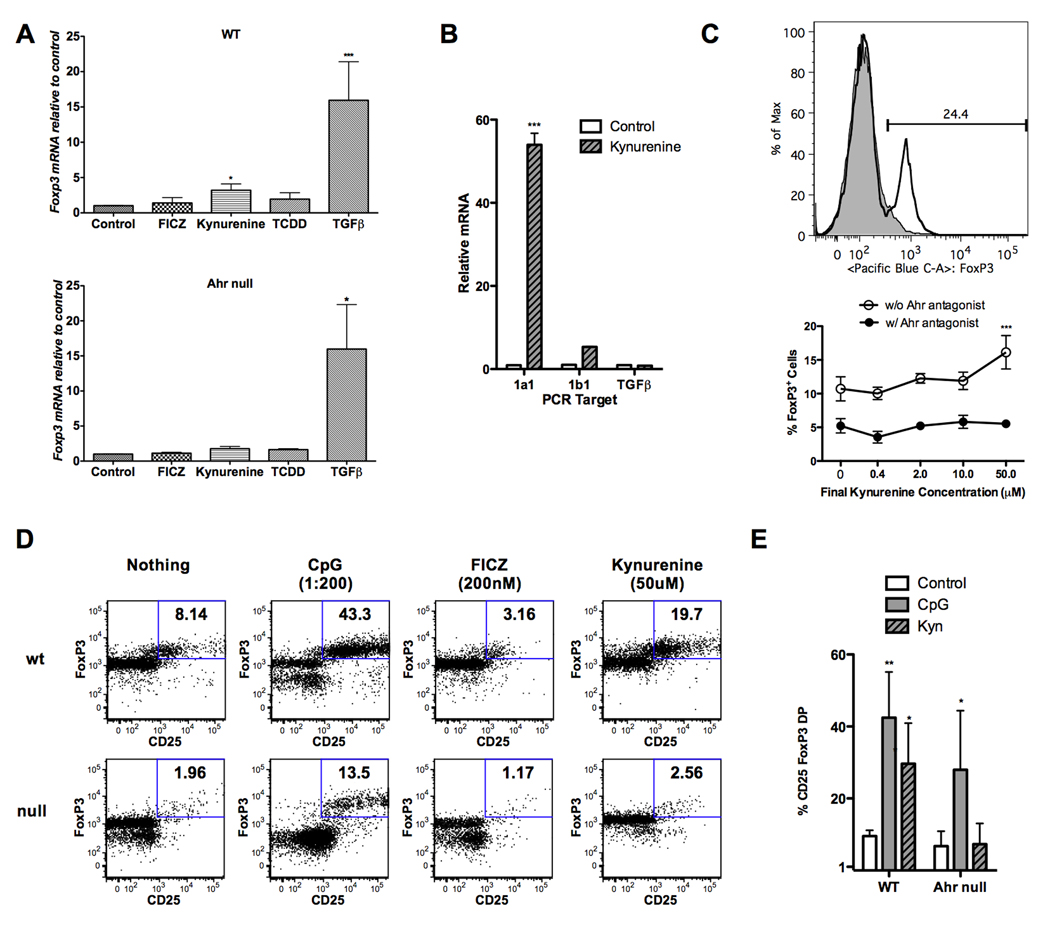
As it is well known that IDO leads to the generation of Tregs, and it appears that kynurenine activates the AHR, the next step was to assess whether kynurenine can directly lead to FoxP3 expression. We first cultured mouse naïve CD4+ T cells with antibody stimulation for 5 days, in the presence of 10 ng/ml of TGF-β, kynurenine, TCDD, or FICZ. RNA was then harvested and tested for the presence of FoxP3. As seen in figure 4A top, only TGF-β and kynurenine led to significant induction of FoxP3 RNA. We analyzed FoxP3 induction in triplicate in 11 separate biological experiments, and achieved a fold change of 3.2, which was significantly increased from control with a p value of 0.017. When AHR null cells were utilized (4A bottom), only TGF-β (10 ng/ml) yielded significant induction of FoxP3 RNA. To further show that kynurenine is leading to FoxP3 mRNA via an interaction with the AHR and not in some indirect way dependent on TGF-β, we cultured mouse naïve CD4+ T cells as above with antibody stimulation, either with or without kynurenine. RNA was then harvested and tested for Cyp1a1, Cyp1b1, and TGF-β. As seen in figure 4B, kynurenine exposure led to significant amounts of Cyp1a1 compared to control, but an increase of TGF-β mRNA over control was not seen, making it unlikely that kynurenine is acting indirectly by generating this cytokine. We then performed a similar experiment exposing naïve T cells to antibody stimulation with and without kynurenine, and after 5 days of culture used flow cytometry to assess for FoxP3. As seen in figure 4C, cells that weren’t exposed to kynurenine showed minimal FoxP3 expression, but those exposed to kynurenine had a significant shift, with 24.4% more FoxP3 expression in analyzed cells in one representative assay. We performed this experiment 6 times, and observed significant FoxP3 protein expression in 4 out of 6 assays. This was also repeated with titrating doses of kynurenine with or without the presence of an AHR antagonist, and this data further confirms that kynurenine at a dose of 50µM leads to FoxP3+ T cells, and the AHR antagonist decreased the percentage of FoxP3+ cells both at baseline and after exposure to kynurenine.
Figure 4. Kynurenine induces generation of FoxP3+ Tregs in an AHR-dependent manner.
A, Naïve CD4+ CD25− T cells were isolated from B6 WT (top) and AHR-null (bottom) mice. Cells at varying cell densities (50 – 200 × 103 cell per well) were cultured in F10 + 5% FCS in wells coated with anti-CD3/ anti-CD28 antibodies for 5 days. Human TGF-β, kynurenine, TCDD and FICZ were added at the start of the culture. mRNA was isolated from harvested cells and qPCR was performed to determine FoxP3 expression levels. Data is relative to WT or AHR-null cells cultured without TGF-β. The WT experiments were conducted 11 times, and are presented as the mean values. Null experiments were conducted 3 times, with mean values presented. Post ANOVA testing comparisons are against the WT or AHR-null control; *, p < 0.05; ***, p < 0.001. B, Naïve T cells were separated and cultured with antibody stimulation as in A, with and without kynurenine (50µM) in the culture. mRNA was isolated after 5 days and qPCR was performed for Cyp1a1, Cyp1b1, and TGF-β. Post ANOVA testing comparisons are against the vehicle control; ***, p < 0.001. C, (top) Naïve CD4+ CD25− T cells were cultured in the presence of immunomagnetic microbeads coated with anti-CD3/ anti-CD28 antibodies with (transparent peak) and without (gray peak) kynurenine (50 µM). Results were measured by flow cytometry with intracellular FoxP3 staining after 5 days of culture. These results are representative of the protein induction obtained in 4 of 6 separate biological assays. C, (bottom) Varying concentrations of kynurenine +/− AHR antagonist were added at the start of culture. Cells were then harvested and subjected to flow cytometry. Percentages are the fraction of gated Live CD4+ cells that are FoxP3 positive. Post ANOVA testing comparisons are against the vehicle control; ***, p < 0.001. D, Naïve CD4+ CD25− T cells were isolated from B6 WT and AHR-null mice and co-cultured with pDCs isolated from BALB/c mice using the Miltenyi mouse pDC isolation kit at a ratio of 20 to 1. CpG, FICZ and kynurenine were added at the start of culture at the concentrations indicated. On day 5, cells were harvested and subjected to flow cytometric analysis. Percentages are the fraction of gated live CD4+ cells that were FoxP3/CD25 double positive. E, The experiments in D were repeated at the same ratio of pDCs to naïve CD4+ T cells (1 to 20), and results are expressed graphically for WT and Null cells. These figures represent 3 independent experiments. Post ANOVA testing comparisons are against the untreated control; *, p < 0.05; **, p < 0.01.
We next utilized the in vitro system separating plasmacytoid DCs and exposed them to allogeneic naïve CD4+ T cells. As seen in figure 4D, in wild-type T cells the addition of CpG exhibited the greatest expression of Tregs, but kynurenine also yielded elevated Treg generation. We also tested FICZ, and this ligand not only did not lead to an increased Treg population, but decreased the percentage of CD4+CD25+FoxP3+ cells compared to untreated control. When AHR-null mice were utilized as the source for naïve T cells, very few Tregs were generated when exposed to pDCs. The addition of kynurenine did not cause any enhancement of Treg formation, presumably due to the lack of the AHR receptor on the T cells. As further confirmation, we repeated the DC/T cell coculture with 1 to 20 ratios of allogeneic BALB/c DC to naïve C57BL/6J T cells multiple times, and present the summarized data graphically. As shown in Figure 4E, the 1 to 20 ratio yielded generation of Tregs by flow, and kynurenine led to significant Treg generation as compared to untreated cells. When repeated with AHR-null T cells, CpG was able to yield Tregs, but kynurenine did not, supporting the dependence of the function of kynurenine on the expression of AHR by the T cell.
In order to assess the importance of the AHR on DCs in this model, we performed this experiment utilizing AHR null cells as the source for pDCs (on a C57BL/6J background), and naïve T cells were taken from wild type mice (BALB/c). As seen in supplementary figure 4, under these circumstances similar generation of Tregs was seen utilizing null pDCs as wild type pDCs, indicating at least in this model that the presence of the AHR is necessary on the T cell, and not the pDC, for optimal Treg generation. The data in this section highlights that the AHR on T cells is activated by kynurenine and leads to Treg induction.
TGF-β upregulates AHR expression, potentiating activation of the DRE by kynurenine
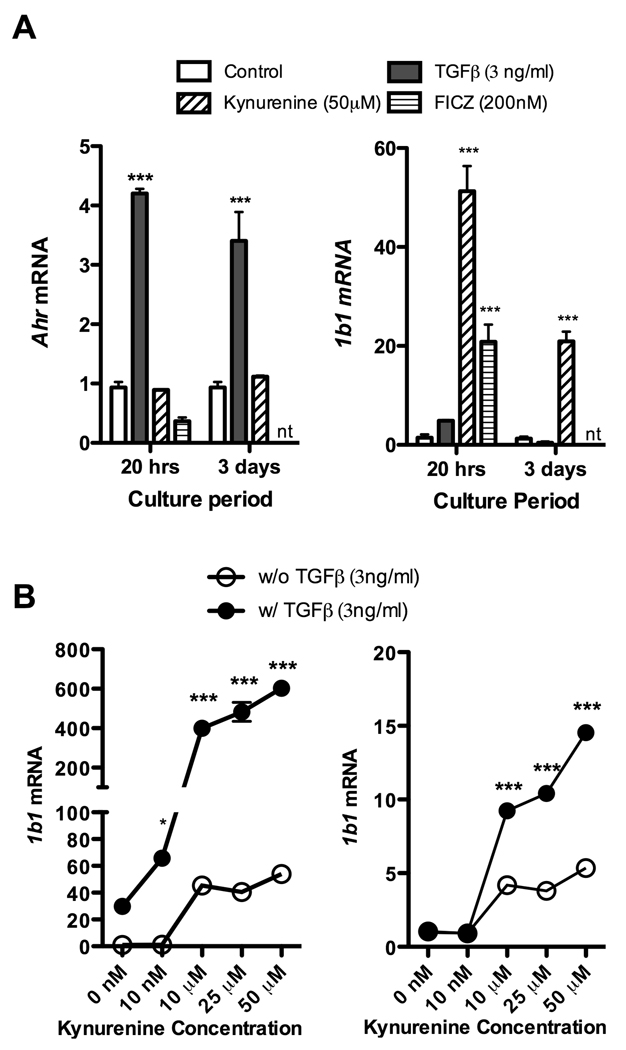
To better understand the role of the AHR in TGF-β dependent Treg generation, we next extracted total RNA from naïve T cells, either fresh or after 20 hours or 3 days of culture, and conducted real time PCR for AHR expression. Culture conditions included antibody stimulation with FICZ, kynurenine, or TGF-β. There is AHR expression at baseline (figure 5A), which increases more than four-fold at 20 hours with exposure to TGF-β, and remains more than three-fold elevated at 3 days. We additionally looked at Cyp1b1 expression at 20 hours and 3 days, and as seen in figure 5A, FICZ and kynurenine led to 20 and 50 times mRNA production over baseline at 20 hours respectively, with Cyp1b1 levels remaining 20 times elevated at 3 days after kynurenine exposure. Culturing in the presence of TGF-β did lead to a small increase in Cyp1b1 (approximately 4 times over baseline at 20 hours), but much less than seen with FICZ or kynurenine. To assess whether AHR upregulation secondary to TGF-β would potentiate binding of ligands to the AHR, we compared the expression of Cyp1a1 and Cyp1b1 after kynurenine exposure with and without TGF-β, which is represented in figure 5B. The response is strongly enhanced after TGF-β exposure, shifting the curve up significantly, implying that TGF-β does potentiate the binding of kynurenine to the AHR when this ligand is present in the culture.
Figure 5. TGF-β upregulates AHR expression, potentiating activation of the DRE by kynurenine.
A, (left) Total RNA was extracted from naïve T cells (separated by magnetic beads), either fresh or after 20 hours or 3 days of culture, and qPCR was performed for AHR expression. Culture conditions included antibody stimulation, FICZ 200nM, kynurenine 50uM, or TGF-β 3ng/ml. A, (right) Cyp1b1 mRNA expression was also examined by qPCR at 20 hours and 3 days after the same culture conditions. The FICZ sample was not tested at 3 days (nt). B, To assess whether AHR upregulation secondary to TGF-β would potentiate binding of ligands to the AHR, Cyp1a1 and Cyp1b1 expression after kynurenine exposure in culture for 3 days with and without TGF-β. The response is strongly enhanced after TGF-β exposure, shifting the curve up significantly, implying that TGF-β does potentiate the binding of kynurenine to the AHR when this ligand is present in the culture. Post ANOVA testing comparisons are against the vehicle control; *, p < 0.05; ***, p < 0.001.
Kynurenine does not lead to TH17 cell generation, whereas FICZ does
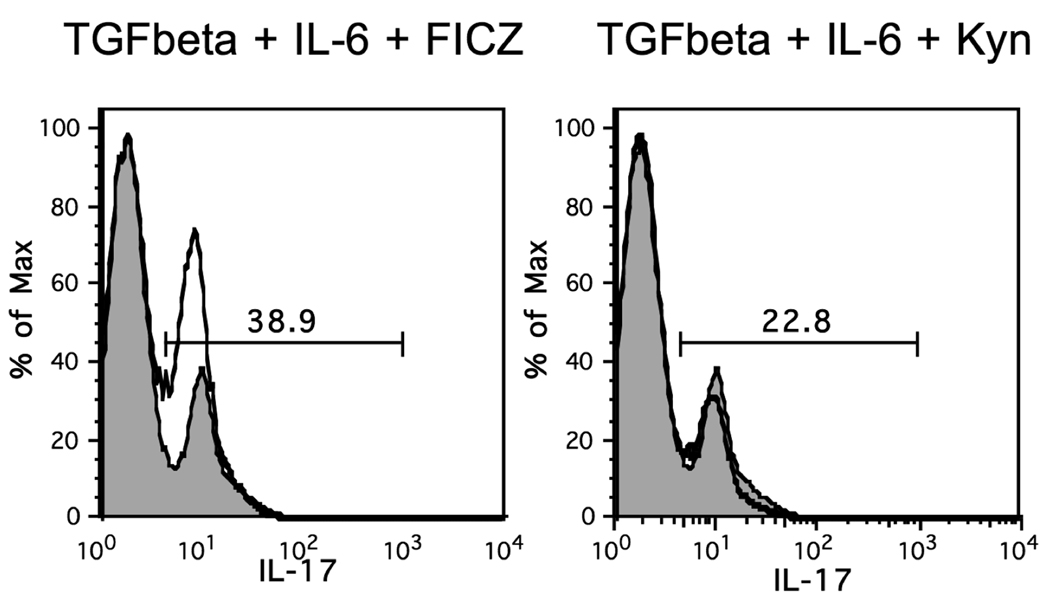
Given that the AHR has also been implicated in the generation of TH17 cells when bound to certain ligands (FICZ), we wondered whether IDO pathway products could also favor TH17 morphology when present in a milieu favoring TH17 generation. We used the TH17 generating conditions described previously, based on exposure of naïve CD4+ T cells to IL-6 and TGF-β (21). We first repeated the finding that FICZ leads to enhancement of IL-17+ cells (23) (figure 6). As mentioned previously, FICZ is thought to act primarily through the AHR, confirming that the AHR can promote T cell differentiation to both Treg and TH17 differentiation depending on the milieu. We then tested kynurenine, and found no effect on the generation of TH17 cells (figure 6). This indicates that activation of the AHR with different ligands can lead to entirely different outcomes depending on the surrounding milieu.
Figure 6. FICZ but not Kynurenine enhances TH17 cell differentiation in vitro.
Naïve CD4+ CD25− T cells were isolated from B6 WT spleens and cultured in the presence of immunomagnetic microbeads coated with anti-CD3/ anti-CD28 antibodies, TGF-β (4 ng/ml) and IL-6 (20ng/ml) for 5 days. FICZ (200 nM) or kynurenine (50 µM) were added at the start of culture. Cells were then stimulated for 6 hours in the presence of PMA, Ionomycin and Brefeldin A at which time they were harvested and surfaced stained for CD4. This was followed by intracellular staining for IL-17. Numbers are the percent of live CD4 T cells expressing IL-17. Solid thick line is experimental histogram and shaded histogram is control (anti-CD3/CD28, TGF-β and IL-6 alone). Number above gate is the percent IL-17 positive of experimental histogram. Percent IL-17 positive of control histogram is 15.4. This figure is representative of 2 independent experiments.
Discussion
Collectively, the data represent a novel way that IDO, via the kynurenine pathway, leads to Treg generation. It is generally believed that T cell differentiation depends on interactions between DCs and T cells (46–48), with IDO playing a role in this. The above findings establish a direct relationship between the AHR and the fate of T cells in vitro. IDO is generated by pDCs. This leads to tryptophan metabolism and kynurenine formation. Kynurenine binds to the AHR in T cells, leading to differentiation to CD25+FoxP3+ T cells. Absence of the AHR in T cells prevents this effect. Kynurenine was previously identified in a review of AHR ligands (35), which corresponds to the knowledge that tryptophan metabolites can lead to ligands of the AHR; the finding that this ligand-receptor interaction leads to Treg generation is novel. While Figure 1 does indicate that IDO induction by DCs can be stimulated via the AHR, the physiologic significance of this needs to be further defined. IFN-γ does stimulate IDO in AHR null DCs (data not shown), and supplemental figure 4 would suggest that Tregs can be induced by AHR null pDCs in coculture assays, but the AHR needs to be present on T cells for optimal Treg generation.
One issue that needs to be addressed is the reliance of TGF-β on the AHR. Figure 3 indicates that optimal generation of Tregs by TGF-β is dependent on the presence of the AHR, similar to a report published previously (21). This decrease in Treg production is demonstrated both in T cells obtained from null mice, and also with the use of the AHR antagonist (figure 3D). It is unlikely that this cytokine binds directly to the AHR, given its structure (we have tested this in a DRE luciferase assay with no response to TGF-β alone). More likely it is secondary to the effect demonstrated in figure 5, which shows that TGF-β with antibody stimulation leads to an upregulation of the AHR in culture. This effect is seen in the first 24 hours in culture, and seems to persist at least 3 days, according to our data and the literature (21, 49). It is important to note that CD4+CD25− T cells do express the AHR prior to its upregulation, which is demonstrated by both western blot (21, 49), DNA microarray (21), and in our own data (figure 5). This is further demonstrated by the fact that exposure of naive T cells to kynurenine or FICZ leads to mRNA transcription of Cyp1a1 and Cyp1b1, which would only occur in the presence of the AHR. It is possible that AHR upregulation alone leads to Treg generation after TGF-β exposure, which would correlate with a previously published experiment where cotransfection of a construct coding for mouse AHR into a bacterial artificial chromosome with FoxP3 tagged with a Renilla luciferase reporter led to upregulation of Renilla activity (18). Another possibility is that the upregulation of the AHR allows endogenous ligands present in the system (either in media, or secreted from cells during inflammation) to bind to the increased receptor with enhanced effect. This is supported by the data in figure 4C, which indicates that the AHR receptor antagonist reduces the amount of FoxP3+ cells seen at baseline, as well as the fact that we do see some Cyp1a1 and Cyp1b1 induction in T cells after exposure to TGF-β (figure 5A, B). Perhaps there is ongoing binding between the AHR and endogenous ligands (which may include kynurenine in an in vivo system), and blocking the receptor blocks this Treg-generating effect. While endogenous ligands may play a role in this differentiation, the effects of kynurenine in our assays far outweigh ligands that may already be present in the media. We tested the role of the AHR with TGF-β in figure 5B, where kynurenine was titrated in culture with CD4+CD25− T cells either in the presence of or without TGF-β. When this cytokine was present, the response of Cyp1a1 and Cyp1b1 was dramatically elevated when exposed to increasing doses of kynurenine, much higher than was seen with TGF-β alone. This would further support that kynurenine is a ligand of the AHR, and that TGF-β potentiates the effect of kynurenine binding by increasing the amount of receptor, far beyond what was seen with any ligand already present in the media.
A second important question is whether kynurenine undergoes catabolism and it is actually a metabolite or breakdown product that is binding the AHR. It is possible that kynurenine in solution, like other small molecules, undergoes breakdown through various mechanisms. We used a 0.5 Molar solution of HCL to get kynurenine into solution, as per the recommendations of the manufacturer regarding maximum solubility. In an effort to test for breakdown of kynurenine, we have conducted HPLC with diode array detection to analyze kynurenine in this solution, as well as in buffered solution at a physiologic pH (50–53). In addition, the manufacturer has tested kynurenine in HCL by HPLC and found it to be greater than 98% pure, which is similar to our findings (supplemental figure 5). As can be seen in the figure, kynurenine dissolved in HCL at one day shows minimal decomposition, whereas kynurenine dissolved in buffer shows decreased purity. It was more difficult to dissolve kynurenine in buffered solution, which took up to 8 hours at 37 degrees, as opposed to kynurenine in HCL which went rapidly into solution, and stayed in solution throughout the assays. We did find that kynurenine in bicarbonate buffer did not strongly activate the DRE in the luciferase assay when made fresh, but after a few days in culture displayed strong activity, which may correlate with solubility issues. When we examined kynurenine in HCL at 3 weeks and 7 months by HPLC, it continued to show minimal breakdown, indicating there is stability when placed in this solution. It is well known in the literature that the isoforms of kynurenine can be modified depending on the surrounding milieu in vivo (50–53), and it is difficult to rule out that this may occur to some degree in our assays. Nevertheless, if it is a metabolite of kynurenine binding to the AHR, it is still formed early in the kynurenine pathway, generated by effects of IDO on tryptophan. This does not diminish the importance of these findings, still linking IDO and the kynurenine pathway to the AHR.
It is fascinating that some ligands (like FICZ) activate the AHR, as seen by the luciferase assays and mRNA analysis, but do not lead to FoxP3 expression. Other ligands (kynurenine) activate this same receptor and do lead to FoxP3 on T cells. We have considered how different ligands might activate the same receptor and lead to disparate outcomes in protein generation. One hypothesis that we are investigating is that kynurenine itself may be enzymatically modified by the cytochrome P450 enzymes that are induced by the AHR, whereas FICZ may not be. The product of this modification may directly lead to FoxP3 induction, as opposed to a direct effect of kynurenine itself. This would explain this differential effect of these AHR ligands. This hypothesis is further supported by the fact that TGF-β can generate FoxP3 in our assays as early as 3 days, whereas the kynurenine-induced generation is typically not seen prior to 5 days (data not shown), despite the fact that the AHR is activated by kynurenine within a few hours. We will test this theory further by utilizing known inhibitors of Cyp1a1 and Cyp1b1 enzymes (trans-stilbenes (54)), and experimenting with our recently generated DRE cluster null mice (55), which have dysfunctional Cyp1a1 and Cyp1a2 enzymes.
Regarding the concentration of kynurenine used in these experiments, the dose chosen was physiologic (44, 56), comparable to levels encountered in humans in areas of inflammation. It is also consistent with observations on the amounts of kynurenine and other tryptophan breakdown products generated in vitro by DCs (57) (5–50uM range). In areas without an ongoing immune response, concentrations of the kynurenines are significantly lower, in the nanomolar range, but increase 1000-fold in microenvironments of inflammation. The fact that kynurenine concentrations in vivo only reach the doses examined in our assays in areas of inflammation could serve as a way to localize the IDO-AHR dependent Treg generation. Conceivably this would allow an immune response to commence, leading to IFN-γ and other inflammatory cytokines, which would then induce DCs to generate IDO. Ultimately kynurenine levels would build up sufficiently to interact with the AHR and generate Tregs, (and AHR expression would be enhanced by TGF-β expression, further potentiating the effects of kynurenine) hence muting the immune response. In areas without inflammation, kynurenine doses would be inadequate to generate significant amounts of AHR dependent Tregs.
This data sheds light on the direct mechanism of IDO, which has been controversial. Two theories on the function of IDO in Treg generation have been proposed. The first is that IDO leads to tryptophan depletion, and this relative starvation leads to cell cycle arrest in some populations, favoring generation of Tregs (32). The second theory is that the tryptophan catabolites themselves have a more direct role in the generation of Tregs, supported by some observational studies but without any clearly understood mechanism (30, 31). The data in this paper strongly supports the second theory, and experiments to elucidate the exact molecular responses elicited after binding of kynurenine or FICZ to the AHR are underway. It is still plausible that tryptophan depletion continues to play a role in the IDO-dependent generation of Tregs, and this paper does not exclude this. In fact, as mentioned in our methods section, we did perform the assays with naïve T cells alone exposed to kynurenine in a low tryptophan media (F10), based on previously published data (32) and our own experience that the yield in this media is superior to when RPMI media (with higher tryptophan content) is utilized. We have contemplated that the reason low tryptophan solutions lead to more regulatory cells is that tryptophan itself is known to form other potential ligands of the AHR, including FICZ. In our in vitro assay we use a low tryptophan solution with kynurenine as the primary AHR ligand. Perhaps this shifts the balance towards generation of regulatory cells, whereas when tryptophan is present to start with, additional ligands can be generated that shift the balance towards an effector response. It is possible that both mechanisms play a role, and in fact may explain why DCs are so potent at generating Tregs in coculture assays (as they generate IDO and deplete the tryptophan present in the assays). Of course other mechanisms, including cell-cell contact and cytokine release may also be involved.
The data in this manuscript furthers our understanding of the emerging role of the AHR as a key player in the differentiation of T cells to Tregs, as well as the ultimate balance of regulatory and effector responses in immunity. As this pathway is further characterized, improved understanding will enhance our knowledge of T cell differentiation and yield new strategies of modulating the balance of regulation and effector response using ligands of the AHR.
Supplementary Material
Footnotes
This work was supported by Supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health (to J.D.M.), grant R37ES005703 (to C.A.B.), grant RO1ES013566 (to C.A.B.), by the American Society of Transplant Surgeons-Astellas Faculty Development Award (to J.D.M.), and an NIH training grant T32ES007015-32 (to B.P.J.).
C.A. Bradfield has served as a scientific consultant to Dow Chemical Co. on issues related to dioxin toxicity.
Throughout this paper, kynurenine is used specifically to delineate this first tryptophan breakdown product in the kynurenine pathway, catalyzed by the enzyme IDO. Those breakdown products downstream to kynurenine are termed kynurenines, and do not include kynurenine itself.
References
- 1.Gershon RK. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- 2.Hedrick SM, Germain RN, Bevan MJ, Dorf M, Engel I, Fink P, Gascoigne N, Heber-Katz E, Kapp J, Kaufmann Y, et al. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985;82:531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 5.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 6.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 7.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt JV, Carver LA, Bradfield CA. Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J Biol Chem. 1993;268:22203–22209. [PubMed] [Google Scholar]
- 11.Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175:221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas RS, Penn SG, Holden K, Bradfield CA, Rank DR. Sequence variation and phylogenetic history of the mouse Ahr gene. Pharmacogenetics. 2002;12:151–163. doi: 10.1097/00008571-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Walker MK, Heid SE, Smith SM, Swanson HI. Molecular characterization and developmental expression of the aryl hydrocarbon receptor from the chick embryo. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126:305–319. doi: 10.1016/s0742-8413(00)00119-5. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol Pharmacol. 2007;72:487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 18.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links T(H)17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 23.Stockinger B, Veldhoen M, Hirota K. Modulation of Th17 development and function by activation of the aryl hydrocarbon receptor--the role of endogenous ligands. Eur J Immunol. 2009;39:652–654. doi: 10.1002/eji.200839134. [DOI] [PubMed] [Google Scholar]
- 24.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen LP, Hsu EL, Chowdhury G, Dostalek M, Guengerich FP, Bradfield CA. D-amino acid oxidase generates agonists of the aryl hydrocarbon receptor from D-tryptophan. Chem Res Toxicol. 2009;22:1897–1904. doi: 10.1021/tx900043s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, Hahn ME, Stegeman JJ. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem Biol Interact. 2009;181:447–454. doi: 10.1016/j.cbi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 28.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 33.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 34.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 36.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieber N, Belohradsky BH. AHR activation by tryptophan--pathogenic hallmark of Th17-mediated inflammation in eosinophilic fasciitis, eosinophilia-myalgia-syndrome and toxic oil syndrome? Immunol Lett. 128:154–155. doi: 10.1016/j.imlet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Matheu MP, Sen D, Cahalan MD, Parker I. Generation of bone marrow derived murine dendritic cells for use in 2-photon imaging. J Vis Exp. 2008 doi: 10.3791/773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 40.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung ID, Lee CM, Jeong YI, Lee JS, Park WS, Han J, Park YM. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581:1449–1456. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 42.Rushing SR, Denison MS. The silencing mediator of retinoic acid and thyroid hormone receptors can interact with the aryl hydrocarbon (Ah) receptor but fails to repress Ah receptor-dependent gene expression. Arch Biochem Biophys. 2002;403:189–201. doi: 10.1016/s0003-9861(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 43.Andrieux L, Langouet S, Fautrel A, Ezan F, Krauser JA, Savouret JF, Guengerich FP, Baffet G, Guillouzo A. Aryl hydrocarbon receptor activation and cytochrome P450 1A induction by the mitogen-activated protein kinase inhibitor U0126 in hepatocytes. Mol Pharmacol. 2004;65:934–943. doi: 10.1124/mol.65.4.934. [DOI] [PubMed] [Google Scholar]
- 44.Heyes MP, Chen CY, Major EO, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326(Pt 2):351–356. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25-precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 46.Razmara M, Hilliard B, Ziarani AK, Chen YH, Tykocinski ML. CTLA-4 × Ig converts naive CD4+CD25− T cells into CD4+CD25+ regulatory T cells. Int Immunol. 2008;20:471–483. doi: 10.1093/intimm/dxn007. [DOI] [PubMed] [Google Scholar]
- 47.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 48.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Negishi T, Kato Y, Ooneda O, Mimura J, Takada T, Mochizuki H, Yamamoto M, Fujii-Kuriyama Y, Furusako S. Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance. J Immunol. 2005;175:7348–7356. doi: 10.4049/jimmunol.175.11.7348. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez S, Truscott RJ, O'Hair RA, Weimann A, Sheil MM. A study of kynurenine fragmentation using electrospray tandem mass spectrometry. J Am Soc Mass Spectrom. 2001;12:786–794. doi: 10.1016/S1044-0305(01)00255-0. [DOI] [PubMed] [Google Scholar]
- 51.Taylor LM, Andrew Aquilina J, Jamie JF, Truscott RJ. UV filter instability: consequences for the human lens. Exp Eye Res. 2002;75:165–175. doi: 10.1006/exer.2002.2012. [DOI] [PubMed] [Google Scholar]
- 52.Taylor LM, Andrew Aquilina J, Jamie JF, Truscott RJ. Glutathione and NADH, but not ascorbate, protect lens proteins from modification by UV filters. Exp Eye Res. 2002;74:503–511. doi: 10.1006/exer.2001.1165. [DOI] [PubMed] [Google Scholar]
- 53.Vazquez S, Aquilina JA, Jamie JF, Sheil MM, Truscott RJ. Novel protein modification by kynurenine in human lenses. J Biol Chem. 2002;277:4867–4873. doi: 10.1074/jbc.M107529200. [DOI] [PubMed] [Google Scholar]
- 54.Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ. Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem. 2002;45:160–164. doi: 10.1021/jm010298j. [DOI] [PubMed] [Google Scholar]
- 55.Nukaya M, Moran S, Bradfield CA. The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci U S A. 2009;106:4923–4928. doi: 10.1073/pnas.0809613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.