Abstract
Erythropoietin (EPO) has shown promise as a neuroprotectant in animal models of ischemic stroke. EPO is thought not only to protect neurons from cell death, but also to promote regeneration after stroke. Here, we report a systematic review and meta-analysis of the efficacy of EPO in animal models of focal cerebral ischemia. Primary outcomes were infarct size and neurobehavioral outcome. Nineteen studies involving 346 animals for infarct size and 425 animals for neurobehavioral outcome met our inclusion criteria. Erythropoietin improved infarct size by 30.0% (95% CI: 21.3 to 38.8) and neurobehavioral outcome by 39.8% (33.7 to 45.9). Studies that randomized to treatment group or that blinded assessment of outcome showed lower efficacy. Erythropoietin was tested in animals with hypertension in no studies reporting infarct size and in 7.5% of the animals reporting neurobehavioral outcome. These findings show efficacy for EPO in experimental stroke, but when the impact of common sources of bias are considered, this efficacy falls, suggesting we may be overestimating its potential benefit. As common human co-morbidities may reduce therapeutic efficacy, broader testing to delineate the range of circumstances in which EPO works best would be beneficial.
Keywords: animal studies, experimental, focal ischemia, neuroprotection, neuroregeneration, trophic factors
Introduction
Of hundreds of interventions that improve outcome in experimental stroke, only recombinant tissue plasminogen activator has found a place in routine clinical practice (O'Collins et al, 2006). This discrepancy between animal and human studies might be due to bias in the conduct or reporting of animal experiments leading to an overstatement of neuroprotective efficacy; to a failure of clinical trials to test interventions under circumstances where efficacy has been observed in animals; or to a failure of animal studies to model human diseases with sufficient fidelity. Systematic review and meta-analyses are techniques that allow an assessment of the likelihood that research conclusions are distorted by bias, and a clear description of the circumstances under which efficacy has been observed in animals.
Erythropoietin is a hematopoietic growth factor used clinically to treat anemia, which has shown promise as an acute treatment in in vitro and in vivo studies in cerebral ischemia (Ruscher et al, 2002; Sinor and Greenberg, 2000; Siren et al, 2001). The mechanisms of this reported protection are not clear, but may include activation of endogenous survival pathways that inhibit apoptosis (Fliser et al, 2006) and decrease inflammatory responses (Villa et al, 2003). Erythropoietin has also been shown to support regeneration by recruiting stem cells and stimulating neoangiogenesis (Brines and Cerami, 2008). To date, the single published clinical trial of EPO in acute ischemic stroke showed limited success in its results (Ehrenreich et al, 2002), the Multicenter-Erythropoietin-Stroke Trial is awaiting analysis and the REGENESIS study (of EPO with recombinant human chorionic gonadotrophin) is in follow-up. The benefits of EPO in human stroke are therefore not known at the present time.
Here, we report a systematic review and meta-analysis of the use of EPO in experimental stroke. Our aim was to investigate whether elements of study design or quality have a significant impact on outcome. These data may assist the development of further preclinical hypothesis to test in animals and in the design of future large-scale clinical trials.
Materials and methods
Systematic Review
We searched three electronic databases (Pubmed, ISI Web of Science, and Embase, in July 2009) using the terms (EPO or erythropoietin) and (stroke or ischemia or ischaemia or cerebrovascular or middle cerebral artery or MCA or MCAO or ACA or ACAO or anterior cerebral artery), limiting results to animals.
Two investigators (MJ, KF) assessed the titles and abstracts of studies and obtained copies of articles that described controlled studies of EPO or EPO analogues in animal models of focal cerebral ischemia and that measured outcome as infarct size or neurobehavioral outcome. The latter was defined to include all methods measuring neurobehavioral outcome or functional behavior where a baseline of normal or pre-stroke function could be clearly established. Articles in foreign languages were translated. Reference lists of the articles were assessed to locate additional studies not identified in the initial search.
Data Extraction
From included studies, we extracted data on study design including method of ischemia induction, species, dose, time and route of administration, and time of assessment of outcome. The number of animals used, mean and variance of treatment and control groups were also extracted. Where data required for meta-analysis were missing, we contacted authors to request additional information. If data were only expressed graphically, values were requested from the authors, and where a response was not received, we measured data from the graphs using digital ruler software. Where data required for meta-analysis were not presented or obtainable, the studies were excluded from the analysis.
Study quality was assessed based on the CAMARADES study quality checklist (Macleod et al, 2004) comprising (1) publication in a peer-reviewed journal, (2) statement of control of temperature, (3) statement of monitoring of physiologic parameters, (4) randomization to treatment or control, (5) blinded induction of ischemia, (6) blinded assessment of outcome, (7) anesthetic without marked intrinsic neuroprotective properties, (8) use of co-morbid animals (9) sample size calculation, (10) statement of compliance with animal welfare regulations, and (11) statement regarding possible conflicts of interest.
Statistical Analysis
A random effects weighted mean difference meta-analysis was used for statistical analysis (DerSimonian and Laird, 1986). The data were stratified according to elements of study quality and aspects of study design to assess their impact on efficacy. We assessed the impact of continuous variables (dose, time of administration, and assessment) on the efficacy of EPO using meta-regression. To allow for multiple comparisons, we set our significance level using Bonferroni correction; P<0.003 for both infarct volume and neurobehavioral outcome.
Results
Our search identified 1565 unique publications of which 19 met our inclusion criteria and were included in this analysis (Tables 1, 2 and 3). One publication was excluded from the analysis because the results were not presented in figures or graphs and another publication was excluded because the number of animals used was not stated (Chang et al, 2008; Yu et al, 2005). In both circumstances, we were unable to obtain this information from the authors.
Table 1. Studies describing the use of EPO administered after induction of focal cerebral ischemia.
| Author, year of publication | Drug | Species | Stroke model | Method of administration | Outcome measures, n (treated/control) | Quality score |
|---|---|---|---|---|---|---|
| Sadamoto et al (1998) | EPO | Rat | Permanent MCAO | Immediately after occlusion, intracerebroventricular infusion, 0.73, 3.64, or 18.18 U/kg daily for 28 days | Morris water maze (24/8) | 6 |
| Brines et al (2000) | EPO | Rat | Permanent MCAO | Immediately, 3, 6, or 9 h after occlusion, intraperitoneal injection, 5,000 U/kg | Infarct volume (32/9) | 4 |
| Dahlberg et al (2004) | EPO | Rat | MCAO (2 h) | 5 mins after occlusion, intraperitoneal injection, 5,000 U/kg | Infarct volume (10/10) | 3 |
| Wang et al (2004) | EPO | Rat | Embolic MCAO | 24 h after occlusion, intraperitoneal injection, 5,000 or 10,000 U/kg | Infarct volume (16/8) Foot Fault test (28/14) Corner test (28/14) | 3 |
| Faure et al (2006) | EPO | Gerbil | Permanent CCAO | 2 and 48 h after occlusion, intraperitoneal injection, 5,000 U/kg | Morris water maze (19/6) Object recognition (19/6) | 5 |
| Aluclu et al (2007) | EPO | Rat | MCAO (2 h) | 2 h after occlusion, intraperitoneal injection, 5,000 U/kg | Neurological score (15/15) | 3 |
| Kolb et al (2007) | EPO | Rat | Permanent focal devascularization | 72 h after occlusion, intracerebroventricular infusion for 7 days, total 927 U/kg | Cylinder test (6/15) Swimming task (6/15) Tray reaching test (6/15) | 3 |
| Villa et al (2007) | EPO | Rat | Permanent MCAO | 3 h after occlusion, intravenous injection, 5,000 U/kg | De Ryck somatosensory score (7/7) Foot-fault test (7/7) | 3 |
| Wang et al (2007) | EPO | Rat | Embolic MCAO | 6, 24, and 48 h after occlusion, intravenous injection, 50, 500, 1150, or 5,000 U/kg | Infarct volume (36/12) Foot-fault test (40/10) | 4 |
| Esneault et al (2008) | EPO | Rat | MCAO (1.5 h) | 24 and 48 h after occlusion, intraperitoneal injection, 1,000 U/kg per injection | Infarct volume (10/12) Neurological score (10/12) Limb placing (10/12) | 5 |
| Belayev et al (2009) | EPO | Rat | MCAO (1.5 h) | 6, 7, and 8 days after occlusion, intravenous injection, 1,440 U/kg per injection | Infarct volume (12/13) Neurological score (12/13) | 7 |
| Fletcher et al (2009) | EPO | Mouse | MCAO (1 h) | Immediately after occlusion, intranasal, 100 U | Infarct volume (16/16) Neurological score (16/16) | 6 |
| Li et al (2009) | EPO | Rat | Embolic MCAO | 24 h after occlusion and then daily for 7 days, intraperitoneal injection, 5,000 U/kg | Infarct volume (11/7) Neurological score (11/7) | 5 |
Table 2. Studies describing the use of EPO analogues administered after induction of focal cerebral ischemia.
| Author, year of publication | Drug | Species | Stroke model | Method of administration | Outcome measures, n (treated/control) | Quality score |
|---|---|---|---|---|---|---|
| Erbayraktar et al (2003) | Asialo EPO | Rat | MCAO (1.5 h) | 1.5 h after occlusion, intravenous injection, 44 μg/kg | Infarct volume (6/6) | 4 |
| Leist et al (2004) | Carbamylated EPO | Rat | MCAO (1 h) | 1 or 4 h after occlusion, intravenous injection, 5 or 50 μg/kg | Infarct volume (8/16 and 8/8) | 1 |
| Belayev et al (2005) | Darepoetin alfa | Rat | MCAO (2 h) | 2 h after occlusion, intraperitoneal injection, 10 μg/kg | Infarct volume (16/13) Limb placing (16/13) | 7 |
| Villa et al (2007) | Caranesp | Rat | Permanent MCAO | 1 h after occlusion, intravenous injection, 50 μg/kg | Infarct volume (9/9) De Ryck somatosensory score (9/9) | 3 |
| Carbamylated EPO | Rat | Permanent MCAO | 1 h after occlusion, intravenous injection, 50 μg/kg | Infarct volume (8/9) De Ryck somatosensory score (8/9) | 3 | |
| Carbamylated EPO | Rat | Permanent MCAO | 3 or 3, 24, and 48 h after occlusion, intravenous injection, 50 μg/kg per injection | De Ryck somatosensory score (5/7 and 5/7) Foot fault (5/7 and 5/7) | 3 | |
| Carbamylated EPO | Rat | Permanent MCAO | 24 and 48 h after occlusion, intravenous injection, 50 μg/kg per injection | De Ryck somatosensory score (5/5) Foot fault (5/5) | 3 | |
| EPO S-100E | Rat | Permanent MCAO | 3 h after occlusion, intravenous injection, 50 μg/kg | De Ryck somatosensory score (8/8) | 3 |
Table 3. Studies describing the use of EPO administered before induction of focal cerebral ischemia.
| Author, year of publication | Drug | Species | Stroke model | Method of administration | Outcome measures, n (treated/control) | Quality score |
|---|---|---|---|---|---|---|
| Bernaudin et al (1999) | EPO | Mouse | MCAO (1.5 h) | 24 h before occlusion, intracerebroventricular infusion, 50 U/kg | Infarct volume (8/9) | 3 |
| Brines et al (2000) | EPO | Rat | Permanent MCAO | 24 h before occlusion, intraperitoneal injection, 5,000 U/kg | Infarct volume (8/9) | 4 |
| Dahlberg et al (2004) | EPO | Rat | MCAO (2 h) | Immediately before occlusion and then once daily for 7 days, subcutaneous injection, 5,000 U/kg | Infarct volume (11/10) | 3 |
| Li et al (2007) | EPO | Mouse | Permanent MCAO | 0.5 h before occlusion and then once daily, intraperitoneal injection, 5,000 U/kg | Infarct volume (6/6) | 5 |
| Wakida et al (2007) | EPO | Mouse | Permanent MCAO | 24 and 1 h before and immediately after occlusion, intraperitoneal injection, 30,000 U/kg per injection | Infarct volume (11/13 and 7/11) | 5 |
For treatment initiated after induction of ischemia, infarct volume was reported in 23 experiments using 346 animals and neurobehavioral outcome was reported in 30 experiments using 425 animals. For treatment initiated before induction of ischemia, infarct volume was reported in 6 experiments using 109 animals and neurobehavioral outcome was not studied.
Overall Efficacy and Impact of Study Design
Erythropoietin and EPO analogues improved infarct volume by 30.0% (95% CI: 21.3 to 38.8) and neurobehavioral outcome by 39.8% (33.7 to 45.9). There was substantial between-study heterogeneity for the analysis of both infarct volume (χ2=63.5, df=22, P<0.003) and neurobehavioral outcome (χ2=67.5, df=29, P<0.003).
For infarct volume, meta-regression suggested that efficacy was reduced by 0.90% (0.39 to 1.41, P<0.003) per hour of delay in treatment, and efficacy was reduced by 2.41% (1.51 to 3.51, P<0.003) per hour of later assessment. No relationship was identified for neurobehavioral outcome.
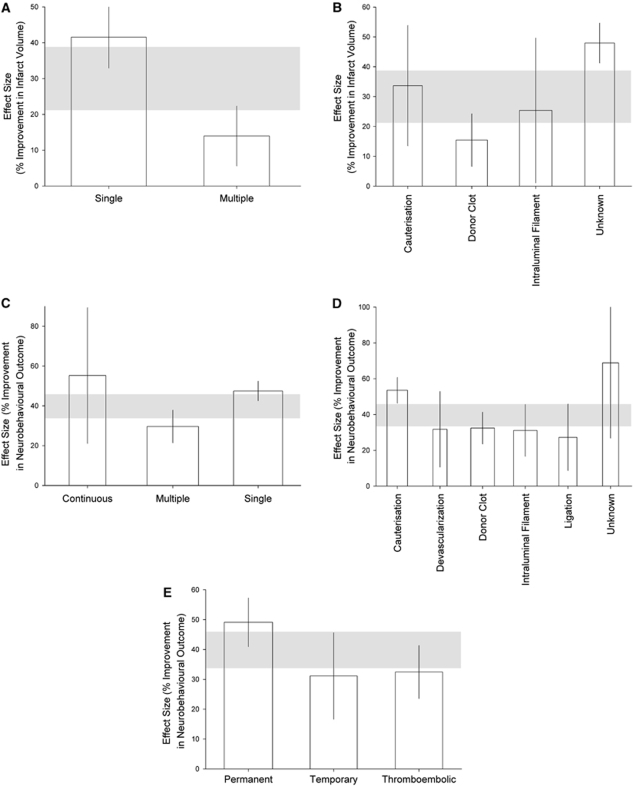
For infarct volume, efficacy was highest when EPO was administered once rather than multiple times (41.6% (32.9% to 50.2%), χ2=33.9, df=1, P<0.003; Figure 1A) and when the method of middle cerebral artery occlusion was not stated (48.0% (41.3% to 54.6%), χ2=35.4, df=5, P<0.003; Figure 1B).
Figure 1.
Effect on the estimate of efficacy for infarct volume of (A) type of delivery, (B) method of artery occlusion, and for neurobehavioral outcome of (C) type of delivery, (D) method of artery occlusion, and (E) model of ischemia, P<0.003. The shaded gray bar represents the 95% confidence limits of the global estimate. The vertical error bars represent the 95% confidence intervals for the individual estimates. The width of each bar reflects the log of the number of animals contributing to that comparison.
For neurobehavioral outcome, efficacy was highest with continuous infusion of EPO rather than single or multiple injections (55.3% (21.1% to 89.4%), χ2=26.7, df=2, P<0.003; Figure 1C); when the method of middle cerebral artery occlusion was not stated (68.8% (26.8% to 110.9%), χ2=28.9, df=5, P<0.003; Figure 1D) and in permanent rather than temporary or thromboembolic models of ischemia (49.1% (41.0% to 57.2%) χ2=18.0, df=2, P<0.003; Figure 1E).
A separate meta-analysis of the efficacy of the eight experiments using EPO analogues only was also performed (Table 2). Infarct volume was reported in 8 experiments using 116 animals and neurobehavioral outcome was reported in 8 experiments using 114 animals. Erythropoietin analogues improved infarct volume by 45.4% (38.7 to 52.2, χ2=8.11, df=7, P=ns) and neurobehavioral outcome by 49.4% (40.5 to 58.3, χ2=11.0, df=7, P=ns).
For treatment initiated before induction of ischemia, EPO improved infarct volume by 33.8% (18.2 to 49.5, χ2=19.5 df=5, P<0.003). Efficacy was highest when EPO was administered once rather than multiple times (50.6% (35.7 to 65.4), χ2=9.5, df=1, P<0.003).
Impact of Study Quality
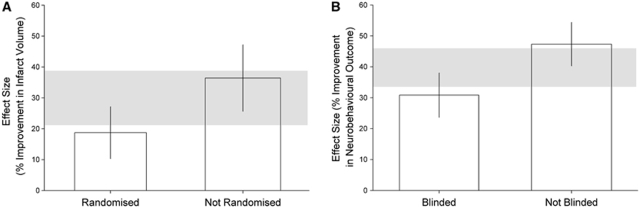
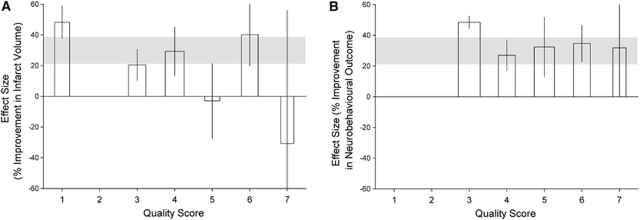
Overall, studies had a median quality score of 4 out of 11 for both outcomes (interquartile range 3 to 5). Study quality accounted for a significant amount of between-study heterogeneity; for infarct volume, studies that reported random allocation to group were associated with lower estimates of efficacy than those that did not (18.7% (10.3 to 27.1, n=146 animals) versus 36.4% (25.6% to 47.2%, n=195), χ2=19.7, df=1, P<0.003; Figure 2A). Neurobehavioral outcome studies that reported blinded assessment of outcome were associated with lower estimates of efficacy (30.8% (23.7% to 38.0%, n=211) versus 47.3% (40.3% to 54.4%, n=186), χ2=24.3, df=1, P<0.003; Figure 2B). Hypertensive animals were only used in studies assessing neurobehavioral outcome (32 animals out of 425), and there was no difference in efficacy between these groups. Significant differences between high and low quality studies were observed, with low quality studies reporting the highest efficacy for both outcomes (P<0.003; Figures 3A and 3B).
Figure 2.
Effect on the estimate of efficacy for infarct volume of (A) random allocation to treatment or control group, and for neurobehavioral outcome of (B) blinded assessment of outcome, P<0.003.
Figure 3.
Effect on the estimate of efficacy of quality score (1=worst, 11=best) for (A) infarct volume and (B) neurobehavioral outcome, P<0.003.
Discussion
Our analysis of 18 publications identified a significant improvement in experimental stroke after EPO treatment. However, these results must be interpreted with caution due to potential sources of bias, such as the lack of allocation concealment and randomization to group of some of the included studies.
Erythropoietin was most effective the earlier it was administered after ischemic stroke induction, although efficacy is still maintained up to treatment initiation of 24 h post-ischemia. This supports the hypothesis that EPO not only has neuroprotective properties but may also support regeneration.
There are limited data using EPO in animals with co-morbidities associated with human stroke; only 7.5% of the animals in the neurobehavioral dataset were hypertensive, all of which were spontaneously hypertensive rats. The efficacy of EPO in the presence of such co-morbidities may have implications for the potential use of EPO in stroke patients.
This review is subject to possible methodological weaknesses. First, the dataset is too small to reliably assess publication bias. In a review of seven different growth factors including EPO (unpublished data), publication bias was identified using a funnel plot and Egger regression, and it is reasonable to expect that this bias extends to these data. Second, analysis can only include data described in the identified articles. This might relate not only to the reporting of items described in the CAMARADES quality checklist (although recent guidelines now recommend this (Macleod et al, 2009), but also incomplete presentation of data, for instance of efficacy at later time points or at lower doses.
This study is a comprehensive systematic review of the use of EPO therapies in experimental stroke. The only clinical trial published to date reported that EPO did not significantly affect stroke mortality, although it did report improvements in functional outcome on the Barthel Index and beneficial trends on the NIH and Scandinavian stroke scales as well as on infarct size as a secondary outcome. The limited animal and human data on the safety and efficacy of EPO in ischemic stroke suggests further testing of EPO in experimental models is warranted before further clinical trials. Elucidating the conditions in which the most benefit is seen in studies with minimal potential sources of bias may provide potential hypotheses to test in clinical trials.
Acknowledgments
We express our gratitude to Professor Geoff Donnan for constructive discussions regarding this paper. This study was supported by The Australian NHMRC, The Swedish Research Council, the region of West Sweden, the ALF Funding Sources Göteborg, the Edit Jakobsson Foundation, the Elsa and Gustav Lindh Foundation, and the Sten A Olsson foundation for Research and Culture.
The authors declare no conflict of interest.
References
- Aluclu MU, Acar A, Guzel A, Bahceci S, Yaldiz M. Evaluation of erythroprotein effects on cerebral ischemia in rats. Neuroendocrinol Lett. 2007;28:170–174. [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Zhao KL, Davidoff AW, Moore AF, Cramer SC. A novel neurotrophic therapeutic strategy for experimental stroke. Brain Res. 2009;1280:117–123. doi: 10.1016/j.brainres.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, Magal E, Ginsberg MD. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36:1071–1076. doi: 10.1161/01.STR.0000160753.36093.da. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, Mackenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D-I, Koh S-H, Yoo A, Oh S, Kim Y, Kim H, Lee K-Y, Lee Y, Kim H-T, Kim J, Kim S.2008Effect of delayed repeated injection of recombinant human erythropoietin in animal model of stroke J Neurol 255(Suppl 2):190 [Google Scholar]
- Dahlberg SA, Xu L, Hess DC, Hohnadel E, Hill WD, Fagan SC. Erythropoietin and erythropoietin mimetic peptide in focal cerebral ischemia. Stroke. 2004;35:279. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Erbayraktar S, Grasso G, Sfacteria A, Xie QW, Coleman T, Kreilgaard M, Torup L, Sager T, Erbayraktar Z, Gokmen N, Yilmaz O, Ghezzi P, Villa P, Fratelli M, Casagrande S, Leist M, Helboe L, Gerwein J, Christensen S, Geist MA, Pedersen LO, Cerami-Hand C, Wuerth JP, Cerami A, Brines M. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esneault E, Pacary E, Eddi D, Freret T, Tixier E, Toutain J, Touzani O, Schumann-Bard P, Petit E, Roussel S, Bernaudin M. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2008;28:1552–1563. doi: 10.1038/jcbfm.2008.40. [DOI] [PubMed] [Google Scholar]
- Faure S, Oudart N, Javellaud J, Fournier A, Warnock DG, Achard JM. Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J Hypertens. 2006;24:2255–2261. doi: 10.1097/01.hjh.0000249704.34607.4c. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Fliser D, Bahlmann FH, deGroot K, Haller H. Mechanisms of disease: erythropoietin--an old hormone with a new mission. Nat Clin Pract Cardiovasc Med. 2006;3:563–572. doi: 10.1038/ncpcardio0609. [DOI] [PubMed] [Google Scholar]
- Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Kapke A, Lu M, Ewing JR, Chopp M. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40:936–941. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu ZY, Keogh CL, Yu SP, Wei L. Erythropoietin-induced neurovascular protection, angiogenesis, and cerebral blood flow restoration after focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:1043–1054. doi: 10.1038/sj.jcbfm.9600417. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, Donnan GA, Howells DW. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- Sinor AD, Greenberg DA. Erythropoietin protects cultured cortical neurons, but not astroglia, from hypoxia and AMPA toxicity. Neurosci Lett. 2000;290:213–215. doi: 10.1016/s0304-3940(00)01361-6. [DOI] [PubMed] [Google Scholar]
- Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, Brines M, Leist M, Ghezzi P, Torup L. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- Wakida K, Shimazawa M, Hozumi I, Satoh M, Nagase H, Inuzuka T, Hara H. Neuroprotective effect of erythropoietin, and role of metallothionein-1 and -2, in permanent focal cerebral ischemia. Neuroscience. 2007;148:105–114. doi: 10.1016/j.neuroscience.2007.04.063. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Wang Y, Zhang RL, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 2005;387:5–10. doi: 10.1016/j.neulet.2005.07.008. [DOI] [PubMed] [Google Scholar]