Abstract
Background
Surgical and perioperative improvements permit earlier repair of partial and transitional atrioventricular septal defects (AVSD). We sought to describe contemporary outcomes in a multicenter cohort.
Methods
We studied 87 patients undergoing primary biventricular repair of partial or transitional AVSD between June 2004 and February 2006 across seven North American centers. One-month and 6-month postoperative data included weight-for-age z-scores, left atrioventricular valve regurgitation (LAVVR) grade, residual shunts, and left ventricular ejection fraction. Paired methods were used to assess 6-month change.
Results
Median age at surgery was 1.8 years; median weight z-score was −0.88. Median days for ventilation were 1, intensive care 2, and hospitalization 5, all independent of age, with 1 in-hospital death. At 1 month, 27% (16 of 73) had ejection fraction less than 55%; 20% (17 of 87) had significant LAVVR; 2 had residual shunts; 1 each had subaortic stenosis and LAVV stenosis. At 6 months (n = 60), there were no interim deaths, reinterventions, or new development of subaortic or LAVV stenosis. Weight z-score improved by a median 0.4 units (p < 0.001), especially for underweight children less than 18 months old. Left atrioventricular valve regurgitation occurred in 31% (change from baseline, p = 0.13), occurring more frequently in patients repaired at 4 to 7 years (p = 0.01). Three patients had ejection fraction less than 55%, and 1 had a residual atrial shunt.
Conclusions
Surgical repair for partial/transitional AVSD is associated with low morbidity and mortality, short hospital stays, and catch-up growth, particularly in underweight children repaired between 3 and 18 months of age. Left atrioventricular valve regurgitation remains the most common residual defect, occurring more frequently in children repaired after 4 years of age.
Children with partial and transitional atrioventricular septal defect (AVSD) are largely asymptomatic so referral for surgical repair is typically delayed to preschool or older ages [1-5]. Recently, single centers have reported good results in younger children [6]. Although there may be theoretical advantages to earlier repair, such as minimizing the child’s exposure to pulmonary overcirculation and right heart volume overload, these factors must be weighed against the potential for unfavorable outcomes in the younger child. The purpose of this study was to describe contemporary outcomes after repair of partial or transitional AVSD in a multicenter cohort.
Patients and Methods
Between June 2004 and February 2006, echocardiographic and clinical data were collected on children undergoing primary biventricular repair of a partial or transitional AVSD uncomplicated by anomalous pulmonary venous connection across the seven North American centers (Appendix) comprising the Pediatric Heart Network. All participating centers received Institutional Review Board approval (clinicaltrials.gov identifier NCT00113698), and this study was funded by the National Heart, Lung, and Blood Institutes (NHLBI). As part of a planned drug trial evaluating the use of angiotensin-converting enzyme (ACE) inhibitors in children with left atrioventricular valve regurgitation (LAVVR) after AVSD repair, consecutive children with parent/guardian consent were enrolled into an observation-only phase for 6 months postoperatively to allow the heart to adapt to surgical intervention. During this 6-month period, prospective clinical and echocardiographic data were collected for 60 of the 87 patients in this analysis. To report outcomes representative of the entire disease spectrum, we added all screened patients in our database from the same time period who met clinical exclusion criteria for the trial (e.g., planned reoperation, left atrioventricular valve [LAVV] stenosis) to the analysis cohort. These patients had demographic characteristics similar to those who had been enrolled in the observation phase of the study. The data for these additional patients were obtained retrospectively under waiver of consent approval. An NHLBI-appointed Data and Safety Monitoring Board monitored the conduct of the study.
Definitions
Published definitions of AVSD subtypes vary. For the present study, partial AVSD was characterized by an ostium primum atrial septal defect (ASD) with intact ventricular septum; a transitional AVSD was characterized by an ostium primum ASD, two-orifice atrioventricular valve, and restrictive inlet ventricular septal defect (VSD) [7, 8]. Since both partial and transitional AVSD result in right heart volume overload with little or no ventricular level shunt, data from children with either defect were combined for this analysis.
Clinical and Surgical Data Collection
Data were collected at surgery, within 1 month of surgery, and at 6 months after surgery. Operative reports from all patients were independently reviewed for uniformity in defect classification and details of repair by three investigators blinded to outcomes. Annuloplasty included any additional left atrioventricular valve surgery beyond cleft closure that involved reduction in the annulus size.
Sex, presence of trisomy 21, associated defects, age and weight at surgery, complications requiring a change in therapy, and duration of mechanical ventilation, intensive care, and total hospitalization were obtained from medical records. Weight-for-age z-scores were calculated using sex-specific reference values for trisomy 21 [9] and normal children [10, 11], as appropriate. For normal children, weight-for-age z-scores were calculated using both World Health Organization [10] and Centers for Disease Control [11] data. Because results were similar and all inferences the same, only Centers for Disease Control data are reported. Patients with weight z-score of −2 or less were considered to have growth failure. Heart failure scores were obtained 6 months after surgery using the Ross heart failure classification [12] or New York Heart Association (NYHA) [13] classification, as age appropriate. Cardiac medications were recorded at the time of the echocardiograms.
Echocardiographic Data
For patients enrolled prospectively, echocardiograms were performed within 1 month after repair and at 6-month follow-up using a standard protocol and sent to a core laboratory where measurements were made by a single observer. For patients enrolled retrospectively, only 1-month echocardiograms were available with all measurements made by each center’s echocardiographers. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s rule or the area/length method [14]. Left ventricular (LV) dysfunction was defined as an LVEF less than 55%. The LAVVR was subjectively graded as none/trace, mild, moderate, or severe based on the appearance of the color Doppler jets in relation to the surrounding chambers [1-5]. The LAVVR was considered significant if graded as moderate or worse. Color Doppler was also used to demonstrate a residual ASD or VSD, and diameters more than 3 mm were considered significant [15]. Pulsed Doppler interrogation was used to evaluate LAVV inflow and LV outflow: LAVV mean gradient of 7.5 mm Hg or more and LV outflow peak instantaneous gradient of 20 mm Hg or more were considered significant.
Statistical Analysis
Patient characteristics by type of defect were compared using Fisher’s exact test for categorical variables, the Wilcoxon rank sum test for positively skewed variables, and a t test for all other continuous variables. Descriptive statistics are reported as mean ± SD unless otherwise noted. Crude analysis of 6-month change in categorical outcomes was conducted with McNemar’s test. Six-month change in continuous outcomes was analyzed using a paired t test. Linear regression was employed to identify independent predictors of 6-month change in weight-for-age z-score. Nonparametric generalized additive modeling was used to identify nonlinear associations between covariates and outcomes. Logistic regression was used to identify surgical correlates of preoperative LAVVR grade and predictors of the presence of moderate or greater LAVVR at 6 months after surgery. All variables significant at the 0.20 level in univariate analysis were evaluated for inclusion in multivariate models for change in weight z-score and for the presence of moderate or greater LAVVR 6 months after surgery.
Results
Subjects
The group for this analysis (Table 1) included 87 patients (60 partial; 27 transitional). Most were white (82%), 33% had trisomy 21, and 51% were male. The median age at repair was 1.8 years, with 26 of 87 (30%) repaired at less than 1 year, and 3 at less than 3 months (Fig 1). Weight z-scores at surgery ranged from −6.8 to 6.2 (median −0.88). Patients with partial AVSD underwent repair significantly later than patients with transitional defects, but their weight z-scores were similar. Weight z-score of −2 or less (growth failure) was present in 17 of 87 (20%). At least moderate LAVVR was present on the preoperative echocardiogram in 31 of 87 (36%). The most common associated defect noted at surgery (Table 2) was an additional atrial level shunt (53 of 87 patients, 61%).
Table 1.
Patient Characteristics by Atrioventricular Septal Defect Subtype
| Variable | n | All | n | Partial | n | Transitional |
|---|---|---|---|---|---|---|
| Number | 87 | 60 | 27 | |||
| Male | 87 | 51% | 60 | 55% | 27 | 41% |
| Median age in years at repair (range)a | 87 | 1.8 (0.01 to 16.7) | 60 | 3.1 (0.01 to 16.7) | 27 | 0.8 (0.3 to 4.8) |
| Median weight-for-age z-score at surgery (range) | −0.88 (−6.8 to 6.2) | −0.75 (−6.8 to 3.0) | −0.88 (−5.7 to 6.2) | |||
| Race | 87 | 60 | 27 | |||
| White | 82% | 87% | 70% | |||
| Black | 9% | 7% | 15% | |||
| Asian | 1% | 2% | 0% | |||
| Other/unknown | 8% | 5% | 15% | |||
| Hispanicb | 86 | 10% | 59 | 10% | 27 | 11% |
| Trisomy 21 | 87 | 33% | 60 | 29% | 27 | 44% |
| Moderate or greater preoperative LAVVR | 87 | 36% | 60 | 32% | 27 | 44% |
Partial versus transitional p < 0.001 for age at repair; no other comparisons significant at the 0.05 level.
Hispanic ethnicity unknown for 1 subject. LAVVR = left atrioventricular valve regurgitation.
Fig 1.
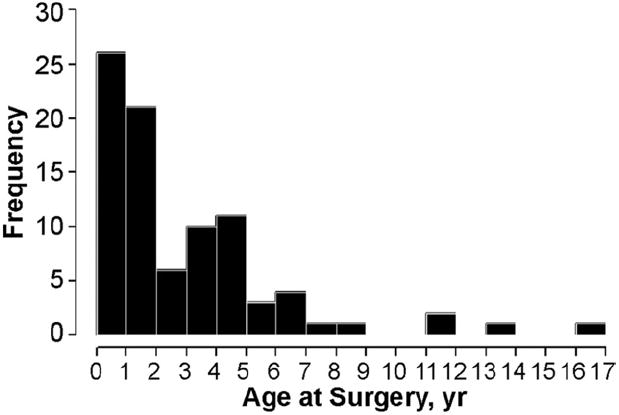
Distribution of age (years) at repair of partial/transitional atrioventricular septal defect. Median age at repair is 1.8 years.
Table 2.
Associated Defectsa Noted at Surgery
| Associated Defect | n | % (n = 87) |
|---|---|---|
| Additional atrial shunt | 53 | 61 |
| Patent ductus arteriosus | 10 | 12 |
| Persistent left superior vena cava | 3 | 3 |
| Subaortic stenosis | 2 | 2 |
| Double orifice mitral valve | 2 | 2 |
| Parachute mitral valve | 2 | 2 |
| Coarctation of the aorta | 1 | 1 |
| Additional ventricular septal defect | 1 | 1 |
| Congenital complete heart block | 1 | 1 |
Defects are not mutually exclusive.
Operative Data
Of the 27 patients with transitional AVSD, 26 underwent VSD closure. Cleft closure was performed in 86 of 87 patients (99%) and annuloplasty in 22 of 87 (25%, including 16 partial and 6 transitional AVSD). Annuloplasty was associated with the presence of significant LAVVR on the preoperative echocardiogram: 42% of patients with moderate or greater LAVVR on the preoperative echocardiogram underwent annuloplasty, compared with 16% who had none/trace or mild preoperative LAVVR (odds ratio [OR] 3.77, 95% confidence interval [CI]: 1.38 to 10.34, p = 0.01). There was no significant variation in annuloplasty use among centers (p = 0.30).
Outcome Data Less Than 1 Month Postoperative
There was 1 in-hospital death (1%), of a 4-day-old infant who underwent arch reconstruction in addition to partial AVSD repair. This infant also had repeat valvuloplasty for low cardiac output and progressive LAVVR and subsequently died of multiorgan failure.
Median days for mechanical ventilation were 1 (range, 0 to 73), intensive care stay 2 (range, 1 to 83), and hospitalization 5 (range, 3 to 90); and all were independent of age at surgery (p = not significant). We identified 20 complications that required change in therapy (Table 3). Within 1 month (6.9 ± 6.7 days) after surgery, 20 of 73 (27%) had LV dysfunction and 17 of 87 (20%) had moderate or greater LAVVR. Three underwent repeat valvuloplasty before discharge. Left atrioventricular valve stenosis (mean gradient 8 mm Hg), subaortic stenosis (peak gradient 39 mm Hg), residual ASD, and residual VSD occurred in 1 patient each. Medications noted at the first postoperative echocardiogram included diuretics in 84%, ACE inhibitor in 8% (5 patients with moderate or greater LAVVR, 2 with LV dysfunction), and digoxin and beta-blocker in 1 child with LV dysfunction (LVEF 47%).
Table 3.
Complications Requiring Change in Therapy
| Complicationa | n | % (n = 87) |
|---|---|---|
| Infection | 6 | 7 |
| Bacterial positive sepsis (3) | ||
| Viral positive (2) | ||
| Pleural effusions | 4 | 5 |
| Nonchylous (3) | ||
| Chylous (1) | ||
| Transient arrhythmias | 4 | 5 |
| Ventricular tachycardia (2) | ||
| Junctional tachycardia (1) | ||
| Complete heart block (1) | ||
| Pericardial effusion | 2 | 2 |
| Intubated >2 weeks postoperatively | 2 | 2 |
| Pneumothorax | 1 | 1 |
| Bloody stool (transfused) | 1 | 1 |
Complications are not mutually exclusive.
Outcome Data 6 Months Postoperative
The 67 prospective patients included 1 in-hospital death and 6 who failed to return for follow-up. In addition, 1 early postoperative echocardiogram was uninterpretable by the core laboratory, preventing paired analysis. Thus, echocardiographic and clinical data pairs were available for 59 and 60 of 87 patients, respectively, with mean follow-up at 6.4 ± 0.8 months. In the interim, there were no deaths or reinterventions, and no additional patients had subaortic or LAVV stenosis. A residual ASD (4 mm) was present in 1 patient, and the 1 residual VSD had spontaneously closed. Six months after surgery, 3 of 59 patients had mild LV dysfunction (LVEF ≥ 51% for all 3). The prevalence of diuretic use decreased to 10% (p < 0.001), ACE inhibitor use (7%) was similar to use less than 1 month after repair, and no patient was using digoxin or beta-blocker. Using Ross (n = 55) or NYHA (n = 5) heart failure scores, 58 patients were functional class I, and 2 were class III—both had transitional AVSD and severe LAVVR.
WEIGHT GAIN AND AGE AT SURGERY
Weight z-scores showed a significant increase (median z-score change of 0.4, p < 0.001), and the 6-month postoperative change was log-linearly related to age at surgery. Children who underwent surgery before age 18 months had the largest gains. We aimed to identify all independent predictors of weight gain and found an interaction between continuous log(age) and preoperative weight z-score (p = 0.002). Weight improvement was only associated with younger age at surgery for children with lower weight z-scores: in particular, those with z-score of −1 or less (Fig 2). There was no association between age at surgery and weight gain for patients with preoperative weight z-scores of −1.0 or greater. Change in weight z-score was unrelated to the presence of trisomy 21 and defect type. At 6 months after surgery, there was also a decrease in the prevalence of growth failure to 8% (5 of 60). Of these 5 children with weight z-scores of −2 or less, 4 had significant LAVVR.
Fig 2.
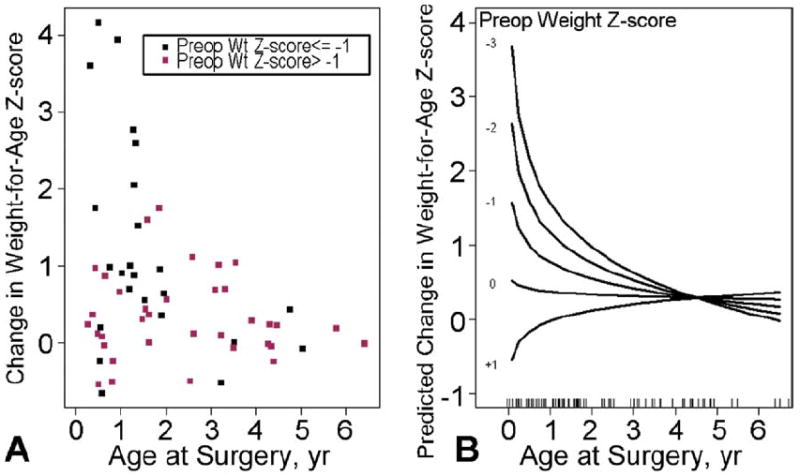
(A) Scatterplot of 6-month change in weight z-score by age at surgery and preoperative weight z-scores. Pearson correlations versus log(age) = 0 (n = 36) and −0.44 (n = 24) for subjects with preoperative weight z-scores −1.0 or less (black) and greater than 1.0 (blue), respectively. Regression was based on all subjects with paired data, but is truncated at age 6.4 years (oldest subject after exclusion of 16-year-old outlier). (B) Predicted change (6 months minus preoperation) in weight-for-age z-score at surgery and preoperative weight z-score. Log(age) by preoperative weight-for-age z-score interaction p = 0.002; R2 = 0.40. The association with log(age) is significant only when weight z-score is −1 or less. The hatch lines at the bottom of the plot represent the observed ages in the dataset. Modeling was based on all subjects with 6-month data, but the plot is truncated at age 6.4 years, (oldest subject after exclusion of 16-year-old outlier).
LAVVR AND AGE AT SURGERY
The prevalence of moderate or greater postoperative LAVVR did not change significantly over time (Fig 3): 20% (12 of 59) at less than 1 month and 31% at 6 months after surgery (18 of 59, p = 0.13) with a higher rate for older children (Fig 4). Significant LAVVR 6 months after surgery occurred in 8 of 9 children (89%) repaired between ages 4 and 7 years compared with 11 of 50 (22%) repaired at less than 4.0 years or at 7 or more years (nonlinearity p = 0.01). This finding persisted even after adjustment for 1-month postoperative LAVVR status. Significant LAVVR at 6 months was associated with moderate or greater LAVVR within 1 month of surgery (OR 4.59, 95% CI: 1.21 to 17.24, p = 0.03) but had no independent association with weight, defect type, double orifice or parachute LAVV, annuloplasty, cleft closure, or trisomy 21. Even after adjustment for significant LAVVR within 1 month of surgery, the presence of moderate to severe LAVVR at 6 months was marginally associated with preoperative moderate to severe LAVVR (adjusted OR 3.18, 95% CI: 0.94 to 10.70, p = 0.06). Of patients with none/trace or mild LAVVR 6 months after surgery, 29% had moderate or greater LAVVR on the preoperative study. In contrast, of patients with moderate or severe LAVVR 6 months postoperatively, 56% had moderate or greater LAVVR preoperatively. Of the 8 children aged 4 to 7 years with moderate to severe LAVVR at 6-month follow-up, 5 had at least moderate LAVVR on the preoperative echocardiogram.
Fig 3.
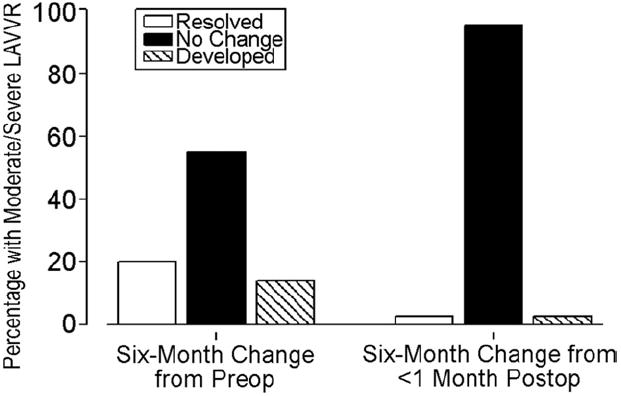
Change in moderate or greater left atrioventricular valve regurgitation (LAVVR) from preoperation and less than 1 month after operation to 6 months after operation (n = 59; McNemar test p values = 0.37 and 0.13). “Resolved,” “no change,” and “developed” refer to the presence of moderate or greater LAVVR.
Fig 4.
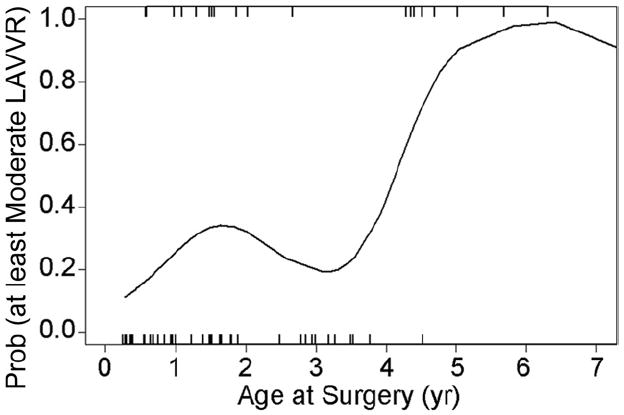
Estimated probability of moderate or greater left atrioventricular valve regurgitation (LAVVR) 6 months after repair (n = 60; test of nonlinearity p = 0.01). Of 9 children repaired at 4 to 7 years of age, 8 had moderate or greater LAVVR (89%) at 6-month follow-up. The hatch lines at the top and bottom of the plot represent the ages at surgery of the patients who had and did not have, respectively, moderate or greater LAVVR. Modeling was based on all subjects with 6-month data, but the plot is truncated at age 7 years (oldest subject aged 16 years not shown).
Comment
Our data indicate that repair of partial or transitional AVSD before preschool ages may be advantageous for improving weight gain and for LAVV function. This multi-institutional study is unique in exploring the improvement in growth after repair of a partial or transitional AVSD. Although poor weight gain is a common indication for surgery in patients with a complete AVSD, it is not the typical reason for repairing the partial or transitional subtypes. Surprisingly, however, 20% of this study population met the definition of growth failure at the time of surgery, and the prevalence dropped to 8% 6 months after repair. In addition, primarily among patients less than 18 months old with preoperative weight z-scores of −1.0 or less, there was a significant increase in weight z-score at 6 months after surgery that was independent of the presence of trisomy 21 and defect type.
Outcomes in this contemporary cohort of children who were repaired at a median of 1.8 years of age were generally good. In-hospital mortality was low (1%) and comparable to recent single-center reports of approximately 2% [1, 3, 8]. Ventilator, intensive care, and hospital days were short and independent of age at repair. Symptomatic infants with partial AVSD who require repair within the first few months of life are known to be higher risk [16], but with only 3 infants younger than 3 months of age, this study was not powered to evaluate the lower bound for repair.
Residual shunts were rare (1% or less) in this cohort, comparing favorably with single-center reports. Residual ASDs have been reported in 0% to 4%, rarely needing reoperation [5, 15] and tend to result from sutures near the atrioventricular conduction system being placed excessively shallow to avoid damaging it. The VSDs may remain after repair of a transitional AVSD when shunts through dense chordal attachments are not addressed in an attempt to avoid distorting valve motion. When surgical closure is attempted and a small ventricular shunt remains, it is likely to close spontaneously [17], as shown here.
One patient had subaortic stenosis during early follow-up. Other investigators report a prevalence of subaortic stenosis of 5% after AVSD repair, with the majority (approximately 60%) of cases occurring in the partial or transitional forms where attachment of the LAVV leaflet to the ventricular crest further encroaches on an already narrowed LV outflow tract [18]. Because subaortic stenosis may develop over time, the 6-month follow-up for this cohort may not be long enough to allow an accurate estimation of the prevalence of this lesion.
Left ventricle dysfunction typically improves after AVSD repair [5], consistent with our finding that only 3 patients had mild LV dysfunction at 6-month follow-up.
Despite the improvement in mortality and other morbidities, there has been little impact on the prevalence of significant postoperative LAVVR in the recent surgical era. The 20% to 31% prevalence of moderate to severe LAVVR after repair of partial/transitional AVSD in our group appears similar to that in other reports where ranges are given from 15% to 50% at a median age of repair ranging from 3.6 to 5.3 years [1-6]. Some reports do not consistently discriminate between partial/transitional and complete AVSD, however, prohibiting direct comparisons with our data. Murashita and colleagues [3] reported 30% of patients with partial/transitional AVSD (median repair age 5.3 years) had at least grade II (scale range, I to IV) LAVVR at hospital discharge that increased to 43% at mean follow-up of almost 9 years. Data from Aubert and colleagues [4] showed LAVVR grade II or higher in 26% of repaired partial AVSD patients (median repair age 5.8 years), with 2.9% having repeat valvuloplasty within 30 days, comparable to our early reoperation rate of 3.4%.
Although the data conflict, potential risk factors reported for significant LAVVR after AVSD repair include partial AVSD, absence of trisomy 21, significant preoperative LAVVR, younger age at repair, incomplete or no cleft closure, technique of repair, and double orifice or parachute LAVV [3, 6, 8,19]. We examined each of these factors and found none predicted significant postoperative LAVVR in this cohort. Although the presence of moderate or greater preoperative LAVVR rendered a threefold risk of significant postoperative LAVVR, the wide confidence interval reflected the clinical variation. We found that moderate or greater LAVVR within 1 month after surgery predicted moderate to severe LAVVR at 6-month follow-up, indicating that early postoperative regurgitation was unlikely to resolve. The prevalence of moderate or greater LAVVR at 6-month follow-up was significantly higher among patients having repair between 4 and 7 years of age. Although annuloplasty has been reported to improve LAVV function [20], it was not associated with a decrease in the prevalence of significant LAVVR in this cohort.
It is not clear why LAVVR remains the most common residual lesion after AVSD repair. Some investigators postulate that despite individualizing each case, alterations in geometry and rotation of the axis of closure combined with deficient and dysplastic subvalvar components may leave some of these valves incompetent, despite the advances in valvuloplasty that have been achieved in the recent era [2, 4, 21]. Because valve regurgitation is progressive, the effects of relatively longstanding significant LAVVR may be another contributing factor. Significant LAVVR results in LV volume overload, annular dilation, and remodeling of the LV from a prolate ellipse to a more spherical and mechanically disadvantaged shape. A positive feedback loop exists between LV dilation and LAVVR severity, leading to parallel augmentation of both [22]. In these circumstances, earlier repair may prevent the geometric alterations of the valve and LV and improve surgical results. It is possible that several factors may play a role in persistent LAVVR after AVSD repair, but this study was not designed or powered to test these hypotheses.
Study Limitations
Because our data were collected both prospectively and retrospectively, all measurements could not be standardized or centrally interpreted, and 6-month postoperative echocardiographic data were not available for some subjects. In addition, the available number of 6-month echocardiographic and clinical data pairs limited our power to detect some associations. Finally, reliable, validated echocardiographic methods for quantitative evaluation of LAVVR grade are not available for children, particularly in the setting of multiple and eccentric jets characteristic of the repaired AVSD valve. We chose to use qualitative assessment of the color Doppler jet, as this was the standard for clinical decision making at all seven centers, and it allowed comparisons with previously published reports where it was the most commonly used method for grading LAVVR.
In conclusion, surgical repair for partial/transitional AVSD in the current era is associated with low morbidity and mortality. Children with preoperative weight z-scores of −1.0 or less repaired between 3 and 18 months had the most catch-up growth, without increasing their risk of significant LAVVR or other morbidities and without prolonging ventilation, intensive care, or hospital days. Significant LAVVR remains the most common adverse outcome, occurring more frequently in patients repaired at 4 years of age or older. Future studies should explore strategies to improve valve function in these patients.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288).
Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- ASD
atrial septal defect
- AVSD
atrioventricular septal defect
- CI
confidence interval
- LAVV
left atrioventricular valve
- LAVVR
left atrioventricular valve regurgitation
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- NHLBI
National Heart, Lung, and Blood Institute
- NYHA
New York Heart Association
- OR
odds ratio
- VSD
ventricular septal defect
Appendix
North American Centers Comprising the Pediatric Heart Network
National Heart, Lung, and Blood Institute
Gail Pearson, Victoria Pemberton, Rae-Ellen Kavey, Mario Stylianou, and Marsha Mathis.
Network Chair: University of Texas Southwestern Medical Center, Lynn Mahony
Data Coordinating Center
New England Research Institutes, Lynn Sleeper (primary investigator), Steven Colan, Gloria Klein, Dianne Gallagher, Minmin Lu, and Paul Mitchell.
Clinical Site Investigators
Children’s Hospital Boston: Jane W. Newburger (primary investigator), Ashwin Prakash, Renee Margossian, Jami Levine, Ellen McGrath, and Carolyn Dunbar-Masterson; Children’s Hospital of New York: Wyman Lai (primary investigator), Seema Mital (currently at Hospital for Sick Children, Toronto), William Hellenbrand, Marc Richmond, Beth Printz (currently at Rady Children’s Hospital, San Diego), Darlene Servedio, and Rosalind Korsin; Children’s Hospital of Philadelphia: Victoria L. Vetter (primary investigator), Meryl Cohen, Sandra DiLullo, and Marisa Nolan; North Carolina Consortium: Duke University, East Carolina University, Wake Forest University: Page A. W. Anderson (primary investigator), deceased, Jennifer Li (primary investigator), Wesley Covitz, Kari Crawford, Michael Hines, James Jaggers, Charlie Sang, Jr, Lori Jo Sutton, and Mingfen Xu; Medical University of South Carolina: J. Philip Saul (primary investigator), Andrew Atz, Girish Shirali, and Jennifer Young; Primary Children’s Medical Center and the University of Utah: L. LuAnn Minich (primary investigator), John A. Hawkins, Linda M. Lambert, and Richard V. Williams; Hospital for Sick Children, Toronto: Brian McCrindle (primary investigator), Fraser Golding, Nancy Slater, and Elizabeth Radojewski.
Echocardiography Core Laboratories
Children’s Hospital of Boston: Steven Colan and Ron Lacro.
Protocol Review Committee
Michael Artman (Chair), Daniel Bernstein, Christopher A. Caldarone, Timothy Feltes, Julie Johnson, Jeffrey Krischer, and G. Paul Matherne.
Data and Safety Monitoring Board
John Kugler (Chair); David J. Driscoll, Kathryn Davis, Sally A. Hunsberger, Mark Galantowicz, Thomas J. Knight, James Tweddell, Catherine L. Webb, and Lawrence Wissow.
References
- 1.Meisner H, Guenther T. Atrioventricular septal defect. Pediatr Cardiol. 1998;19:276–81. doi: 10.1007/s002469900309. [DOI] [PubMed] [Google Scholar]
- 2.Tlaskal T, Hucin B, Marek J, et al. Individualized repair of the left atrioventricular valve in spectrum of atrioventricular septal defect. J Cardiovasc Surg. 1997;38:233–9. [PubMed] [Google Scholar]
- 3.Murashita T, Kubota T, Ob J, Aoki T, Matano J, Yasuda K. Left atrioventricular valve regurgitation after repair of incomplete atrioventricular septal defect. Ann Thorac Surg. 2004;77:2137–62. doi: 10.1016/j.athoracsur.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Aubert S, Henaine R, Raisky, et al. Atypical forms of isolated partial atrioventricular septal defect increase the risk of initial valve replacement and reoperation. Eur J Cardiothorac Surg. 2005;28:223–8. doi: 10.1016/j.ejcts.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury UK, Diplomate NB, Airan B, et al. Specific issues after surgical repair of partial atrioventricular septal defect: actuarial survival, freedom from reoperation, fate of the left atrioventricular valve, prevalence of left ventricular outflow obstruction, and other events. J Thorac Cardiovasc Surg. 2009;137:548–55. doi: 10.1016/j.jtcvs.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Harkel AD, Cromme-Dijkhuis AH, Heinerman BC, Hop WC, Boggers AJ. Development of left atrioventricular valve regurgitation after correction of atrioventricular septal defect. Ann Thorac Surg. 2005;79:607–12. doi: 10.1016/j.athoracsur.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C. Congenital heart surgery nomenclature and database project: atrioventricular canal defect. Ann Thorac Surg. 2000;69:536–43. doi: 10.1016/s0003-4975(99)01235-7. [DOI] [PubMed] [Google Scholar]
- 8.Michielon G, Stellin G, Rizzoli G, et al. Left atrioventricular valve incompetence after repair of common atrioventricular canal defects. Ann Thorac Surg. 1995;60(Suppl):604–9. doi: 10.1016/0003-4975(95)00851-9. [DOI] [PubMed] [Google Scholar]
- 9.Styles ME, Cole DJ, Dennis J, Preece MA. New cross sectional stature, weight, and head circumference reference for Down’s syndrome in the UK and Republic of Ireland. Arch Dis Child. 2002;87:104–8. doi: 10.1136/adc.87.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO multicenter growth reference study. [October 17, 2008]; Available at: http://www.who.int/childgrowth/software/en/index.html.
- 11.Z-score data files for growth. [May 12, 2009]; Available at: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/zscore/zscore.htm.
- 12.Ross RD, Bolinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–5. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 13.New York Heart Association classification for congestive heart failure. [May 24, 2009]; Available at: http://www.hcoa.org/hcoacme/chf-cme/chf00070.htm.
- 14.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 15.Larrazabal LA, del Nido PJ, Jenkins KJ, et al. Measurement of technical performance in congenital heart surgery: a pilot study. Ann Thorac Surg. 2007;83:179–84. doi: 10.1016/j.athoracsur.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Manning PB, Mayer JE, Sanders SP, et al. Unique features and prognosis of primum ASD presenting in the first year of life. Circulation. 1994;90:II30–5. [PubMed] [Google Scholar]
- 17.Dodge-Khatami A, Knirsch W, Tomaske M, et al. Spontaneous closure of small residual ventricular septal defects after surgical repair. Ann Thorac Surg. 2007;83:902–6. doi: 10.1016/j.athoracsur.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 18.Van Arsdell GS, Williams WG, Boutin C, et al. Subaortic stenosis in the spectrum of atrioventricular septal defects. J Thorac Cardiovasc Surg. 1995;110:1534–42. doi: 10.1016/S0022-5223(95)70077-3. [DOI] [PubMed] [Google Scholar]
- 19.Al-hay AA, Lincoln CR, Shore DF, Shinebourne EA. The left atrioventricular valve in partial atrioventricular septal defect; management strategy and surgical outcome. Eur J Cardiothorac Surg. 2004;26:754–61. doi: 10.1016/j.ejcts.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Padala M, Vasilyev NV, Owen JW, et al. Cleft closure and undersizing annuloplasty improve mitral repair in atrioventricular canal defects. J Thorac Cardiovasc Surg. 2008;136:1243–9. doi: 10.1016/j.jtcvs.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanani M, Elliott M, Cook A, Juraszek A, Devine W, Anderson RH. Late incompetence of the left atrioventricular valve after repair of atrioventricular septal defects: the morphologic perspective. J Thorac Cardiovasc Surg. 2006;132:640–6. doi: 10.1016/j.jtcvs.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 22.Sasayma S, Takahashi M, Osakada G, et al. Dynamic geometry of the left atrium and left ventricle in acute mitral regurgitation. Circulation. 1977;56:106–13. doi: 10.1161/01.cir.60.1.177. [DOI] [PubMed] [Google Scholar]


