Summary
To understand the T cell response to prostate cancer, we created transgenic mice that express a model antigen in a prostate-restricted pattern and crossed these animals to TRAMP mice that develop spontaneous prostate cancer. Adoptive transfer of prostate-specific CD4 T cells shows that, in the absence of prostate cancer, the prostate gland is mostly ignored. Tumorigenesis allows T cell recognition of the prostate gland—but this recognition is tolerogenic, resulting in abortive proliferation and ultimately in hyporesponsiveness at the systemic level. Androgen ablation (the most common treatment for metastatic prostate cancer) was able to mitigate this tolerance—allowing prostate-specific T cells to expand and develop effector function after vaccination. These results suggest that immunotherapy for prostate cancer may be most efficacious when administered after androgen ablation.
Introduction
One of the major obstacles to the development of vaccine strategies for treating cancer is that the T cell compartment often develops tolerance to tumor antigens (Staveley-O’Carroll et al., 1998; Morgan et al., 1998; Hu et al., 1993). This difficulty is likely to be most pronounced for antigens that are shared between tumors and normal tissues, as the immune system will have access to these antigens long before tumors develop. Antigens that are sequestered from the immune system might have some advantage in this regard, remaining unrecognized until tumorigenesis occurs. A potential example is the prostate gland, which gives rise to prostate cancer—the most common malignancy in American men (Jemal et al., 2003). The nonvital nature of the prostate gland, coupled with the observation that specialized prostate epithelial cells share antigens with prostate tumors, makes prostate cancer an attractive target for immunotherapy. This concept was borne out by experiments that showed vaccination using irradiated prostate tumor cells combined with CTLA-4 blockade led to immune responses that were restricted to both normal and malignant prostate tissues (Hurwitz et al., 2000).
To understand the immune response to the prostate gland and investigate prostate cancer immunotherapy, we created transgenic mice that express the model antigen influenza hemagglutinin under the control of the prostate-specific minimal rat probasin promoter (Pro-HA) (Rennie et al., 1993). We found that naive prostate-specific CD4 T cells are mostly ignorant of the prostate gland, suggesting that, in the absence of tumorigenesis, prostate-specific tolerance might not be generated. When Pro-HA mice were crossed with TRAMP mice that develop spontaneous prostate cancer, naive T cells were able to recognize the prostate gland. However, this recognition was tolerogenic—leading to abortive proliferation, an absence of effector cytokine production, and impaired responsiveness to vaccination.
Androgen ablation is the most common therapy for prostate cancer that cannot be treated with surgery or radiation (Denmeade and Isaacs, 2002). In mice without prostate cancer, we found that androgen ablation leads to a transient increase in T cell recognition of the prostate gland, followed by a return to baseline recognition levels. In mice with prostate cancer, a progressive decrease in T cell recognition of the prostate gland was noted after androgen ablation. At the systemic level, this decreased recognition was sufficient to abrogate CD4 T cell tolerance and allow these cells to develop effector function in response to vaccination. These data suggest that immunotherapy approaches for prostate cancer may prove most efficacious when applied after androgen ablation.
Results
Generation and characterization of transgenic mice expressing HA on prostate epithelia
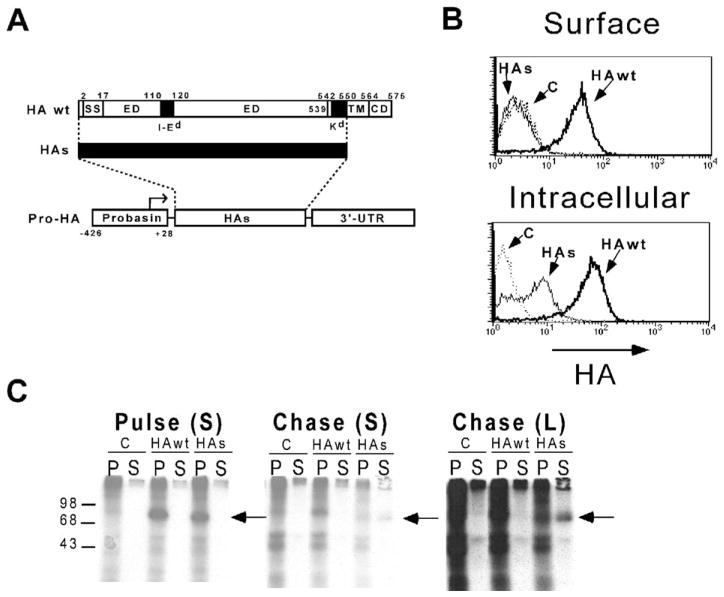
To generate transgenic mice that express HA exclusively on prostate epithelia, the minimal rat Probasin promoter (−426 to +28 bp) (Greenberg et al., 1994, 1995; Rennie et al., 1993) was used to drive HA expression. Since the prostate epithelia produce numerous proteins that are secreted into the prostatic lumen and since many of these proteins are potential targets for immunotherapy because they are also expressed on prostate tumors, we engineered a secreted form of HA (HAs) that was truncated to remove the cytoplasmic domain as well as the C-terminal half of the transmembrane domain. The resulting HAs protein retains a Kd-restricted epitope located in the N-terminal half of the transmembrane domain that is recognized by clone 4 TCR transgenic CD8 cells (Morgan et al., 1996), as well as an I-Ed-restricted epitope located in the extracellular domain that is recognized by 6.5 TCR transgenic CD4 cells (Kirberg et al., 1994) (Figure 1A). To verify that the HAs peptide is secreted rather than expressed on the plasma membrane, we employed two strategies. In the first, P815 cells were infected with recombinant vaccinia viruses expressing either the full-length or truncated HA sequence and subsequently analyzed by FACS using the HA-specific mAb H-18 (Figure 1B). Fixation and permeabilization prior to staining resulted in intracellular detection of both the HA wt and HAs peptides (although the HAs peptide was present at lower levels); however, cells that were not fixed and permeabilized only exhibited surface staining of the HA wt peptide. Thus, it appeared that the HAs peptide was translated, but not expressed on the cell surface. To verify that this lack of surface expression was the result of secretion, COS-7 cells were transiently transfected with expression vectors containing the HA wt and HAs sequences, metabolically labeled with 35S-methionine, and both solubilized cell pellets and media supernatants were immunoprecipitated with the H-18 mAb following either a 1 hr pulse or 3 hr chase period (Figure 1C). Following the pulse, both the HA wt and HAs peptides were immunoprecipitated from the cell pellet fractions, but not from supernatants. The HAs (but not HA wt) peptide was subsequently detected in the supernatant following the chase period, confirming that it was secreted.
Figure 1. The Pro-HA expression construct.
A: The Pro-HA transgene, with the structure of the secreted HA (HAs) protein shown above. A map of the HA wt protein shows the locations of the ER signal sequence (SS, amino acids 2–17), the I-Ed epitope (amino acids 110–120, located within the extracellular domain [ED]), the Kd epitope (amino acids 542–550, located within the trans-membrane domain [TM, amino acids 539–564]), and the cytoplasmic domain (CD). The structure of the HAs protein is shown by a thick black line designating the portion of the wild-type protein that has been retained.
B: Subcellular localization of HA wt and HAs proteins. FACS histogram plots of P815 cells infected with the indicated recombinant vaccinia (or not infected as a background staining control [C]) and stained for HA either directly (Surface) or after membrane fixation and permeabilization (Intracellular).
C: Immunoprecipitation of HA wt and HAs proteins. COS-7 cells were transiently transfected with expression plasmids encoding the HA wt or HAs constructs, or the empty expression plasmid (C). Forty-eight hours later, they were labeled with [35S]methionine for 1 hr (pulse) and then cultured an additional 3 hr with an excess of cold methionine (chase). Following both the pulse and chase periods, cell pellets (P) as well as media supernatants (S) were harvested for immunoprecipitation using the anti-HA mAb H-18. Samples were analyzed by SDS-PAGE, with the location of molecular weight standards shown on the left, and the location of the HA proteins marked by an arrow to the right of each autoradiogram. Both short (S) as well as long (L) time exposures of the chase autoradiogram are shown.
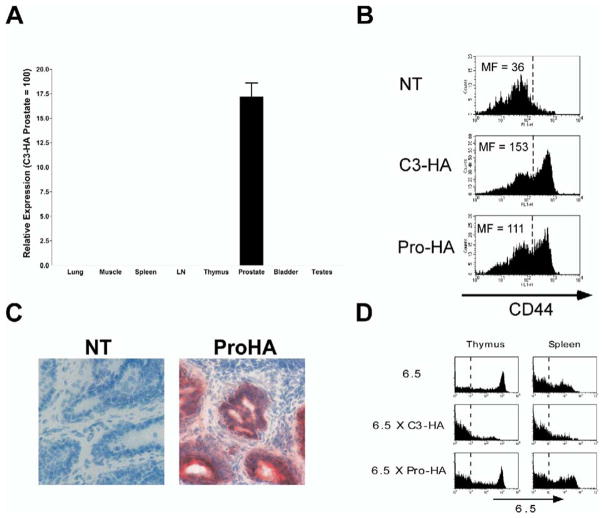
The Pro-HA expression cassette was microinjected into fertilized B10.D2 embryos, and the founder line 9972 was established and used in subsequent studies. Southern analysis indicated the integration of a single intact transgene copy (data not shown), and quantitative RT-PCR analysis demonstrated prostate-specific HA expression (Figure 2A). Two approaches were used to assess whether Pro-HA transgenic prostates express HA protein. First, Pro-HA prostate homogenates were injected into the footpads of nontransgenic (NT) recipients that had also received adoptive transfers of naive 6.5 clonotypic CD4 cells. If the homogenates contain HA protein, the I-Ed-restricted HA epitope should be presented by APCs in the draining popliteal LNs, and the clonotypic CD4 cells should lose their naive phenotype (Adler et al., 1998). Five days following injection of Pro-HA prostate homogenate, the recovered clonotypic CD4 cells exhibited increased expression levels of the activation marker CD44 relative to control recipients. The magnitude of CD44 expression induced by Pro-HA homogenates was roughly comparable to homogenates from C3-HA transgenic prostates (Figure 2B), which express HA in multiple tissues including the prostate (Adler et al., 1998). To further assess whether Pro-HA mice express sufficient levels of prostatic HA to be recognized by HA-specific T cells in situ, Pro-HA and NT (control) Thy1.2+ recipients received adoptive transfers of naive Thy1.1+ HA-specific clonotypic CD8 T cells and were simultaneously inoculated with a recombinant vaccinia virus expressing HA (vacc-HA) to prime the clonotypic CD8 cells. Two weeks later, frozen sections prepared from dorsal prostate lobes were stained with anti-Thy1.1 to mark the clonotypic CD8 cells (Figure 2C). In Pro-HA prostates, but not in NT prostates, the primed clonotypic CD8 cells infiltrated the prostatic ducts as evidenced by staining that colocalized with the epithelium (the presumed site of transgene expression [Greenberg et al., 1994, 1995]).
Figure 2. Pro-HA transgenic mice express prostate-restricted hemagglutinin.
A: Quantitative RT-PCR was performed on the indicated tissues of Pro-HA mice using HA-specific primers and probe. Relative expression is shown normalized to C3-HA prostate (100), which was used as a positive control for HA expression (Adler et al., 1998, 2000). Reactions were run in triplicate and repeated two times.
B: Pro-HA mice express prostatic HA protein. Prostate homogenates from the indicated mice were injected into the footpads of NT recipients that had received adoptive transfers of naive 6.5 clonotypic CD4 cells the previous day. Five days later, single-cell suspensions prepared from the draining popliteal LNs were analyzed by FACS. Histogram plots of CD44 expression on gated clonotypic CD4 cells are presented with the mean fluorescence (MF) values shown to the left of the dashed reference line. The data shown is representative of several replicates.
C: Infiltration of primed clonotypic CD8 cells into Pro-HA prostates. Pro-HA (right panel) and NT (control, left panel) mice expressing the Thy1.2 congenic marker received adoptive transfers of Thy1.1+ naive clone 4 clonotypic CD8 cells and were simultaneously inoculated with vacc-HA to prime the clonotypic CD8 cells. Two weeks later, frozen sections of dorsal prostate lobes were stained with an anti-Thy1.1 mAb (red stain) and counterstained with hematoxylin. The sections shown are representative of several mice.
D: Thymic deletion of clonotypic CD4 cells does not occur in Pro-HA transgenic mice. 6.5 TCR single transgenic, 6.5 × C3-HA and 6.5 × Pro-HA double transgenic mice were analyzed for clonotypic TCR (i.e., 6.5) expression on CD4 single positive thymocytes and mature splenic CD4 cells. A dotted reference line indicates background 6.5 staining (determined from a NT control). Histogram plots are representative of multiple mice.
Prostatic HA is ignored by cognate naive CD4 cells in Pro-HA mice
Some transgenes under the control of “tissue-specific” promoters can be expressed at low levels in the thymus (Jolicoeur et al., 1994). In fact, a number of “peripheral” proteins are expressed at low levels in thymus, suggesting that tolerance to some peripheral antigens may be generated thymically (Jolicoeur et al., 1994; Derbinski et al., 2001; Anderson et al., 2002). To determine whether the Pro-HA transgene induces thymic deletion of HA-specific T cells, we generated double transgenic mice that expressed both the 6.5 TCR and Pro-HA transgenes (Figure 2D). Single transgenic 6.5 mice express high levels of the clonotypic TCR on a large proportion of their CD4 single positive thymocytes, as well as on mature splenic CD4 cells. When the 6.5 mice were crossed with C3-HA transgenics that express HA in a number of tissues (including the thymus [Adler et al., 1998]), the frequency of 6.5+ CD4 cells in both the thymus and spleen were greatly reduced. In contrast, thymic deletion of clonotypic CD4 cells did not occur in 6.5 × Pro-HA double transgenics (consistent with the lack of thymic transgene mRNA expression [Figure 2A]). Given that the 6.5 clonotypic TCR has a relatively high affinity for the I-Ed-HA peptide complex and is sensitive to the presence of thymic HA (Jordan et al., 2001), this result suggests that a normal HA-specific T cell repertoire is exported from the thymus of Pro-HA transgenic mice. It is interesting that thymic deletion of HA-specific T cells does not occur in Pro-HA mice, given that the Probasin promoter driving SV40 T antigen expression in TRAMP transgenic mice is expressed thymically and results in thymic deletion of T antigen-specific T cells (Zheng et al., 2002). Since the endogenous murine Probasin gene is also expressed at low levels in the thymus (Zheng et al., 2002), the lack of deletion in Pro-HA mice might result from a fortuitous integration site of the Pro-HA transgene that limits thymic mRNA expression.
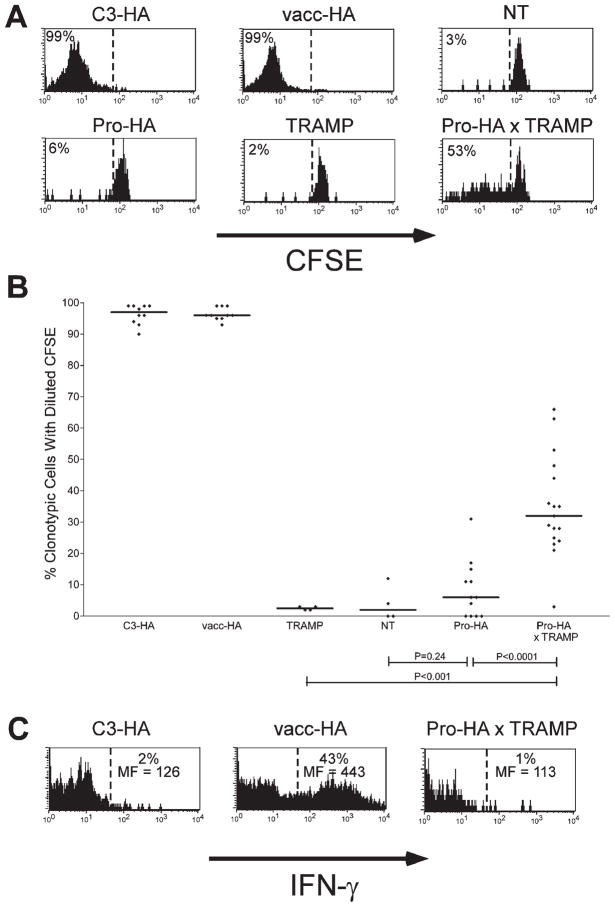
To assess whether mature naive CD4 cells recognize prostatic HA in the periphery of Pro-HA mice, we performed adoptive transfer experiments using CFSE-labeled HA-specific clonotypic CD4 T cells (Figures 3A and 3B). As positive controls for antigen recognition, we used C3-HA recipients in which clonotypic CD4 cells undergo a vigorous proliferative response prior to reaching a tolerant state, as well as NT recipients infected with vacc-HA in which the clonotypic CD4 cells proliferate and develop Th1 effector function (Adler et al., 2000; Higgins et al., 2002b). Six days post-transfer, clonotypic CD4 cells recovered from the prostate-draining periaortic LNs of Pro-HA recipients had not undergone significant division (i.e., CFSE dilution), nor had they divided in prostate-non-draining lymphoid organs such as the spleen and the axillary and mesenteric LNs (data not shown). Thus, the expression of prostatic HA (Figures 2B and 2C) neither induces thymic deletion (Figure 2D), nor peripheral recognition by mature CD4 cells (Figures 3A and 3B).
Figure 3. Prostate tumorigenesis alters presentation of prostate-epithelial antigen.
Age-matched (6-month-old) Thy1.2+ NT, C3-HA, Pro-HA, TRAMP, Pro-HA x TRAMP, and vacc-HA-infected NT (vacc-HA) recipients received adoptive transfers of Thy1.1+ CFSE-labeled naive clonotypic CD4 cells, which were recovered from the prostate-draining periaortic LNs 6 days later.
A: Representative proliferative responses (i.e., CFSE-dilutions) of clonotypic CD4 cells. The percentage of clonotypic CD4 cells (Thy1.1+ and 6.5+) exhibiting diluted CFSE fluorescence is shown to the left of the dashed reference line.
B: Scatter plot showing the effect of tumorigenesis on clonotypic CD4 cell proliferation. The percentage of CFSE-diluted clonotypic CD4 cells are shown for individual mice, with horizontal lines designating the median values. p values were calculated using an unpaired two-tailed t test.
C: Representative histogram plots of IFN-γ staining, with the percentage of cytokine-positive clonotypic CD4 cells, as well as the level of cytokine expression on these positive cells (expressed as mean fluorescence [MF]) shown above and below the reference bar, respectively.
Prostate tumorigenesis leads to increased tolerogenic recognition of prostatic HA in the prostate-draining LN
Prostatic HA might be ignored by cognate naive CD4 cells because under normal circumstances secreted HA is not directed toward the bloodstream or draining lymphatics (Whitmore and Gittes, 1977) and hence is unavailable to tolerogenic APCs (Adler et al., 1998; Higgins et al., 2002b). Thus, we analyzed HA-specific T cell responses in the setting of tumorigenesis, where altered prostatic architecture and/or metastases might allow HA to gain access to draining LNs. Pro-HA mice were crossed to TRAMP transgenic mice that develop spontaneous prostate tumors resulting from SV40 T antigen expression driven under the control of the minimal rat Probasin promoter (Greenberg et al., 1995). In these double transgenic mice, HA and T antigen should be coexpressed during the transformation process since they are both under the control of an identical promoter. In contrast to Pro-HA and TRAMP single transgenic recipients in which adoptively transferred CFSE-labeled naïve clonotypic CD4 cells remained undivided, a significant fraction of these cells underwent division in Pro-HA × TRAMP double transgenic recipients. Although the extent of proliferation was less in Pro-HA × TRAMP recipients than in C3-HA and vacc-HA-infected NT control recipient groups, it was significantly greater than in either Pro-HA or TRAMP single transgenic recipients (Figures 3A and 3B). This increased antigen recognition appeared to be tolerogenic; the clonotypic CD4 cells that did divide only underwent a limited number of divisions, and there was not an increased frequency of cells at later relative to earlier divisions, suggesting that the divided cells were prone to deletion.
To further assess the functional capacity of the divided clonotypic CD4 cells recovered from the prostate-draining LNs of Pro-HA × TRAMP double transgenic recipients, we performed intracellular IFN-γ staining following in vitro restimulation with HA peptide-pulsed APCs (Figure 3C). Consistent with our previous observations (Higgins et al., 2002a, 2002b), clonotypic CD4 cells primed in vacc-HA-infected NT recipients expressed high levels of IFN-γ following in vitro restimulation, while counterparts tolerized in C3-HA recipients did not. The divided clonotypic CD4 cells recovered from Pro-HA × TRAMP recipients exhibited negligible intracellular IFN-γ expression, consistent with a nonimmunogenic response. Furthermore, these cells did not express IL-4 or IL-10, arguing against a Th2 or regulatory response (data not shown).
Androgen ablation transiently increases immunological recognition of a prostate-restricted antigen
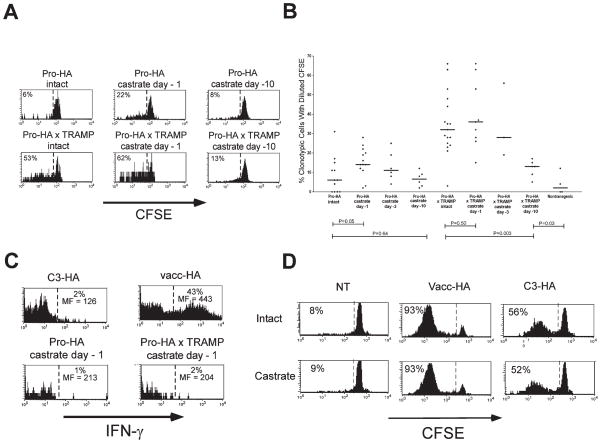
Castration (i.e., androgen ablation) induces apoptotic degeneration of the prostate epithelia (Sugimura et al., 1986; Furuya et al., 1995). Apoptotic cells can be cleared by phagocytic APCs, a process that has been hypothesized to be a mechanism by which tolerogenic APCs acquire parenchymal self-antigens (Steinman et al., 2000; Liu et al., 2002). To assess whether androgen-blockade-induced prostate epithelial degeneration influences the quantity or quality of antigen recognition in the draining LNs, single transgenic Pro-HA recipients were castrated prior to receiving CFSE-labeled naive clonotypic CD4 cells. Castration 1 day prior to adoptive transfer increased the amount of clonotypic CD4 cell proliferation relative to intact single transgenic Pro-HA recipients, albeit to a lesser degree than did tumorigenesis (Figures 4A and 4B), and these divided cells expressed negligible levels of IFN-γ following in vitro re-stimulation (Figure 4C). When adoptive transfers were performed 3 or 10 days following castration, the resulting CFSE-dilution profiles approached those of intact Pro-HA recipients, indicating that androgen ablation is only able to transiently increase antigen recognition (presumably due to either loss of HA-expressing prostate epithelia or to decreased androgen-mediated HA expression). Similarly, when Pro-HA × TRAMP double transgenic recipients were castrated 1 day prior to adoptive transfer, CD4 cell proliferation was slightly enhanced compared to intact counterparts; however, the CD4 cell proliferative response was markedly diminished when castration was performed 10 days prior to adoptive transfer (Figures 4A and 4B). Castration did not completely eliminate antigen recognition in ProHA × TRAMP mice—even 10 days after androgen ablation, significantly greater CD4 T cell proliferation was detected in ProHA × TRAMP animals than NT controls (Figure 4B). To assess whether the altered T cell response elicited by androgen ablation was due to the removal of a direct immunosuppressive effect of androgen on T cell function (Roden et al., 2004; Viselli et al., 1995), we adoptively transferred clonotypic CD4 cells into either intact or castrated NT and C3-HA mice, or intact or castrated NT mice infected with vacc-HA. As shown in Figure 4D, division of HA-specific T cells was nearly identical between castrated and intact animals for all three recipient groups, suggesting that the effects of androgen ablation on T cell recognition of the prostate gland (Figures 4A–4C) were mediated by changes in the quantity and/or quality of antigen recognition, rather than direct effects of androgens on T cell function.
Figure 4. Androgen ablation transiently increases presentation of prostate-epithelial antigen.
Pro-HA and Pro-HA × TRAMP mice castrated at the indicated time points received adoptive transfers of Thy1.1+ CFSE-labeled naive clonotypic CD4 cells, which were recovered from the prostate-draining periaortic LNs 6 days later.
A: Representative proliferative responses (i.e., CFSE dilutions) of clonotypic CD4 cells.
B: Scatter plot showing the effect of androgen ablation on clonotypic CD4 cell proliferation is presented as in Figure 3B.
C: Representative histogram plots of IFN-γ staining, with the percentage of cytokine-positive clonotypic CD4 cells, as well as the level of cytokine expression on these positive cells (expressed as mean fluorescence [MF]) shown above and below the reference bar, respectively.
D: Comparison of CFSE dilution in clonotypic CD4 cells recovered from intact and castrated NT, vacc-HA-infected NT (vacc-HA), and C3-HA recipients.
Androgen ablation mitigates systemic tolerance to a prostate/prostate tumor-restricted antigen
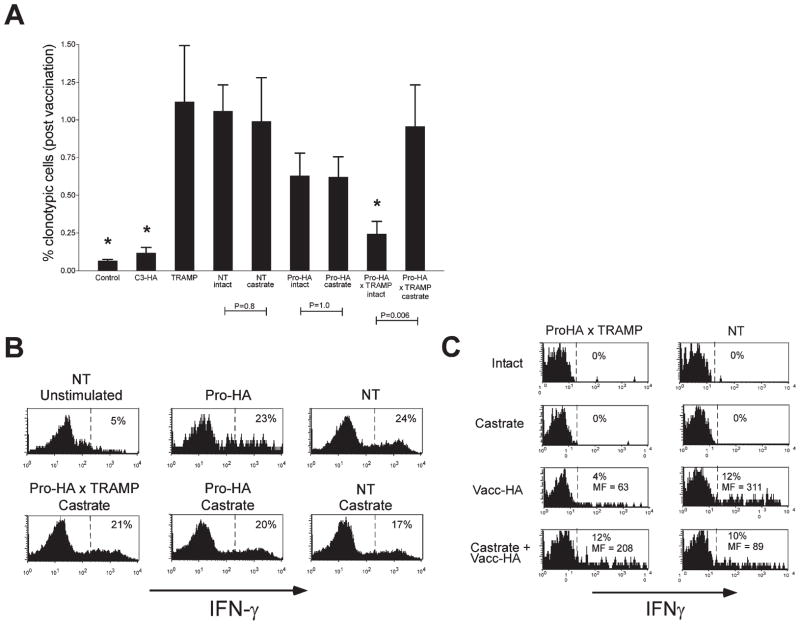
These results suggested that androgen ablation in the setting of prostate cancer eventually converts a tolerance-inducing environment to one that is neutral. We therefore queried whether this conversion would reverse the tolerization process at the systemic level—permitting HA-specific T cells to expand following vaccination. After allowing the adoptively transferred clonotypic CD4 cells to remain in the various recipient groups for a longer period (2 weeks), we assessed whether they could undergo clonal expansion in prostate-non-draining LNs following challenge with vacc-HA (Figure 5A). If NT recipients of 1 × 105 naive clonotypic CD4 cells are challenged with vacc-HA, the frequency of clonotypic CD4 cells expands to approximately 1% of the total lymphocyte population 6 days later (without vacc-HA challenge this frequency is less than 0.1% in NT mice, as well as in all other nonvaccinated control groups [data not shown]). As a positive control for systemic tolerization (i.e., impaired responsiveness to vaccination), naive clonotypic CD4 cells parked for 2 weeks in C3-HA recipients exhibited a greatly impaired ability to expand following vacc-HA challenge. In Pro-HA single transgenic recipients, vacc-HA induced clonotypic CD4 cell expansion to a level that was slightly less than NT controls (although not statistically different), consistent with the minimal level of HA recognition that was occasionally observed in Pro-HA single transgenic mice (Figure 3B). These data further confirm that prostatic HA is mostly ignored in the absence of tumorigenesis. In contrast, vacc-HA-induced clonotypic CD4 cell expansion was significantly reduced in Pro-HA × TRAMP double transgenics. Since clonotypic CD4 cell expansion was not impaired in TRAMP single transgenics, these data indicate that tumorigenesis promotes systemic tolerization to HA, rather than inducing an antigen-nonspecific immune suppression. Castration did not alter clonotypic CD4 cell expansion in NT or Pro-HA single transgenic recipients, but was able to markedly mitigate the tolerogenic effect of tumorigenesis (Figure 5A), most likely due to the diminution of tolerogenic antigen presentation over the 2 week period prior to immunization with vacc-HA (Figure 4B). Intracellular staining showed that clonotypic T cells from castrated Pro-HA × TRAMP mice that had expanded following vacc-HA challenge produced IFN-γ in response to in vitro restimulation at levels comparable to control recipients, demonstrating that castration truly mitigated tolerization (Figure 5B). In the above experiments, T cells were adoptively transferred 24 hr after androgen ablation. In clinical vaccination settings, prostate-specific T cells would most likely be in circulation prior to either chemical or surgical castration. To model this scenario, we adoptively transferred clonotypic CD4 cells to 12- to 14-week-old ProHA × TRAMP or NT mice 1 week prior to androgen ablation. Following an additional week, the systemic immune response to HA was assessed by challenge with vacc-HA as above. While the frequency of prostate-specific T cells was not dramatically altered by androgen ablation in these animals (data not shown), their ability to produce IFN-γ was restored to levels observed in NT control animals (Figure 5C). Interestingly, this mitigation of tolerance was not as pronounced in 18- to 22-week-old animals with more advanced disease (data not shown). Taken together, these data demonstrate that prostate tumorigenesis impairs the systemic T cell response to prostate-specific vaccination (i.e., promotes tolerance), while androgen ablation mitigates this effect.
Figure 5. Tumorigenesis induces systemic tolerization to prostate-epithelial antigens, while castration mitigates this effect.
A: Age-matched (6-month-old) NT (n = 10), NT castrated at day −1 (n = 6), C3-HA (n = 10), Pro-HA (n = 10), Pro-HA castrated at day −1 (n = 10), TRAMP (n = 9), Pro-HA × TRAMP (n = 12), and Pro-HA × TRAMP castrated at day −1 (n = 6) Thy1.2+ recipients received adoptive transfers of 1 × 105 unlabeled Thy1.1+ naive clonotypic CD4 cells, and 2 weeks later were challenged with 1 × 106 pfu of vacc-HA. Control NT mice received T cells, but not vacc-HA (n = 7). Six days later, the frequency of clonotypic CD4 cells (identified as CD4+ and Thy1.1+) was measured in the prostate-non-draining axillary LNs. Asterisks indicate groups that are significantly different (p < 0.05, unpaired two-tailed t test) from the NT control group.
B: Representative histogram plots of intracellular IFN-γ staining, with the percentage of cytokine-positive clonotypic CD4 cells shown.
C: 12- to 14-week-old NT or ProHA × TRAMP mice were adoptively transferred with 1 × 107 clonotypic CD4 cells 1 week prior to castration. After 1 additional week, animals were challenged with vacc-HA and harvested 5 days later. Representative histograms of clonotypic CD4 cell intracellular IFN-γ staining from irrelevant (systemic) LNs are shown.
Discussion
Peripheral T cell tolerance can be mediated by a variety of mechanisms. While active forms of tolerization such as deletion (Jones et al., 1990) or functional inactivation (i.e., anergy) (Mueller et al., 1989) are commonly observed, tolerance can also be manifested passively, i.e., naive T cells may simply ignore the presence of their cognate peripheral self-antigens (Ohashi et al., 1991; Oldstone et al., 1991). In some cases, ignorance may result from low levels of self-antigen expression (Kurts et al., 1998). In our Pro-HA system, ignorance most likely occurs because HA is sequestered from the draining lymphatics (Whitmore and Gittes, 1977) and, hence, tolerogenic APCs (Adler et al., 1998; Higgins et al., 2002a, 2002b). Thus, both prostate tumorigenesis and castration, which alter prostatic architecture, significantly increase the level of cognate T cell recognition of a defined HA epitope that is presented in the draining LNs. Tumorigenesis might also increase T cell recognition of HA by directly delivering HA to the draining LNs via metastatic HA-expressing tumor cells or by increasing the overall quantity of HA expression. Nonetheless, compromised sequestration likely plays a critical role, since the amount of HA recognition in the prostate-draining LNs did not directly correlate with either the size of the primary prostate tumors or with the presence of enlarged draining LNs that would be indicative of metastatic tumor (data not shown).
The functional consequence of the increased prostate-epithelial antigen recognition by cognate T cells associated with prostate tumorigenesis appears to be tolerance; cognate naive CD4 cells undergo an abortive proliferative response and fail to develop effector function (i.e., the ability to express Th1 or Th2 cytokines). Even though tolerogenic presentation appeared to be limited to the prostate-draining LNs, over time there was a decrease in the ability of cognate CD4 cells to undergo clonal expansion in response to vaccination, indicating that this presentation leads to systemic tolerization. This probably occurs after all of the cognate CD4 cells have circulated through the prostate-draining LN, as has been previously described when self-antigen expression is limited to the pancreas (Morgan et al., 1999). In another transgenic system, the development of insulinomas also enhances cross-presentation of a tumor-associated antigen, but interestingly, the outcome is not tolerance (Nguyen et al., 2002). Differences in the immunological properties of transplantable tumors have also been observed; in some cases these tumors are tolerogenic (Staveley-O’Carroll et al., 1998; Bogen, 1996; Shrikant et al., 1999), and in others immunogenic (Ochsenbein et al., 2001; Hanson et al., 2000; Spiotto et al., 2002). These data underscore the notion that different types of tumors are likely to have different immunological properties. The tolerogenic nature of prostate tumors might be conferred in part by their ability to produce the immunosuppressive cytokine TGF-β (Barrack, 1997).
The observation that prostate tumorigenesis promotes tolerization toward tumor-associated antigens has implications for designing immunotherapy strategies to treat prostate cancer. Given that tolerance does not appear to occur prior to tumorigenesis, vaccines targeting antigens that are also expressed on primary tumors might be effective when administered prophylactically or at early stages of disease progression. This would not necessarily be the case for other types of tumors where the targeted tumor-associated antigens might promote tolerance prior to tumorigenesis (Morgan et al., 1998; Hu et al., 1993). At later stages of prostate tumor progression, when tolerization becomes more apparent, vaccine efficacy would likely decline. Consistent with this possibility, the ability of vaccines to impede disease progression in TRAMP mice declines with age (Hurwitz et al., 2000), although factors other than tolerance (e.g., the establishment of an immunosuppressive tumor microenvironment [Radoja et al., 2001]) might also contribute to this phenomenon.
Since androgen blockade is a standard therapy for prostate cancer (Denmeade and Isaacs, 2002), understanding its effect on tolerance is important in designing therapeutic strategies that combine androgen blockade with vaccines. A recent study has shown that prostate cancer patients undergoing androgen ablation develop prostatic infiltration of T cells that appear to exhibit a Th1 effector phenotype, suggesting that androgen ablation induces T cell priming to prostatic antigens (Mercader et al., 2001). We find that in the absence of tumorigenesis, androgen ablation appears to induce prostate-specific naive CD4 cells to undergo tolerogenic differentiation. These CD4 cells exhibit a CFSE-dilution profile indicative of an abortive proliferative response and also fail to develop the potential to express effector cytokines. This result is consistent with studies showing that cell death under noninfectious conditions promotes tolerization (Hugues et al., 2002; Liu et al., 2002; Ferguson et al., 2002) as well as the observation that castration enhances prostatic expression of the immunosuppressive cytokine TGF-β (Kyprianou and Isaacs, 1989). Nonetheless, androgen blockade does not lead to systemic T cell tolerance, apparently because the synchronous apoptosis of HA-expressing prostate epithelia results in transient HA presentation in the draining LNs that is insufficient in duration for all of the cognate CD4 cells to encounter HA.
Androgen ablation-induced prostate epithelial cell and tumor cell apoptosis in Pro-HA × TRAMP double transgenic mice also appears to lead to a transient but insignificant increase in the level of HA recognition. Over time, HA recognition by cognate T cells decreases, but even 10 days after castration, significantly greater T cell recognition is observed in castrated Pro-HA × TRAMP mice than nontransgenic controls. Interestingly, systemic tolerance to ProHA × TRAMP tumors is significantly mitigated around this time point. These data suggest that attenuation of systemic tolerance is probably not mediated by the complete elimination of tolerogenic antigen, but by either a reduction to levels below a threshold required to maintain nonresponsiveness or possibly by other mechanisms affecting the context of antigen recognition (Ramsdell and Fowlkes, 1992; Pape et al., 1998).
Our data suggest that one approach to immunotherapy for prostate cancer might be an adoptive immunotherapy strategy (Yee et al., 1997) in which tumor-reactive effector T cells are administered after androgen ablation. In this case, the decreased antigen burden mediated by castration (Figure 4B) might prove particularly advantageous, since effector T cells are highly susceptible to antigen-mediated tolerization (Higgins et al., 2002a; Long et al., 2003; Mihalyo et al., 2004). For vaccine approaches, our data suggest that vaccination after androgen ablation may augment efficacy (Figure 5C). It is important to note that targeted vaccine approaches are dependent upon sufficient expression of the target antigen by tumor cells. While decreased target antigen expression secondary to androgen ablation is a major concern, it should be noted that most patients with prostate cancer who undergo androgen ablation eventually relapse with a rising PSA, a canonical androgen-regulated transcript (Gretzer and Partin, 2003; Prins, 2000). In TRAMP mice, a similar phenomena occurs: tumors that progress in castrated animals express the SV40 large T antigen, which is under the control of the androgen-regulated probasin promoter (Eng et al., 1999), and in Pro-HA × TRAMP mice, castration appears to reduce but not eliminate HA expression. Expression of these androgen-regulated genes in castrate patients and TRAMP mice is most likely due in part to alterations in either the expression or activity of the androgen receptor, allowing activation in the absence of normal androgen levels (Taplin and Balk, 2004; Chen et al., 2004; Edwards et al., 2003; Hakimi et al., 1996; Zhao et al., 2000). Thus, progressing tumors would most likely continue to express the targeted antigens, rendering them potentially susceptible to immune eradication. Preliminary data in Pro-HA × TRAMP mice support a sequential androgen-ablation → vaccination treatment strategy (data not shown). However, extensive analyses optimizing vaccine formulation and the timing of administration relative to androgen ablation will be necessary to maximize the clinical benefit of this combined-modality approach.
Experimental procedures
Mice
All mice were on the B10.D2 (H-2d) background. C3-HA transgenic mice (founder line 142) that express influenza HA under the control of the rat C3(1) promoter have been described (Adler et al., 1998, 2000). 6.5 (Kirberg et al., 1994) and clone 4 (Morgan et al., 1996) TCR transgenic mice express clonotypic TCRs that recognize either an I-Ed-restricted HA epitope (110SFERFEIFPKE120) or a Kd-restricted HA epitope (542IYSTVASSL550), respectively, and were both backcrossed to a Thy1.1 congenic B10.D2 background. TRAMP transgenic mice (Greenberg et al., 1995) on the C57BL/6J background were backcrossed to the B10.D2 background. Castration was performed as previously described (Eng et al., 1999).
The Pro-HA expression cassette was constructed by first PCR amplifying the minimal Probasin promoter fragment (−426 bp to +28 bp) (Rennie et al., 1993) from rat genomic DNA using the proofreading enzyme Pfu polymerase (Stratagene, La Jolla, CA) and the primers 5′-ATCCTGAGCTCAAG CTTCCAAAGTGCATTTAGC-3′ and 5′-GAGGAGGATGACCCTCATCG-3′. The purified product was digested with SacI and ligated into the SacI site of pGem3 (Promega, Madison, WI). A secreted form of the HA gene derived from the wild-type (wt) HA sequence of influenza virus A/PR/8/34 (Mount Sinai strain) (Townsend et al., 1984) was constructed by PCR-based mutagenesis using Pfu polymerase and the primers 5′-TTTCAAGATCTGCAGGGGAAAATAAAAACAACC-3′ and 5′-AGACCGGATCCTACAGTGAACTGG CGACAGTTG-3′, which introduce a termination codon following amino acid 550. The 1.6 kb modified HA fragment was ligated downstream of the Probasin promoter using StuI and BamHI sites. Finally, a 1 kb fragment containing the SV40 small T antigen 3′-untranslated region was PCR amplified from the plasmid 3xHRE-3 (Adler et al., 1991) using Pfu polymerase and the primers 5′-CGGGATCCATGTCGGCAGAATGC-3′ and 5′-GAAGATCTGACACTATAGAATACAAGC-3′, and ligated downstream of HA using BamHI and BglII sites. The entire 3.1 kb Pro-HA expression cassette was gel isolated following digestion with HindIII and was microinjected into fertilized B10.D2 embryos (performed at the Baylor College of Medicine transgenic core facility). Transgenic progeny were identified by PCR analysis of DNA extracted from tail biopsies using the primers 5′-GAATGGATAATAGTCAT CATG-3′ and 5′-GGTTTCCCAAGAGCCATC-3′.
Transient transfection and immunoprecipitation assay
COS-7 cells (1 × 105 plated in a 6-well plate 1 day earlier in DME media containing 10% FCS) were transiently transfected with 5 μg of mammalian expression plasmids (i.e., pcDNA3) containing the indicated HA sequence under the control of the viral CMV promoter using the DEAE-dextran method as previously described (Adler et al., 1993). Forty-eight hours post-transfection, the cells were given media containing DME without methionine, 10% dialysed FCS, and 100 μCi [35S]methionine. After 1 hr, one group of cells were harvested into 0.5 ml RIPA buffer (40 mM Tris [pH 8.0], 1% deoxycholate, 150 mM NaCl, 1% NP40, 2 mM EDTA, 0.1% SDS, 1 mM NaVO4, 120 μM PMSF, 5 ug/ml aprotinin, 1 μg/ml pepstatin, and 10 μg/ml leupeptin) (i.e., pulse), while the second group were washed, given media containing an excess of cold methionine, and incubated an additional 3 hr before harvesting into RIPA buffer (i.e., chase). Media samples were also collected at the end of both the pulse and chase periods and mixed 1:1 with RIPA buffer. Subsequently, the cell lystates were spun at top speed in a microcentrifuge at 4°C, and the supernatants precleared with 50 μl protein A sepharose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 10 min at 4°C. The cleared supernatants were then incubated with the anti-HA mAb H-18 for 2 hr at 4°C, immunoprecipitated with 50 μl protein A Sepharose, extracted with SDS-containing gel loading buffer, and run on SDS-PAGE gels containing 7.5% acrylamide. Dried gels were exposed to film for autoradiography.
Recombinant vaccinia viruses
A previously established protocol (Earl and Moss, 1993) was used to recombine the HAs sequence into the TK gene of wild-type vaccinia virus using the appropriate pSC11MCS1 vectors (in which the modified HA sequences have been placed under the control of the early/late p7.5 promoter) in HuTK− cells. Recombinant vaccinia expressing the wild-type HA sequence has previously been described (Staveley-O’Carroll et al., 1998). The recombinant vaccinia viruses were amplified in HuTK-cells, purified over 36% sucrose, and titered using HuTK− cells.
FACS staining of vaccinia-infected cells
P815 cells were infected in vitro with the indicated recombinant vaccinia viruses at a multiplicity of infection (MOI) of 5 plaque forming units (pfu) per cell. Sixteen hours later, the samples were split and either stained directly with the HA-specific mAb H-18 followed by a PE-conjugated goat anti-mouse Ig secondary stain (for surface HA staining) or fixed in PBS containing 4% formaldehyde for 5 min at 37°, followed be permeabilization in FACS buffer (PBS containing 2% calf serum and 0.1% azide) supplemented with 0.25% Saponin, and then staining with H-18 and PE-conjugated goat anti-mouse Ig (for intracellular HA staining).
Quantitative RT-PCR
RNA was extracted from the indicated tissues using the RNeasy kit (Qiagen, Chatsworth, CA) and DNase treated. cDNA was transcribed using the SuperScript II system (Life Technologies, Rockville, MD), and PCR reactions were run in triplicate wells in an ABI 7700 instrument (PE Applied Biosystems, Foster City, CA). In each reaction well, 18S rRNA-specific primers and VIC-labeled probe (Applied Biosystems) were run concurrently to normalize input cDNA. Relative HA expression was calculated using the delta-delta CT method (18), with RNA from C3-HA prostate serving as a standard (set at 100). The HA-specific primers were 5′-CGCCGGATGGCTCTTG-3′ (forward) and 5′-ACAATGTAGGACCATGATCTCACTG-3′ (reverse). The HA-specific probe was 5′-FAM-AAACCCAGAATGCGACCCACTGCTT-TAMRA-3′. No amplification was detected in the absence of template.
Adoptive transfers and flow cytometry
Adoptive transfers of Thy1.1+ naive clonotypic TCR transgenic T cells into male Thy1.2+ recipients and intracellular cytokine staining of CFSE-labeled Thy1.1+ clonotypic CD4 cells following in vitro restimulation with HA peptide were performed as previously described (Higgins et al., 2002a, 2002b; Long et al., 2003), except that restimulation was performed with 2 × 106 LN cells in 200 μl CTL media per well in v-bottom 96-well plates.
Prostatic HA expression bioassay
Whole prostates were homogenized in 500 μl PBS using an Omni TH hand-held homogenizer (Omni International, Warrenton, VA), and 100 μl of the homogenates (containing approximately 60 μg protein) were injected into the footpads of NT mice that had received adoptive transfers of naive 6.5 clonotypic CD4 cells 1 day earlier. Five days later, single-cell suspensions prepared from the draining popliteal LNs were analyzed by FACS to determine CD44 expression on the clonotypic CD4 cells as previously described (Adler et al., 1998, 2000; Higgins et al., 2002b).
Histological analysis of prostate-infiltrating clonotypic CD8 cells
Two weeks following adoptive transfer of Thy1.1+ clone 4 clonotypic CD8 cells and vacc-HA inoculation (1 × 107 pfu administered i.p.), dorsal prostate lobes were removed from the indicated Thy1.2+ recipients, embedded in Tissue-Tek OCT freezing compound (Sakura Kinetek, Torrance, CA), and frozen in liquid N2. Four micron sections were cut using a cryostat, placed onto poly-lysine-coated slides, air-dried for 1 hr, fixed in acetone for 5 min at 4°C, and air dried for 10 min at RT. All subsequent steps were performed at RT. Dried sections were soaked in PBS for 10 min, incubated with Peroxo-Block (Zymed, South San Francisco, CA) for 45 s, washed 3× with PBS, incubated sequentially with avidin and biotin block (Vector Laboratories, Burlingame, CA) for 15 min with an intervening wash (3× with PBS), blocked for 10 min in binding buffer (PBS + 5% FCS), incubated with biotin-conjugated anti-Thy1.1 (BD Pharmingen, San Diego, CA) (0.5 μg/ml in binding buffer) for 30 min to mark the primed clonotypic CD8 cells, developed with streptavidin-peroxidase followed by AEC (producing a red stain), and then counterstained with hemotoxylin prior to mounting (using the Histomouse-SP Kit as per the manufacturers instructions [Zymed]).
SIGNIFICANCE.
In 2004, approximately 30,000 men will die of metastatic prostate cancer. While androgen ablation has a high initial response rate, most patients who develop progressive disease eventually die of cancer. To assess prostate/prostate cancer-specific immune responses, we developed a transgenic mouse model that expresses a well-characterized antigen exclusively in the prostate gland and in spontaneously arising prostate tumors. Using this transgenic model, we show that immunotherapy for prostate cancer may be limited by the propensity for prostate-specific T cells to rapidly develop tolerance. Unexpectedly, we found that androgen ablation mitigated T cell tolerance at a systemic level, suggesting that immunotherapy for prostate cancer may be augmented when applied in conjunction with this common intervention.
Acknowledgments
We thank Dr. Linda Sherman for providing the clone 4 mice, as well as Drs. Norman Greenberg and Franco DeMayo for assistance in generating the Pro-HA mice. This work was supported by National Institutes of Health Grants CA109339 and AI49813 as well as Research Scholar Grant RSG-02-235-01-LIB from the American Cancer Society (to A.J.A.), a National Cancer Institute Prostate SPORE grant (CA-58236, to D.M.P and C.G.D.), and NCI grant CA096948 (to C.G.D.). C.G.D. is a Damon Runyon-Lilly Clinical Investigator and was the recipient of a Faculty Recruitment grant from the Maryland Cigarette Restitution Fund.
References
- Adler AJ, Scheller A, Hoffman Y, Robins DM. Multiple components of a complex androgen-dependent enhancer. Mol Endocrinol. 1991;5:1587–1596. doi: 10.1210/mend-5-11-1587. [DOI] [PubMed] [Google Scholar]
- Adler AJ, Scheller A, Robins DM. The stringency and magnitude of androgen-specific gene activation are combinatorial functions of receptor and nonreceptor binding site sequences. Mol Cell Biol. 1993;13:6326–6335. doi: 10.1128/mcb.13.10.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J Exp Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31:61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur J Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B. Generation of recombinant vaccinia viruses. Curr Prot Mol Biol. 1993;2:17–21. doi: 10.1002/0471142727.mb1617s43. [DOI] [PubMed] [Google Scholar]
- Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng MH, Charles LG, Ross BD, Chrisp CE, Pienta KJ, Greenberg NM, Hsu CX, Sanda MG. Early castration reduces prostatic carcinogenesis in transgenic mice. Urology. 1999;54:1112–1119. doi: 10.1016/s0090-4295(99)00297-6. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Lin XS, Walsh JC, Nelson WG, Isaacs JT. Androgen ablation-induced programmed death of prostatic glandular cells does not involve recruitment into a defective cell cycle or p53 induction. Endocrinology. 1995;136:1898–1906. doi: 10.1210/endo.136.5.7720636. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretzer MB, Partin AW. PSA markers in prostate cancer detection. Urol Clin North Am. 2003;30:677–686. doi: 10.1016/s0094-0143(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Hakimi JM, Rondinelli RH, Schoenberg MP, Barrack ER. Androgen-receptor gene structure and function in prostate cancer. World J Urol. 1996;14:329–337. doi: 10.1007/BF00184606. [DOI] [PubMed] [Google Scholar]
- Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cells are tolerized upon exposure to parenchymal self-antigen. J Immunol. 2002a;169:3622–3629. doi: 10.4049/jimmunol.169.7.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins AD, Mihalyo MA, McGary PW, Adler AJ. CD4 cell priming and tolerization are differentially programmed by APCs upon initial engagement. J Immunol. 2002b;168:5573–5581. doi: 10.4049/jimmunol.168.11.5573. [DOI] [PubMed] [Google Scholar]
- Hu J, Kindsvogel W, Busby S, Bailey MC, Shi YY, Greenberg PD. An evaluation of the potential to use tumor-associated antigens as targets for antitumor T cell therapy using transgenic mice expressing a retroviral tumor antigen in normal lymphoid tissues. J Exp Med. 1993;177:1681–1690. doi: 10.1084/jem.177.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science. 1990;250:1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989;3:1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. 1. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Higgins AD, Mihalyo MA, Adler AJ. Effector CD4 cell tolerization is mediated through functional inactivation and involves preferential impairment of TNF-alpha and IFN-gamma expression potentials. Cell Immunol. 2003;224:114–121. doi: 10.1016/j.cellimm.2003.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- Morgan DJ, Kreuwel HT, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Elford AR, Murakami K, Garza KM, Schoenberger SP, Odermatt B, Speiser DE, Ohashi PS. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J Exp Med. 2002;195:423–435. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- Prins GS. Molecular biology of the androgen receptor. Mayo Clin Proc. 2000;75:S32–S35. [PubMed] [Google Scholar]
- Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167:5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- Ramsdell F, Fowlkes BJ. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- Rennie PS, Bruchovsky N, Leco KJ, Sheppard PC, McQueen SA, Cheng H, Snoek R, Hamel A, Bock ME, MacDonald BS. Characterization of two cis-acting DNA elements involved in the androgen regulation of the probasin gene. Mol Endocrinol. 1993;7:23–36. doi: 10.1210/mend.7.1.8446105. [DOI] [PubMed] [Google Scholar]
- Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–342. [PMC free article] [PubMed] [Google Scholar]
- Whitmore WF, Gittes RF. Studies on the prostate and testis as immunologically privileged sites. Cancer Treat Rep. 1977;61:217–222. [PubMed] [Google Scholar]
- Yee C, Riddell SR, Greenberg PD. Prospects for adoptive T cell therapy. Curr Opin Immunol. 1997;9:702–708. doi: 10.1016/s0952-7915(97)80052-0. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- Zheng X, Gao JX, Zhang H, Geiger TL, Liu Y, Zheng P. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens over-expressed in solid tumors. J Immunol. 2002;169:4761–4769. doi: 10.4049/jimmunol.169.9.4761. [DOI] [PubMed] [Google Scholar]