Abstract
Stroke is a sexually dimorphic disease, with differences between males and females observed both clinically and in the laboratory. While males have a higher incidence of stroke throughout much of the lifespan, aged females have a higher burden of stroke. Sex differences in stroke result from a combination of factors, including elements intrinsic to the sex chromosomes as well as the effects of sex hormone exposure throughout the lifespan. Research investigating the sexual dimorphism of stroke is only in the beginning stages, but early findings suggest that different cell death pathways are activated in males and females after ischemic stroke. A greater understanding of the mechanisms underlying sex differences in stroke will lead to more appropriate treatment strategies for patients of both sexes.
Keywords: brain, cerebral ischemia, gender differences, hypoxia–ischemia, middle cerebral artery occlusion, sex differences, stroke
Stroke is the third leading cause of death and the major cause of long-term disability, striking almost 800,000 people in the USA each year [1]. While strokes are most profoundly linked with increasing age [2], stroke’s aftermath affects patients of all ages, ranging from neonates through to the elderly. The annual incidence of first-time stroke for patients of all ages is 158 per 100,000 [3]. Patients younger than 50 years of age are ten-times less likely to have a stroke, with an estimated annual incidence of approximately 10 per 100,000 in this age group [4]. In adults, 87% of strokes result from ischemia and 13% from hemorrhages [1]. In childhood, the distribution between stroke etiologies is more equal, with 50% of strokes in children resulting from hemorrhage and the other 50% from ischemia [5].
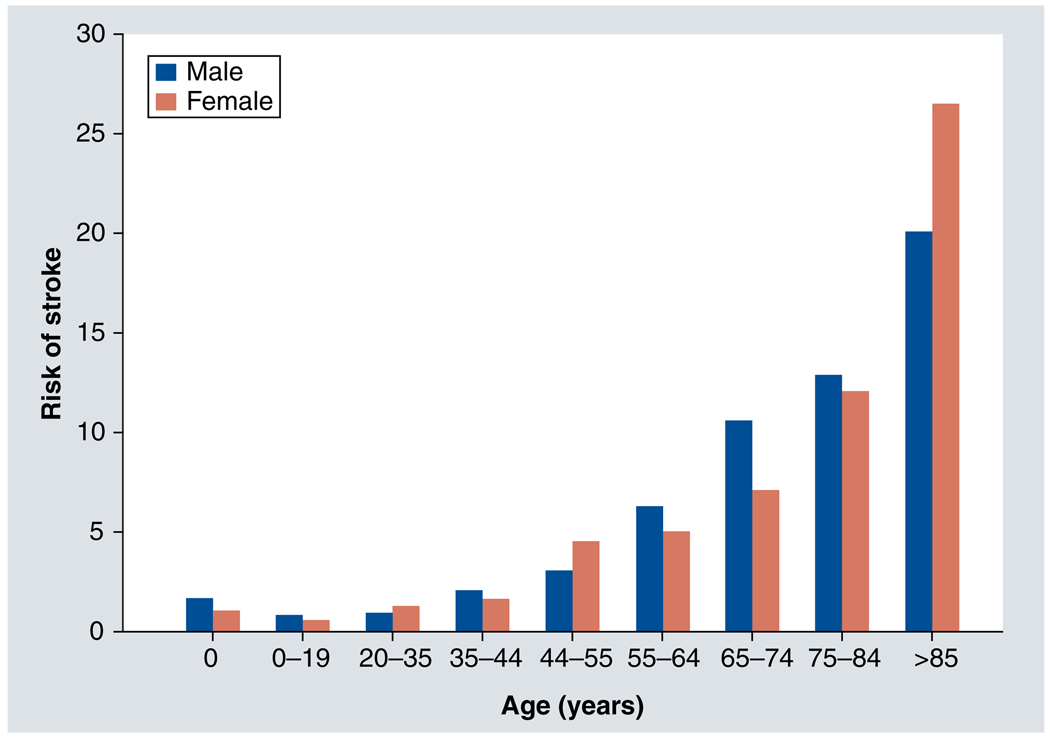
Review of epidemiological data indicates that stroke risk is affected by biological sex. However, whether an individual is at higher risk of stroke as a male or a female depends upon the individual’s age (Figure 1). The overall incidence rate of stroke in men is 33% higher than in women, with both ischemic and hemorrhagic strokes more common in men [6]. Men experience a higher risk of stroke over most of the lifespan, with several notable exceptions. There is a stroke surge in women between the ages of 19 and 30 years [4,7], which may be associated with pregnancy. A second increase in stroke risk occurs in women aged between 45 and 54 years [8], a time-frame during which menopause occurs. After the age of 50 years, the risk of stroke for both sexes rises dramatically with age, with a higher incidence in men compared with women until after 85 years of age [1]. Beyond the age of 85 years, women are more likely to experience stroke than men [1].
Figure 1. Approximate risk of stroke by age and sex.
Approximate risk is estimated from data compiled from available sources [1,4,8,25,58,134–136].
The etiology of strokes also differs in men and women, with cardioembolic strokes occurring more frequently in women, owing to higher rates of atrial fibrillation, whereas large and small vessel atherosclerosis is more common in men [9]. Despite the higher incidence of stroke in men, women have more severe strokes [6] with poorer recovery and greater long-term disability [10–12]. A more detailed discussion of the clinical evidence for sex differences in stroke can be found in several detailed reviews [13–15], including a recent systematic review [6].
Preclinical studies in animal models confirm that the effects of stroke vary based on sex and age. A long-term study of spontaneously hypertensive, genetically stroke-prone rats documented that males had greater ‘sensitivity’ to ischemia, and that cerebral hemorrhage developed later in females than in males [16]. Decreased ischemic damage for equal insults occurs in adult female versus male rodents in models of global [17] and focal [18] cerebral ischemia (for further reviews, see [19,20]). Interestingly, just as in humans, with increasing age (16 months; average lifespan in mice is 2–3 years), female mice become more susceptible to stroke damage than males, an effect opposite to that observed in younger mice (aged 2–3 months) [21].
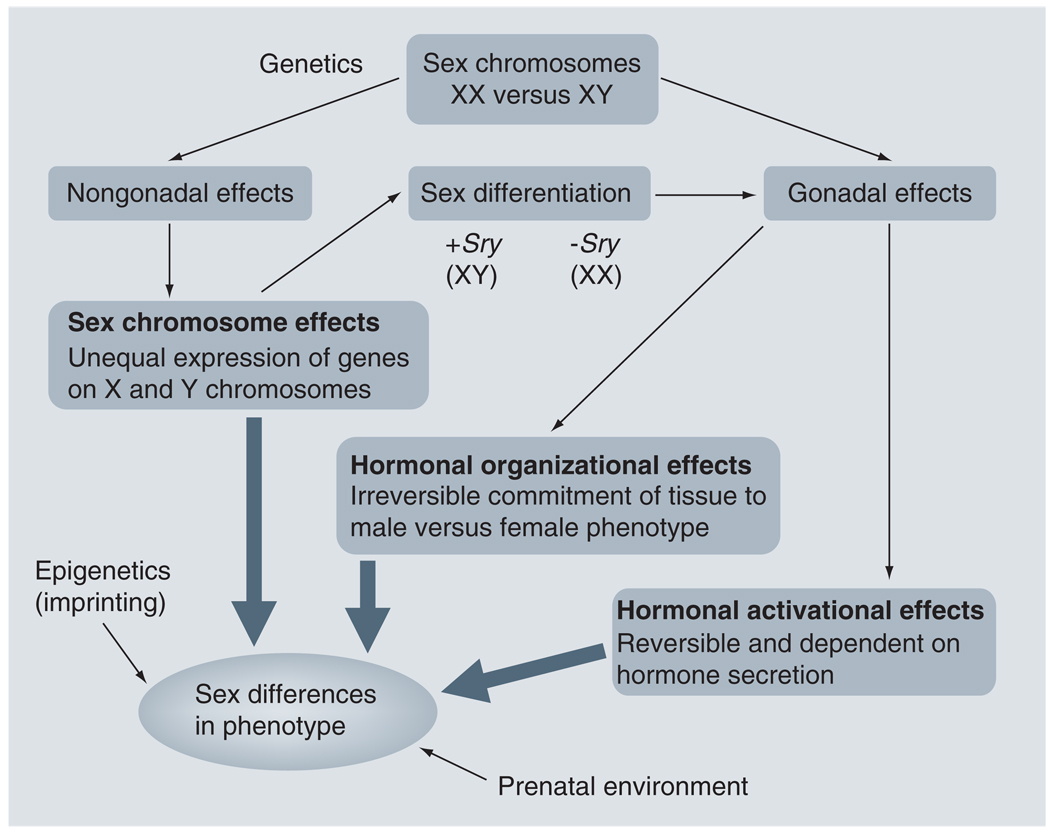
Why does stroke display such profound sexual dimorphism at different ages? The answer to that question is the subject of intense investigation in both the laboratory and the clinic. The leading candidates for influencing sex differences in stroke are intrinsic factors, such as the sex chromosomes themselves (XX for females and XY for males), which are invariant throughout an individual’s lifetime, and the effects of sex steroid hormones, such as estrogen, progesterone, and testosterone, the levels of which fluctuate at various life stages. Sex steroid hormones can act via organizational effects, which irreversibly commit tissues to a male or female phenotype, or activational effects, which are reversible effects that are dependent on the continued presence of the hormone [22–24]. In addition, recent evidence suggests that chromosomal sex may influence familial risk of stroke. Any combination of these factors may influence the final phenotype (Figure 2). This review discusses the evidence for roles of each of these elements in stroke.
Figure 2. Model of how various factors result in sex differences in stroke phenotype.
An individual’s genetic inheritance determines biologic sex. The Sry gene is normally inherited on the Y chromosome. If Sry is present, the individual differentiates into a phenotypic male (XY) and develops testes. If Sry is absent, the individual differentiates into a phenotypic female (XX) and develops ovaries. Sex steroid hormones released by the testes (i.e., testosterone) and ovaries (i.e., estrogen and progesterone) subsequently trigger both organizational effects (irreversible changes) and activational effects (reversible effects dependent on the continued presence of the hormone). The sex chromosomes themselves may also trigger nongonadal effects and differential gene expression, further affecting phenotype. Prenatal environment and epigenetics can also influence phenotype. The combination of these factors results in sex differences in phenotype. Model based on concepts developed by AP Arnold [23,24].
Intrinsic sex differences in stroke
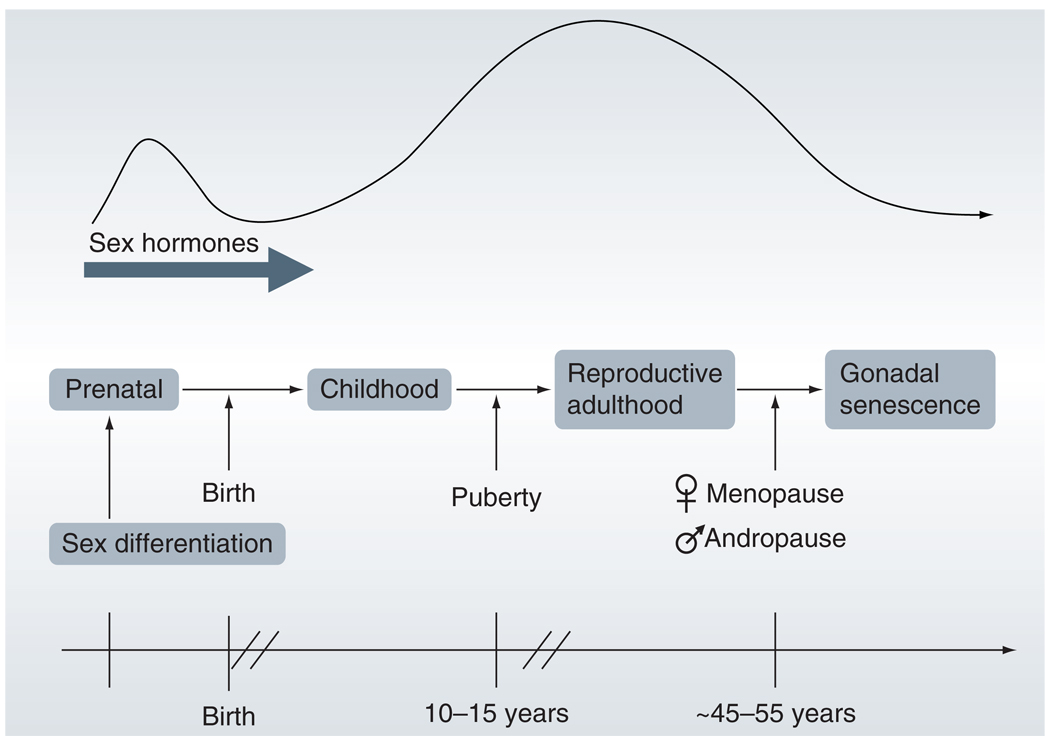
Clinical evidence that suggests intrinsic sex differences play a role in the response to stroke comes from the extreme ends of the lifespan – in the very young and in the elderly. In children and the elderly, the levels of circulating sex steroid hormones are minimal (Figure 3), and any observed differences in response to stroke in males and females could result from intrinsic sex effects. However, the elderly have already experienced a surge of sex steroid hormones during puberty, as well as elevated levels of circulating hormones for many years prior to reproductive senescence. Long exposure to higher levels of sex steroid hormones may have triggered permanent organizing effects on the neurovasculature that confound experimental design and data analysis. Therefore, the best clinical representation of the effects of intrinsic sex differences comes from the neonatal and preadolescent pediatric population, as pre-pubertal children have not yet had years of exposure to unequal levels of sex steroid hormones. However, even the human pediatric population is not an ideal model to display the effects of intrinsic sex differences alone, since differing prenatal or postnatal environmental exposure to endogenous and exogenous androgens and estrogens may result in additional sexual dimorphism.
Figure 3. Relative changes in sex steroid hormone levels over the lifespan.
Exposure to sex steroid hormone levels spikes during prenatal development and puberty. After puberty, sex steroid hormones remain elevated until late middle age.
Childhood stroke
In the perinatal, neonatal and childhood population, males are at higher risk of both hemorrhagic and ischemic stroke [5,25–29]. Approximately 60% of childhood ischemic stroke occurs in boys, both in neonates and in later childhood [26]. The majority of childhood hemorrhagic strokes also occur in males, with an odds ratio for perinatal hemorrhagic stroke of 2.4 (95% CI: 0.87–6.7) in boys [5]. This male predominance continues into adolescence, as demonstrated by the latest results of the International Pediatric Stroke Study [26], despite adolescence being a time during which sex steroid hormone expression spikes differentially between girls and boys (Figure 3).
Cerebral palsy, which usually results from an acquired injury, such as perinatal stroke, occurs more often in males than females [30], with 30% higher incidence in males [31]. A sex disparity in outcomes after preterm birth also exists, with female infants faring better than males [32–35]. Low birthweight male infants are more likely to die than females, and are also more likely to have intraventricular hemorrhage [36]. Considering that sex hormone levels are low in both females and males at this age, the discrepancy in outcomes may be influenced by sex-specific, hormone-independent factors. In both adult humans [37,38] and mice [39,40], a variety of genes are differentially expressed between males and females. This differential expression is also present in fetal mouse brain [41], but difficulties in obtaining normal human fetal tissue samples have prevented similar studies in humans [42].
A recent randomized, double-blinded, controlled trial published in the Journal of the American Medical Association suggests that sex differences in preterm infants may lead to variable neurodevelopmental responses to clinical interventions [43]. In this trial, infants born at less than 33 weeks’ gestation were fed either standard doses of docosahexaenoic acid (DHA; 0.3% fatty acids) or high DHA (1% fatty acids) diets from day 2 to 4 of life until their term-corrected age, and were then assessed for their mental development at 18 months’ corrected age. While male preterm infants that were fed the high DHA diet showed no change relative to controls, female preterm infants that had been fed the high DHA diet had higher neurodevelopmental scores than standard DHA diet female controls. These results strongly suggest that intrinsic sex differences exist in the immature brain and can influence the response to therapeutic interventions.
Modeling neonatal stroke: hypoxia–ischemia
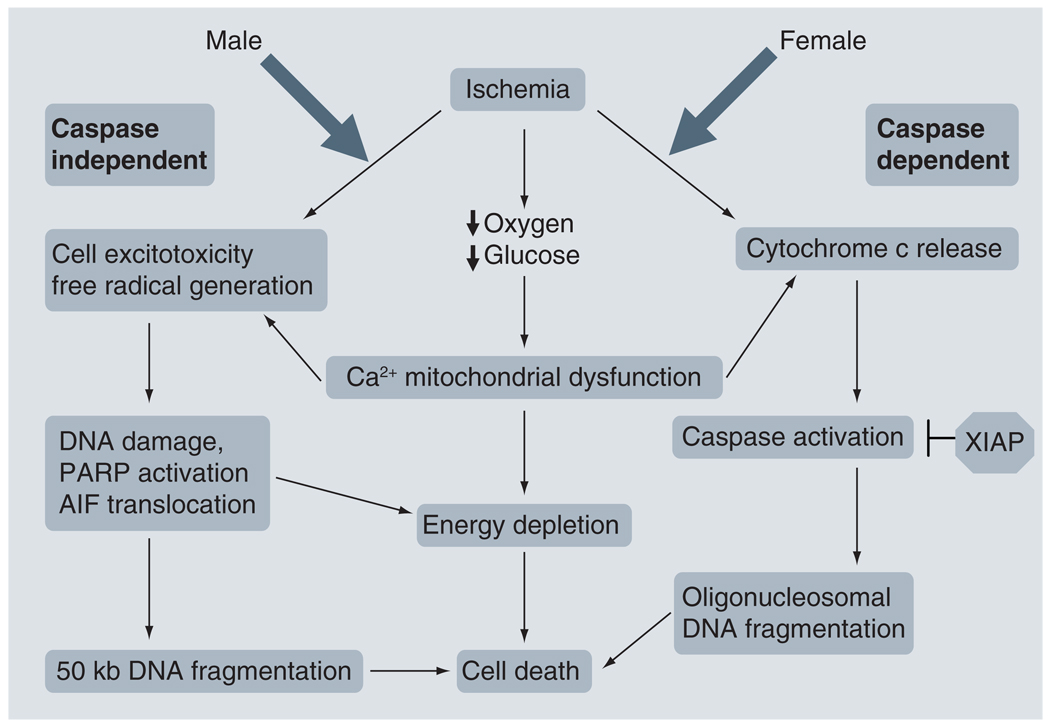
Preclinical evidence of sex-specific responses to pharmacological interventions in the neonatal population also exists, specifically in the neonatal hypoxic–ischemic (HI) model of neonatal stroke. To better appreciate the implications of these studies, a brief overview of the ischemic cell death pathways observed in male and female animals is presented here (Figure 4). In essence, current experimental evidence indicates that male ischemic cell death preferentially occurs through a caspase-independent pathway in which DNA damage, activation of poly(ADP-ribose) polymerase-1 (PARP-1) and apoptosis-inducing factor (AIF) translocation are crucial [44–46]. By contrast, in females, ischemic cell death is proposed to occur predominantly via a caspase-dependent pathway in which there is early release of cytochrome c from the mitochondria, caspase activation, and subsequent DNA damage and cell death [47,48]. The sex-specific pathways implicated in ischemic cell death are discussed in several excellent recent preclinical reviews [49,50]. Whether the same sex-specific cell death pathways play a role in hemorrhagic stroke is unknown at present, as hemorrhagic stroke has been significantly less well studied in animal models at any age.
Figure 4. Sex-specific ischemic cell death pathways.
Ischemic cell death in males occurs predominantly through a caspase-independent pathway involving PARP activation and AIF translocation. The female ischemic cell death pathway is caspase dependent, with early release of cytochrome c. XIAP can block caspase activation. Mitochondrial dysfunction and energy depletion occurs in both cell death pathways.
PARP: Poly(ADP-ribose) polymerase; AIF: Apoptosis-inducing factor; XIAP: X-linked inhibitor of apoptosis protein.
Investigations of candidate neuroprotective agents in the neonatal HI model indicate that these sex-specific ischemic cell death pathways can affect the response to pharmacological intervention. Administration of the broad-spectrum pan-caspase inhibitor, Q-VD-OPh, to postnatal day 7 (P7) rat pups after HI resulted in neuroprotection in female pups, but not in male pups [47], suggesting that a caspase-dependent cell death pathway is critical in the female response to ischemia. In contrast to original predictions that neuronal nitric oxide synthase (nNOS) inhibition would benefit males and worsen outcomes in females, 2-iminobiotin, a putative selective inhibitor of neuronal and inducible nNOS, induced neuroprotection in females, but not in males, after HI in P7 rat pups [51]. Surprisingly, 2-iminobiotin had a different mechanism of action than predicted. Nitric oxide formation was not inhibited by 2-iminobiotin in these studies; instead 2-iminobiotin inhibited the cytochrome c-caspase 3 pathway in females but not in males, resulting in female neuroprotection, consistent with the hypothesis that females are inherently more sensitive to caspase-mediated cell death. Hypothermia, induced after neonatal HI, is also more protective in female rat pups than in males, although the mechanism of this effect is still unclear [52]. By contrast, genetic knockout of PARP-1, a critical component of the caspase-independent cell death pathway, protected male neonates from HI-induced injury, but had no effects in female pups [44]. These preclinical studies are of great translational importance, as they provide evidence that sex-specific therapies may be critical in the treatment of stroke.
Sex-specific cell cultures
Additional confirmation that intrinsic sex differences exist between males and females is provided by in vitro experiments using cells typed as male (XY) versus female (XX). One critical advantage of studying cells in culture is that sex steroid hormones (and agents that may activate sex steroid responses, such as phenol red) can be eliminated from the cell culture medium. If sex-typed cells are used, any differences in behavior observed between male and female cells must result from inherent sex differences within the cells, from prenatal hormone exposure, or from a combination of the two.
The two most common methods of mimicking an ischemic insult in cell culture are either to subject cells to oxygen–glucose deprivation or to expose them to N-methyl-d-aspartic acid (NMDA). In cultures of hippocampal slices, slices from female P7 pups demonstrated intrinsic protection against oxygen–glucose deprivation or NMDA exposure, relative to those from male pups [53]. Similarly, primary rat hippocampal neuron male cultures were more sensitive to hypoxic insult than female cultures [54]. Sex differences in ischemic sensitivity have also been reported in cultured astrocytes [55] and splenocytes [56], demonstrating that sex dimorphism is present in multiple cell types. As in the neonatal HI studies, these effects appear to be mediated via a caspase-independent, AIF-dependent pathway in XY cells, and a cytochrome c, caspase-dependent pathway in XX cells [56].
These data from preclinical studies and from pediatric populations demonstrate the existence of sex differences in the response to stroke in the developing brain. As the neonatal and pre-adolescent populations have extremely low exposure to circulating sex steroid hormones, the observed sex differences are likely to be independent of hormonal activational effects. In this population, any sex differences will reflect: intrinsic sex differences associated with factors related to the sex chromosomes themselves; the organizational effects of prenatal sex hormone exposure; or, most likely, a combination of the two.
The four core genotype mouse model, in which chromosomal sex can be segregated from gonadal sex, will be a useful tool in future studies to better segregate the effects of intrinsic sex differences and prenatal hormonal organization effects [23,24]. In this model, the sex determining gene Sry, which is normally located on the Y chromosome, has been relocated to an autosome. Four possible genotypes of mice result in this model: XX gonadal females (XXF), XX gonadal males (XXM; resulting from inheritance of Sry on an autosome and subsequent ‘male’ levels of testosterone), XY gonadal females (XYF; resulting from the lack of inheritance of Sry and female levels of estrogen) and XY gonadal males (XYM). Comparison of mice with similar gonads (XXF vs XYF; XYM vs XXM) but different sex chromosomes facilitates determination of the effects of XX versus XY sex chromosomes in the absence of hormonal variation. Comparison of mice with different gonads but similar sex chromosomes (XXF vs XXM; XYF vs XYM) segregates hormonal effects from variation in sex chromosome complement. Investigation of experimental cerebral ischemia in the four core genotypes model will be invaluable in future studies of sex differences in stroke in our laboratory and others.
Sex hormone effects
During adulthood, the relationship between sex and stroke becomes even more complicated as variations in sex steroid hormone levels are now in play, in addition to any underlying intrinsic sex differences. While men have an overall higher incidence of stroke than women [6], the sex that is more at risk of stroke varies depending on age, as discussed previously (Figure 1).
During young adulthood, the risk of stroke for both men and women is much lower than at advanced age, but between the ages of 19 and 35 years, females have a greater risk of stroke [4,57]. The reasons for this are not completely understood. However, this age group encompasses the prime child-bearing years for women, and the peripartum and immediate postpartum periods encompass the highest risk period for stroke for a young woman [58–61]. The peripartum and postpartum period confers more than a 12-fold higher risk of ischemic and hemorrhagic stroke in women [14].
This age range also covers a time period during which many women are taking oral contraceptives (OCPs). An association between high-dose OCP use and ischemic stroke was first noted shortly after the introduction of OCPs in the 1960s [62], with a relative risk of ischemic stroke ranging from 1.2 to 3.1 in users of high-dose OCPs [63–65]. While the link between high-dose OCP use and ischemic stroke is strong across studies, the absolute risk of stroke is low (4.1 per 100,000) among nonsmoking, normotensive women on low-dose OCPs [66,67]. However, cigarette-smoking women taking OCPs are at even higher risk of ischemic stroke, with a relative risk ranging from 4 to 7, in comparison to nonsmokers not taking OCPs [63,65]. The increased risk of stroke during pregnancy and during OCP use in smokers suggests that hormonal factors play a role in the increased risk of stroke in young women between the ages of 19 and 35 years. For more information, a detailed clinical review of stroke during pregnancy and other situations unique to women was recently published [14].
Between the ages of 35 and 85 years, men have a higher incidence of stroke compared with women [1,4,6]. However, a recent study found a surge in stroke risk in women between the ages of 45 and 55 years, as well as increasing prevalence of obesity and metabolic syndrome [8]. This is the time period during which most women undergo natural menopause, suggesting that the withdrawal of estrogen and/or progesterone may have detrimental effects. Further support for the influence of ovarian hormones comes from a prospective study that investigated the relationship between the risk of stroke and the age of natural menopause. Women with natural menopause prior to age of 42 years had twice the risk of ischemic stroke compared with women who underwent natural menopause above the age of 42 years [68]. The lower risk of stroke in women until the age of 85 years has, in general, been ascribed to the neuroprotective effects of gonadal hormones. However, since women undergo menopause at an average age of 51 years, with subsequent low levels of ovarian hormones, hormones alone cannot account for the relative neuroprotection of women until advanced age.
Modeling adult ischemic stroke: middle cerebral artery occlusion
Estrogen
Animal models of ischemic stroke demonstrate that estrogen is a potent neuroprotective agent in both global and focal stroke (for reviews, see [20,69]). In the middle cerebral artery occlusion model of ischemic stroke, females are more protected against stroke relative to males [18,70,71]. This effect vanishes in ovariectomized females, but is rescued by estrogen supplementation in ovariectomized young females, suggesting that estrogen is critical in neuroprotection [18,70,71]. Chronic estrogen supplementation also reduces infarct volume in aging female and male mice [21]. Acute administration of estrogen after stroke reduces ischemic damage in both male and female rodents [72,73].
Evidence that intrinsic sex differences and sex steroid hormone effects interplay in the response to stroke exists in animal models. In corollary to the neonatal HI data, genetic deletion of either PARP-1 or nNOS protects adult male mice from focal ischemic damage [45]. Intriguingly, however, injury in adult female mice is exacerbated after stroke in PARP-1 or nNOS knockouts [45]. The tetracycline derivative, minocycline, is a PARP inhibitor that is neuroprotective in stroke in both open-label clinical trials [74] and animal models [75]. In the mouse middle cerebral artery occlusion model, minocycline’s neuroprotective effects only occur in males, with no benefit in ovariectomized females [76], again suggesting the presence of important and therapeutically relevant sex differences in stroke. Whether minocycline will have similar sex-specific effects in human stroke is currently unknown, as the clinical trials to date were not designed to investigate sex differences.
Ovariectomized female PARP-1 or nNOS-knockout mice are also no longer rescued by estrogen supplementation, suggesting that interference with the caspase-independent ischemic cell death pathway negates estrogen’s neuroprotective effects by mechanisms that are not yet understood [45]. This effect does not appear to be mediated via polymer of ADP-ribose formation or AIF translocation as these are equivalent in both sexes after stroke, yet this reduction in AIF levels is neuroprotective only in males (Figure 4) [46].
Progesterone
Progesterone is neuroprotective in a variety of animal models of neuronal injury (for review see [20,77,78]), including ischemia [79,80]. Similar to estrogen, acute progesterone administration reduces infarct volume in both male and female animals, in both young animals [81–85] and in reproductively senescent animals [86]. Chronic progesterone treatment in animal models of stroke has shown no benefit [87] or worsened stroke outcomes [88]. Clinical trials to date involving progesterone in stroke have involved chronic hormone therapy (HT), demonstrating either no benefit or greater risk of cardiovascular disease and stroke [89,90]. Early clinical trials suggest that acute administration of progesterone after traumatic brain injury may be beneficial [91], but no clinical studies have investigated whether acute progesterone therapy has similar effects after stroke. As with estrogen, the discrepancies between results in animal models and clinical trials suggests that timing and duration of progesterone exposure may be critical in whether it is beneficial or harmful after stroke. Progesterone’s effects upon the sex-specific pathways of ischemic cell death, modeled in Figure 4, are not yet known.
Testosterone
After stroke, low testosterone levels in men are associated with poorer clinical outcomes [92]. However, as acute brain injury triggers a stress response that lowers testosterone levels, these data are not simple to interpret [20]. Studies of the role of testosterone in stroke are only just beginning in experimental models of stroke. Young adult castrated male rats have decreased injury relative to intact males and testosterone-supplemented castrated rats [93]. Androgen supplementation in young castrated male rats prior to stroke results in increased damage [94–96] and also increases glutamate-associated neurotoxicity in vitro [95]. However, testosterone supplementation in middle-aged rats to levels observed in young intact males reduces damage after stroke [93]. Young castrated male rats administered acute androgen supplementation after stroke experience improved functional recovery [97]. The differential effects of androgen supplementation prior to stroke and after stroke in animal models suggests that, as with estrogen and progesterone, the timing and duration of androgen administration may be critical to whether it harms or helps after stroke. As with progesterone, investigation into testosterone’s role in modifying sex-specific cell death pathways is only just beginning. The conversion of testosterone into estrogen by aromatase (for review see [98]) adds an additional level of complexity regarding which effects are secondary to estrogen and which result directly from testosterone.
Clinical trials of hormonal therapy in stroke
Observational trials initially documented a reduction in cardiovascular disease in postmenopausal women on estrogen or combined estrogen–progestin HT [99,100]. However, subsequent major randomized clinical trials of chronic HT in primary and secondary stroke prevention have shown either no benefit or a greater risk of cardiovascular disease and stroke in postmenopausal women on HT [89,90,101,102]. A single case report of stroke in a previously healthy genetic male taking chronic estrogen and progesterone replacement as preparation for gender reassignment surgery suggests that initiation of chronic HT may be detrimental in both sexes [103].
One major criticism of HT trials in stroke prevention carried out to date has been the age at which HT has been initiated. The randomized, blinded, placebo-controlled Kronos Early Estrogen Prevention Study (KEEPS) is currently underway to address this criticism, by initiating HT close to the time of menopause, rather than years later [104]. Several recent reviews discuss the clinical literature on HT and stroke prevention [13,15].
To date, no clinical trials have focused on any acute effects of sex steroid hormones in stroke treatment, despite evidence from preclinical studies that acute estrogen therapy may be most neuroprotective for both sexes [69]. In the laboratory, acute estrogen therapy after experimental stroke reduces injury in both male and female animals [72,73]. The best translation of this preclinical data to clinical trials may be to test the effects of acute HT in stroke patients randomized to treatment or placebo within 6 h of stroke onset.
The higher risk of stroke in men is most marked between the ages of 55 and 85 years – a period beyond the time at which a woman with an intact uterus and ovaries will have undergone natural menopause. Considering this, and the failure of clinical trials of chronic HT, the simple presence or absence of ovarian hormones cannot be the sole answer. Otherwise, immediately after undergoing natural menopause, women would have a stroke risk equivalent to that of men.
One possible explanation for the sexual dimorphism of stroke involves the organizational effects of sex steroid hormones. The cyclical effects of estrogen and progesterone exposure over the course of a woman’s reproductive years may trigger long-term organizational changes that result in neuroprotection, the effects of which do not fully dissipate in women until long after menopause. Another possibility is that lowered testosterone levels in middle-aged males increases their stroke risk. A third possibility is that, as seen in the neonatal and pediatric populations, intrinsic sex differences based on sex chromosome complement (XX versus XY) may play a role in the sexual dimorphism of stroke throughout the lifespan. However, the mechanism behind sex differences in stroke most likely results from some combination of all of these factors.
Sex-specific drug effects in humans
Both sex steroid hormones and biological sex probably influence the response to certain clinical treatments. However, few clinical trials to date have addressed whether or not sex-specific responses to treatment exist. The different effects of aspirin in men and women in the primary prevention of cardiovascular disease and stroke provide a striking example of how influential these responses may be. The Physicians Health Study found that aspirin substantially reduced the risk of cardiovascular disease, but not stroke, in men [105]. By contrast, in the Women’s Health Study, aspirin reduced the risk of stroke by 24%, but had no effect on cardiovascular disease, in women [106]. A subsequent sex-specific meta-analysis confirmed the differential effects of aspirin in men and women with respect to stroke and coronary artery disease risk [107]. The mechanism underlying these differences in aspirin’s effects between men and women is currently under investigation [108].
Oral anticoagulation with warfarin also affects men and women differently, as demonstrated in a recent prospective study of 780 atrial fibrillation patients [109]. Although men and women were anticoagulated to equal degrees in this study, anticoagulated women had a relative risk of 2.0 for ischemic stroke versus anticoagulated men, even after age correction. Women with atrial fibrillation are also more likely than men to have thromboembolic complications when not taking warfarin [110]. These studies suggest that bridging therapy may be more critical in women than in men.
Biological sex also affects response to acute thrombolytic therapy after stroke (for a more extensive review see [13]). After stroke, women have worse functional outcomes compared with men [10,111,112], yet several studies have demonstrated an absence of sex differences in functional outcome after acute thrombolytic therapy [113–115]. This normalization of baseline differences in outcome suggests that women may experience a greater benefit from tissue plasminogen activator (tPA) treatment than men [115]. By contrast, women with metabolic syndrome are more likely to be resistant to intravenous tPA than men with metabolic syndrome, suggesting a possible mechanistic pathway by which the sex-specific effects of tPA may be mediated [116].
The different responses to stroke therapy in men and women in these studies demonstrate the importance of proper trial design. Stroke is a sexually dimorphic disease, and the potential influence of biological sex on the response to treatment must be considered. This is why it is crucial that any putative neuroprotective agents be evaluated properly, in studies that are designed to evaluate responses in both males and females. Based on the sex-specific pathways involved in ischemic cell death, agents that target the more male-specific parts of the pathway may be ineffective in females, and vice versa.
Sex differences in the heritability of stroke
In addition to influencing stroke risk, as discussed previously, biological sex may play a role in hereditary forms of stroke. While rare genetic mutations following Mendelian or mitochondrial inheritance patterns can cause stroke (i.e., the X-linked disorder Fabry’s disease or mitochondrial encephalopathy, lactic acidosis and stroke-like episodes syndrome [MELAS]) [117], the degree to which hereditary factors contribute to stroke risk is still under active investigation.
Several studies indicate that some degree of ischemic stroke is hereditary [118,119], although some of these genetic investigations into stroke are probably confounded by the inheritance of intermediate phenotypes, such as hypertension, which elevate stroke risk [119,120]. Many of the initial studies of the heritability of stroke were also confounded by the investigators studying ischemic and hemorrhagic strokes together, while the genetic influences on these subtypes of stroke are likely to be quite distinct [121]. A study that distinguished between types of stroke found that the incidence of family history of ischemic stroke in patients with ischemic stroke was 2.14 (95% CI: 1.21–3.74), and of family history of hemorrhagic stroke in patients with hemorrhagic stroke was 1.82 (95% CI: 1.21–2.75), with no association of family history between groups [121].
To ascertain whether the heritability of ischemic stroke might follow a more complex mode of inheritance, one group of investigators conducted a more detailed family history study of probands with ischemic stroke using data from the Oxford Vascular Study (OXVASC) [122]. In this study of 806 probands with ischemic stroke, women had a much higher degree of heritability of ischemic stroke than men (odds ratio [OR]: 1.4; 95% CI: 1.1–2.0), with the majority of affected relatives being female. A history of maternal stroke was common in affected females, but not in males with stroke, and no association was found between paternal stroke and probands of either sex. The authors found these results to be independent of traditional risk factors for stroke, and confirmed that similar results could be found on analysis of two databases independent of the OXVASC data.
A systematic review and meta-analysis subsequently evaluated both published and unpublished data from 18 studies that included details about family history in stroke patients [123]. In this analysis, women with stroke were more likely than men to have a family history of stroke, particularly a maternal history of stroke (OR: 1.47; 95% CI: 1.27–1.70 for women; OR: 1.02; 95% CI: 0.88–1.17 for men).
The mechanism that accounts for this excess maternal history of stroke in females, but not in males, is currently unclear. A similar excess maternal history has recently been noted in coronary artery disease [121]. Epigenetic factors, such as imprinting, may also play a role [123,124], perhaps in a manner dependent on offspring sex, as has been suggested for Huntington’s disease [125]. Environmental factors, or interactions between genes and environment, are also likely to be important.
Different paternal and maternal influences, including prenatal environment, are known to affect the risk of developing cardiovascular disease [126–128]. Similarly, maternal pre-eclampsia (hazard ratio [HR]: 1.9; 95% CI: 1.2–3.0) and gestational hypertension (HR: 1.4; 95% CI: 1.0–1.8) are each associated with an increased risk of stroke in offspring [129]. Both low birthweight [130–132] and low weight gain during the first 2 years of life are associated with an increased risk of stroke in adulthood [133].
Elucidating the degree to which genetics, environment and epigenetic factors affect stroke risk will entail further investigation. However, the studies performed to date show intriguing evidence that sex influences stroke risk in a manner dependent upon the sex of the offspring. The exact mechanisms by which this occurs are likely to be complex, but a greater understanding of them is crucial to our understanding of the interplay between sex and stroke.
Conclusion
Both biological sex and sex steroid hormones affect the risk of and response to stroke. Overall, males have a greater incidence of stroke, although elderly females are more affected by stroke. In genetic epidemiology studies, women with stroke are more likely to have a history of maternal stroke. While the mechanisms underlying the ways that sex and hormones interact in stroke are not yet understood, evidence suggests that there are sex-specific cell death pathways in the response to ischemia. Therapies aimed at prevention or acute treatment of stroke must consider the possibility of sex-specific effects in stroke. To date, no neuroprotective agents have been found to be useful in clinical trials, perhaps partly because many of the trials have not taken the possibility of sex-specific responses into account.
Future perspective
Over the next 10 years the importance of sex-specific responses in stroke will impact the design and implementation of clinical trials for stroke therapies. Further elucidation of the different pathways involved in the response to stroke in men and women will result in the development of more targeted treatments. Pharmaceutical agents targeted at specific aspects of the ischemic cell death pathways will be tested in trials designed to elucidate their effects in groups of males and females, rather than focusing on populations of convenience. Researchers will translate the results of preclinical experiments and clinical trials more appropriately so that better experiments and trials can be designed to investigate gaps in our understanding. In order to present the best model of stroke, which is predominantly a disease of the aged, animal studies will be performed in aged animals and in postmenopausal females. Investigators will incorporate both female and male animals into their preclinical trial design, rather than utilizing animals solely of one sex or another. Rather than assuming that one medicine fits all patients, the era of genomic medicine will begin.
The next 10 years will also lead to progress on answering the following questions:
Does hemorrhagic stroke also involve sex-specific pathways of cell death and, if so, is it by similar or different mechanisms to those in ischemia?
How do biological sex and sex steroid hormones interact in the response to stroke? Are there specific genes on the X and Y chromosome that influence stroke risk or outcome? The use of the four core genotype mice, in which biological sex can be separated from gonadal sex [23,24], may help address this at the preclinical level.
Is acute administration of sex steroid hormones after stroke as neuroprotective in humans as it is in animal models?
Executive summary
Introduction
The burden of stroke is different between males and females. While the overall incidence of both ischemic and hemorrhagic stroke is higher in men, women experience several periods of high risk for stroke, including pregnancy, perimenopause and beyond the age of 85 years.
Sex differences in stroke result from a combination of intrinsic factors (inherent to the sex chromosomes themselves) and the influence of sex steroid hormones via both organizational effects (permanent) and activational effects (reversible).
Prenatal environment and epigenetics (i.e., parental imprinting) can also influence the sex-specific response to stroke.
Intrinsic sex differences in stroke
Evidence for the existence of intrinsic sex differences comes from cell culture experiments, in which male (XY) and female (XX) cells, grown in sex steroid hormone-free environments, display different responses to ischemia.
Clinical evidence for the role of sex differences in stroke comes from studies of neonatal and preadolescent children, in whom a higher incidence of strokes is observed in boys compared with girls.
Sex hormone effects
Prior to the onset of menopause, women are at a lower risk of stroke compared with men.
Animal studies demonstrate that females are protected against stroke relative to males, unless all ovarian hormones have been removed by ovariectomy.
Acute administration of sex steroid hormones after stroke is neuroprotective in animal models.
In clinical trials, chronic administration of the sex steroid hormones estrogen and progesterone to postmenopausal women had no benefit and increased risk of stroke and heart disease.
No clinical trials to date have investigated the effects of acute sex steroid hormone administration in stroke.
Sex differences in the heritability of stroke
Women with stroke are more likely to have a history of stroke, particular of maternal stroke, compared with men.
Future perspective
Greater understanding of the mechanisms underlying sex differences in stroke will result in the implementation of sex-specific treatments for stroke.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants 5R01NS050505 and 5R01NS055215 to Louise D McCullough. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
L Christine Turtzo, Departments of Neurology and Neuroscience, University of Connecticut Health Center, 263 Farmington Avenue, MC 1840, Farmington, CT 06030, USA, Tel.: +1 860 679 8939, Fax: +1 860 679 1181, lturtzo@uchc.edu.
Louise D McCullough, Departments of Neurology and Neuroscience, University of Connecticut Health Center, 263 Farmington Avenue, MC 1840, Farmington, CT 06030, USA, Tel.: +1 860 679 2271, Fax: +1 860 679 1181, lmccullough@uchc.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Rojas JI, Zurru MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old – risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur. J. Neurol. 2007;14(8):895–899. doi: 10.1111/j.1468-1331.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37(10):2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 4.Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40(4):1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser Pediatric Stroke Study. Pediatrics. 2009;123(3):823–828. doi: 10.1542/peds.2008-0874. [DOI] [PubMed] [Google Scholar]
- 6. Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. ▪ Most recent systematic review to examine the literature for sex differences at the clinical level.
- 7.Rasura M, Spalloni A, Ferrari M, et al. A case series of young stroke in Rome. Eur. J. Neurol. 2006;13(2):146–152. doi: 10.1111/j.1468-1331.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 8.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69(20):1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- 9.Forster A, Gass A, Kern R, et al. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40(7):2428–2432. doi: 10.1161/STROKEAHA.109.548750. [DOI] [PubMed] [Google Scholar]
- 10.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24(3):123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 11.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 12.Fukada M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern. Med. 2009;48(12):967–973. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- 13.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc. Dis. 2008;26(5):462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushnell CD. Stroke and the female brain. Nat. Clin. Pract. Neurol. 2008;4(1):22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 15.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasagawa S, Yamori Y, Sato M, Horie R, Fukushima S. Proceedings: experimental studies on sex difference in stroke-prone SHR (SHRSP): hypoxic vulnerability and aortic brittleness. Jpn Heart J. 1976;17(3):399–400. doi: 10.1536/ihj.17.399. ▪ One of a landmark series of papers on stroke published in the mid 1970s in the spontaneously hypertensive rat.
- 17.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J. Cereb. Blood Flow Metab. 1991;11(2):292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 18. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165. doi: 10.1161/01.str.29.1.159. discussion 166. ▪ One of the first papers to investigate the role of sex differences in experimental stroke.
- 19.Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36(2):193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- 20.Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin. Reprod. Med. 2009;27(3):229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J. Cereb. Blood Flow Metab. 2009;29(4):792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 23.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J. Neuroendocrinol. 2009;21(4):377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju TN, Nelson KB, Ferriero D, Lynch JK. Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics. 2007;120(3):609–616. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- 26.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- 27.Golomb MR, Dick PT, MacGregor DL, Curtis R, Sofronas M, deVeber GA. Neonatal arterial ischemic stroke and cerebral sinovenous thrombosis are more commonly diagnosed in boys. J. Child Neurol. 2004;19(7):493–497. doi: 10.1177/08830738040190070301. [DOI] [PubMed] [Google Scholar]
- 28.Salih MA, Abdel-Gader AG, Al-Jarallah AA, et al. Stroke in Saudi children. Epidemiology, clinical features and risk factors. Saudi. Med. J. 2006;27 Suppl. 1:S12–S20. [PubMed] [Google Scholar]
- 29.Bonduel M, Sciuccati G, Hepner M, et al. Arterial ischemic stroke and cerebral venous thrombosis in children: a 12-year Argentinean registry. Acta Haematol. 2006;115(3–4):180–185. doi: 10.1159/000090932. [DOI] [PubMed] [Google Scholar]
- 30.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev. Med. Child Neurol. 2007;49(1):74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis S, Glinianaia SV, Arnaud C, et al. Case gender and severity in cerebral palsy varies with intrauterine growth. Arch. Dis. Child. 2005;90(5):474–479. doi: 10.1136/adc.2004.052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110 Suppl. 20:34–38. doi: 10.1016/s1470-0328(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 33.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD. The Nichd Neonatal Research Network: Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–1248. doi: 10.1080/08035250600599727. [DOI] [PubMed] [Google Scholar]
- 34.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn. Ther. 2004;19(4):366–369. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 35.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend. Med. 2007;4(1):19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 36.Tioseco JA, Aly H, Essers J, Patel K, El-Mohandes AA. Male sex and intraventricular hemorrhage. Pediatr. Crit. Care Med. 2006;7(1):40–44. doi: 10.1097/01.pcc.0000192341.67078.61. [DOI] [PubMed] [Google Scholar]
- 37.Nishida Y, Yoshioka M, St-Amand J. Sexually dimorphic gene expression in the hypothalamus, pituitary gland, and cortex. Genomics. 2005;85(6):679–687. doi: 10.1016/j.ygeno.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Vawter MP, Evans S, Choudary P, et al. Gender-specific gene expression in postmortem human brain: localization to sex chromosomes. Neuropsychopharmacology. 2004;29(2):373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Grisham W, Arnold AP. X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur. J. Neurosci. 2009;29(4):768–776. doi: 10.1111/j.1460-9568.2009.06610.x. [DOI] [PubMed] [Google Scholar]
- 41.Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003;118(1–2):82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- 42.Davies W, Wilkinson LS. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126(1):36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 43. Makrides M, Gibson RA, McPhee AJ, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301(2):175–182. doi: 10.1001/jama.2008.945. ▪ First paper to demonstrate a sex difference in neurological outcomes among infants after dietary supplementation.
- 44.Hagberg H, Wilson MA, Matsushita H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 2004;90(5):1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 45.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005;25(4):502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 46.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp. Neurol. 2009;217(1):210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Renolleau S, Fau S, Goyenvalle C, et al. Specific caspase inhibitor Q-VD-OPH prevents neonatal stroke in P7 rat: a role for gender. J. Neurochem. 2007;100(4):1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. ▪ Demonstrates differential effects of caspase inhibition in females versus males after neonatal hypoxic–ischemic injury.
- 48.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J. Transl. Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14(1):46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- 51.Nijboer CH, Groenendaal F, Kavelaars A, Hagberg HH, van Bel F, Heijnen CJ. Gender-specific neuroprotection by 2-iminobiotin after hypoxia–ischemia in the neonatal rat via a nitric oxide independent pathway. J. Cereb. Blood Flow Metab. 2007;27(2):282–292. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- 52.Bona E, Hagberg H, Loberg EM, Bagenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia–ischemia: short- and long-term outcome. Pediatr. Res. 1998;43(6):738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann. Neurol. 2005;58(2):317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- 54.Heyer A, Hasselblatt M, von Ahsen N, Hafner H, Siren AL, Ehrenreich H. In vitro gender differences in neuronal survival on hypoxia and in 17β-estradiol-mediated neuroprotection. J. Cereb. Blood Flow Metab. 2005;25(4):427–430. doi: 10.1038/sj.jcbfm.9600056. [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of p450 aromatase in sex-specific astrocytic cell death. J. Cereb. Blood Flow Metab. 2007;27(1):135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 56.Du L, Bayir H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 2004;279(37):38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 57.Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol. Clin. 1992;10(1):113–124. [PubMed] [Google Scholar]
- 58.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N. Engl. J. Med. 1996;335(11):768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salonen Ros H, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology. 2001;12(4):456–460. doi: 10.1097/00001648-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 60.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet. Gynecol. 2005;106(3):509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 61.Bashiri A, Lazer T, Burstein E, et al. Maternal and neonatal outcome following cerebrovascular accidents during pregnancy. J. Matern. Fetal Neonatal Med. 2007;20(3):241–247. doi: 10.1080/14767050601135030. [DOI] [PubMed] [Google Scholar]
- 62.Vessey MP, Doll R. Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report. Br. Med. J. 1969;2(5658):651–657. doi: 10.1136/bmj.2.5658.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Investigators W WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case–control study. Lancet. 1996;348(9026):498–505. [PubMed] [Google Scholar]
- 64.Kemmeren JM, Tanis BC, van den Bosch MA, et al. Risk of arterial thrombosis in relation to oral contraceptives (ratio) study: oral contraceptives and the risk of ischemic stroke. Stroke. 2002;33(5):1202–1208. doi: 10.1161/01.str.0000015345.61324.3f. [DOI] [PubMed] [Google Scholar]
- 65.Tanis BC, Rosendaal FR. Venous and arterial thrombosis during oral contraceptive use: risks and risk factors. Semin. Vasc. Med. 2003;3(1):69–84. doi: 10.1055/s-2003-38334. [DOI] [PubMed] [Google Scholar]
- 66.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284(1):72–78. doi: 10.1001/jama.284.1.72. [DOI] [PubMed] [Google Scholar]
- 67.Bushnell CD. Oestrogen and stroke in women: assessment of risk. Lancet Neurol. 2005;4(11):743–751. doi: 10.1016/S1474-4422(05)70220-9. [DOI] [PubMed] [Google Scholar]
- 68.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: The Framingham Heart Study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol. Metab. 2003;14(5):228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 70.Simpkins JW, Rajakumar G, Zhang YQ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J. Neurosurg. 1997;87(5):724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 71.Dubal DB, Kashon ML, Pettigrew LC, et al. Estradiol protects against ischemic injury. J. Cereb. Blood Flow Metab. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29(8):1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 73.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32(3):796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 74.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69(14):1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 75.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl Acad. Sci. USA. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J. Cereb. Blood Flow Metab. 2009;29(4):670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties? Ann. Emerg. Med. 2008;51(2):164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 2007;116(1):77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735(1):101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 80.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138(2):246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 81.Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J. Cereb. Blood Flow Metab. 2004;24(7):805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 82.Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp. Neurol. 2005;193(2):522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J. Cereb. Blood Flow Metab. 2002;22(10):1181–1188. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- 84.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann. Emerg. Med. 2006;47(4):381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 87.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J. Cereb. Blood Flow Metab. 2004;24(10):1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 88.Murphy SJ, Traystman RJ, Hurn PD, Duckles SP. Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke. 2000;31(5):1173–1178. doi: 10.1161/01.str.31.5.1173. [DOI] [PubMed] [Google Scholar]
- 89.Hulley S, Grady D, Bush T, et al. Heart and Estrogen/Progestin Replacement Study (HERS) research group. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 90.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 91.Jasavala R, Martinez H, Thumar J, et al. Identification of putative androgen receptor interaction protein modules: cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Mol. Cell Proteomics. 2007;6(2):252–271. doi: 10.1074/mcp.M600169-MCP200. [DOI] [PubMed] [Google Scholar]
- 92.Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler. Thromb. Vasc. Biol. 1996;16(6):749–754. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- 93.Cheng J, Hu W, Toung TJ, et al. Age-dependent effects of testosterone in experimental stroke. J. Cereb. Blood Flow Metab. 2009;29(3):486–494. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796(1–2):296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 95.Yang SH, Perez E, Cutright J, et al. Testosterone increases neurotoxicity of glutamate in vitro and ischemia–reperfusion injury in an animal model. J. Appl. Physiol. 2002;92(1):195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 96.Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J. Cereb. Blood Flow Metab. 2007;27(9):1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043(1–2):195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 98.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin. Reprod. Med. 2009;27(3):207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paganini-Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas. 2001;38(3):243–261. doi: 10.1016/s0378-5122(01)00167-0. [DOI] [PubMed] [Google Scholar]
- 100.Langer RD. Hormone replacement and the prevention of cardiovascular disease. Am. J. Cardiol. 2002;89(12A):E36–E46. doi: 10.1016/s0002-9149(02)02411-6. discussion E46. [DOI] [PubMed] [Google Scholar]
- 101.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 102.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW The Stroke Prevention in Atrial Fibrillation (SPAF) investigators. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAFF I–III clinical trials. Stroke. 1999;30(6):1223–1229. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 103.Egan RA, Kuyl JM. Ischemic stroke in a man using estrogen. J. Stroke Cerebrovasc. Dis. 2002;11(2):117–118. doi: 10.1053/jscd.2002.126693. [DOI] [PubMed] [Google Scholar]
- 104.Harman SM, Brinton EA, Cedars M, et al. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8(1):3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 105.Group SCotPHSR. Final report on the aspirin component of the ongoing physicians’ health study. Steering Committee of the Physicians’ Health Study Research Group. N. Engl. J. Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 106. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. ▪ First study to demonstrate that women can respond differently to medication compared with men with respect to cardiovascular disease and stroke prevention.
- 107.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 108.Zuern CS, Lindemann S, Gawaz M. Platelet function and response to aspirin: gender-specific features and implications for female thrombotic risk and management. Semin. Thromb. Hemost. 2009;35(3):295–306. doi: 10.1055/s-0029-1222608. [DOI] [PubMed] [Google Scholar]
- 109.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb. Haemost. 2009;101(5):938–942. [PubMed] [Google Scholar]
- 110.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (atria) study. Circulation. 2005;112(12):1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke. 2005;36(1):62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- 112.Kapral MK, Fang J, Hill MD, et al. Sex differences in stroke care and outcomes: results from the registry of the Canadian stroke network. Stroke. 2005;36(4):809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 113.Shah SH, Liebeskind DS, Saver JL, et al. Influence of gender on outcomes after intra-arterial thrombolysis for acute ischemic stroke. Neurology. 2006;66(11):1745–1746. doi: 10.1212/01.wnl.0000218208.31305.84. [DOI] [PubMed] [Google Scholar]
- 114.Hill MD, Kent DM, Hinchey J, et al. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke. 2006;37(9):2322–2325. doi: 10.1161/01.STR.0000237060.21472.47. [DOI] [PubMed] [Google Scholar]
- 115.Kent DM, Buchan AM, Hill MD. The gender effect in stroke thrombolysis. Of CASES, controls, and treatment-effect modification. Neurology. 2008;71(14):1080–1083. doi: 10.1212/01.wnl.0000316191.84334.bd. [DOI] [PubMed] [Google Scholar]
- 116.Arenillas JF, Sandoval P, Perez de la Ossa N, et al. The metabolic syndrome is associated with a higher resistance to intravenous thrombolysis for acute ischemic stroke in women than in men. Stroke. 2009;40(2):344–349. doi: 10.1161/STROKEAHA.108.531079. [DOI] [PubMed] [Google Scholar]
- 117.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123(Pt 9):1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 118.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35(1):212–227. doi: 10.1161/01.STR.0000107187.84390.AA. [DOI] [PubMed] [Google Scholar]
- 119.Flossmann E, Rothwell PM. Family history of stroke in patients with transient ischemic attack in relation to hypertension and other intermediate phenotypes. Stroke. 2005;36(4):830–835. doi: 10.1161/01.STR.0000158920.67013.53. [DOI] [PubMed] [Google Scholar]
- 120.Flossmann E, Schulz UG, Rothwell PM. Potential confounding by intermediate phenotypes in studies of the genetics of ischaemic stroke. Cerebrovasc. Dis. 2005;19(1):1–10. doi: 10.1159/000081905. [DOI] [PubMed] [Google Scholar]
- 121.Sundquist K, Li X, Hemminki K. Familial risk of ischemic and hemorrhagic stroke: a large-scale study of the Swedish population. Stroke. 2006;37(7):1668–1673. doi: 10.1161/01.STR.0000227409.59195.d1. [DOI] [PubMed] [Google Scholar]
- 122.Touze E, Rothwell PM. Heritability of ischaemic stroke in women compared with men: a genetic epidemiological study. Lancet Neurol. 2007;6(2):125–133. doi: 10.1016/S1474-4422(06)70683-4. [DOI] [PubMed] [Google Scholar]
- 123.Touze E, Rothwell PM. Sex differences in heritability of ischemic stroke: a systematic review and meta-analysis. Stroke. 2008;39(1):16–23. doi: 10.1161/STROKEAHA.107.484618. [DOI] [PubMed] [Google Scholar]
- 124.Jiang YH, Bressler J, Beaudet AL. Epigenetics and human disease. Annu. Rev. Genomics Hum. Genet. 2004;5:479–510. doi: 10.1146/annurev.genom.5.061903.180014. [DOI] [PubMed] [Google Scholar]
- 125.Kovtun IV, Therneau TM, McMurray CT. Gender of the embryo contributes to CAG instability in transgenic mice containing a Huntington’s disease gene. Hum. Mol. Genet. 2000;9(18):2767–2775. doi: 10.1093/hmg/9.18.2767. [DOI] [PubMed] [Google Scholar]
- 126.Barker DJ, Lackland DT. Prenatal influences on stroke mortality in England and Wales. Stroke. 2003;34(7):1598–1602. doi: 10.1161/01.STR.0000077257.27430.7E. [DOI] [PubMed] [Google Scholar]
- 127.Barker DJ. The developmental origins of adult disease. Eur. J. Epidemiol. 2003;18(8):733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- 128.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(1):R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 129.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki Birth Cohort study. Stroke. 2009;40(4):1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 130.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association stroke council. Stroke. 2006;37(6):1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 131.Lawlor DA, Leon DA. Association of body mass index and obesity measured in early childhood with risk of coronary heart disease and stroke in middle age: findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;111(15):1891–1896. doi: 10.1161/01.CIR.0000161798.45728.4D. [DOI] [PubMed] [Google Scholar]
- 132.Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen children of the 1950s prospective cohort study. Circulation. 2005;112(10):1414–1418. doi: 10.1161/CIRCULATIONAHA.104.528356. [DOI] [PubMed] [Google Scholar]
- 133.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki Birth Cohort study. Stroke. 2007;38(2):264–270. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- 134.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J. Clin. Epidemiol. 1995;48(11):1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 135.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke Workshop on Perinatal and Childhood Stroke. Pediatrics. 2002;109(1):116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 136.Bernard TJ, Goldenberg NA. Pediatric arterial ischemic stroke. Pediatr. Clin. North Am. 2008;55(2):323–338. doi: 10.1016/j.pcl.2008.01.002. [DOI] [PubMed] [Google Scholar]