Abstract
The PCR primers commonly used to detect Plasmodium knowlesi infections in humans were found to cross-react stochastically with P. vivax genomic DNA. A nested primer set that targets one of the P. knowlesi small-subunit rRNA genes was validated for specificity and for sensitivity of detection of <10 parasite genomes.
In 2004, it was reported that infections (in ca. 60% of the malaria cases) recorded initially as being caused by Plasmodium malariae in the Kapit division of Malaysian Borneo were in fact caused by P. knowlesi (8), a parasite of long-tailed (Macaca fascicularis) and pig-tailed (M. nemestrina) macaques. More recent studies have described P. knowlesi infections in several southeast Asian countries: 5 in the Philippines (5), 11 in Thailand (3, 7), and 1 in Singapore (6). Most cases have been reported to occur in Sarawak and the neighboring state of Sabah in Borneo (2), with some also recorded in peninsular Malaysia (11). Most cases have been detected in forest dwellers living in close proximity to the natural monkey hosts. In Sarawak, Anopheles latens is the principal vector (12). The role of human-to-human transmission is uncertain. The parasite's potential to disseminate far from its zone of endemicity was highlighted by the detection of P. knowlesi in a traveler returning to China from Myanmar (14) and in two travelers returning to Finland (4) and Sweden (1) from Borneo. P. knowlesi has a quotidian cycle and can reach high parasite densities rapidly in humans, and infection is potentially fatal (2). Accurate identification in cases of human malaria is essential.
Upon microscopy analysis, young ring stages of P. knowlesi resemble P. falciparum, but older forms are similar to the band forms of P. malariae. The recorded P. knowlesi infections in humans were discovered and confirmed by a PCR assay using a set of oligonucleotide primers (Pmk8 and Pmkr9) that target one of the parasite's small-subunit rRNA (ssrRNA) genes (8). Most Plasmodium parasites have two, and some species have three, distinct ssrRNA genes that are differentially expressed during the parasite's life cycle (13), and the Pmk8-Pmkr9 primers target the gene (ssrRNA-S) expressed during the sexual stages (8). In the course of nested PCR screening of Thai isolates for the presence of P. knowlesi by using the Pmk8-Pmkr9 primers, we noted an unexpected number of positive patient samples that had been identified microscopically as P. vivax and in which parasites resembling P. knowlesi were not observed. This raised the possibility that the Pmk8-Pmkr9 primers could be cross-reactive with some P. vivax isolates.
In order to assess the specificity of Pmk8-Pmkr9, we carried out nested amplification (8) with a broad panel of genomic DNA samples from the four human malaria parasites, including isolates from Thai patients infected with P. falciparum (n = 30), P. vivax (n = 30), P. malariae (n = 19), and P. ovale (n = 4). The diagnoses were confirmed by a species-specific nested PCR assay (10). We also included samples of DNA obtained from MR4 (Malaria Research and Reference Resource Center [http://www.mr4.org/]) which were purified from Aotus monkeys infected with strains of P. vivax collected from Nicaragua, Panama, and Thailand (MRA340G, MRA343G, and MRA342G) or from P. knowlesi Malayan, H, Philippine, and Hackeri strains (MRA487F, MRA456G, MRA457, and MRA547) and with other malarial species that infect primates: P. cynomolgi ceylonensis (MRA484F), P. cynomolgi bastianellii (MRA350G), P. cynomolgi Smithsonian and Cambodian strains (MRA351G and MRA597G), P. inui OS (MRA486F), P. simiovale Sri Lanka (MRA488F), P. brasilianum (MRA349G), P. fragile (MRA352G), and P. simium (MRA353G).
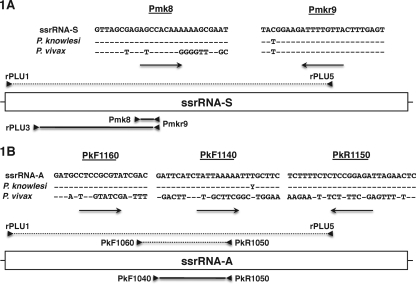
Nested PCR revealed that the Pmk8-Pmkr9 primers yielded positive amplification not only for the 4 P. knowlesi control strains but also for 8 P. vivax isolates (6 of 30 from Thailand and the isolates from Nicaragua and Panama). Amplification was negative for all the other parasite samples described above. When the P. knowlesi and P. vivax ssrRNA-S gene sequences from GenBank were aligned, the sequences of the region targeted by the Pmkr9 primer were found to be identical (Fig. 1A); in the region corresponding to the Pmk8 primer, the P. vivax sequence showed dissimilarity at the 3′ end but presented only two mismatches in the first 19 bases (Fig. 1A). Thus, it seems unlikely that the Pmk8-Pmkr9 pair would support efficient priming for the P. vivax ssrRNA-S gene. In order to establish whether the positive amplification observed for some of the P. vivax samples was due to stochastic cross-reactivity or to a hitherto unknown polymorphism in the P. vivax ssrRNA-S gene, the rPLU3-Pmkr9 primer fragments (Fig. 1A) bearing the Pmk8 regions from three P. vivax samples that gave positive amplification with Pmk8-Pmkr9 were cloned into the Topo vector (Invitrogen, The Netherlands). Bacterial clones harboring the rPLU3-Pmkr9 fragment were then screened with Pmk8-Pmkr9, and in each case, a positive clone and a negative clone were picked and the insert was sequenced. All the sequences obtained were aligned with the published P. vivax ssrRNA-S gene sequences, and no sequence variations in the region that corresponds to the Pmk8 primer were observed. Moreover, when the six sequenced plasmids were subjected to PCR analysis using Pmk8-Pmkr9, positive amplification was observed randomly. These results demonstrate that stochastic cross-reaction with the Pmk8-Pmkr9 primers led to false-positive amplification when P. vivax genomic DNA was used.
FIG. 1.
(A) Alignments of the target sequences for Pmk8 and Pmkr9 primers in the ssrRNA-S genes of P. vivax (n = 2; accession numbers U03080 and U07368) and P. knowlesi (n = 8; accession numbers DQ350256 to DQ350262) and schematic representation of the Plasmodium ssrRNA-S gene. The Pmkr9 sequence differs from the published sequences by a single base close to the 3′ end of the primer. (B) Alignments of the target sequences for the PkF1060, PkF1140, and PkR1550 primers in the ssrRNA-A genes of P. vivax (n = 2; accession numbers U03079 and U07367) and P. knowlesi (n = 11; accession numbers L07560AY580317 and AY327549 to AY327557) and schematic representation of the Plasmodium ssrRNA-A gene. The relative positions of the different primers employed for nested PCR amplification, Topo cloning, and screening and those of the amplified fragments are indicated above (dotted lines, primary reaction) and below (solid lines, secondary reaction) the gene representations. It should be noted that the reverse primers (indicated by the arrows pointing to the left below the sequences) are presented as their reverse complemented sequences.
It is important to be clear that the unsuspected cross-reactivity of the Pmk8-Pmkr9 primers described here does not put into question the results published to date for P. knowlesi infections discovered in humans by using these primers. In all such cases, the presence of P. vivax in the tested samples was convincingly excluded and/or confirmation of P. knowlesi was obtained through amplification and sequencing of another P. knowlesi gene.
Although cross-reactivity of Pmk8-Pmkr9 with P. vivax may be reduced by further optimization of the amplification conditions, it was felt that a new set of primers truly specific for P. knowlesi would be a better alternative. Three primers suitable for seminested PCR amplification of a fragment of the P. knowlesi ssrRNA gene expressed during the asexual stages (ssrRNA-A) were designed (Fig. 1B) to target regions that differ in the corresponding related P. vivax gene. These primers were tested using the same panel of genomic DNA described above. All amplification reactions were carried out with a total volume of 20 μl, in the presence of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 250 nM (each) oligonucleotide primers, MgCl2 at the concentration suitable for each primer pair (1 mM for PkF1060-PkR1550 and 2 mM for PkF1140-PkR1550), 125 μM (each) deoxynucleoside triphosphates, and 4 U of Taq polymerase (Invitrogen). The cycling conditions were an initial denaturation step at 95°C for 5 min, followed by cycles of annealing at 55°C for PkF1060-PkR1550 and 50°C for PkF1140-PkR1550 for 1 min, extension at 72°C for 1 min, and finally, denaturation at 94°C for 1 min; 30 cycles for the primary amplification reaction and 35 for the secondary amplification reaction were carried out before a final annealing step, followed by 5 min of extension. When the primers were used for seminested amplification or when PkF1140-PkR1550 primers were used in the secondary reaction after primary amplification with rPLU1-rPLU5 (8, 9), the primers were found to be specific to P. knowlesi, with no amplification observed for any of the other parasite species. This result confirmed that the eight P. vivax samples for which cross-reactivity with Pmk8-Pmkr9 was noted were indeed free of P. knowlesi. The sensitivity of the nested reaction was found to be equivalent to that obtained using the rPLU3-rPLU4 primers, which consistently detect 1 to 10 parasite genomes per sample (9).
Acknowledgments
We thank MR4 for the provision of parasite material.
This study was financed in part by the Wellcome Trust of Great Britain. M.I. is a Wellcome Trust intermediate fellow (recipient of grant no. 080867/Z/06/Z).
Footnotes
Published ahead of print on 7 October 2009.
REFERENCES
- 1.Bronner, U., P. C. S. Divis, A. Färnert, and B. Singh. 2009. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malaria J. 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox-Singh, J., T. M. E. Davis, K.-S. Lee, S. S. G. Shamsul, A. Matusop, S. Ratnam, H. A. Rahman, D. J. Conway, and B. Singh. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jongwutiwes, S., C. Putaporntip, T. Iwasaki, T. Sata, and H. Kanbara. 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg. Infect. Dis. 10:2211-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantele, A., H. Marti, I. Felger, D. Müller, and T. S. Jokiranta. 2008. Monkey malaria in a European traveler returning from Malaysia. Emerg. Infect. Dis. 14:1434-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchavez, J., F. Espino, P. Curameng, R. Espina, D. Bell, P. L. Chiodini, D. Nolder, C. J. Sutherland, K.-S. Lee, and B. Singh. 2008. Human infections with Plasmodium knowlesi, the Philippines. Emerg. Infect. Dis. 14:811-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng, O. T., E. E. Ooi, C. C. Lee, P. J. Lee, L. C. Ng, P. S. Wong, T. M. Tu, J. P. Loh, and Y. S. Leo. 2008. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg. Infect. Dis. 14:814-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putaporntip, C., T. Hongsrimuang, S. Seethamchai, T. Kobasa, K. Limkittikul, L. Cui, and S. Jongwutiwes. 2009. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J. Infect. Dis. 199:1143-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh, B., L. Kim Sung, A. Matusop, A. Radhakrishnan, S. S. G. Shamsul, J. Cox-Singh, A. W. Thomas, and D. J. Conway. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017-1024. [DOI] [PubMed] [Google Scholar]
- 9.Snounou, G., and B. Singh. 2002. Nested PCR analysis of Plasmodium parasites. Methods Mol. Med. 72:189-203. [DOI] [PubMed] [Google Scholar]
- 10.Snounou, G., S. Viriyakosol, X. P. Zhu, W. Jarra, L. Pinheiro, V. E. Do Rosário, S. Thaithong, and K. N. Brown. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315-320. [DOI] [PubMed] [Google Scholar]
- 11.Vythilingam, I., Y. M. NoorAzian, T. C. Huat, A. I. Jiram, Y. M. Yusri, A. H. Azahari, I. Norparina, A. NoorRain, and S. LokmanHakim. 2008. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasites Vectors 1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vythilingam, I., C. H. Tan, A. Matusop, S. T. Chan, K.-S. Lee, and B. Singh. 2006. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans. R. Soc. Trop. Med. Hyg. 100:1087-1088. [DOI] [PubMed] [Google Scholar]
- 13.Waters, A. P. 1994. The ribosomal RNA genes of Plasmodium. Adv. Parasitol. 34:33-79. [DOI] [PubMed] [Google Scholar]
- 14.Zhu, H.-M., J. Li, and H. Zheng. 2006. [Human natural infection of Plasmodium knowlesi.] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 24:70-71. (In Chinese.) [PubMed]