Abstract
The semen plasma virus load is measured to ensure the safety of sperm processing during medically assisted procreation (MAP) for couples with a human immunodeficiency virus type 1 (HIV-1)-infected man. A practical, automated protocol using the COBAS Ampliprep CAP/CTM kit in the COBAS TaqMan96 system was developed to measure the HIV-1 load in semen plasma samples. HIV-1 was detected in 13.4% of the semen samples processed at our MAP center. Of the eight patients having a detectable semen HIV-1 load, five had no detectable virus in their blood plasma. This highlights the residual risk of HIV-1 transmission during unprotected intercourse and raises the question of the possible consequences of ineffective highly active antiretroviral therapy in the genital tract.
Detection of the human immunodeficiency virus (HIV) genome in semen samples is commonly used to check the safety of semen-processing procedures before medically assisted procreation (MAP) and to monitor processed samples. This practice is now widespread in European MAP centers, helping serodiscordant HIV type 1 (HIV-1)-infected couples to conceive without HIV transmission (4). Since residual virus replication within the male genital tract can occur despite highly active antiretroviral therapy (HAART) being chronically effective in controlling the blood virus content (3, 15, 18), testing the semen HIV-1 load (SVL) could also be useful for studying the dynamics of virus replication and the efficacy of antiretroviral treatment in the male genital tract reservoir. Thus, SVL may help us to assess the risk of transmission during unprotected sexual intercourse before giving a “license to love” (31).
The development and automation of real-time PCR over the past few years have greatly improved the performance and practicability of kits for measuring the HIV-1 load. But these kits were designed to work with blood plasma or serum samples, and laboratories measuring the HIV-1 load in semen plasma were obliged to use manual techniques or to adapt one of the commercially available kits for use with semen plasma. This led to the development of many different protocols and hence discrepancies between the results in different laboratories, as shown in a multicenter quality control (16). We have developed a standardized protocol for semen samples based on the COBAS Ampliprep TNAI kit and the COBAS TaqMan48 HIV-1 system (19). New reagents, particular for the COBAS Ampliprep CAP/CTM kit and the new COBAS TaqMan96 system, have become available for the fully automated testing of the HIV-1 blood plasma viral load (BPVL). This system may be used to test semen samples by including a short specific preanalytical step.
We have assessed the performances of the COBAS Ampliprep CAP/CTM kit and the COBAS TaqMan96 system for testing semen samples for HIV-1 RNA with reference to the former protocol and prospectively evaluated its potential for laboratory practice.
MATERIALS AND METHODS
Clinical specimens.
The semen samples used for sensitivity studies were produced by HIV-positive and HIV-negative volunteers by masturbation. The samples were pooled to provide sufficient seminal plasma volumes and cell counts. The correlation study was done on consecutive semen samples routinely collected at the Laboratory of Spermiology, CECOS Midi-Pyrénées (Toulouse University Hospital). The prospective evaluation of the assay was done on 82 semen samples given by 37 HIV-1-infected men attending our MAP center. All semen samples were processed in the laboratory of spermiology as previously described (3, 17).
Standard samples.
Aliquots of processed seminal plasma (200 μl) and harvested spermatozoa (3 × 106/vial) from HIV-negative donors were placed in 1.5-ml Eppendorf vials and spiked with known quantities of HIV-1.
A pool of blood plasma from HIV-1 subtype B-positive patients that was stored at −80°C was diluted in HIV-negative blood plasma and used for HIV-1 spiking. This pool was used as an independent quality control and tested daily (COBAS TaqMan96 system; Roche Diagnostics, Meylan, France); it had a mean viral load of 260,000 RNA copies/ml in three measurements.
Quantifying HIV genomes in semen with the COBAS Ampliprep TNAI and TaqMan HIV-1 HPS kits in the COBAS TaqMan48 HIV-1 system.
Samples were prepared with the Ampliprep Total Nucleic Acid Isolation (TNAI) kit and the COBAS TaqMan48 HIV-1 system (19). This extraction kit is not specific for any particular virus and so needs specific reagents for the internal quality standard (IQS) and PCR mixture. Briefly, aliquots of seminal plasma (340 μl) were placed in the COBAS Ampliprep instrument (COBAS Ampliprep system; Roche Diagnostics, Meylan, France) and nucleic acids were extracted from 200 μl of each sample with the TNAI reagent and protocol. The IQS (50 μl HIV) was added to each sample before extraction, as recommended by the manufacturer. A sample of extract (50 μl) was mixed manually with 50 μl COBAS TaqMan HIV-1 HPS test premix reagent and amplified with the real-time PCR COBAS TaqMan 48 instrument (COBAS Ampliprep analyzer; Roche Diagnostics, Meylan, France). The default results were multiplied by 5/2 (COBAS Ampliprep input of 200 μl instead of the normal 500 μl) and multiplied by 2, because of the initial twofold dilution, to obtain the corrected results.
Quantifying HIV genomes in semen with the COBAS Ampliprep CAP/CTM kit in the COBAS TaqMan96 system.
This kit was designed to specifically measure HIV-1 loads and contains all of the necessary reagents, including an IQS, ready for use with COBAS machines. Samples were prepared with the COBAS Ampliprep CAP/CTM kit and the COBAS TaqMan96 system with 200 μl of seminal plasma diluted in 850 μl of HIV-negative blood plasma (dilution, 0.19). The seminal plasma was well mixed by micropipetting, and bubbles were removed by a short centrifugation. The total volume was placed in COBAS Ampliprep entry tubes, extracted, and amplified as recommended by the manufacturer for blood plasma samples. Since this protocol did not modify the IQS used, the default results were divided by 0.19 to obtain the corrected viral loads.
RESULTS
Performance of the COBAS Ampliprep CAP/CTM kit in the COBAS TaqMan96 system.
Sensitivity was assessed by testing semen samples containing various concentrations of HIV-1 particles (National Institute for Biological Standards and Control standard). Detection was 100% positive for 10 semen plasma samples containing 400 RNA copies/ml (10/10). Detection was 96.7% positive for 30 semen plasma samples containing 200 RNA copies/ml (29/30).
Specificity was assessed with negative controls in each run. Specificity was 100% (27/27 runs), and there was no cross contamination in 10 samples with alternation of positive and negative controls.
Intraassay reproducibility was tested in three measurements within the same run for three SVLs (Table 1). The coefficients of variation were 2.2% for 10,000 RNA copies/ml, 6.9% for 1,000 RNA copies/ml, and 14.9% for 500 RNA copies/ml.
TABLE 1.
Intra- and interassay reproducibility
| Target no. or parameter | Assay reproducibilitya at SVL of:
|
||
|---|---|---|---|
| 4 log/ml | 3 log/ml | 2.7 log/ml | |
| Intraassay test | |||
| 1 | 3.90 | 2.90 | 2.51 |
| 2 | 3.80 | 2.77 | 2.42 |
| 3 | 3.73 | 2.77 | 2.44 |
| Median | 3.80 | 2.77 | 2.44 |
| CV (%) | 2.36 | 2.63 | 1.89 |
| Interassay test | |||
| 1 | 3.59 | 2.47 | 2.43 |
| 2 | 3.69 | 2.83 | 2.54 |
| 3 | 3.75 | 2.65 | 1.90 |
| Median | 3.69 | 2.65 | 2.43 |
| CV (%) | 2.19 | 6.88 | 14.94 |
Reproducibility values represent the coefficients of variation calculated using inter- and intra-assay measurements.
Interassay reproducibility was tested in three measurements performed in three different runs at three SVLs (Table 1). The coefficients of variation were 2.4% for 10,000 RNA copies/ml, 2.6% for 1,000 RNA copies/ml, and 1.8% for 500 RNA copies/ml.
Linearity of quantification was assessed by using the results of the reproducibility analyses; the coefficient of correlation was R2 = 0.998 (y = 1.06x − 0.31).
Comparison of the COBAS Ampliprep CAP/CTM kit in the COBAS TaqMan96 system with the COBAS Ampliprep TNAI kit in the COBAS TaqMan48 system.
The two protocols were compared by using 11 clinical samples, 10 quantified standards, and 14 negative controls. All of the negative samples gave negative results with both protocols, except for one sample that had an invalid IQS by the TaqMan96 system protocol.
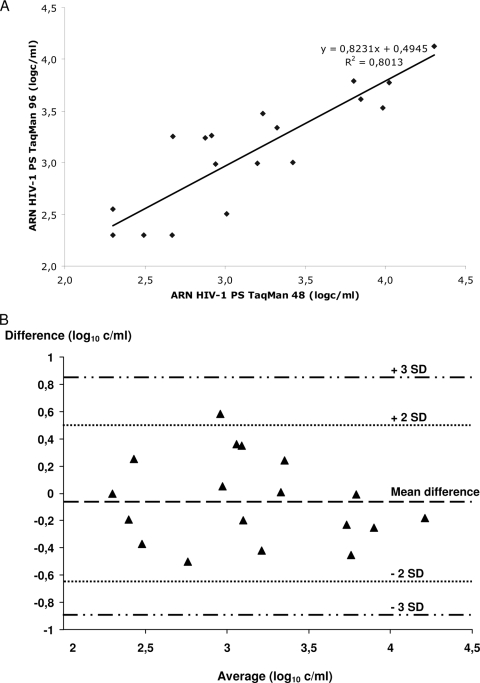
We found no significant differences between the protocols for clinical samples (3.33 ± 0.66 versus 3.24 ± 0.57, P = 0.45), for quantified standards (2.76 ± 0.5 versus 2.76 ± 0.5, P = 0.74), or in a global analysis (3.06 ± 0.63 versus 3.01 ± 0.59, P = 0.52). Spearman's coefficient of correlation between the two protocols was r = 0.89 (P < 0.0001) (Fig. 1A). There was no systematic bias of measurement in the Bland-Altman plots used to graphically assess the magnitude of disagreement between the two protocols; all values were within the mean ± 3 standard deviations (SD) (Fig. 1B).
FIG. 1.
Comparison of the COBAS Ampliprep CAP/CTM kit in the COBAS TaqMan96 system with the COBAS Ampliprep TNAI kit in the COBAS TaqMan48 system. (A) Correlation between HIV-1 RNA quantifications in semen plasma by the COBAS TaqMan48 system with the former protocol and by the COBAS TaqMan96 system with the new protocol. (B) SVLs obtained for all specimens with the CAP/CTM and TNAI/HPS assays. Difference = CAP/CTM value − TNAI/HPS value (log10 copies/ml). Average = (CAP/CTM value − TNAI/HPS value)/2 (log10 copies/ml).
Prospective SVL testing of patient samples with the COBAS Ampliprep CAP/CTM kit.
The new protocol was used to assay routine semen samples from 1 January to 15 July 2008. A total of 31 SVL assays were performed. An independent internal quality control sample was included in each run and went through all of the analytical steps. The mean independent internal quality control was 2.75 ± 0.13 log copies/ml, and the coefficient of variation (CV) was 4.76%. Twenty-nine (93.5%) of 31 measurements were within the mean ± 2 SD, and 100% were within the mean ± 3 SD.
A total of 82 SVL measurements were performed on samples provided by 37 patients (mean CD4 cell count, 605 ± 210/mm3). The semen from each patient was tested by using one to eight samples (mean number of samples per patient, 2.22 ± 1.5). One semen sample gave an invalid IQS (1.2%), and two samples had to be diluted twofold before testing because the volume of semen plasma was too small. Of the 82 SVL samples tested, 11 (13.4%) had detectable HIV-1 RNA. The median SVL was 2.78 log RNA copies/ml, with a maximum of 6.26 log RNA copies/ml. Of the 82 BPVL samples tested, 18 (22%) had detectable HIV-1 RNA. The median BPVL was 2.45 log RNA copies/ml, with a maximum of 4.84 log RNA copies/ml. There were 66 samples from patients on HAART and 16 from four untreated patients. The BPVLs measured in samples taken the same day as the 11 semen samples with detectable SVLs were undetectable (<40 HIV-1 RNA copies/ml) in seven cases (54%) (Table 2). Eight patients (21.6%) had a detectable SVL in at least one sample during follow-up, and three of these eight patients had a detectable BPVL, while five had an undetectable BPVL on the day of sampling. The five patients who had an undetectable BPVL and a detectable SVL were under efficient HAART for more than 6 months (7 to 48 months). The CD4 cell counts of the patients with a detectable BPVL and HIV-1 shedding (mean, 512 ± 256) and those with no shedding (mean, 622 ± 199, P = 0.11) were similar. The CD4 cell counts of patients with undetectable BPVLs with HIV-1 shedding (mean, 576 ± 288) and without shedding (mean, 609 ± 199, P = 0.76) were similar.
TABLE 2.
Characteristics of the 11 samples from eight patients with detectable SVLs
| Patient (sample no.) | SVLa | BPVLa | CD4 cell count/mm3 | HAARTb |
|---|---|---|---|---|
| 1 (1) | 2.607 | 2.297 | 389 | |
| 2 (1) | 2.907 | 3.239 | 282 | TDF + FTC + ATZ/r |
| 3 (1) | 2.775 | <1.7 | 984 | TDF + FTC + LPV/r |
| 4 (1) | 2.779 | 3.817 | 536 | |
| 4 (2) | 4.349 | 3.724 | 457 | |
| 5 (1) | 6.257 | <1.7 | 237 | TDF + FTC + LPV/r |
| 5 (2) | 5.017 | <1.7 | 159 | TDF + FTC + LPV/r |
| 6 (1) | 2.373 | <1.7 | 651 | ABC + 3TC + LPV/r |
| 7 (2) | 2.981 | <1.7 | 703 | ABC + 3TC + NVP |
| 8 (1) | 2.322 | <1.7 | 782 | AZT + 3TC + LPV/r |
| 8 (2) | 2.344 | <1.7 | 580 | AZT + 3TC + LPV/r |
Log number of RNA copies per milliliter.
Abbreviations: TDF, tenofovir; FTC, emtricitabine; ATZ/r, atazanavir plus ritonavir; LPV/r, lopinavir plus ritonavir; ABC, abacavir; 3TC, lamivudine; NVP, nevirapine.
DISCUSSION
The analytical performance of the protocol assessed in this study was similar to that of the previous protocol, but the new protocol is much more practical (19). As there is only one simple manual step in the sample preparation, the procedure is fully automated and blood and semen plasma samples can be included in the same run of extraction and amplification. This reduces the cost per test (number of controls) and shortens the wait for results. The detection threshold of 200 RNA copies/ml is higher than for BPVL (50 RNA copies/ml) and is directly linked to the sample input volume (200 μl versus 1 ml). The sensitivity of a protocol is important for safety reasons, particularly for MAP centers. We believe that this sensitivity of 200 HIV-1 RNA copies/ml is sufficient to ensure the safety of semen samples. It first reduces the risk of false-negative results that are mainly due to defective sampling, nucleic acid extraction, or amplification. These risks are well controlled throughout the automated analysis, in particular by the IQC. This avoids missing high-SVL samples (more than 1,500 RNA copies/ml) that indicate a real risk of transmission (6, 22). Second, if the effective HIV-1 SVL of the sample is lower than 200 RNA copies/ml, the risks of infection and HIV transmission are probably very low and not significantly different than those for samples with 50 RNA copies/ml. All of the studies that have assessed the rate of HIV transmission during a single instance of sexual intercourse have correlated it with the BPVL or HAART (5, 21, 34) but not with the SVL. This sensitivity can be increased by using a larger volume of semen plasma, but this has not been checked for PCR inhibitors. This protocol can also be used to test semen cells for the HIV-1 genome with a sensitivity of 200 RNA copies per 5 × 106 cells (data not shown).
Despite the efficiency of HAART at reducing the BPVLs of most patients, there may still be an HIV-1 SVL. The majority of patients shedding HIV into their semen attending our MAP center had undetectable BPVLs and no indication of a risk of sexually transmitted disease. This raises the questions of the sexual transmission of HIV in this population and the efficiency of HAART in the male genital tract and its potential consequences. It is now widely agreed by both physicians and patients that HAART reduces the genital shedding of HIV and its transmission during unprotected intercourse (7, 28). But how safe is it, and should we recommend it (1, 33)? The residual risk of sexually transmitted HIV is probably very low, since only one case has been documented (26), and it is still being discussed (32). In contrast, there have been reports of natural conceptions under HAART with no transmission of HIV to the partner (2, 30). Nevertheless, most studies of HIV shedding seem to have looked at a short period after HAART initiation in which patients reached undetectable levels after 6 months on a HAART regimen containing protease inhibitors (11, 15). HIV shedding may continue for up to 2 years in a few patients and may be high enough for sexual transmission (18). The different rates at which HAART acts on the two compartments seems to be directly linked to the poor diffusion of some antiretroviral drugs in the male genital tract, as indicated by drug concentrations in semen (20, 23, 25, 27, 29) and to intracellular active drug concentrations that are too low (8). Somewhat surprisingly, there have been no reports of impaired HAART efficiency (14) or local selection of resistant HIV (9, 10, 12, 13, 18) despite poor local drug concentrations and residual HIV shedding. Short-term residual shedding may be linked to HIV replication in genital tract cells that lack effective drugs with a poor yield of cell reinfection. There may also be a gradual decrease in local inflammation within the genital tract as immune cells become infected and shed HIV. Inflammation has been identified as the main source of HIV shedding in semen (3, 24). As the genital tract is not impermeable to blood components, the gradual reduction of shedding to extinction may be due to HAART interrupting the supply of virus from the blood.
In summary, we have described an automated and very convenient method for quantifying HIV-1 RNA in semen. This protocol can be used to assay both blood and semen samples in the same run. The quantification of HIV in semen is a key assay in the context of HIV infection and procreation, but its use may be extended to evaluation of the risk of HIV sexual transmission in cases of unprotected intercourse and to general assessment of the efficiency of HAART in the genital tract.
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Barreiro, P., J. A. Castilla, P. Labarga, and V. Soriano. 2007. Is natural conception a valid option for HIV-serodiscordant couples? Hum. Reprod. 222353-2358. [DOI] [PubMed] [Google Scholar]
- 2.Barreiro, P., J. del Romero, M. Leal, V. Hernando, R. Asencio, C. de Mendoza, P. Labarga, M. Nunez, J. T. Ramos, J. Gonzalez-Lahoz, and V. Soriano. 2006. Natural pregnancies in HIV-serodiscordant couples receiving successful antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 43324-326. [DOI] [PubMed] [Google Scholar]
- 3.Bujan, L., M. Daudin, T. Matsuda, L. Righi, L. Thauvin, L. Berges, J. Izopet, A. Berrebi, P. Massip, and C. Pasquier. 2004. Factors of intermittent HIV-1 excretion in semen and efficiency of sperm processing in obtaining spermatozoa without HIV-1 genomes. AIDS 18757-766. [DOI] [PubMed] [Google Scholar]
- 4.Bujan, L., L. Hollander, M. Coudert, C. Gilling-Smith, A. Vucetich, J. Guibert, P. Vernazza, J. Ohl, M. Weigel, Y. Englert, and A. E. Semprini. 2007. Safety and efficacy of sperm washing in HIV-1-serodiscordant couples where the male is infected: results from the European CREAThE network. AIDS 211909-1914. [DOI] [PubMed] [Google Scholar]
- 5.Castilla, J., J. Del Romero, V. Hernando, B. Marincovich, S. Garcia, and C. Rodriguez. 2005. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J. Acquir. Immune Defic. Syndr. 4096-101. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty, H., P. K. Sen, R. W. Helms, P. L. Vernazza, S. A. Fiscus, J. J. Eron, B. K. Patterson, R. W. Coombs, J. N. Krieger, and M. S. Cohen. 2001. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 15621-627. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, M. S., C. Gay, A. D. Kashuba, S. Blower, and L. Paxton. 2007. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann. Intern. Med. 146591-601. [DOI] [PubMed] [Google Scholar]
- 8.Dumond, J. B., Y. S. Reddy, L. Troiani, J. F. Rodriguez, A. S. Bridges, S. A. Fiscus, G. J. Yuen, M. S. Cohen, and A. D. Kashuba. 2008. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. J. Acquir. Immune Defic. Syndr. 48156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eron, J. J., P. L. Vernazza, D. M. Johnston, F. Seillier-Moiseiwitsch, T. M. Alcorn, S. A. Fiscus, and M. S. Cohen. 1998. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS 12F181-F189. [DOI] [PubMed] [Google Scholar]
- 10.Eyre, R. C., G. Zheng, and A. A. Kiessling. 2000. Multiple drug resistance mutations in human immunodeficiency virus in semen but not blood of a man on antiretroviral therapy. Urology 55591. [DOI] [PubMed] [Google Scholar]
- 11.Ghosn, J., M. L. Chaix, G. Peytavin, J. L. Bresson, J. Galimand, P. M. Girard, F. Raffi, I. Cohen-Codar, J. F. Delfraissy, and C. Rouzioux. 2008. Absence of HIV-1 shedding in male genital tract after 1 year of first-line lopinavir/ritonavir alone or in combination with zidovudine/lamivudine. J. Antimicrob. Chemother. 611344-1347. [DOI] [PubMed] [Google Scholar]
- 12.Ghosn, J., J. P. Viard, C. Katlama, M. de Almeida, R. Tubiana, F. Letourneur, L. Aaron, C. Goujard, D. Salmon, M. Leruez-Ville, C. Rouzioux, and M. L. Chaix. 2004. Evidence of genotypic resistance diversity of archived and circulating viral strains in blood and semen of pre-treated HIV-infected men. AIDS 18447-457. [DOI] [PubMed] [Google Scholar]
- 13.Liuzzi, G., A. Chirianni, M. Zaccarelli, D. Zinzi, V. Esposito, V. Guadagnino, A. Antinori, and M. Piazza. 2004. Differences between semen and plasma of nucleoside reverse transcriptase resistance mutations in HIV-infected patients, using a rapid assay. In Vivo 18509-512. [PubMed] [Google Scholar]
- 14.Lowe, S. H., A. M. Wensing, J. A. Droste, R. W. ten Kate, S. Jurriaans, D. M. Burger, J. C. Borleffs, J. M. Lange, and J. M. Prins. 2006. No virological failure in semen during properly suppressive antiretroviral therapy despite subtherapeutic local drug concentrations. HIV Clin. Trials 7285-290. [DOI] [PubMed] [Google Scholar]
- 15.Marcelin, A. G., R. Tubiana, S. Lambert-Niclot, G. Lefebvre, S. Dominguez, M. Bonmarchand, D. Vauthier-Brouzes, F. Marguet, N. Mousset-Simeon, G. Peytavin, and C. Poirot. 2008. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS 221677-1679. [DOI] [PubMed] [Google Scholar]
- 16.Pasquier, C., D. Anderson, C. Andreutti-Zaugg, R. Baume-Berkenbosch, F. Damond, A. Devaux, Y. Englert, J. Galimand, C. Gilling-Smith, O. Guist'hau, L. Hollander, M. Leruez-Ville, B. Lesage, A. Maillard, A. G. Marcelin, M. P. Schmitt, A. Semprini, M. Vourliotis, C. Xu, and L. Bujan. 2006. Multicenter quality control of the detection of HIV-1 genome in semen before medically assisted procreation. J. Med. Virol. 78877-882. [DOI] [PubMed] [Google Scholar]
- 17.Pasquier, C., M. Daudin, L. Righi, L. Berges, L. Thauvin, A. Berrebi, P. Massip, J. Puel, L. Bujan, and J. Izopet. 2000. Sperm washing and virus nucleic acid detection to reduce HIV and hepatitis C virus transmission in serodiscordant couples wishing to have children. AIDS 142093-2099. [DOI] [PubMed] [Google Scholar]
- 18.Pasquier, C., N. Moinard, K. Sauné, C. Souyris, M. Lavite, M. Daudin, J. Izopet, and L. Bujan. 2008. Persistent differences in the antiviral effects of highly active antiretroviral therapy on blood and male genital tract. AIDS 221894-1896. [DOI] [PubMed] [Google Scholar]
- 19.Pasquier, C., C. Souyris, N. Moinard, L. Bujan, and J. Izopet. 2006. Validation of an automated real-time PCR protocol for detection and quantitation of HIV and HCV genomes in semen. J. Virol. Methods 137156-159. [DOI] [PubMed] [Google Scholar]
- 20.Pereira, A. S., L. M. Smeaton, J. G. Gerber, E. P. Acosta, S. Snyder, S. A. Fiscus, R. R. Tidwell, R. M. Gulick, R. L. Murphy, and J. J. Eron, Jr. 2002. The pharmacokinetics of amprenavir, zidovudine, and lamivudine in the genital tracts of men infected with human immunodeficiency virus type 1 (AIDS Clinical Trials Group study 850). J. Infect. Dis. 186198-204. [DOI] [PubMed] [Google Scholar]
- 21.Powers, K. A., C. Poole, A. E. Pettifor, and M. S. Cohen. 2008. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect. Dis. 8553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342921-929. [DOI] [PubMed] [Google Scholar]
- 23.Reddy, Y. S., S. K. Gotzkowsky, J. J. Eron, J. Y. Kim, W. D. Fiske, S. A. Fiscus, L. Petch, M. S. Cohen, and A. D. Kashuba. 2002. Pharmacokinetic and pharmacodynamic investigation of efavirenz in the semen and blood of human immunodeficiency virus type 1-infected men. J. Infect. Dis. 1861339-1343. [DOI] [PubMed] [Google Scholar]
- 24.Sadiq, S. T., S. Taylor, S. Kaye, J. Bennett, R. Johnstone, P. Byrne, A. J. Copas, S. M. Drake, D. Pillay, and I. Weller. 2002. The effects of antiretroviral therapy on HIV-1 RNA loads in seminal plasma in HIV-positive patients with and without urethritis. AIDS 16219-225. [DOI] [PubMed] [Google Scholar]
- 25.Solas, C., A. Lafeuillade, P. Halfon, S. Chadapaud, G. Hittinger, and B. Lacarelle. 2003. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stürmer, M., H. W. Doerr, A. Berger, and P. Gute. 2008. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antivir. Ther. 13729-732. [PubMed] [Google Scholar]
- 27.Taylor, S., D. J. Back, S. M. Drake, J. Workman, H. Reynolds, S. E. Gibbons, D. J. White, and D. Pillay. 2001. Antiretroviral drug concentrations in semen of HIV-infected men: differential penetration of indinavir, ritonavir and saquinavir. J. Antimicrob. Chemother. 48351-354. [DOI] [PubMed] [Google Scholar]
- 28.van der Straten, A., C. A. Gomez, J. Saul, J. Quan, and N. Padian. 2000. Sexual risk behaviors among heterosexual HIV serodiscordant couples in the era of post-exposure prevention and viral suppressive therapy. AIDS 14F47-F54. [DOI] [PubMed] [Google Scholar]
- 29.van Praag, R. M., S. Repping, J. W. de Vries, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2001. Pharmacokinetic profiles of nevirapine and indinavir in various fractions of seminal plasma. Antimicrob. Agents Chemother. 452902-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernazza, P. 2008. HAART improves quality of life: should we care about the quality of spermatozoa? AIDS 22647-648. [DOI] [PubMed] [Google Scholar]
- 31.Vernazza, P., I. Brenner, and I. Graf. 2007. Pre-exposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child, abstr. MOPDC01. In 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention. International AIDS Society, Geneva, Switzerland. http://www.ias2007.org/PAG/AbstractPlus/AbsDoc_3139_1.pdf.
- 32.Vernazza, P. L., and B. Hirschel. 2008. HIV transmission hunting—the chase for low risk events. Antivir. Ther. 13641-642. [PubMed] [Google Scholar]
- 33.Vernazza, P. L., L. Hollander, A. E. Semprini, D. J. Anderson, and A. Duerr. 2006. HIV-discordant couples and parenthood: how are we dealing with the risk of transmission? Aids 20635-636. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, D., M. G. Law, A. E. Grulich, D. A. Cooper, and J. M. Kaldor. 2008. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet 372314-320. [DOI] [PubMed] [Google Scholar]