Abstract
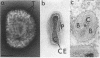
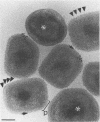
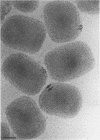
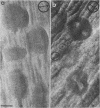
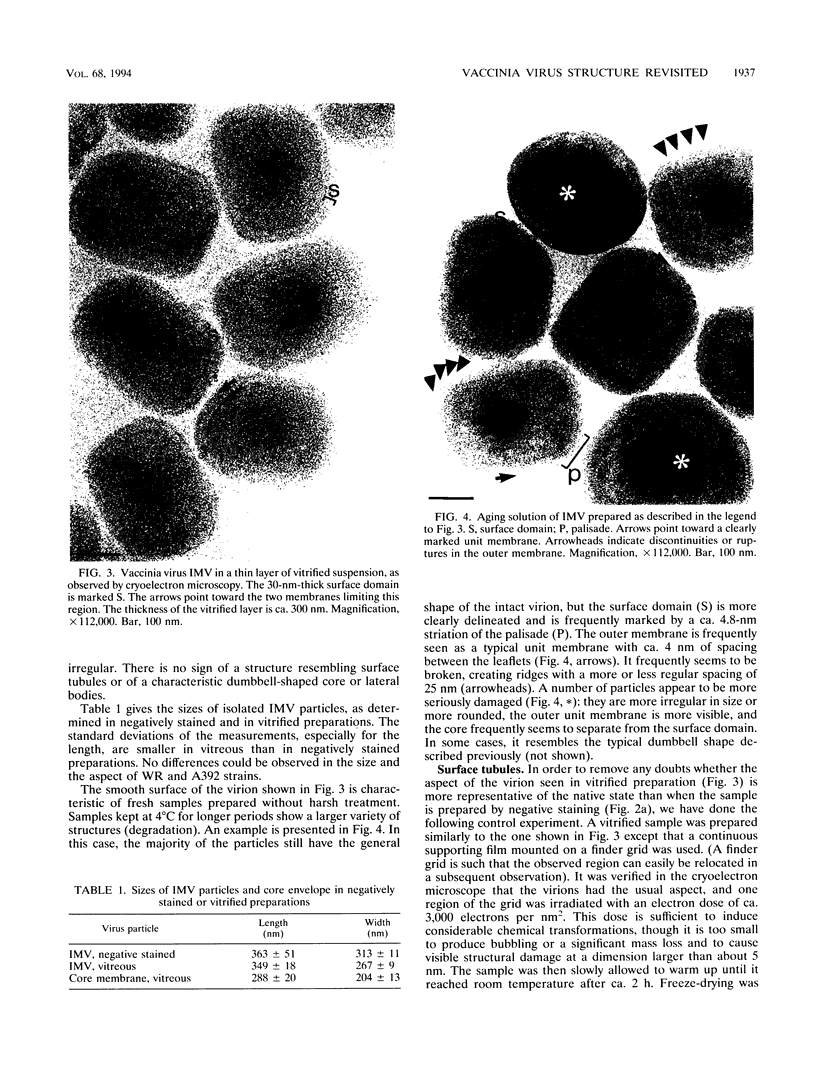
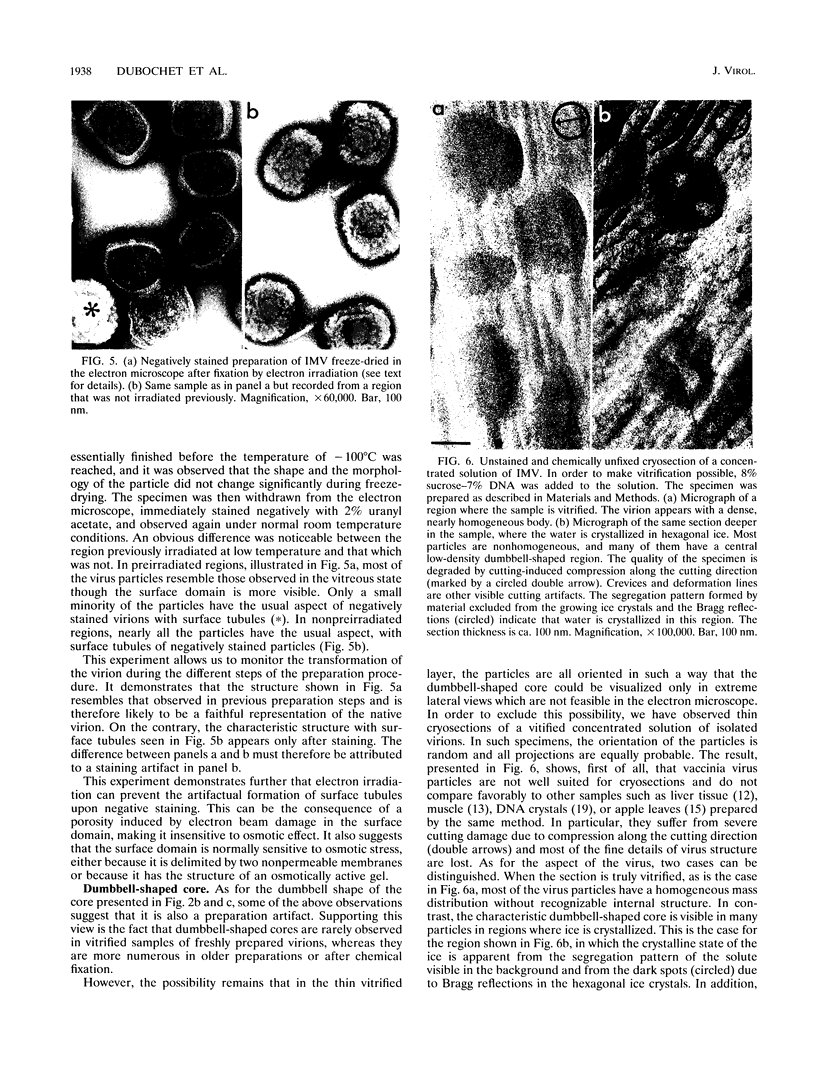
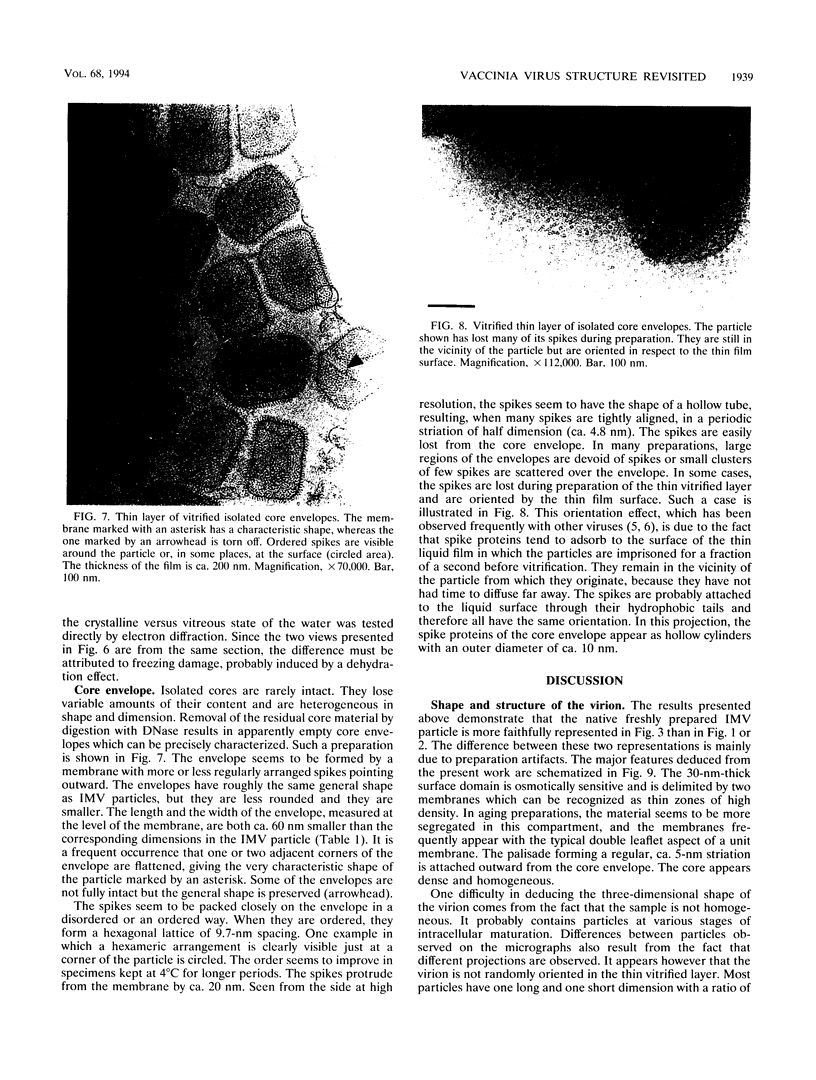
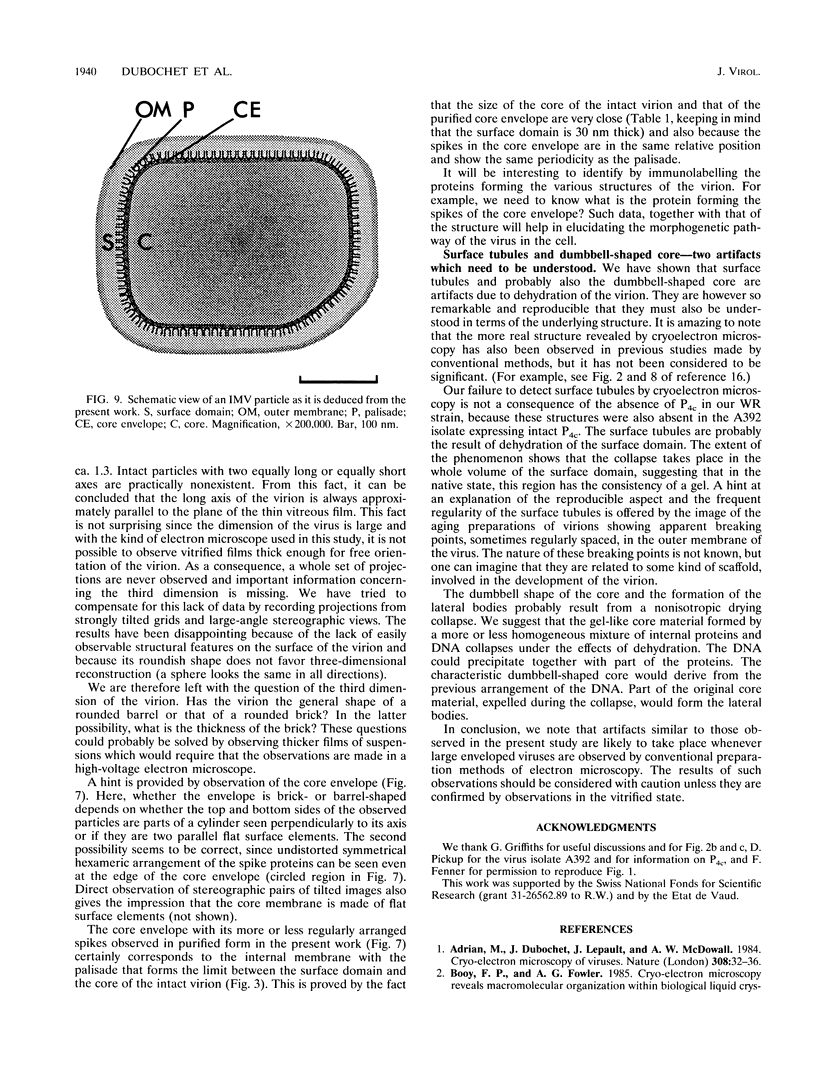
Intracellular mature vaccinia virus, also called intracellular naked virus, and its core envelope have been observed in their native, unfixed, unstained, hydrated states by cryoelectron microscopy of vitrified samples. The virion appears as a smooth rounded rectangle of ca. 350 by 270 nm. The core seems homogeneous and is surrounded by a 30-nm-thick surface domain delimited by membranes. We show that surface tubules and most likely also the characteristic dumbbell-shaped core with the lateral bodies which are generally observed in negatively stained or conventionally embedded samples are preparation artifacts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Bremer A., Henn C., Engel A., Baumeister W., Aebi U. Has negative staining still a place in biomacromolecular electron microscopy? Ultramicroscopy. 1992 Oct;46(1-4):85–111. doi: 10.1016/0304-3991(92)90008-8. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- McDowall A. W., Chang J. J., Freeman R., Lepault J., Walter C. A., Dubochet J. Electron microscopy of frozen hydrated sections of vitreous ice and vitrified biological samples. J Microsc. 1983 Jul;131(Pt 1):1–9. doi: 10.1111/j.1365-2818.1983.tb04225.x. [DOI] [PubMed] [Google Scholar]
- McDowall A. W., Hofmann W., Lepault J., Adrian M., Dubochet J. Cryo-electron microscopy of vitrified insect flight muscle. J Mol Biol. 1984 Sep 5;178(1):105–111. doi: 10.1016/0022-2836(84)90233-x. [DOI] [PubMed] [Google Scholar]
- Metcalf P., Cyrklaff M., Adrian M. The three-dimensional structure of reovirus obtained by cryo-electron microscopy. EMBO J. 1991 Nov;10(11):3129–3136. doi: 10.1002/j.1460-2075.1991.tb04874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S. Magnification calibration and the determination of spherical virus diameters using cryo-microscopy. Ultramicroscopy. 1989 Jul-Aug;30(3):281–297. doi: 10.1016/0304-3991(89)90057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Sodeik B., Doms R. W., Ericsson M., Hiller G., Machamer C. E., van 't Hof W., van Meer G., Moss B., Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993 May;121(3):521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]