Abstract
Delivery of newly synthesized membrane-spanning proteins to the apical plasma membrane domain of polarized MDCK epithelial cells is dependent on yet unidentified sorting signals present in the luminal domains of these proteins. In this report we show that structural information for apical sorting of transmembrane neurotrophin receptors (p75NTR) is localized to a juxtamembrane region of the extracellular domain that is rich in O-glycosylated serine/threonine residues. An internal deletion of 50 amino acids that removes this stalk domain from p75NTR causes the protein to be sorted exclusively of the basolateral plasma membrane. Basolateral sorting stalk-minus p75NTR does not occur by default, but requires sequences present in the cytoplasmic domain. The stalk domain is also required for apical secretion of a soluble form of p75NTR, providing the first demonstration that the same domain can mediate apical sorting of both a membrane-anchored as well as secreted protein. However, the single N-glycan present on p75NTR is not required for apical sorting of either transmembrane or secreted forms.
In polarized epithelial cells, newly synthesized plasma membrane proteins are sorted into distinct transport vesicles during transit through the TGN (Wandinger-Ness et al., 1990). Whereas all basolateral sorting signals described to date reside in cytoplasmic domains, apical signals appear to be luminal. This conclusion is supported by findings that viral protein chimeras (McQueen et al., 1986; Roth et al., 1987), tail-minus mutants of basolateral membrane proteins (Mostov et al., 1986; Hunziker et al,. 1991; Prill et al., 1993), and soluble mutants of apical and basolateral membrane proteins (Mostov et al., 1987; Brown et al., 1989; Lisanti et al., 1989; Powell et al., 1991: Corbeil et al., 1992; Vogel et al., 1992b ; Weisz et al., 1992; Prill et al., 1993; Le Gall et al., 1997), are all targeted to the apical membrane of polarized MDCK epithelial cells. In addition, basolateral signals are frequently composed of short, homologous sequences, many of which bear remarkable similarity to sequences involved in endocytosis (Matter and Mellman, 1994). No such sequence homology has been found among apically sorted proteins, whose luminal domains tend to be large and highly folded. This makes the task of defining apical sorting information particularly daunting.
It is clear that some type of apical sorting signal must exist, and that proteins are not transported to the apical surface by default simply because they lack a basolateral targeting signal. Membrane proteins are targeted to the apical surface of MDCK cells with nearly perfect fidelity (Lisanti et al., 1989; Le Bivic et al., 1990; Wessels et al., 1990; Casanova et al., 1991; Jalal et al., 1991; Wollner et al., 1992). In contrast, secretory proteins lacking sorting information are released from these cells in a nonpolar fashion (Kondor-Koch et al., 1985; Gottleib et al., 1986; Stephens and Compans, 1986; Soole et al., 1992; Vogel et al., 1992b ). Certain membrane-anchored proteins also appear to lack sorting information and are randomly inserted into both apical and basolateral membranes (Hunziker et al., 1991; Haller and Alper, 1993; Mays et al., 1995; Odorizzi et al., 1996). Furthermore, mutations that inactivate basolateral sorting signals promote random delivery of several proteins to both the apical and basolateral membranes, suggesting that transport to the apical membrane does not occur by default (Geffen et al., 1993; Prill et al., 1993; Matter et al., 1994; Thomas and Roth, 1994; Le Gall et al., 1997).
Attention has focused on asparagine-linked carbohydrates as potential apical sorting signals (for review see Fiedler and Simons, 1995). Apical secretion of several proteins appears to require the presence of N-glycans and their removal by tunicamycin treatment or site-directed mutagenesis results in nonpolar secretion (Urban et al., 1987; Musto, 1993; Kitigawa et al., 1994). In addition, when novel N-glycanation sites were inserted into rat growth hormone, this normally nonglycosylated and randomly secreted protein was targeted to the apical membrane (Scheiffele et al., 1995). For transmembrane proteins, the role of N-glycans in sorting remains an open issue. Early studies concluded that N-glycans are not required for apical sorting of influenza virus hemagglutinin (Roth et al., 1979; Green et al., 1981). However, hemagglutinin requires N-glycans for proper folding and, in the presence of tunicamycin, only a minor fraction exits the ER. Furthermore, these early studies relied on morphological techniques and, consequently, failed to show that the minor pool of hemagglutinin reaching the cell surface was nonglycosylated.
In this paper, we have reexamined the role of N-glycosylation in apical sorting. The p75 neurotrophic receptor (p75NTR) is a type I membrane protein that belongs to a family of cell surface receptors that share structural homology within four extracellular cysteine-rich repeat domains. Stably transfected MDCK cells sort p75NTR in the TGN and deliver the protein primarily to the apical plasma membrane (Le Bivic et al., 1991). The protein has a single N-glycosylation site, located within the first cysteine-rich repeat (Johnson et al., 1986; Radeke et al., 1987). This single N-glycan is not required for transport to the plasma membrane. When N-glycosylation is inhibited, p75NTR folds and exits the ER efficiently (Grob et al., 1985; Baldwin and Shooter, 1995). Here, we show that N-glycan minus p75NTR is correctly sorted to the apical membrane. However, deletion of a juxtamembrane region that contains several O-glycosylated residues reverses the polarity of this protein so that the mutant receptor is targeted to the basolateral membrane domain. Our results demonstrate that N-glycans are not involved in apical sorting of p75NTR, and that a distinct juxtamembrane region rich in O-glycans contains an apical targeting signal.
Materials and Methods
Reagents
Cell culture reagents were obtained from Gibco Laboratories (Grand Island, NY). [35S]cysteine and 125I-goat anti–rabbit IgG were purchased from ICN Biomedicals (Irvine, CA). Protein A– Sepharose was purchased from Pharmacia Biotech. (Piscataway, NJ). Staphylococcus aureus cells (Pansorbin) were obtained from Calbiochem-Novabiochem (La Jolla, CA). Affinity-purified rabbit anti–mouse IgG was purchased from Cappel Laboratories (Westchester, PA). Sulfo-NHS-SS-Biotin (sulfosuccinimidyl 2-[biotinamido]ethyl-1,3-dithiopropionate), NHS-LC-Biotin (sulfosuccinimidyl-6-[biotinamido]hexanoate), and avidin-agarose were from Pierce Chemical Co. (Rockville, IL). Fluorescein-labeled goat anti–mouse IgG or anti–rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Propidium iodide was from Molecular Probes (Eugene, OR). Molecular biology products and enzymes for oligosaccharide digestions were from Boehringer-Mannheim Biochemicals (Indianapolis, IN). All other reagents, including tunicamycin, were obtained from Sigma Chemical Co. (St. Louis, MO).
Antibodies
mAb ME 20.4 against the extracellular domain of human p75NTR (Ross et al., 1984) was produced as ascites and used at a dilution of 1:300 for immunoprecipitation and 1:500 for immunofluorescence. Rabbit polyclonal antisera against a fusion protein encoding a large portion of the cytoplasmic domain of p75NTR were produced (kindly provided by M. Chao, Cornell University Medical College, New York). This antibody recognizes both human and rat p75NTR and was used at a dilution of 1:250 for immunoprecipitation and 1:1,000 for immunofluorescence. Immunoprecipitation of neural cell adhesion molecule (N-CAM)1 sol was achieved using a 1:300 dilution of a mouse anti–chicken N-CAM mAb (mAb 5e) (Developmental Studies Hybridoma Bank, Johns Hopkins University, Baltimore, MD).
Constructs
Native human p75NTR was expressed in MDCK cells using a replication-defective recombinant adenovirus vector (kindly provided by S.-O. Yoon, M. Chao, and E. Falck-Pedersen, Cornell University Medical College). Human p75NTR deletion mutants p75NTR sol, ΔBS (Δ168–218), ΔBSp75NTR tail-minus, and ΔBSp75NTR sol were constructed using polymerase chain reaction with full-length human p75NTR cDNA in pBluescript as template (provided by B. Hempstead, Cornell University Medical College). The 5′ polymerase chain reaction primer GGG GGT ACC AGC TTG GGC TGC AGG TCG ACT was used to introduce a KpnI site 5′ to the start of the p75NTR coding sequence. Unique 3′ primers inserted a stop codon (TAG) at position 167 (for ΔBSp75NTR sol), 219 (for p75NTR sol), and 250 (for ΔBSp75NTR tail-minus). Gel-purified amplification products were subcloned into pCMV5 and sequences were verified by dideoxynucleotide sequencing performed by Cornell University DNA Services (Ithaca, NY). Δ193– 215 p75NTR was constructed by inserting an XhoI linker after partial digestion with HaeIII as described previously (Yan and Chao, 1991).
Plasmid pBJ5 vectors containing sequences encoding native rat p75NTR and mutant forms lacking N- and/or O-glycosylation sites have been described previously (Baldwin et al., 1992; Baldwin and Shooter, 1995).
Cell Culture and Transfection
MDCK cells, strain II, were maintained in DME supplemented with 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). Cells were transfected using the DNA calcium phosphate procedure (Graham and Van der Eb, 1973). Stable transformants were selected in 500 μg/ ml active G418 (Gibco Laboratories). G418-resistant clones were isolated with cloning rings and screened for p75NTR expression by immunofluorescent staining or by immunoprecipitation of p75NTR from [35S]cysteine labeled cells (see below). For expression of native human p75NTR in polarized MDCK cells, the recombinant adenovirus vector AdCMVp75 was applied apically to filter-grown cultures at a multiplicity of infection of 10 plaque formation units (pfu)/cell, as described previously (Yeaman et al., 1996).
Immunofluorescence and Confocal Microscopy
MDCK cells, cultured on 12-mm-diam Transwell filters (Costar Corp., Cambridge, MA) for 4 or 5 d, were fixed in 2% paraformaldehyde in Dulbecco's PBS solution containing 1.8 mM Ca2+ and 0.5 mM Mg2+ (CM-PBS). Cells were permeabilized with 0.075% saponin in CM-PBS containing 0.2% BSA, incubated with rabbit anti-p75NTR cytoplasmic domain antibodies, and diluted 1:1,000 in the same buffer. After washes with CM-PBS–saponin–BSA, filters were incubated with fluorescein-conjugated goat anti– rabbit IgG and RNase (DNase-free; Boehringer-Mannheim Biochemicals), each diluted 1:200 in CM-PBS–saponin–BSA. Nuclei were subsequently labeled with propidium iodide. Immunofluorescent images were collected using a confocal microscope (Molecular Dynamics, Sunnyvale, CA).
Steady State Localization of Native and Mutant p75NTR
Apical and basolateral plasma membrane proteins were biotinylated as described previously (Sargiacomo et al., 1989). Briefly, confluent monolayers of MDCK cells on filters (Transwell; Costar Corp.) were washed thrice in ice-cold CM-PBS. 500 μg/ml sulfo-NHS-SS-biotin, diluted from a 200-mg/ml stock in DMSO, was added to either apical (0.67 ml) or basolateral (1.33 ml) chambers. For biotinylation of ΔBStail-minus p75NTR, nonreducible NHS-LC-biotin was used to facilitate later detection with radioiodinated streptavidin. Surfaces not receiving biotin were incubated in CM-PBS alone. Filters were incubated 20 min on ice, and then fresh buffer and biotin was applied and filters were incubated another 20 min. Biotinylation reactions were quenched by washing cells in five changes of CM-PBS containing 50 mM NH4Cl and 0.2% BSA.
Filters were excised from plastic collars and monolayers solubilized by incubation for 1 h at 4°C in lysis buffer (150 mM NaCl, 20 mM TrisHCl, pH 8.0, 5 mM EDTA, 1% Triton X-100, 0.2% BSA, and a protease inhibitor cocktail including 1 mM PMSF and 10 μg/ml each of antipain, pepstatin A, and leupeptin). Extracts were precleared by incubation for 1 h with fixed Staphylococcus aureus cells (Pansorbin), and cleared supernatants were incubated with avidin-agarose for 2 h at 4°C with end-over-end rotation. Avidin-agarose precipitates were washed as described previously for immunoprecipitates (Le Bivic et al., 1989). Bound proteins were released from beads by boiling in SDS-PAGE sample buffer containing 100 mM dithiothreitol, separated by SDS-PAGE, and transferred to membranes (Immobilon-P; Millipore Corp., Waters Chromatography, Milford, MA). Blots were incubated with rabbit anti-p75NTR cytoplasmic domain antiserum followed by 125I-goat anti–rabbit IgG. Bound secondary antibody was quantified using a phosphorimager (Molecular Dynamics, Inc.).
Targeting of Metabolically Labeled Proteins
To examine initial delivery of proteins to apical and basolateral membrane domains, a biotin targeting assay was used (Le Bivic et al., 1989). Briefly, 4 or 5-d-old filter-grown cultures were pulse labeled for 20 min in [35S]cysteine and chased for various times in medium containing excess unlabeled cysteine. At each chase time, filters were placed on an ice bath, biotinylated from the apical or basolateral surface, lysed, and precleared as described above. For immunoprecipitation of rat p75NTR proteins, lysates were incubated overnight with rabbit anti-p75NTR antiserum (diluted 1:250) and immune complexes were collected by incubation for 2 h with protein A–Sepharose (5 mg/ml). Immunoprecipitations of human p75NTR proteins were performed with a mouse mAb (ME 20.4, diluted 1:300), and immune complexes were collected by incubation for 2 h with protein A–Sepharose (5 mg/ml) precoupled to rabbit anti–mouse IgG. Immunoprecipitates were eluted by boiling in 5% SDS, diluted 50-fold with lysis buffer, and then biotinylated proteins were recovered by reprecipitation with avidin-agarose. Proteins were separated by SDS-PAGE and analyzed with a phosphorimager (Molecular Dynamics Inc.).
Endoglycosidase Digestion
After immunoprecipitation, proteins were eluted from protein A–Sepharose beads by boiling in 0.1% SDS and then diluted 10-fold in digestion buffer (20 mM sodium phosphate, pH 7.2, containing 1% Triton X-100 and protease inhibitors). Deglycosylation was achieved by addition of 0.4 U N-glycosidase F or 2.5 mU O-glycosidase (EC 3.2.1.97) and 2 mU neuraminidase (EC 3.2.1.18) in a total volume of 100 μl. Digests were incubated at 37°C overnight and received fresh enzyme after the first 6 h.
Results
P75NTR Lacking N-glycans Is Correctly Sorted to the Apical Domain of Polarized MDCK Cells
P75NTR has a single glycosylated asparagine residue located in the first cysteine repeat and a cluster of serine/ threonine-linked sugars in a juxtamembrane stalk region (Fig. 1). This protein is sorted in the TGN and targeted to the apical membrane of polarized MDCK cells (Le Bivic et al., 1991). To determine whether the N-glycan is necessary for apical sorting of p75NTR, we monitored surface delivery of the N-glycan minus protein using a biotin targeting assay (Le Bivic et al., 1989). In these experiments, human p75NTR was introduced into MDCK cells by adenovirus-mediated gene transfer, but the polarity and kinetics with which protein is delivered to the plasma membrane are identical to those previously reported for stably transfected MDCK cells (Le Bivic et al., 1991).
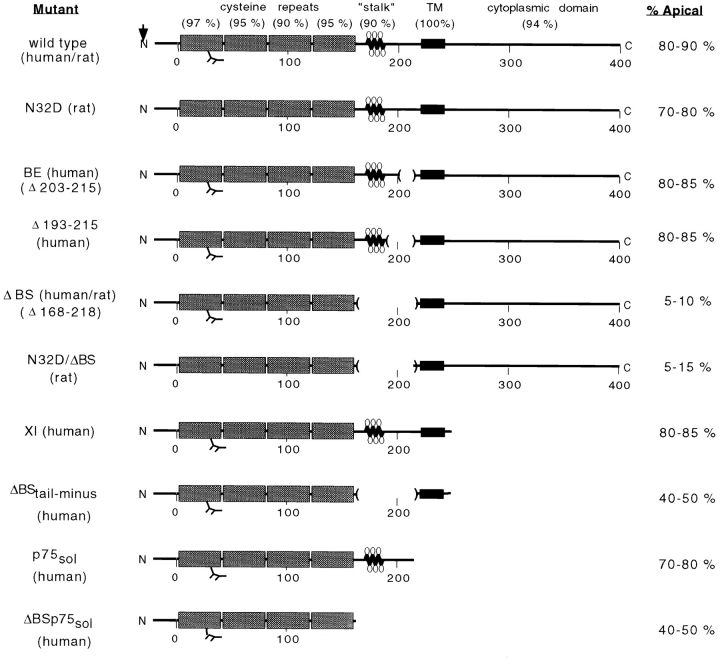
Figure 1.
Mutants constructed to characterize apical sorting information in p75NTR and their membrane distribution upon expression in MDCK cells. Degrees of amino acid conservation (percent identity) between human and rat p75NTR are shown in parentheses above each domain. Site of signal peptide cleavage is indicated by an arrow. Stippled boxes represent cysteine-rich repeat domains and black boxes represent the transmembrane (TM) domain. The N-linked glycosylation site in the first cysteine repeat is indicated. Lollipop structures represent O-linked carbohydrates in the juxtamembrane stalk domain. Internal deletions constructed within this domain are marked by parentheses. Amino acid residues are numbered, from amino to carboxy terminus, beneath schematic models. % Apical values are based on steady state biotinylation experiments discussed in the text.
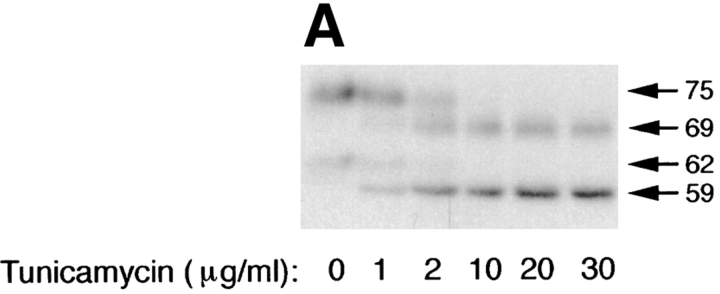
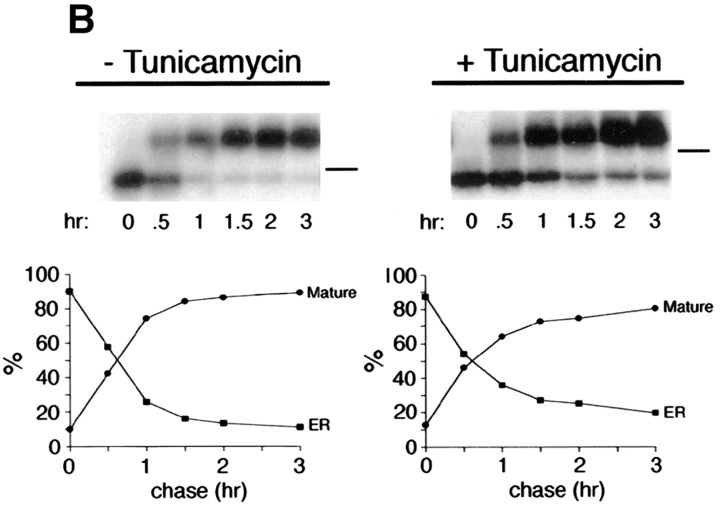
Treatment of cells with 10 μg/ml tunicamycin reduces the apparent mass of precursor (62 kD; ER) and mature (75 kD; Golgi) forms of p75NTR by ∼3–6 kD, compatible with the loss of one high mannose or completely processed N-glycan, respectively (Fig. 2 A). The tunicamycin-insensitive difference in molecular weight between precursor and mature forms, ∼10 kD, corresponds to a cluster of O-linked carbohydrates added in the Golgi (Figs. 1 and 3). By monitoring the shift in mol wt that accompanies acquisition of O-linked carbohydrates, it is possible to measure the kinetics with which p75NTR is transported from the ER to the Golgi in the absence or presence of tunicamycin. This analysis reveals that in untreated cells, p75NTR is converted from ER to Golgi form with a half-time of ∼40 min after a 20-min pulse label (Fig. 2 B). In the presence of tunicamycin, N-glycan minus p75NTR is transported to the Golgi with nearly identical kinetics, although a slightly greater amount of immature ER form remains after a 3-h chase (Fig. 2 B; 18% of total p75, compared with 12% in the control cells). Therefore, the N-glycan does not appear to be required for correct folding and ER-to-Golgi transport of the major pool of p75NTR, and tunicamycin treatment does not cause a significant retention of misfolded p75NTR in the ER during short pulse label conditions used in targeting assays. These results are in close agreement with the previously published kinetics of p75NTR transport in A875 cells, in which the Golgi form appeared after a chase of 30 min in the absence or presence of 10 μg/ml tunicamycin (Grob et al., 1985). We attribute the accumulation of immature protein observed in Fig. 2 A to the fact that cells were pretreated for 2.5 h and then labeled for 4 h in the continued presence of tunicamycin in this experiment. This prolonged treatment likely caused accumulation of other misfolded proteins in the ER that may reduce the efficiency with which p75NTR is transported to the Golgi as a secondary consequence.
Figure 2.
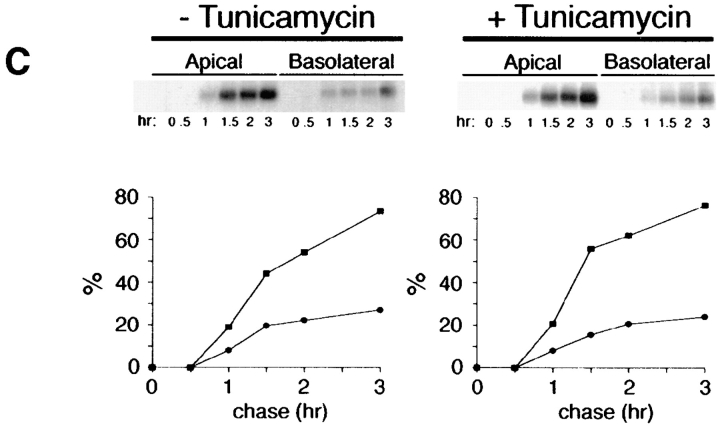
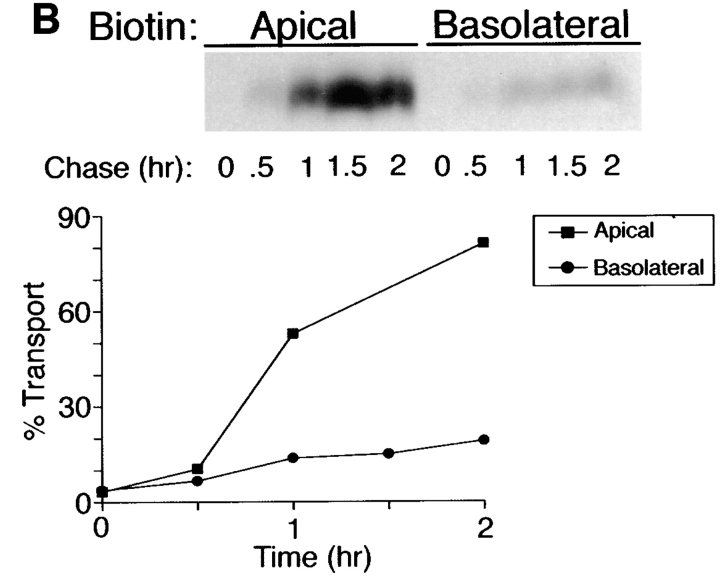
Inhibition of N-glycosylation does not perturb apical sorting of p75NTR. (A) Tunicamycin inhibits N-glycosylation of p75NTR in a concentration-dependent manner. Adenovirus-mediated gene transfer into filter-grown MDCK with 50 pfu/cell AdCMVp75 was performed 48 h before experiment. Cells were pretreated 2.5 h with indicated concentrations of tunicamycin and then labeled with [35S]cysteine for 4 h in the continued presence of tunicamycin. p75NTR was immunoprecipitated from cell lysates and subjected to SDS-PAGE as described in Materials and Methods. (B) ER-to-Golgi transport and (C) polarized targeting of p75NTR in the absence or presence of tunicamycin. Adenovirus-mediated gene transfer into filter-grown MDCK with 50 pfu/cell AdCMVp75 was performed 48 h before experiment. Cells were pretreated 2 h with or without 10 μg/ml tunicamycin, starved in cysteine-free medium for 30 min and then pulse labeled with [35S]cysteine for 20 min in the continued presence of tunicamycin, where indicated. After the pulse, cultures were chased in the continued presence or absence of tunicamycin. At indicated chase times, filters were placed on ice and subjected to domain-selective biotinylation. p75NTR was immunoprecipitated from cell lysates and surface-labeled protein was recovered on streptavidin-agarose. Aliquots of immunoprecipitates from apically biotinylated cells are shown in B, and streptavidin-agarose precipitates from the same samples and from basolaterally biotinylated cells are shown in C. Position of albumin standard (66 kD) is indicated in B. Quantitation reflects the average of duplicate experiments, one of which is represented by gels shown in figure.
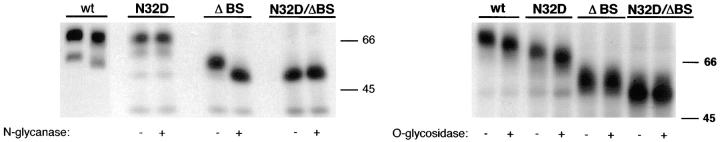
Figure 3.
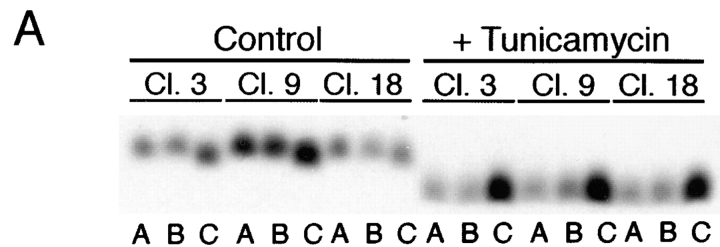
Rat p75NTR mutants lack N- and/ or O-glycans. MDCK cells stably expressing native (wt) and mutant forms of rat p75NTR were labeled overnight with [35S]cysteine. p75NTR was immunoprecipitated from cell lysates with anti-cytoplasmic domain antibodies and deglycosylated with indicated enzymes as described in Materials and Methods. Note that N32D p75NTR lacks N-glycans but is O-glycosylated; ΔBS p75NTR lacks O-glycans but is N-glycosylated; and N32D/ΔBS p75NTR lacks both N- and O-glycans.
To determine whether N-glycan minus p75NTR is sorted to the apical membrane domain, arrival of newly synthesized proteins at the cell surface was monitored by domain-selective biotinylation. During the first hour of chase after pulse labeling of cells, little surface p75NTR was detected in cells treated with or without tunicamycin (Fig. 2 C). Over the subsequent 2-h chase, labeled p75NTR was inserted into plasma membrane with similar kinetics and efficiencies in cells cultured in the absence or presence of tunicamycin, as determined by assessing the relative amount of labeled p75NTR that is accessible to biotinylation at each time point. These results are consistent with previously published studies (Grob et al., 1983, 1985). Moreover, p75NTR is primarily delivered to the apical membrane domain at each time point, irrespective of N-glycosylation (Fig. 2 C). Therefore, the N-glycan does not appear to be necessary for apical sorting of p75NTR.
To confirm results from experiments using tunicamycin, the distribution of rat p75NTR was determined after the native N-glycosylation site was removed by site-directed mutagenesis (Fig. 1). N-glycosidase treatment of p75NTR immunoprecipitated from cells expressing this mutant (N32D) confirmed that the protein lacks N-glycans (Fig. 3). The point mutation does not promote misfolding and retention in the ER because kinetics of ER-to-Golgi transport, measured as the acquisition of O-glycans in the Golgi, are identical for native rat and N32D p75NTR proteins (half-time = 35–40 min). Furthermore, immunofluorescent staining of cells expressing N32D p75NTR shows clear surface localization; no ER staining has been observed (Fig. 4). Finally, it was shown previously that COS 7 cells expressing N32D p75NTR showed wild-type levels of NGF binding to the cell surface as assessed by cross-linking and equilibrium binding experiments (Baldwin and Shooter, 1995).
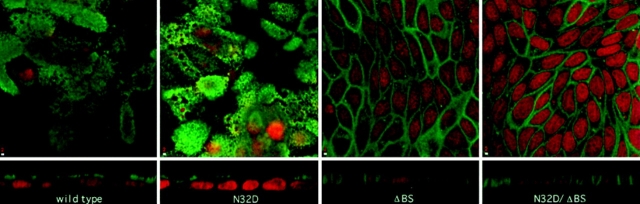
Figure 4.
Immunolocalization of rat p75NTR glycosylation mutants. Stably transfected MDCK cultures expressing native and mutant forms of rat p75NTR were fixed and permeabilized as described in Materials and Methods. p75NTR was detected by indirect immunofluorescence using anti- cytoplasmic domain polyclonal antibodies. Each panel shows a single 1-μm optical section in the XY-plane (top) as well as a vertical section through the sample (bottom). Bar, 2 μm.
Site-directed mutagenesis of the single N-glycosylation site does not significantly alter apical polarity of p75NTR. Immunofluorescent staining is primarily confined to the apical surface of MDCK cells expressing both native and N32D p75NTR forms (Fig. 4). Quantitation of steady state distribution of N32D p75NTR by domain-selective biotinylation, streptavidin precipitation, and immunoblotting reveals that 70–80% of the protein is localized to the apical surface (Fig. 5). Since neither tunicamycin treatment of cells nor mutation of the native N-glycosylation site compromises apical sorting of p75NTR, it is unlikely that the N-glycan is the main apical sorting determinant for this protein.
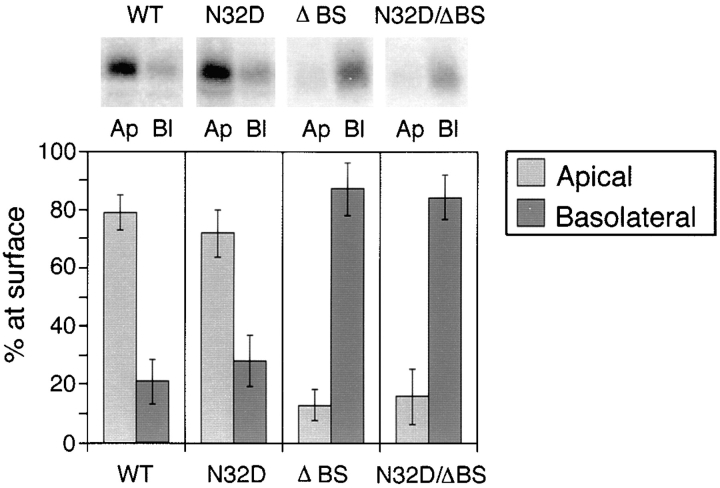
Figure 5.
Steady state biotinylation of rat p75NTR glycosylation mutants. Stably transfected MDCK clones expressing native and mutant forms of rat p75NTR were grown on filters for 5 d and then subjected to domain-selective biotinylation with NHS-SS-biotin as described in Materials and Methods. Biotinylated proteins were precipitated from cell lysates with avidin-agarose and resolved by SDS-PAGE. Electrophoretic protein transfers were probed with rabbit polyclonal antibodies against the cytoplasmic domain of p75NTR and detected with 125I-anti–rabbit IgG. Blots were analyzed with a Molecular Dynamics phosphorimager. Bars represent the mean ± SD of multiple determinations from three separate experiments (n = 8).
Apical Sorting of p75NTR in the TGN Is Dependent on the O-glycosylated Membrane Stalk Domain
The mature form of p75NTR contains O-glycoside–linked oligosaccharides, clustered in the serine/threonine-rich stalk domain immediately adjacent to the membrane-spanning segment (Johnson et al., 1986; Radeke et al., 1987). Sugars exposed at this location could interact with putative membrane-sorting receptors or lipid domains involved in sorting. To determine whether this juxtamembrane region is responsible for apical sorting of p75NTR, mutant receptors lacking most of this domain (ΔBS, deleted of amino acids 168–218) or both the O-glycan domain and the N-glycan (N32D/ΔBS) were expressed by stable transfection into MDCK cells (Fig. 1). These proteins were previously shown to retain NGF binding activity and to be recognized by two conformation-sensitive mAbs directed to the NGF binding domain (Baldwin et al., 1992; Baldwin and Shooter, 1995). Therefore, proper folding and export of p75NTR from the ER does not appear to require the serine/threonine-rich stalk domain.
Assessment of glycanation status of the mutant receptors (Fig. 3) confirmed that both native- and stalk-deleted forms (ΔBS) were sensitive to N-glycosidase. Replacement of asparagine 32 with aspartic acid eliminated the native glycosylation site and caused proteins carrying this mutation (N32D and N32D/ΔBS) to become resistant to N-glycosidase treatment. Presence of O-glycans was evaluated by digestion with a combination of O-glycosidase and neuraminidase. Both native and N32D p75NTR forms (Fig. 3) were sensitive to O-glycosidase. However, the electrophoretic mobility of proteins in which the region between amino acids 168–218 was deleted was unaffected by O-glycosidase/neuraminidase treatment, indicating that all potential serine/threonine glycosylation sites had been removed.
Indirect immunofluorescence and laser-scanning confocal microscopy of cells stained with antibodies against p75NTR demonstrates that neither N- nor O-glycosylation is required for surface expression of this protein (Fig. 4). However, the membrane domain in which p75NTR accumulates is profoundly affected by the presence of the O-glycosylated membrane stalk domain. En face images reveal a clear punctate apical staining in cells expressing native and N32D p75NTR. Vertical sections clearly show intense, specific fluorescence on the apical membrane. In striking contrast, cells expressing either ΔBS p75NTR or N32D/ΔBS p75NTR show most intense, specific fluorescent staining on the basolateral membrane. Little or no specific apical staining is detected in these cells.
To confirm and quantify these steady state distributions, domain-selective biotinylation and immunoblotting were performed as described in Materials and Methods. Native rat p75NTR was predominantly (80%) located on the apical membrane domain, in agreement with results obtained with the human receptor (Fig. 5). On the other hand, <10% of surface p75NTR was biotinylated on the apical membrane in cells expressing either ΔBS p75NTR or N32D/ ΔBS p75NTR (Fig. 5). Most of the protein at the surface of these cells (>90%) was labeled from the basolateral surface.
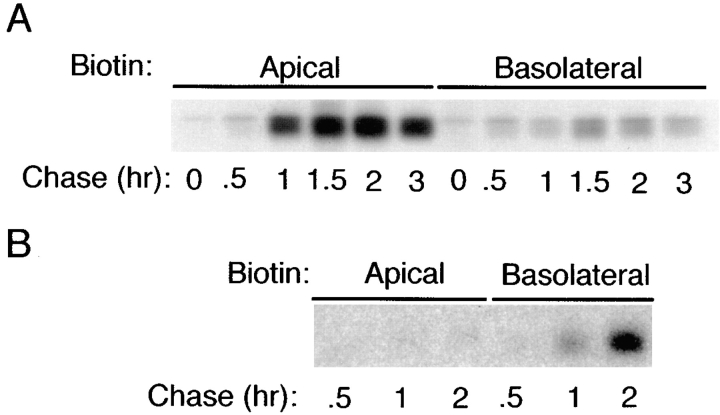
The reversed polarity of ΔBS p75NTR observed at steady state could be a consequence of a change in TGN sorting or in events occurring after arrival at the plasma membrane, such as selective stabilization of the mutant form on the basolateral surface or selective removal from the apical surface. Targeting of metabolically labeled protein was examined to determine whether a change in TGN sorting contributes to this reversed polarity (Fig. 6). In cells expressing native rat p75NTR, <20% of newly synthesized receptor could be detected upon basolateral membrane biotinylation, whereas >80% was labeled during biotinylation of the apical membrane domain (Fig. 6 A). In contrast, >90% of newly synthesized ΔBS p75NTR was labeled from the basolateral surface and almost none was labeled by apically applied biotin (Fig. 6 B). This result indicates that ΔBS p75NTR is vectorially targeted to the basolateral membrane domain from the TGN, and suggests that structural information for apical sorting of p75NTR is contained in a region between residues 168–218 in the extracellular domain.
Figure 6.
ΔBS p75NTR is targeted directly to the basal-lateral domain of MDCK cells. Polarized MDCK cultures stably expressing (A) native rat p75NTR or (B) ΔBS rat p75NTR were starved in cysteine-free medium for 30 min and then pulse labeled with [35S]cysteine for 20 min. After the pulse, cultures were chased in medium containing an excess of unlabeled cysteine. At indicated chase times, filters were placed on ice and incubated with NHS-SS-biotin either in the apical or basolateral medium. p75NTR was immunoprecipitated from cell lysates and surface-labeled protein was recovered on streptavidin-agarose. Images shown were obtained after exposure of the gel to Kodak XAR-5 film (A) or Molecular Dynamics phosphorimager screen (B).
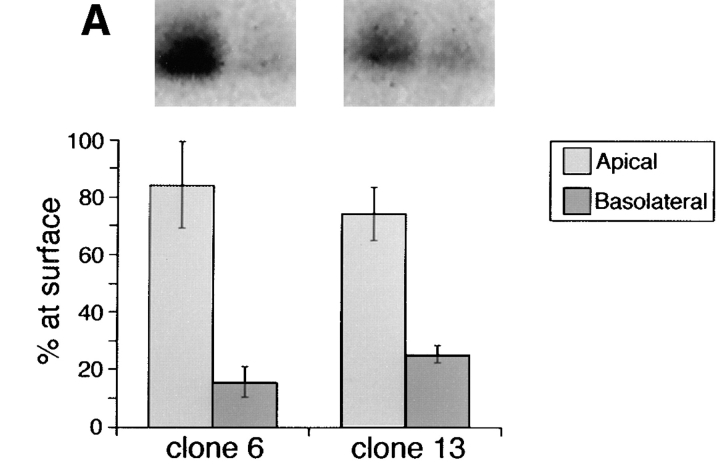
We reported previously that deletion of amino acids 203–215 within the stalk domain did not affect apical sorting of human p75NTR (Le Bivic et al., 1991). Although it is true that deletion of residues 203–215 removes a cluster of potentially glycosylated amino acids, the mutant receptor (BE p75NTR) still retains nine serine/threonine residues within the region spanning residues 168–218 (Table I). To further define the size of the domain involved in apical sorting, a third mutant was constructed, in which amino acids 193–215 were deleted (Fig. 1). This protein retains 10 of the 16 serine/threonine residues present in the native receptor and contains O-linked oligosaccharides (Table I). Cell surface biotinylation revealed that the steady state distributions of Δ193–215 p75NTR in two independently isolated clones were 80–85% apical and 15–20% basolateral (Fig. 7 A). These distributions reflect direct delivery from the TGN, because targeting of metabolically labeled protein occurs primarily to the apical surface domain (Fig. 7 B). Therefore, apical sorting information is likely contained within a region spanning residues 168–193 or 216–218.
Table I.
Mutations within Serine/Threonine-rich Stalk Domain
| Mutant | Stalk length (amino acids) | Ser/Thr residues | Potential O-glycans* | Polarity (Ap/Bl) | ||||
|---|---|---|---|---|---|---|---|---|
| Native | 60 | 16 | 11 | 85:15 | ||||
| BE (Δ203–215)‡ | 47 | 12 | 9 | 85:15 | ||||
| Δ193–215 | 37 | 10 | 7 | 80:20 | ||||
| ΔBS (Δ168–218) | 9 | 2 | 0 | 10:90 |
Prediction based on Hansen et al. (1995).
Data from Le Bivic et al. (1991).
Figure 7.
Amino acids 193–215 can be deleted from the juxtamembrane stalk domain without compromising apical sorting of p75NTR. (A) Stably transfected MDCK clones expressing Δ193–215 p75NTR were grown on filters for 5 d and then subjected to domain-selective biotinylation with NHS-SS-biotin as described in Materials and Methods. Biotinylated proteins were precipitated from cell lysates with avidin-agarose and resolved by SDS-PAGE. Electrophoretic protein transfers were probed with rabbit polyclonal antibodies against the cytoplasmic domain of p75NTR and detected with 125I-anti–rabbit IgG. Blots were analyzed with a Molecular Dynamics phosphorimager. Bars represent the mean ± SD of triplicate determinations from a representative experiment. (B) Stably transfected MDCK cultures expressing Δ193–215 p75NTR were starved in cysteine-free medium for 30 min and then pulse labeled with [35S]cysteine for 20 min. After the pulse, cultures were chased in medium containing an excess of unlabeled cysteine. At indicated chase times, filters were placed on ice and incubated with NHS-SS-biotin either in the apical or basolateral medium. Δ193–215 p75NTR was immunoprecipitated from cell lysates with an antibody against the cysteine-rich domain (ME 20.4) and surface-labeled protein was recovered on streptavidin-agarose.
The O-glycosylated Domain Is Required for Apical Secretion of Soluble p75NTR
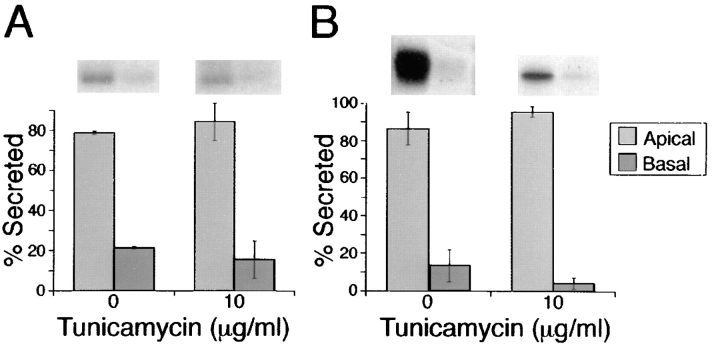
Apical sorting of p75NTR is associated with possession of an O-glycosylated juxtamembrane domain but not with possession of an N-glycan. However, N-glycans are proposed to be involved in apical sorting of some secretory proteins (Urban et al., 1987; Kitigawa et al., 1994; Scheiffele et al., 1995). It is possible that N-glycans serve as sorting determinants for secreted apical proteins, but that membrane-anchored proteins rely on other types of apical sorting signals, such as an O-glycosylated domain or a glycosyl-phosphatidylinositol anchor (Lisanti et al., 1991). If this were true for apical secretion in general, then a soluble form of p75NTR would be expected to use an N-glycan as an apical sorting signal. To address this possibility, MDCK cells were stably transfected with a cDNA encoding p75NTR sol, a truncated protein that lacks both the transmembrane and cytoplasmic domains (Fig. 1). p75NTR sol is secreted largely (80%) into the apical medium (Fig. 8 A). When cells are treated with tunicamycin, nonglycosylated p75NTR sol continues to be secreted apically (85%). A truncated form of the basolateral protein N-CAM is also apically secreted (85%) in transfected MDCK cells (Fig. 8 B; Le Gall et al., 1997). In cells treated with tunicamycin, N-CAMsol exits the ER poorly (data not shown). However, the fraction of N-CAMsol that is secreted (5–10% of control levels) is directed apically (90%). Therefore, for anchor-minus forms of two glycoproteins, routing into the apical pathway occurs independently of N-glycosylation.
Figure 8.
N-glycosylation is not required for apical secretion of soluble forms of p75NTR or N-CAM. Stably transfected MDCK clones expressing truncated ectodomains of either (A) p75NTR or (B) N-CAM were pretreated for 2.5 h with or without 10 μg/ml tunicamycin, starved for 30 min in medium lacking methionine and cysteine, and then pulse labeled with [35S]cysteine (for p75NTR labeling) or 35S-express (for N-CAM labeling) for 20 min. After the pulse, cultures were chased in the continued presence or absence of tunicamycin for 2 h. p75NTR and N-CAM were immunoprecipitated from apical and basolateral chase medium samples and resolved by SDS-PAGE. Fluorograms were analyzed by scanning densitometry. Bars represent mean ± SD for triplicate determinations from a representative experiment.
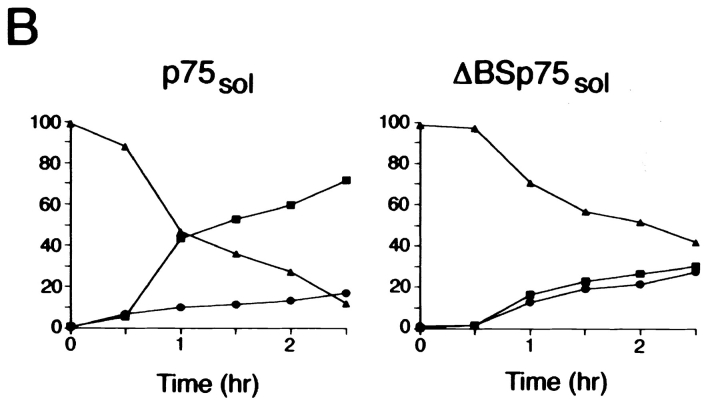
Since the O-glycosylated membrane stalk domain is required for apical sorting of membrane-anchored p75NTR, it is possible that this motif is also involved in apical secretion of the soluble protein. To test this hypothesis, a soluble form of p75NTR lacking the O-glycosylated domain was expressed in MDCK cells (Fig. 1). The resulting protein, ΔBS p75NTR sol, consisting of just the cysteine-rich domain, is secreted in a nonpolarized fashion in three independent clones (Fig. 9 A). A time course of secretion showed an equivalent level of ΔBS p75NTR sol recovered in apical and basolateral media over a 2.5-h chase period (Fig. 9 B). This is in contrast with soluble p75NTR containing the serine/ threonine domain, which is approximately fivefold more abundant in the apical medium at time points later than 30 min (Fig. 9 B). It is worth noting that ΔBS p75NTR sol is N-glycosylated because tunicamycin treatment of cells results in expression of protein with an increased electrophoretic mobility (Fig. 9 A). Nevertheless, the N-glycan does not mediate apical sorting of this protein.
Figure 9.
O-glycosylated domain is required for apical secretion of soluble p75NTR. (A) Clones of MDCK cells stably expressing human p75NTR truncated at amino acid 168 were pretreated for 2.5 h with or without 10 μg/ml tunicamycin. Cells were starved in cysteine-free medium for 30 min then pulse labeled with [35S]cysteine for 20 min. After the pulse, cultures were chased in the continued presence or absence of tunicamycin for 3 h. ΔBS p75NTR sol was immunoprecipitated from apical and basolateral chase medium and from cell lysates with a mAb against the cysteine-rich domain (ME 20.4). After SDS-PAGE, p75NTR was detected by fluorography. Results obtained with three independent clones are shown. (B) Time course of secretion of soluble p75NTR with and without O-glycosylated domain. Cells were starved in cysteine-free medium for 30 min, pulse labeled with [35S]cysteine for 20 min, and then chased in medium containing excess unlabeled cysteine for indicated times. p75NTR sol and ΔBS p75NTR sol were immunoprecipitated from apical and basolateral chase medium and from cell lysates with a mAb against the cysteine-rich domain (ME 20.4). Proteins were resolved by SDS-PAGE and gels were analyzed with a Molecular Dynamics phosphorimager. Apical, basolateral, and cells indicated by closed squares (▪), closed circles (•), and closed triangels (▴), respectively.
A second consequence of removing the juxtamembrane serine/threonine-rich segment from p75sol is that ER-to-Golgi transport is slowed. In cells expressing ΔBS p75NTR sol, protein is secreted from cells with a half-time of ∼2 h, and ∼40% of the protein remains associated with the cell layer after 2.5 h of chase (Fig. 9, A and B). These kinetics are significantly slower than the exit time observed for p75NTR sol containing the serine/threonine domain. This protein is secreted from cells with a half-time of ∼1 h, and only 12% of the protein remains in the cells after 2.5 h of chase (Fig. 9 B). Cell-associated ΔBS p75NTR sol has a slightly faster electrophoretic mobility than that of the secreted protein, and is sensitive to tunicamycin, suggesting that it bears an N-glycan that has not received terminal modifications (Fig. 9 A). This protein likely represents a precursor form in the ER because it is sensitive to digestion with Endo H, and immunofluorescent staining of cells shows a clear ER distribution of the protein (data not shown). Therefore, removal of the serine/threonine-rich domain from p75NTR sol (but not from native p75NTR), apparently reduces the efficiency of ER-to-Golgi transport relative to proteins containing this domain. This may be due to protein misfolding or to removal of sequences required for efficient export from the ER. It must be stressed, however, that two-thirds of pulse-labeled ΔBS p75NTR sol is secreted into the medium during a 3-h chase. This appears in equal amounts in apical and basolateral mediums, demonstrating that the major pool of p75NTR sol is secreted but not sorted upon removal of the serine/threonine-rich domain.
Basolateral Transport of Stalk-Minus p75NTR Is Dependent on Cytoplasmic Domain Sequences
A surprising aspect of these observations is that the consequence of removing the O-glycosylated stalk domain is not identical for transmembrane and soluble forms of p75NTR. Stalk-minus p75NTR lacking transmembrane and cytoplasmic domains is secreted from cells in a nonpolar fashion, but the same protein containing these domains is targeted to the basolateral membrane (Fig. 1). This suggests that sequences present in either the transmembrane or cytoplasmic domain are required for basolateral sorting of p75NTR when the O-glycosylated stalk is deleted. To address this possibility, we examined the polarity of ΔBS p75NTR lacking the cytoplasmic domain.
ΔBStail-minus p75NTR has a deletion of the entire cytoplasmic domain with the exception of five amino acids closest to the membrane (Fig. 1). In earlier published results, it was shown that p75NTR lacking the cytoplasmic domain was apically sorted, presumably because the O-glycan domain was present (Fig. 1; XI p75NTR; Le Bivic et al., 1991). However, when the cytoplasmic domain and the O-glycosylated membrane stalk domain are simultaneously deleted, the resulting protein is expressed in a nonpolarized fashion (Fig. 10 A). In two independent clones, steady state distributions of ΔBStail-minus p75NTR were 40–45% apical and 55–60% basolateral. These distributions likely reflect direct insertion of newly synthesized protein into both the apical and basolateral membrane domains at roughly equivalent levels. Metabolic targeting of newly synthesized ΔBStail-minus p75NTR shows that approximately equal levels of protein appear on apical and basolateral membranes after 2 h of chase, although the protein is detected on the basolateral surface earlier than the apical surface (Fig. 10 B). Therefore, basolateral sorting of ΔBS p75NTR appears to require sequences present in the cytoplasmic domain.
Figure 10.
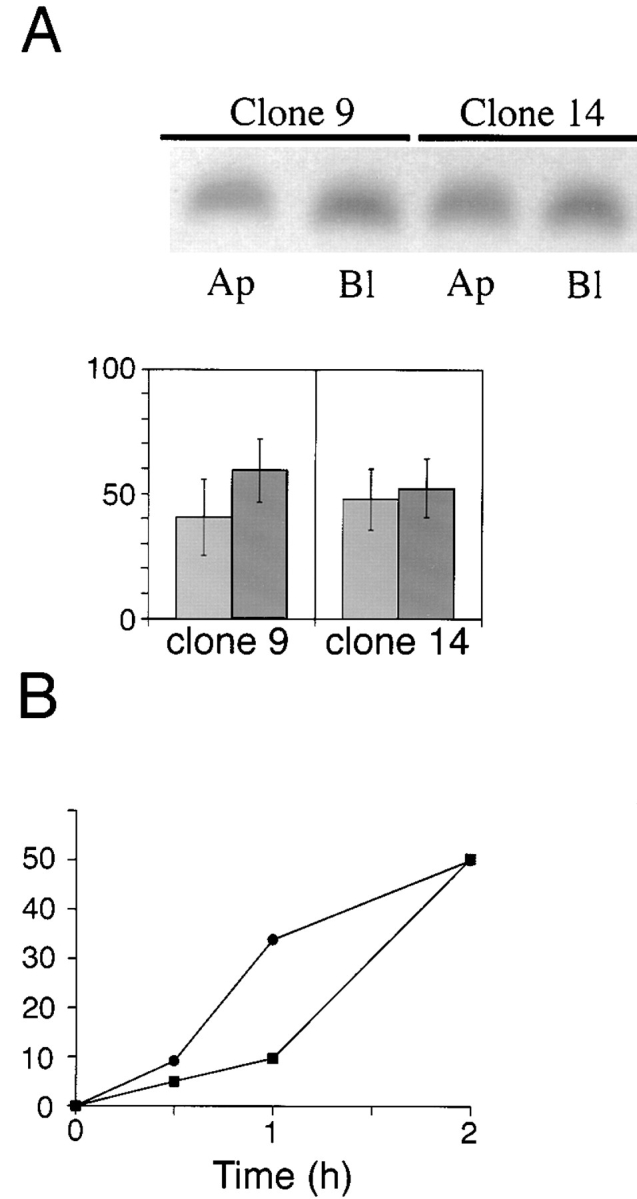
Basolateral expression of ΔBS p75 is dependent on the cytoplasmic domain. Clones of MDCK cells expressing human p75NTR with an internal deletion of the O-glycosylated membrane stalk domain (amino acids 168–218) and truncated at amino acid 248 were grown on filters for 5 d. (A) Cultures were subjected to domain-selective biotinylation with NHS-LC-biotin. ΔBS p75tail-minus was immunoprecipitated from cell lysates with a mAb against the cysteine-rich domain (ME 20.4) and resolved by SDS-PAGE. Electrophoretic protein transfers were probed with 125I-streptavidin. Blots were analyzed with a Molecular Dynamics phosphorimager. Bars (% apical, light grey, and % basolateral, dark grey, respectively) represent the mean ± SD of triplicate determinations in a representative experiment. Results obtained with two independent clones are shown. (B) Stably transfected MDCK cultures expressing ΔBS p75tail-minus were starved in cysteine-free medium for 30 min and then pulse labeled with [35S]cysteine for 20 min. After the pulse, cultures were chased in medium containing an excess of unlabeled cysteine. At indicated chase times, filters were placed on ice and incubated with NHS-SS-biotin either in the apical or basolateral medium. ΔBS p75tail-minus was immunoprecipitated from cell lysates and surface-labeled protein was recovered on streptavidin-agarose. % apical and % basolateral indicated by closed squares (▪) and closed circles (•), respectively.
Discussion
Very solid evidence supports the involvement of a mannose-6-phosphate N-glycan in lysosomal targeting (Kornfield and Mellman, 1989). In contrast, evidence in support of N-glycan participation in protein targeting to the cell surface is very controversial. Recent work has highlighted a possible role of N-glycans in apical targeting of certain secretory proteins (for review see Fiedler and Simons, 1995). It was proposed that N-glycans may serve as apical sorting determinants for influenza hemagglutinin nearly two decades ago (Rodriguez-Boulan and Sabatini, 1978). However, when this hypothesis was tested directly, it was concluded that specific sorting information for hemagglutinin was not inherent in the N-glycans, but was likely contained in the polypeptide (Roth et al., 1979; Green et al., 1981). Since that time, no data has been reported to suggest otherwise for transmembrane apical proteins.
In the current study, we have reconsidered a potential role for N-glycans in apical sorting of one membrane-spanning protein, the neurotrophin receptor p75NTR. The principle advantage in using this protein for our studies is that it has a single N-glycan, that facilitates mutational analysis. It is also clear from the current study, as well as previous studies, that N-glycosylation is not required for proper folding and export of p75NTR from the ER (Grob et al., 1985; Baldwin et al., 1992). Therefore, consequences of N-glycosylation on apical protein sorting per se can be studied independently of general effects on folding and secretion. Results of these studies are inconsistent with a role for N-glycans in apical sorting of p75NTR. Instead, an apical sorting determinant appears to be located in a region of the external domain of the protein spanning residues 168–193. This region is rich in serine/threonine residues, many of which are O-glycosylated, but it must be stressed that there is insufficient data at present to propose that the sugars themselves mediate apical sorting of p75NTR.
N-glycans Are Not Necessary for Apical Sorting of Membrane-anchored or Soluble Forms of p75NTR
Quantitative analysis of the effects of N-glycosylation on polarized sorting of apical membrane glycoproteins has never been reported. The data presented here demonstrate conclusively that N-glycans are not involved in apical sorting of p75NTR. First, newly synthesized p75NTR is inserted into the apical membrane domain irrespective of N-glycanation status. Tunicamycin treatment neither slows the rate nor alters the polarity with which receptors are delivered to the apical membrane. Site-directed mutagenesis of the single N-glycanation site on p75NTR does not affect its apical distribution. Second, receptors lacking the juxtamembrane stalk are efficiently targeted to the basolateral surface even though they are N-glycosylated. Finally, receptors lacking both the O-glycosylated stalk and the cytoplasmic domain are inserted randomly into apical and basolateral surfaces with no polarity, even though they still possess N-glycans. Therefore, the single N-glycan is not an apical sorting determinant for p75NTR.
It is well documented that anchor-minus forms of both apical and basolateral proteins are secreted apically (Mostov et al., 1987; Brown et al., 1989; Lisanti et al., 1990; Powell et al., 1991; Corbeil et al., 1992; Vogel et al., 1992b ; Weisz et al., 1992; Prill et al., 1993). It was recently speculated that N-glycans represent a general signal for apical sorting of these proteins (Fiedler and Simons, 1995). However, the present report is the first study in which a role for N-glycans in apical sorting of anchor-minus forms of membrane proteins was tested directly. Soluble forms of both p75NTR and N-CAM were apically sorted and secreted by cells cultured in the absence or presence of tunicamycin. In cultures treated with tunicamycin, p75NTR was efficiently secreted. In contrast, soluble N-CAM was poorly secreted, most likely due to misfolding in the ER. However, the polarity with which each of these proteins was secreted was nearly identical in tunicamycin treated and untreated cultures. These data suggest that signals other than N-glycans direct these proteins into the apical pathway. In the case of p75NTR, the signal appears to be contained within the region spanning residues 168–218, the same domain that is associated with apical sorting of the membrane-anchored protein. N-CAM does not possess a serine/threonine-rich region and is not O-glycosylated (Cunningham et al., 1987). Therefore, it is likely that a novel type of signal mediates apical sorting of this protein.
These results confirm and extend conclusions that we and others presented previously, that N-glycans are not necessary for apical sorting of influenza virus hemagglutinin (Roth et al., 1979; Green et al., 1981). Our findings are also consistent with reports that apical sorting of several other proteins is independent of N-glycosylation (Lisanti et al., 1989, 1990; Ullrich et al., 1991; Ragno et al., 1992; Gonzalez et al., 1993), and that many N-glycosylated proteins are poorly sorted in MDCK cells (Stephens and Compans, 1986; Soole et al., 1992; Geffen et al., 1993; Haller and Alper, 1993; Thomas et al., 1993; Le Gall et al., 1997). However, these data are not inconsistent with the possible involvement of N-glycans in sorting of a subset of apical proteins (Urban et al., 1987; Kitigawa et al., 1994; Scheiffele et al., 1995). Nevertheless, it is clear that N-glycans do not represent a general signal for apical sorting.
A Juxtamembrane Region in the External Domain of p75NTR is Required for Apical Sorting
The extracellular domain of p75NTR is composed of four cysteine-rich repeat segments that constitute the NGF-binding domain, which are connected to the membrane-spanning domain by a spacer, or stalk, of 60 amino acids that are not required for NGF binding (Baldwin et al., 1992). Deletion of the cysteine-rich repeats does not compromise apical sorting of the receptor in MDCK cells (Monlauzer L. and A. Le Bivic, unpublished observations). Furthermore, a construct comprising only the four cysteine-rich repeats is poorly sorted and secreted from both apical and basolateral surfaces equivalently (Fig. 9). Therefore, structural information for apical sorting of p75NTR does not appear to be contained within the cysteine-rich repeats.
In contrast, deletion of 51 amino acids from within the juxtamembrane stalk domain reverses apical sorting of the protein in transfected MDCK cells. Since the mutant receptor is targeted directly to the basolateral membrane, it is likely that apical sorting information is contained within the region spanning residues 168–218. Nearly one-third of the residues within this region are serines or threonines, many of which are O-glycosylated. Previously, we reported that receptors deleted of amino acids 203–215 were sorted and delivered to the apical surface of MDCK cells with the same efficiency as native p75NTR (Le Bivic et al., 1991). Although deletion of residues 203–215 was considered sufficient to remove a cluster of sites for attachment of O-linked sugars and suggested to us that these moieties were not required for apical sorting of p75NTR, it is now evident that the deletion left intact a structural domain necessary for apical sorting of this protein. We attribute this oversight to the fact that the focus of our previous study was on a novel cytoplasmic sequence mediating basolateral sorting and rapid endocytosis of p75NTR.
A third deletion mutant lacking residues 193–215 was constructed to further define the boundaries of the apical sorting determinant. This protein has a truncated stalk of 37 amino acids between the last cysteine residue and the membrane-spanning segment. Like native p75NTR, proteins deleted of residues 193–215 are sorted and delivered primarily (80%) to the apical membrane domain. Therefore, we conclude that the apical sorting signal is likely contained within the region spanning either amino acids 168–193 or 216–218.
The precise structural determinants within the juxtamembrane stalk region that are required for apical sorting have not yet been identified. The most conspicuous feature of this domain is the presence of 16 serines and threonines (Table I). Most of these likely serve as attachment sites for O-glycans (Hansen et al., 1995). Therefore, either specific amino acids or sugar moieties within the stalk potentially contribute to the activity of the apical signal. One possibility is that a minimum number of glycosylated serine/threonine residues is required within the juxtamembrane domain to form a critical conformation. O-glycosylated regions adopt extended conformations resembling semiflexible rods with a length of ∼2.5 angstroms per amino acid residue (Jentoft, 1990). Such a structure could be required to hold the cysteine-rich neurotrophin binding domain away from the lipid bilayer and surrounding glycocalyx, possibly to allow apical sorting machinery to interact with signals present in the stalk domain. In the absence of such spacing, apical signals may not be recognized for steric reasons, and the protein is sorted basolaterally.
Juxtamembrane serine/threonine-rich domains are present in several apical membrane proteins, including sucrase-isomaltase (Hunziker et al., 1986), aminopeptidase N (Olsen et al., 1988), and decay-accelerating factor (Medof et al., 1987). Other apical membrane proteins, including maltase-glucoamylase (Naim et al., 1988), γ-glutamyltranspeptidase (Blochberger et al., 1989), and mucins such as gp40 (Zimmer et al., 1995) are O-glycosylated, although serine/threonine-rich domains have not been described on these proteins. However, there is currently no evidence that a stalk region is required for apical sorting of any protein with the exception of p75NTR. Indeed, there are data in the literature that are inconsistent with the model that this motif serves as a general apical signal. An anchor-minus form of aminopeptidase N is secreted in a polarized manner into the apical medium of MDCK cells (Vogel et al., 1992b ). Deletion of the serine/threonine-rich stalk does not inhibit apical sorting of the catalytic head group, suggesting either that the stalk does not contain an apical sorting signal or that redundant sorting information is contained in both the stalk and the catalytic head group (Vogel et al., 1992a ). Furthermore, mutant transferrin receptors lacking a basolateral sorting signal are randomly distributed on apical and basolateral surfaces of MDCK cells despite the presence of O-glycans in the external domain (Odorizzi et al., 1996). Although these observations do not exclude the possibility that a juxtamembrane region rich in glycosylated serine/ threonine residues mediates apical sorting of a family of proteins, it is clear that further studies are required to address this issue.
A Hierarchy of Sorting Signals Determines the Polarized Distribution of p75NTR in MDCK Cells
Deletion of residues 168–218 from the external domain of p75NTR reverses its sorting from apical to basolateral in polarized MDCK cells. Stalk-minus p75NTR is targeted to the basolateral membrane domain with nearly perfect fidelity, suggesting either that a basolateral sorting signal is present in the mutant receptor or that ΔBS p75NTR is sent basolaterally by default. Two observations support the former explanation: (a) truncation of stalk-minus receptors at residue 251 causes random insertion of proteins into both apical and basolateral membrane domains; and (b) the default pathway in MDCK cells is not basolateral. Proteins that lack any sorting information are sent to both apical and basolateral membrane domains at roughly equivalent levels (Kondor-Koch et al., 1985; Gottleib et al., 1986; Stephens and Compens, 1986; Soole et al., 1992; Haller and Alper, 1993; Mays et al., 1995; Odorizzi et al., 1996). Therefore, the cytoplasmic domain of p75NTR appears to contain structural information to specify basolateral sorting of receptors lacking juxtamembrane serine/threonine-rich domains. Since the native receptor is sorted apically with ∼85% fidelity, it is clear that basolateral sorting signals do not function efficiently in receptors containing O-glycosylated stalks. Therefore, it seems likely that cytoplasmic sequences are recessive to apical sorting information contained within the stalk domain.
Although the apical sorting signal contained in the juxtamembrane stalk domain of p75NTR appears to be dominant over basolateral sorting information in the cytoplasmic domain, several observations indicate that this apical sorting signal is recessive to other known basolateral sorting signals. First, we reported previously that an internal deletion in the cytoplasmic domain of p75NTR that places a tyrosine in a charged environment next to the membrane reverses the polarity of the protein so that mutant receptors are targeted basolaterally, even though these proteins contain an O-glycosylated domain (Le Bivic et al., 1991). This suggests that tyrosine-dependent basolateral signals likely have a higher affinity for sorting machinery than the apical sorting signal on p75NTR. Furthermore, the tyrosine-independent basolateral sorting signal in the cytoplasmic domain of N-CAM can redirect chimeras containing this signal fused to the transmembrane and luminal domains of p75NTR into the basolateral pathway (Le Gall et al., 1997). Finally, receptors for both polymeric immunoglobulin and low density lipoprotein contain juxtamembrane serine/ threonine-rich domains similar to that of p75NTR, but are nonetheless targeted basolaterally due to the presence of dominant basolateral sorting signals in the cytoplasmic domain (Casanova et al., 1991; Hunziker et al., 1991).
On the basis of these observations, we propose that there exists a hierarchy of three types of sorting determinants in transmembrane proteins: dominant basolateral signals, consisting of tyrosine-based motifs present in many proteins including low density lipoprotein (LDL) receptor and vesicular stomatitis virus glycoprotein (VSV G) and nontyrosine-based signals in polymeric immunoglobulin receptor (pIgR) and N-CAM; apical sorting signals, contained within the O-glycosylated stalk of p75NTR; and recessive basolateral signals, contained in cytoplasmic domains of p75NTR and dipeptidyl peptidase IV (Weisz et al., 1992). Only when none of these signals is present, as in the case of ΔBStail-minus p75NTR, is the protein randomly delivered to the plasma membrane. However, it must be stressed that recessive basolateral sorting signals are not weak in activity, since ΔBS p75NTR is targeted to the basolateral surface with a fidelity of >90%. Also, the cytoplasmic domain of dipeptidyl peptidase IV is capable of directing a lysozyme fusion protein into the basolateral pathway with a fidelity of >90% (Weisz et al., 1992). We suggest, rather, that this type of basolateral signal competes poorly for sorting machinery when a functional apical sorting determinant is present.
The nature of the recessive basolateral signal in the cytoplasmic domain of p75NTR is unknown. It must be structurally distinct from tyrosine-dependent basolateral sorting signals because these are dominant over the apical signal contained within the serine/threonine-rich domain. This also suggests that the recessive basolateral signal interacts with a different set of basolateral sorting machinery than tyrosine-dependent signals. One type of sorting signal that could mediate targeting of stalk-minus p75NTR is the dileucine motif, which mediates basolateral sorting of macrophage Fc receptors (FcRII-B2) in MDCK cells (Hunziker and Fumey, 1994; Matter et al., 1994). This type of signal appears to utilize a distinct mechanism than that used by tyrosine-based sorting signals (Marks et al., 1996). The cytoplasmic domain of p75NTR contains three dileucine motifs (Johnson et al, 1986; Radeke et al., 1987). If these mediate basolateral sorting of stalk-minus p75NTR, then the dileucine motif might represent a type of basolateral sorting signal present on naturally occuring membrane proteins that are targeted basolaterally because they lack apical sorting information.
Acknowledgments
This work was supported by National Institutes of Health grants GM 34107 and GM 41771 (to E. Rodriguez-Boulan), and the Jules and Doris Stein Professorship (to E. Rodriguez-Boulan) from the Research to Prevent Blindness Foundation and by the Dyson Foundation. C. Yeaman is supported by a postdoctoral fellowship from the National Kidney Foundation. A. Le Bivic and L. Monlauzeur were supported by grants from the Centre National de la Recherche Scientifique (UMR 6545), the Association pour la Recherche sur le Cancer (6421), the Institut National de la Sante et de la Recherche Medicale (930203), and the Association Francaise de Lutte contre la Mucoviscidose.
Abbreviations used in this paper
- N-CAM
neuronal cell adhesion molecule
- pfu
plaque formation units
Footnotes
Address all correspondence to Enrique Rodriguez-Boulan, Dyson Vision Research Institute, Room LC-300, Department of Ophthalmology, Cornell University Medical College, 1300 York Avenue, New York, NY 10021. Tel.: (212) 746-2272. Fax: (212) 746-8175. E-mail: boulan@med.cornell.edu
We are very grateful to W.J. Nelson (Stanford University Medical School, Stanford, CA) for critically reading the manuscript.
References
- Baldwin AN, Shooter EM. Zone mapping of the binding domain of the rat low affinity nerve growth factor receptor by the introduction of novel N-glycosylation sites. J Biol Chem. 1995;270:4594–4602. doi: 10.1074/jbc.270.9.4594. [DOI] [PubMed] [Google Scholar]
- Baldwin AN, Bitler CM, Welcher AA, Shooter EM. Studies on the structure and binding properties of the cysteine-rich domain of rat low affinity nerve growth factor receptor (p75NGFR) J Biol Chem. 1992;267:8352–8359. [PubMed] [Google Scholar]
- Blochberger TC, Sabatine JM, Lee YC, Hughey RP. O-linked glycosylation of rat renal g-glutamylpeptidase adjacent to its membrane anchor domain. J Biol Chem. 1989;264:20718–20722. [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Corbeil D, Boileau G, Lemay G, Crine P. Expression and polarized apical secretion in Madin-Darby canine kidney cells of a recombinant soluble form of neutral endopeptidase lacking the cytosolic and transmembrane domains. J Biol Chem. 1992;267:2798–2801. [PubMed] [Google Scholar]
- Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Geffen I, Fuhrer C, Leitinger B, Weiss M, Huggel K, Griffiths G, Spiess M. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993;268:20772–20777. [PubMed] [Google Scholar]
- Gonzalez A, Nicovani S, Juica F. Apical secretion of hepatitis B surface antigen from transfected Madin-Darby canine kidney cells. J Biol Chem. 1993;268:6662–6667. [PubMed] [Google Scholar]
- Gottlieb TA, Baudry G, Rizzolo L, Colman A, Rindler M, Adesnik M, Sabatini DD. Secretion of endogenous and exogenous proteins from polarized MDCK cell monolayers. Proc Natl Acad Sci USA. 1986;83:2100–2104. doi: 10.1073/pnas.83.7.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F, Van der Eb A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green RF, Meiss HK, Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981;89:230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob PM, Berlot CH, Bothwell MA. Affinity labeling and partial purification of nerve growth factor receptors from rat pheochromocytoma and human melanoma cells. Proc Natl Acad Sci USA. 1983;80:6819–6823. doi: 10.1073/pnas.80.22.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob PM, Ross AH, Koprowski H, Bothwell M. Characterization of the human melanoma nerve growth factor receptor. J Biol Chem. 1985;260:8044–8049. [PubMed] [Google Scholar]
- Haller C, Alper SL. Nonpolarized surface distribution and delivery of human CD7 in polarized MDCK cells. Am J Physiol. 1993;265:C1069–C1079. doi: 10.1152/ajpcell.1993.265.4.C1069. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Lund O, Engelbrecht J, Bohr H, Nielsen JO, Hansen JE. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GalNac:polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A dileucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO (Eur Mol Biol Organ) J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Spiess M, Semenza G, Lodish HF. The sucrase–isomaltase complex: primary structure, membrane orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986;46:227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Jalal F, Lemay G, Zollinger M, Berteloot A, Boileau G, Crine P. Neutral endopeptidase, a major brush border protein of the kidney proximal nephron, is directly targeted to the apical domain when expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991;266:19826–19832. [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? . Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Kitigawa Y, Sano Y, Ueda M, Higashio K, Narita H, Okano M, Matsumoto S-I, Sasaki R. N-glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp Cell Res. 1994;213:449–457. doi: 10.1006/excr.1994.1222. [DOI] [PubMed] [Google Scholar]
- Kondor-Koch C, Bravo R, Fuller SD, Cutler D, Garoff H. Exocytic pathways exist to both the apical and the basolateral cell surface of the polarized epithelial cell MDCK. Cell. 1985;43:297–306. doi: 10.1016/0092-8674(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Mostov K, Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990;110:1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991;115:607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall AH, Powell SK, Yeaman CA, Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J Biol Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- Lisanti M, Caras IP, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Gilbert T, Hanzel D, Rodriguez-Boulan E. Vectorial apical delivery and slow endocytosis of a glycolipid-anchored fusion protein in transfected MDCK cells. Proc Nat Acad Sci USA. 1990;87:7419–7423. doi: 10.1073/pnas.87.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Woodruff L, Ohno H, Bonifacino J. Protein targeting by tyrosine- and dileucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc Receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen NL, Nayak DP, Stephens EB, Compans RW. Polarized expression of a chimeric protein in which the transmembrane and cytoplasmic domains of the influenza virus hemagglutinin have been replaced by those of the vesicular stomatitis virus G protein. Proc Natl Acad Sci USA. 1986;83:9318–9322. doi: 10.1073/pnas.83.24.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof ME, Lublin DM, Holers VM, Ayers DJ, Getty RR, Leykam JF, Atkinson JP, Tykocinski ML. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci USA. 1987;84:2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov KE, Kops AB, Deitcher DL. Deletion of the cytoplasmic domain of the polymeric immunoglobulin receptor prevents basolateral localization and endocytosis. Cell. 1986;47:359–364. doi: 10.1016/0092-8674(86)90592-1. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Breitfeld P, Harris JM. An anchor-minus form of the polymeric immunoglobulin receptor is secreted predominantly apically in Madin-Darby canine kidney cells. J Cell Biol. 1987;105:2031–2036. doi: 10.1083/jcb.105.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto N. Polarized secretion of human corticosteoid binding globulin by MDCK and BeWo cells. Exp Cell Biol. 1993;209:271–276. doi: 10.1006/excr.1993.1311. [DOI] [PubMed] [Google Scholar]
- Naim HY, Sterchi EE, Lentze MJ. Structure, biosynthesis, and glycosylation of human small intestinal maltase-glucoamylase. J Biol Chem. 1988;263:19709–19717. [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge I, Hopkins C. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Cowell GM, Konigshofer E, Danielsen EM, Moller J, Lausten L, Hansen OC, Welinder KG, Engberg J, Hunziker W, et al. Complete amino acid sequence of human intestinal aminopeptidase N as deduced from cloned cDNA. FEBS (Fed Eur Biochem Soc) Lett. 1988;238:307–314. doi: 10.1016/0014-5793(88)80502-7. [DOI] [PubMed] [Google Scholar]
- Powell SK, Lisanti MP, Rodriguez-Boulan E. Thy-1 expresses two signals for apical localization in epithelial cells. Am J Physiol. 1991;260:C715–C720. doi: 10.1152/ajpcell.1991.260.4.C715. [DOI] [PubMed] [Google Scholar]
- Prill V, Lehmann L, von Figura K, Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO (Eur Mol Biol Organ) J. 1993;12:2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Ragno P, Estreicher A, Gos A, Wohlwend A, Belin D, Vassalli J-D. Polarized secretion of urokinase-type plasminogen activator by epithelial cells. Exp Cell Res. 1992;203:236–243. doi: 10.1016/0014-4827(92)90060-l. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Sabatini DD. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AH, Grob P, Bothwell M, Elder DE, Ernst CS, Marano N, Ghrist BFD, Slemp CC, Herlyn M, Atkinson B, Koprowski H. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies. Proc Natl Acad Sci USA. 1984;81:6681–6685. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MG, Fitzpatrick JP, Compans RW. Polarity of influenza and vesicular stomatitis virus maturation MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci USA. 1979;76:6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MG, Gundersen D, Patil N, Rodriguez-Boulan E. The large external domain is sufficient for the correct sorting of secreted or chimeric influenza virus hemagglutinins in polarized monkey kidney cells. J Cell Biol. 1987;104:769–782. doi: 10.1083/jcb.104.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. Integral and peripheral protein compositions of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989;107:277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Soole KL, Hall J, Jepson MA, Hazlewood GP, Gilbert HJ, Hirst BH. Constitutive secretion of a bacterial enzyme by polarized epithelial cells. J Cell Sci. 1992;102:495–504. doi: 10.1242/jcs.102.3.495. [DOI] [PubMed] [Google Scholar]
- Stephens EB, Compans RW. Nonpolarized expression of a secreted murine leukemia virus glycoprotein in polarized epithelial cells. Cell. 1986;47:1053–1059. doi: 10.1016/0092-8674(86)90820-2. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Ullrich O, Mann K, Haase W, Koch-Brandt C. Biosynthesis and secretion of an osteopontin-related 20-kD polypeptide in the Madin-Darby canine kidney cell line. J Biol Chem. 1991;266:3518–3525. [PubMed] [Google Scholar]
- Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel LK, Noren O, Sjostrom H. The apical sorting signal on human aminopeptidase N is not located in the stalk but in the catalytic head group. FEBS (Fed Eur Biochem Soc) Lett. 1992a;308:14–17. doi: 10.1016/0014-5793(92)81039-o. [DOI] [PubMed] [Google Scholar]
- Vogel LK, Spiess M, Sjostrom H, Noren O. Evidence for an apical sorting signal on the ectodomain of human aminopeptidase N. J Biol Chem. 1992b;267:2794–2797. [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz OA, Machamer CE, Hubbard AL. Rat liver dipeptidylpeptidase IV contains competing apical and basolateral targeting information. J Biol Chem. 1992;267:22282–22288. [PubMed] [Google Scholar]
- Wessels HP, Hansen GH, Fuhrer C, Look AT, Sjostrom H, Noren O, Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990;111:2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Krzeminski KA, Nelson WJ. Remodeling the cell surface distribution of membrane proteins during the development of epithelial cell polarity. J Cell Biol. 1992;116:889–899. doi: 10.1083/jcb.116.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Chao M V. Disruption of cysteine-rich repeats of the p75 nerve growth factor receptor leads to loss of ligand binding. J Biol Chem. 1991;266:12099–12104. [PubMed] [Google Scholar]
- Yeaman C, Heinflink M, Falck-Petersen E, Rodriguez-Boulan E, Gershengorn MC. Polarity of TRH receptors in transfected MDCK cells is independent of endocytosis signals and G protein coupling. Am J Physiol. 1996;270:C753–C762. doi: 10.1152/ajpcell.1996.270.3.C753. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Klenk H-D, Herrler G. Identification of a 40-kD cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J Biol Chem. 1995;270:17815–17822. doi: 10.1074/jbc.270.30.17815. [DOI] [PubMed] [Google Scholar]