Abstract
Numerous studies have indicated a protective effect of male circumcision against acquisition of human immunodeficiency virus (HIV)-1. We investigated mechanisms responsible for the possible increased HIV-1 susceptibility of human foreskin. Foreskins from eight pediatric and six adult patients with (n = 3) and without (n = 11) histories of sexually transmitted disease were evaluated. Six cervical biopsies from HIV-1-seronegative women were included as controls. CD4+ T cells, macrophages, and Langerhans’ cells (LCs) were quantified using image analysis. Cells expressing HIV-1 co-receptors CCR5 and CXCR4 were quantified using immunofluorescence and image analysis. Foreskin biopsies were infected ex vivo in organotypic culture with HIV-1. HIV-1 DNA copies in foreskin and cervical mucosal tissue were compared and the infected cell phenotype was determined. Foreskin mucosa contained higher mean proportions of CD4+ T cells (22.4%), macrophages (2.4%), and LCs (11.5%) in adults than in children (4.9%, 0.3%, and 6.2%, respectively) or in cervical mucosa (6.2%, 1.4%, and 1.5%, respectively). The highest proportions of CD4+ T cells and LCs occurred in patients with a history of infection. Foreskin immune cells expressed predominantly the CCR5 HIV-1 co-receptor. Adult foreskin mucosa had greater susceptibility to infection with HIVbal than cervical mucosa or the external surface of foreskin tissue. Circumcision likely reduces risk of HIV-1 acquisition in men by decreasing HIV-1 target cells.
By December 2001, ∼40 million people worldwide were infected with the human immunodeficiency virus (HIV-1). Of those, ∼80% of the infections occurred during sexual activity. 1 In men, an estimated 70% of HIV-1 infections have been acquired through vaginal intercourse and a smaller number through insertive anal sex. 2 Thus the route of infection for most HIV-1 infected men is via the penis. More than 35 observational epidemiological studies have reported a significant association between lack of male circumcision and HIV-1 acquisition with a twofold to eightfold increased risk of infection for uncircumcised men. 3-5 These findings have led to speculation concerning how HIV-1 enters the penis and the role of the foreskin in HIV-1 acquisition. 2,6
The major HIV-1 target cells [CD4+ T cells, macrophages, and Langerhans’ cells (LCs)] exist in animal and human genital tract mucosa, and chemokine receptors (CCR5, CXCR4) are expressed on these target cells in human genital tissue. 7 In the cervix, susceptibility to HIV-1 infection is associated with the number of HIV-1 target cells and the expression of the appropriate HIV-1 co-receptors. 7 However, little is known about the presence or density of HIV-1 target cells in the human foreskin, and there is no evidence to date of HIV-1 binding to target cells in human male preputial tissue. Here we report results from studies investigating the biological mechanisms of HIV-1 acquisition in the human prepuce. Using foreskin tissue acquired after elective or corrective surgery from infants, children, and adults in the United States, we assess the proportions of all cells that are major HIV-1 target cells, the expression of the chemokine receptors, and the infectability of these target cells with HIV-1. We compare results from foreskin tissue to equivalent studies of cervical tissue.
Materials and Methods
Patients
Foreskin and cervical tissues were obtained through urology practices and a women’s health clinic at two major medical centers in Chicago, IL. Female patients were 21 to 42 years of age in the proliferative phase of their menstrual cycle with no history of sexually transmitted diseases. Biopsies were obtained from women undergoing hysterectomy for benign conditions (eg, fibroid tumors). Male patients ranged in age from 10 months to 69 years. The medical reasons for circumcision were phimosis, with or without balinitis, adhesions, or redundant foreskin. Others were circumcised for religious, cultural, and cosmetic reasons. Informed consent was obtained from the parents of patients under the age of 18 years and from the adult patients directly. A brief questionnaire was administered to parents, in the case of patients under the age of 18, to obtain the reported reason for circumcision. The questionnaire administered to adult patients provided additional information on sexual history and history of sexually transmitted infections. The reason for the circumcision was also provided by the surgeon.
After surgery, foreskin tissues were held at 4°C and processed in the laboratory within 3 hours. Duplicate sample sections were fixed in Streck Tissue Fixative (Streck Laboratories, Omaha, NE), and paraffin-embedded or frozen in OCT tissue-freezing compound (Fisher Scientific, Pittsburgh, PA). Foreskin tissue was processed fresh for in vitro organ culture.
Immunohistochemistry/Immunofluorescence
Tissue sections were cut to 5 μm, adhered to silanized slides, and deparaffinized through xylenes and graded alcohols. After peroxidase quenching and blocking with mouse serum in phosphate-buffered saline (PBS), pH 7.4 with 5% nonfat dry skim milk, immunohistochemistry was performed using the Vectastain ABC-HP kit (Vector Laboratories, Burlingame, CA) as per the manufacturer’s recommendations. Diaminobenzidine was used as substrate with hematoxylin counterstain. Frozen tissue sections for quantitative image analysis were allowed to air-dry for 5 minutes, followed by postfixation in cold acetone for 20 minutes. Sections were washed in PBS and an optimized dilution of primary antibody was applied. Commercially available antibodies to CD4, CD45RO, CD68, CD1a, CCR5, and CXCR4 (PharMingen, San Diego, CA) were used at concentrations optimized on control tissues. Quantitative image analysis was performed on both immunohistochemically stained and immunofluorescence stained slides using MetaMorph software version 4.5 (Universal Imaging Corp., Pittsburgh, PA). Percentages were based on examination of the entire mucosal surface containing 50% epithelium and 50% submucosa within the field.
Foreskin Organotypic Culture
Organ culture conditions, HIV-1 infection of tissue, and tissue harvesting were generally performed as previously described. 8 Briefly, inner mucosal foreskin tissue samples as well as external foreskin tissue samples and cervical tissue samples were soaked in a concentrated antibiotic wash (20,000 U/ml penicillin/streptomycin, 250 μg/ml Fungizone, and 120 U/ml Nystatin) for 10 minutes. The tissues were then washed three times in Raft media to wash away any remaining antibiotics. A 4.0-mm Acu-punch biopsy scalpel (Acuderm, Ft. Lauderdale, FL) was used to provide a number of contiguous samples from each tissue, which were then measured for thickness. Three 4.0-mm biopsies from the inner mucosal surface and three from the outer external surface were cultured and infected in parallel in the same 12-well plate. Tissue biopsies were placed with the epithelial side up on a 3.0-μm membrane in the top chamber of a 12-well Transwell (Costar, Cambridge, MA). A 3% solution of agarose (SeaKem Agarose; FMC BioProducts, Rockland, ME) in Hanks’ balanced salt solution (Life Technologies, Inc., Grand Island, NY) was added to the area surrounding the tissue in the top well exposing only the epithelium. After 1 day in culture, the foreskin biopsies were infected with either 1000 TCID50 of the CCR5-using (R5) HIV-1Bal or the CXCR4-using (X4) HIV-1Lai. One day after infection, the tissues were harvested and infectivity quantified using real-time quantitative polymerase chain reaction for HIV-1 pol DNA. A qualitative assessment of the cell types infected was performed using simultaneous immunophenotyping for CD4, CD68, and/or CD1a and UFISH for HIV-1 gag-pol mRNA.
Real-Time Quantitative Polymerase Chain Reaction for HIV-1 pol DNA
Real-time quantitative DNA polymerase chain reaction was performed by adding 45 μl of reaction mix [1× Taqman polymerase chain reaction buffer (PE Applied Biosystems, Foster City, CA), 4.0 mmol/L MgCl2, 200 μmol/L dATP, 200 μmol/L dCTP, 200 μmol/L dGTP, 200 μmol/L dTTP, 200 nmol/L forward primer (5′-AACTCCATCCTGACAAATGGACA-3′), 200 nmol/L reverse primer (5′-TGCTTTGGTTCCCCTAAGGAG-3′), 100 nmol/L internally conserved, sequence-specific, fluorogenic probe (5′-CTGCCAGAAAAAGACAGCTGGACTGTCAA-3′) labeled at the 5′ end with FAM and at the 3′ end with TAMRA, 10 U AmpliTaq Gold polymerase] to ∼500 ng of DNA in 5 μl of water. Thermal amplification was performed as previously described. 7 Total DNA from peripheral blood of HIV-1-seronegative individuals and total DNA from the HIV-1 uninfected T-cell line CEM were used as negative controls in each run.
Simultaneous Immunophenotyping/Ultrasensitive Fluorescence in Situ Hybridization
HIV-1 mRNA was detected in the tissues using the ViroTect HIV-1 In Cell Detection System (Invirion, Inc., Frankfort, MI) as previously described. 9 Briefly, the cell types of interest were labeled with optimized concentrations of antibodies specific for T cells, macrophages, and LCs. After fixation and permeabilization in PermiFlow (Invirion, Inc.) for 1 hour at room temperature, the probe was hybridized to the target sequence for 60 minutes at 43°C, in a GeneAmp 1000 slide cycler (PE Applied Biosystems). Multiparameter analyses of cell surface phenotype and HIV-1 gag-pol mRNA were performed on a laser confocal microscope (Olympus, Melville, NY).
Statistical Analyses
Correlations of age and proportions of target cells were computed using Spearman’s rank correlation coefficient r, and distributions were compared using the Mann Whitney U-test.
Results
Quantification of HIV-1 Target Cells in Human Foreskin
To test the hypothesis that the number of HIV-1 target cells and the expression of HIV-1 co-receptors is associated with susceptibility to HIV-1 infection in the foreskin, we analyzed foreskin specimens from 14 individuals ranging in age from 10 months to 65 years (Table 1) ▶ . Samples were stained for HIV-1 target cells with antibodies against CD4 (T cells), CD68 (macrophages), and CD1a (LCs) (Figure 1) ▶ . In all foreskin samples, quantitative immunohistochemistry revealed 0.4 to 3.1% CD4+ T cells, 1.9 to 15.6% LCs, whereas macrophages were less abundant (0.1 to 2.7%). These percentages were based on the number of brown staining cells indicating a specific immunophenotypic marker divided by the total number of nucleated cells. The overwhelming majority of the T cells were found in the submucosa whereas the majority of LCs were found in the epithelium. Additional studies using frozen tissue confirmed that the T cells were memory cells by expression of the CD45RO antigen (data not shown). The proportions of all three types of HIV-1 target cells increased with the age of the patients: CD4, r = 0.69, P = 0.007; CD68, r = 0.61, P = 0.02; CD1a, r = 0.40, P = 0.16, despite the two oldest men (ages 56 and 65 years) having lower proportions of target cells than some children. The mean proportions of each cell type were greater for adults than for children and infants. Adult men had approximately four times as many CD4+ T cells, five times as many macrophages, and 50% more LCs than children. These differences between adults and children were significant for CD4+ T cells (P < 0.01) and macrophages (P < 0.002), but not for LCs (P < 0.11). Two adult patients had notably elevated CD4+ T cells, macrophage and LC proportions: patient no. 12 had a recent history of balanitis and patient no.9 reported more than 10 lifetime sexual partners, had a history of chlamydia, and had genital warts at time of circumcision. The pediatric patient (Table 1 ▶ , no. 6) with the highest proportion of CD4+ T cells also had a documented history of balanitis.
Table 1.
Percent of Total Cells in Foreskin Tissues that are T Cells (CD4), Macrophages (CD68), and Langerhans’ Cells (CD1a) by Age of Subjects and Reported History of Infection
| Age (yrs) | Cell types | Infectious history | |||
|---|---|---|---|---|---|
| CD4%* | CD68% | CD1a% | |||
| Male subjects | |||||
| 1 | 0.75 | 1.7 | 0.2 | 3.9 | No |
| 2 | 1.4 | 0.4 | 0.2 | 4.3 | No |
| 3 | 1.8 | 0.9 | 0.1 | 5.1 | No |
| 4 | 2 | 11.1 | 0.8 | 3.7 | No |
| 5 | 2 | 3.2 | 0.7 | 4.8 | No |
| 6 | 6 | 13.9 | 0.3 | 10.4 | Yes |
| 7 | 9 | 3.4 | 0.1 | 9.3 | No |
| 8 | 15 | 4.6 | 0.1 | 7.8 | No |
| 9 | 18 | 37.1 | 2.7 | 11.2 | Yes |
| 10 | 39 | 15.8 | 2.2 | 15.6 | No |
| 11 | 42 | 21.3 | 0.7 | 9.8 | No |
| 12 | 49 | 43.1 | 1.8 | 13.2 | Yes |
| 13 | 56 | 7.9 | 1.3 | 1.9 | No |
| 14 | 65 | 9.2 | 0.9 | 6.3 | No |
| Female subjects | |||||
| 1 | 28 | 3.4 | 2.1 | 0.9 | No |
| 2 | 21 | 9.1 | 0.7 | 2.1 | No |
| 3 | 34 | 2.1 | 0.3 | 1.2 | No |
| 4 | 23 | 8.9 | 0.4 | 1.5 | No |
| 5 | 42 | 0.9 | 0.4 | 0.1 | No |
| 6 | 36 | 5.4 | 2.4 | 0.6 | No |
Percentages were based on the examination of the entire mucosal surface containing 50% epithelium and 50% submucosa within the field.
*% of total cells; Spearman rank correlation between age and target cell percent: CD4− r = 0.69, P = 0.007; CD68− r = 0.61, P = 0.02, CD1a− r = 0.4, P = 0.16.
Difference between means of pediatric (1 to 8) versus adult subjects (9 to 14): CD4− U = 44, P < 0.01; CD68− U = 51.5, P < 0.002; CD1a− U = 37, P < 0.11.
Figure 1.
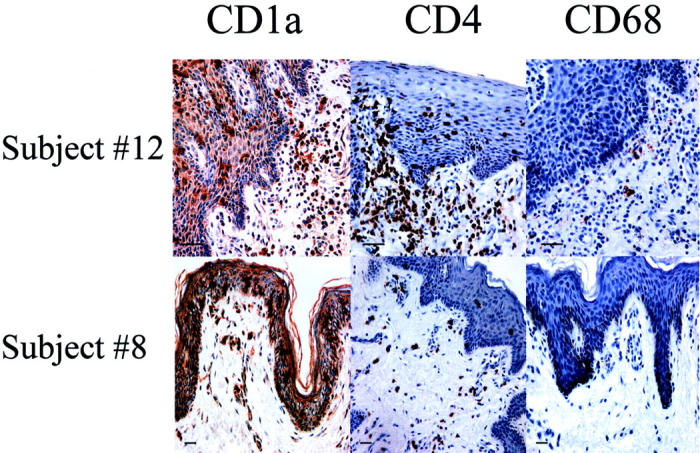
Immunohistochemical staining of LCs (CD1a), T cells (CD4), and macrophages (CD68) in foreskin tissue from an adult (Table 1 ▶ , no. 12) and pediatric (Table 1 ▶ , no. 8) patient. Cells expressing the appropriate antigen appear brown. Tissues were counterstained with hematoxylin. Images were stored and scanned for quantitative image analysis. Scale bar, 50 μm.
In a subset of individuals with foreskins that included the external surface (adults), quantitative immunohistochemistry was performed as well. As shown in Table 2 ▶ , the external foreskin mucosa contained fewer CD4+ T cells (P = 0.002) and LCs (P = 0.007) than the inner, mucosal surface as shown in Table 1 ▶ . The percentage of macrophages was similar in the external foreskin compared to the inner, mucosal surface. Further, the extent of keratinization was much greater in the external foreskin surface compared to the inner mucosal surface (data not shown). Taken together, a decrease in the number of HIV-1 target cells and an increase in the keratinization of the external foreskin suggest that susceptibility to HIV-1 infection should be significantly less in the outer compared to the inner surface of foreskin.
Table 2.
Percent of Total Cell in the External Surface of Foreskin Tissues that Are T Cells (CD4), Macrophages (CD68), and Langerhans’ Cells (CD1a) by Age of Subjects and Reported History of Infection
| Subject | CD4%* | CD68% | CD1a% |
|---|---|---|---|
| Male | |||
| 9 | 0.9 | 0.6 | 1.2 |
| 10 | 1.2 | 1 | 0.5 |
| 11 | 3.4 | 0.2 | 1.4 |
| 12 | 2.4 | 1.1 | 2.8 |
| 13 | 0.8 | 0.3 | 0.3 |
| 14 | 4.1 | 0.9 | 1.8 |
Percentages were based on the examination of the entire external mucosal surface containing 50% epithelium and 50% submucosa within the field.
*% of total cells.
Compared to the ectocervix, an HIV-1 susceptible, mucosal tissue with similar types of HIV-1 target cells, the proportion of CD1a+ LCs in adult foreskin (9.7%) is significantly greater (1.1%, P = 0.003). The proportion of LCs in pediatric foreskin was also greater than in the cervix, with a mean of 6.1% compared to 1.1% (P < 0.001). The percentage of LCs in the cervix was similar to our previous estimates of HIV-1 target cells in the cervix from a sample of 12 adult women from our Chicago cohort. 7 These data suggest that foreskin may be more susceptible to HIV-1 infection than the cervix because of an increased percentage of LCs.
HIV-1 Co-Receptor Expression in Human Foreskin
To determine chemokine receptor expression, we stained six adult foreskin specimens and six cervical samples simultaneously with phycoerythrin-labeledanti-CCR5 and fluorescein isothiocyanate-labeled anti-CXCR4 (Table 3) ▶ . In six of six adult male foreskin specimens and five of six cervical specimens, the majority of cells expressed CCR5 while a minority of cells expressed CXCR4 (Table 2) ▶ . Cells expressing the highest levels of CCR5 were found in the submucosa both on small round cells of 10-μm diameter consistent with lymphocytes and on long processes consistent with LCs (Figure 2) ▶ . The percentage of cells expressing CCR5 was significantly increased compared to the number of cells expressing CCR5 in the cervix (P < 0.05), however the percentage of cells expressing CXCR4 was greater in the foreskin but not to statistically significant levels. These data demonstrate high level expression of the necessary HIV-1 co-receptors in the foreskin.
Table 3.
Percent of Total Cells in Foreskin Tissue Expressing the Major HIV-1 Co-Receptors CCR5 and CXCR4
| Subject | CCR5%* | CXCR4% |
|---|---|---|
| Male | ||
| 9 | 13.6 | 4.5 |
| 10 | 29.6 | 3.4 |
| 11 | 9.8 | 5.6 |
| 12 | 12.7 | 2.9 |
| 13 | 4.1 | 3.7 |
| 14 | 18.4 | 1.7 |
| Female | ||
| 1 | 5.8 | 2.1 |
| 2 | 11.6 | 8.9 |
| 3 | 2.1 | 0.5 |
| 4 | 7.9 | 2.3 |
| 5 | 0.8 | 1.1 |
| 6 | 6.8 | 3.9 |
*% of total cells.
Figure 2.
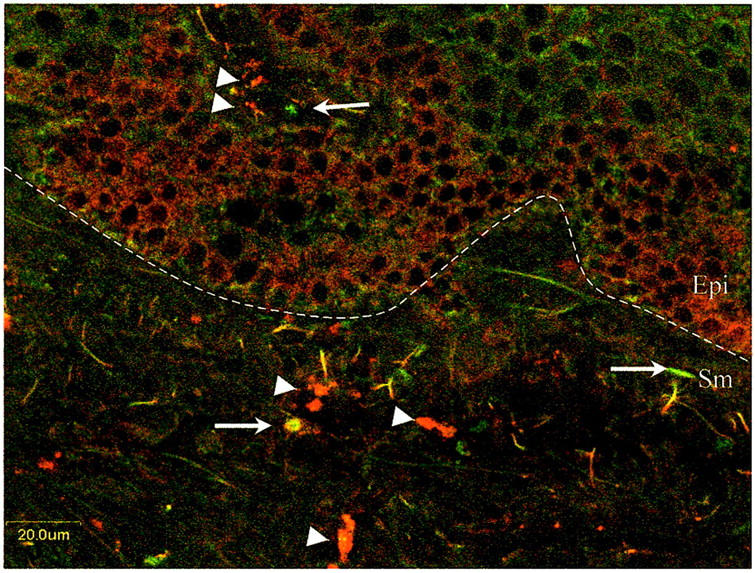
Representative laser confocal image of two-color immunofluorescence staining for the HIV-1 co-receptors CCR5 and CXCR4 in adult male foreskin. Red, CCR5+ cells (arrowheads); green, CXCR4+ cells (arrows). The dotted line represents the epithelial (Epi)/submucosa (Sm) junction. Scale bar, 20 μm.
Susceptibility of Human Foreskin to HIV-1 Infection
To investigate the infectivity of target cells by HIV-1, we cultured fresh, 4-mm foreskin and ectocervical biopsies in organotypic culture, infected with cell-free HIV-1, and quantified HIV-1 pol DNA copies per 1000 cells as previously described for cervical tissue culture. 8 The foreskin samples from two adults (no. 10 and no. 11) exhibited much higher levels of HIV-1 DNA than the foreskin of the 22-month-old infant (no. 3, Table 4 ▶ ). The mean HIV-1 DNA in adult foreskin tissue with no known previous exposure to sexually transmitted infections was nine times greater than in cervical tissue. Qualitative assessment of immunophenotypically identified, HIV-1-infected cells showed that infection was predominant in CD4+ T cells and in LCs at the base of the epithelial layer (Figure 3) ▶ . Consistent with our finding few HIV-1 target cells and increased keratinization in the external foreskin (Figure 4) ▶ , HIV-1 DNA was below the limits of detection in the outer surfaces of foreskin tissues infected in vitro. Consistent with the percentage of target cells and the expression of HIV-1 co-receptors on the inner mucosal surface, foreskin was more susceptible to HIV-1 infection than cervical tissue. Compatible with the chemokine receptor expression determined in this study (Table 3) ▶ and the known predilection for memory T cells and LCs to express CCR5, 10 foreskin tissue explants were far more infectable by the R5 isolate HIV-1Bal than with the X4 isolate HIV-1Lai (Table 4) ▶ .
Table 4.
Quantification of HIV-1 DNA in Foreskin and Cervical Tissues 1 Day after ex Vivo Infection in Explant Culture
| Subject | HIV-1Bal* | HIV-1Lai* |
|---|---|---|
| Male | ||
| 3 | 81 ± 24 | 12 ± 7 |
| 10 | 492 ± 234 | 17 ± 5 |
| 11 | 112 ± 57 | 42 ± 29 |
| Female | ||
| 1 | 22 ± 6 | <10 |
| 2 | 52 ± 31 | <10 |
| 3 | <10 | <10 |
| 4 | 89 ± 34 | 22 ± 12 |
| 5 | 15 ± 10 | <10 |
| 6 | <10 | <10 |
DNA was purified from 10–20 uM scrolls from the frozen tissue oriented to contain the entire cross-section of the biopsy (i.e., including epithelium and submucosa). The numbers represent the mean ± SE of three DNA preparations from each tissue performed in triplicate. HIV-1 DNA was below the limits of detection in the outer surface of the foreskin tissues.
*HIV-1 DNA copies (per 1000 cells), mean ± SE.
Figure 3.
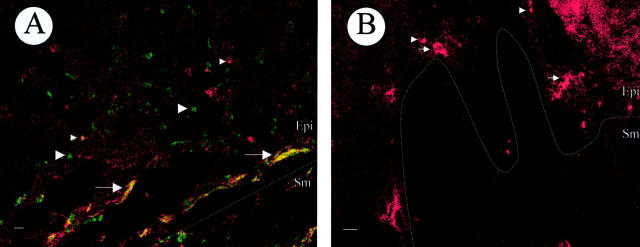
A: Representative laser confocal image from the inner (mucosal) surface of foreskin (Table 1 ▶ , no. 10) infected by HIV-1Bal in explant culture demonstrating infection of T cells (small, green, round cells; large arrowheads) and LCs (large, wavy, yellow cells; arrows). Uninfected CD4 T cells are red (small arrowheads). The majority of LCs in this field are infected. B: Representative laser confocal image from the outer surface of foreskin (Table 1 ▶ , no. 10) infected by HIV-1Bal in explant culture showing uninfected T cells (small, red, round cells; small arrowheads) and uninfected LCs (large, wavy, orange cells; arrows). T cells and LCs expressing HIV-1 gag-pol mRNA were identified using ultrasensitive fluorescence in situ hybridization with simultaneous CD4-APC and CD1a-PE immunofluorescence in a three-color laser confocal microscope. The green color in A represents red CD4 plus blue-green HIV-1. The yellow color in A represents orange CD1a plus blue-green HIV-1. The red color in A and B represents CD4 alone and the orange color in B represents CD1a alone. Dotted lines represent the epithelial (Epi)/submucosa (Sm) junction. Scale bar, 20 μm.
Figure 4.
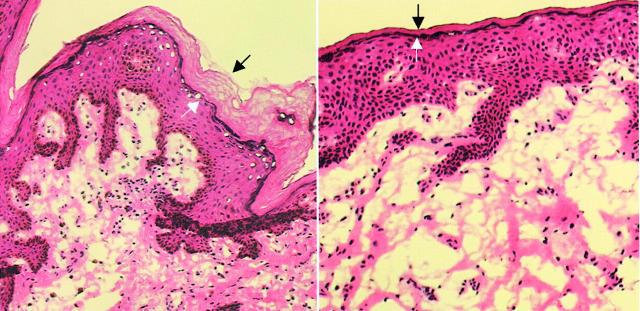
Representative photomicrograph of H&E-stained foreskin tissue from the outer surface (left) and the inner surface (right) of the foreskin. The extent of the keratin layer is identified by the black and white arrows.
Discussion
Repeated epidemiological studies from Africa, India, and North America have reported significant elevated risks of HIV-1 acquisition in uncircumcised compared to circumcised men. 3,4 A reason that the international health community has so far been hesitant to endorse male circumcision as a potential HIV-1 prevention strategy despite calls for its consideration 3,11,12 is that the biological mechanisms by which the presence of a foreskin increases susceptibility to HIV-1 infection in men has not been previously evaluated. Hussain and Lehner 6 previously described high densities of LCs in the mucosal surface of the foreskin, and others have suggested that the thin keratin layer on the inner mucosal surface predisposes the foreskin to infection. 13
In this study, we have confirmed that LCs are present at high densities in the mucosal epithelium of the foreskin—several times greater than in cervical tissue. Further, previously activated memory CD4+ T cells are abundant in the foreskin’s mucosal surface. These HIV-1 target cells expressed both the CCR5 and CXCR4 HIV-1 co-receptors, with CCR5 being predominant. When HIV-1 was introduced to the foreskin tissue, there was extensive infection of both the CD4+ T cells and LCs by the CCR5-using isolate HIV-1Bal, whereas infection with HIV-1Lai was ineffective in all tissue samples. Paralleling the differences in target cell proportions, the infectivity of the inner mucosal surface of the adult foreskin was several times greater than in cervical tissue. Remarkably, there was no HIV-1 infiltration of tissue taken from the outer surface of the foreskin. These results demonstrate that the inner surface of the human foreskin is highly susceptible to HIV-1 infection, much more susceptible even than the cervix, which is a known site of HIV-1 acquisition in women. 14 In addition, these results suggest that the highly keratinized outer surface of the foreskin is an unlikely site for efficient HIV-1 acquisition in men, as evidenced by the absence of detectable HIV-1 DNA in this portion of the tissue infected in explant culture.
A limitation of this study is that comparable results are not available for tissue from the circumcised penis. Thus it is not possible to demonstrate that the foreskin increases risk of HIV-1 infection compared to skin from the penile shaft. In the absence of biopsies of fresh penile shaft tissue, we used the outer surface of the foreskin for comparison, and no infiltration by HIV-1 of this tissue was detected. In so far as the penile shaft is likely to be covered by a keratinized stratified squamous epithelium similar to what we found in the outer surface of the foreskin, 13 this comparison is likely to be valid. Some have speculated that the route for HIV-1 infection in the uncircumcised male is through the epithelium of the glans, which, because it is protected by the foreskin, is likely to be less keratinized in adults than the glans of the circumcised penis. However, in a study of Australian cadavers, the epithelia of the glans in circumcised and uncircumcised men were equally keratinized. 2 A logical but as yet unproven conclusion concerning routes of HIV-1 acquisition in the circumcised penis is that infection occurs through the urethral mucosa or through disruptions of the penile shaft epithelia caused by genital ulcer disease or trauma.
The two adult patients who had recent penile infections (patients no. 9 and no. 12) had highly elevated proportions of HIV-1 target cells. Because the levels of infiltration by HIV-1 of foreskin tissue in this study paralleled the levels of target cells in the tissue, active infection with sexually transmitted infections or other pathogens would likely elevate target cell activity and result in increased risk of HIV-1 acquisition. This is consistent with epidemiological evidence that infection with a sexually transmitted infection increases risk of HIV-1 acquisition more in uncircumcised than in circumcised men. 15 That HIV-1 target cells increased with age in the patients suggests that there is a developmental trajectory of the immune response. However, these findings are also consistent with the hypothesis that exposure to pathogens stimulates increases in target cell recruitment. The levels of CD4+ T cells, macrophages, and LCs were variable in the children, and the highest levels of CD4+ T cells and LCs were in sexually active adult men. Remarkably, the two oldest patients, who reported little sexual activity, had target cell levels comparable to children.
Previous studies report HIV-1 in women to be genetically heterogeneous, but the virus detected in men soon after sexual contact is homogeneous. 16 Here, we have demonstrated that, at least in this small sample, the adult male foreskin contains much greater numbers of LCs and memory T cells than the ectocervix of women. These two cell types have previously been demonstrated to express almost exclusively CCR5 on their surfaces, 10,17 a finding also supported by results in this study. The predominant expression of CCR5 in foreskin tissue is consistent with infection by homogeneously macrophage-tropic isolates of HIV-1. Further, recent studies have demonstrated that the interactions of dendritic cells and T cells through the lectin-receptor DCSIGN enhance productive HIV-1 infection and dissemination of the virus to regional lymph nodes. 18
A recent study of 187 HIV-1 serodiscordant couples in Uganda in which females were infected and males were initially uninfected, reported that, during just less than 2 years of follow-up, 40 of 137 uncircumcised men seroconverted, while 0 of 50 circumcised men seroconverted. 19 Those results combined with our findings showing vigorous infiltration by HIV-1 of target cells in foreskin tissue suggest that the presence of a foreskin is a true biological cause of increased HIV-1 susceptibility in uncircumcised men. The implications of these findings are that strong consideration should be given to integrating male circumcision information and services with the currently limited armamentarium of other HIV-1 prevention tools. This will require considerations of the acceptability and operational feasibility of introducing safe, affordable services in currently noncircumcising societies where most infections are transmitted heterosexually. 20 Attention must be given to counseling parents and men against increasing sexual risk behaviors in the belief that circumcision fully protects against HIV-1 acquisition. In addition, the development of topically active agents capable of blocking HIV-1 binding sites and that can be applied to the penis or vagina should proceed. 21
Footnotes
Address reprint requests to Bruce K. Patterson, M.D., Department of Pediatrics, Division of Infectious Diseases, Children’s Memorial Hospital, Northwestern University Medical School, 2300 Children’s Plaza #51, Chicago, IL 60614. E-mail: bpatterson@childrensmemorial.org.
Supported in part by grants AI47065-02 and PO1HD40539-01 from the National Institutes of Health.
References
- 1.: Joint United Nations Programme on HIV-1/AIDS (UNAIDS) and World Health Organization (WHO): AIDS Epidemic Update—December 2001 2002. UNAIDS, Geneva
- 2.Szabo R, Short R: How does male circumcision protect against HIV-1 infection? Br Med J 2000, 320:1592-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey RC, Plummer FA, Moses S: Male circumcision and HIV prevention: current knowledge and future research directions. Lancet Infect Dis 2002, 1:223-231 [DOI] [PubMed] [Google Scholar]
- 4.Moses S, Bailey R, Ronald A: Male circumcision: assessment of health benefits and risks. Sexually Transmitted Infections 1998, 74:368-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss HA, Quigley MA, Hayes RJ: Male circumcision and risk of HIV-1 infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS 2000, 14:2261-2370 [DOI] [PubMed] [Google Scholar]
- 6.Hussain L, Lehner T: Comparative investigation of Langerhans’ cells and potential receptors for HIV-1 in oral, genitourinary and rectal epithelia. Immunology 1995, 85:475-484 [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson BK, Landay A, Anderson Brown C, Behbanhani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski M, Garcia P: Repertoire of chemokine receptor expression in the female genital tract. Am J Pathol 1998, 153:481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins K, Patterson BK, Naus G, Landers D, Gupta P: Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med 2000, 6:475-479 [DOI] [PubMed] [Google Scholar]
- 9.Behbahani H, Popek E, Garcia P, Andersson J, Spetz A-L, Landay A, Flener Z, Patterson BK: Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am J Pathol 2000, 157:1811-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul CC, WU L, Hoxie JA, Springer TA, Mackay CR: The HIV coreceptors CXCR4, and CCR5 are differentially expressed and regulated on human T-lymphocytes. Proc Natl Acad Sci USA 1997, 94:1925-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink A: A possible explanation for heterosexual male infection with AIDS. N Engl J Med 1986, 314:1167. [PubMed] [Google Scholar]
- 12.Buve A, Auvert B, Langarde E, Kahindo M, Hayes R, Carael M: Male circumcision and HIV-1 spread in sub-Saharan Africa. In Abstracts, XIII International Conference on AIDS. (MoOrC192). Durban, South Africa, August, 2000
- 13.Fussell EN, Kaak MB, Cherry R, Roberts J: Adherence of bacteria to human foreskin. J Urol 1988, 140:997-1001 [DOI] [PubMed] [Google Scholar]
- 14.Alexander NJ: Sexual transmission of human immunodeficiency virus: virus entry into the male and female genital tract. Fertil Steril 1990, 54:1-18 [DOI] [PubMed] [Google Scholar]
- 15.Cameron D, Simonsen J, D’Costa L, Ronald A, Maitha G, Gakinya M, Cheang M, Ndinya-Achola J, Piot P, Brunham R, Plummer F: Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion. Lancet 1989, 2:403-407 [DOI] [PubMed] [Google Scholar]
- 16.Long EM, Martin HL, Kreiss JK, Rainwater SMJ, Lavreys L, Jackson DJ, Rakwar J, Mandaliya K, Overbaugh J: Gender differences in HIV-1 diversity at time of infection. Nat Med 2000, 6:71-75 [DOI] [PubMed] [Google Scholar]
- 17.Zaitseva M, Blauvelt A, Lee S, Lapham CK, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H: Expression and function of CCR5 and CXCR4 on human Langerhan’s cells and macrophages: implications for HIV-1 primary infection. Nat Med 1997, 3:1369-1375 [DOI] [PubMed] [Google Scholar]
- 18.Geijtenbeek T, Kwon D, Torensma R, van Vliet S, van Duijnhoven G, Middel J, Cornelissen I, Nottet H, Kewalromani V, Littman D, Figdor C, van Kooyk Y: DC-SIGN, a dendritic cell-specific HIV-1 binding protein that enhances trans-infection of T cells. Cell 2000, 100:587-597 [DOI] [PubMed] [Google Scholar]
- 19.Quinn T, Wawer M, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan M, Lutalo T, Gray RH: For the Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000, 342:921-929 [DOI] [PubMed] [Google Scholar]
- 20.Bailey RC, Muga R, Poulussen, R. Trial intervention introducing male circumcision to reduce HIV-1/STD infections in Nyanza Province, Kenya: baseline results. In Abstract Book, Volume I, XIII International Conference on AIDS, Durban, South Africa, August, 2000
- 21.UNAIDS: Microbicides for HIV infection: UNAIDS technical update. UNAIDS Best Practice Collection: Technical Update. Geneva, UNAIDS, April, 1998