Abstract
Telomerase activity and telomerase reverse transcriptase (hTERT), the key component of the telomerase complex, are tightly proliferation regulated in normal and malignant cells both in vitro and in vivo; however, underlying mechanisms are unclear. In the present study, we identified mitogen-activated protein kinase (MAPK) cascade-mediated histone H3 ser10 phosphorylation to be a molecular link between proliferation and induction of hTERT/telomerase activity. In normal human T lymphocytes and fibroblasts, growth or stress stimuli known to drive H3 phosphorylation through the MAPK signaling induce hTERT expression and/or telomerase activity that was preceded by phosphorylated histone H3 (ser10) at the hTERT promoter. Blockade of the MAPK-triggered H3 phosphorylation significantly abrogates hTERT induction and ser10 phosphorylation at this promoter. However, H3 ser10 phosphorylation alone resulted in low, transient hTERT induction, as seen in fibroblasts, whereas H3 phosphorylation followed by its acetylation at lys14 robustly trans-activated the hTERT gene accompanying constitutive telomerase activity in normal and malignant T cells. H3 acetylation without phosphorylation similarly exerted weak effects on hTERT expression. These results define H3 phosphorylation as a key to hTERT transactivation induced by proliferation and reveal a fundamental mechanism for telomerase regulation in both normal human cells and transformed T cells.
Telomerase, an RNA-dependent DNA polymerase responsible for de novo elongation of telomere repeats at the chromosome termini, is composed of two core components, the rate-limiting catalytic unit telomerase reverse transcriptase (hTERT) and ubiquitously expressed telomerase RNA template (18, 21, 24). It has been widely accepted that hTERT induction and telomerase activation are crucial for transformed cells to stabilize their telomere length and to acquire infinite replicative potentials during the oncogenic process, whereas most normal human somatic cells lack telomerase activity due to the stringent repression of the hTERT gene and thereby undergo progressive telomere shortening, by which cellular senescence is eventually triggered (2, 34). However, as a striking exception, substantial levels of hTERT/telomerase activity are seen in highly proliferating normal human and mouse cells and tissues, both in vitro and in vivo (1, 5, 13, 14). For instance, human T or B lymphocytes, once entering cell cycle pools in response to mitogenic stimuli, undergo rapid up-regulation of hTERT expression and telomerase activity (3, 16). A recent study even shows the presence of hTERT expression and telomerase activity in normal cycling human diploid fibroblasts (HDFs), a cell type where hTERT was previously believed to be tightly repressed at the transcriptional level (30, 31). Moreover, abolishing the hTERT/telomerase expression led to the disruption of telomere structure, accelerated replicative senescence, and impaired DNA damage response in these HDFs (30, 31). These observations strongly suggest the presence of a physiological controlling pathway and functional roles of hTERT expression in most proliferative human cells, challenging the widespread concept of the stringent repression of the hTERT gene in normal cells. On the other hand, proliferation-regulated hTERT/telomerase activity similarly occurs in cancer cells: abundant when actively proliferating while repressed when in a quiescent state (13, 17). So far, however, such tightly proliferation-regulated hTERT/telomerase expression in both normal and tumor cells has been poorly understood.
In eukaryotic cells, DNA is compacted with histones and other proteins to form chromatin, which is nonpermissive for transcription by preventing transcription factors access to promoters. Covalent modifications of histones including acetylation, phosphorylation, and methylation have recently emerged as key mechanisms to modulate chromatin configuration and gene expression (20). Acetylation of histones, currently the best studied of these modifications, has been shown to transcriptionally target the hTERT gene, suggesting a role for chromatin remodeling in controlling telomerase activity (9, 11, 19, 22, 25, 36, 39). In earlier investigations of hTERT induction mediated by histone acetylation, we noticed that cycloheximide (CHX) alone was capable of inducing hTERT mRNA expression (unpublished data) and synergistically transactivated the hTERT gene with the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) (19). It is known that CHX, in addition to inhibiting protein synthesis, activates the p38 mitogen-activated protein kinase (MAPK) cascade, thereby leading to a fraction of the histone H3 ser10 phosphorylation through activated MSK1 and MSK2, the downstream effectors of the MAPK pathway (10). Similarly, extracellular signal-regulated kinase (ERK), once activated by growth factors, targets MSKs that, in turn, phosphorylate histone H3 at ser10 (10, 35). The rapid ser10 phosphorylation of H3 mediated by the MAPK cascade, named the “nucleosomal response”, is a requisite step for induction of “immediately early” (IE) genes, including proto-oncogenes c-fos and c-jun in mammalian cells, when exposed to mitogenic or stress signals (7, 28, 35). With this in mind, we sought to elucidate whether proliferation stimuli can signal to the hTERT chromatin through the MAPK pathway, or, more specifically, whether the H3 phosphorylation event plays roles in proliferation-induced hTERT expression/telomerase activity. In the present study, we have utilized two types of normal human cells, T lymphocytes and HDFs, and a malignant T-cell line to address this issue.
MATERIALS AND METHODS
Cells and reagents.
Normal human T lymphocytes were isolated from buffy coats of healthy individuals, and HDFs including lung fetal (LF1) (37) and dermal-derived fibroblasts (19) were kindly provided by J. Sedivy (Brown University) and Z. Yan (Karolinska Institutet). The above cells as well as Jurkat cells, a T-cell lymphoma line, were all maintained in RPMI 1640 medium (Life Technologies, Paisley, Scotland) supplemented with 10% of fetal calf serum (FCS), 2 mM l-glutamine, and antibiotics. Epidermal growth factor (EGF), concanavalin A (ConA), anisomycin, CHX, PD98095, 12-O-tetradecanoylphorbol-13-acetate (TPA), and TSA were purchased from Sigma, and H89 was from Alexis Biochemicals. Prior to the treatment of HDFs with the above reagents, the cells were cultured in 0.5% FCS-containing RPMI 1640 medium for 48 to 72 h to induce quiescence. T lymphocytes were incubated with 15 μg/ml of ConA to stimulate their proliferation or activation.
RNA extraction and RT-PCR.
Total cellular RNA was extracted using the ULTRASPEC-II RNA kit (Biotecx Laboratories, Houston, TX). cDNA was synthesized using random primers (N6) (Pharmacia, Uppsala, Sweden) and Moloney murine leukemia virus reverse transcriptase. The reverse transcription-PCR (RT-PCR) primers and conditions for hTERT mRNA were as described previously (32), and the PCR cycles were 32 and 37 for T or Jurkat cells and fibroblasts, respectively. β2-microglobulin (β2-M) expression was used as a control for RNA loading and RT efficiency and was amplified with its specific primers for 25 cycles. With the above amplification conditions, PCR for both hTERT and β2-M mRNA was in a linear phase, which allowed a semiquantitative evaluation for the level of hTERT transcript, as shown in our previous study (38).
Chromatin immunoprecipitation (ChIP).
The ChIP assay was carried out according to the protocol from Upstate Biotechnology (Lake Placid, NY). All the ChIP reagents, including antibodies against phospho-ser10 H3 and acetylated (K14) H3, were from Upstate Biotechnology. Briefly, the cells with different treatments were cross-linked by incubating them in 1% (vol/vol) formaldehyde-containing medium for 7 to 8 min at 37°C and then sonicated (four times for 8 s each) to make soluble chromatin. With these conditions, the produced DNA fragments were between 200 and 800 bp (data not shown). The antibodies against phospho-ser10 H3 and acetylated (K14) H3 were used to precipitate DNA fragments bound by phosphorylated and acetylated histone H3, respectively. The protein-DNA complex was collected with protein A Sepharose beads, eluted, and reverse cross-linked. The samples were then extracted with phenol-chloroform and precipitated with ethanol. The recovered DNA was resuspended in double-distilled H2O and used for the PCR amplification. The PCR primer (TERT-p) sequences for the hTERT promoter (see Fig. 2B) were 5′ CCA GGC CGG GCT CCC AGT GGA T 3′ (forward) and 5′ GGC TTC CCA CGT GCG CAG CAG GA 3′ (reverse), and for the downstream region of the hTERT gene (TERT-np) they were 5′ GCT TGC AGA GGT GGC TCT AA 3′ (forward) and 5′ GCT GTG GTT TGG GAG ACT AAA 3′ (reverse). The GAPDH gene was amplified with the primers 5′ AAA GGG CCC TGA CAA CTC TT 3′ (forward) and 5′ GGT GGT CCA GGG GTC TTA CT 3′ (reverse), which resulted in the production of a 117-bp DNA fragment.
FIG. 2.
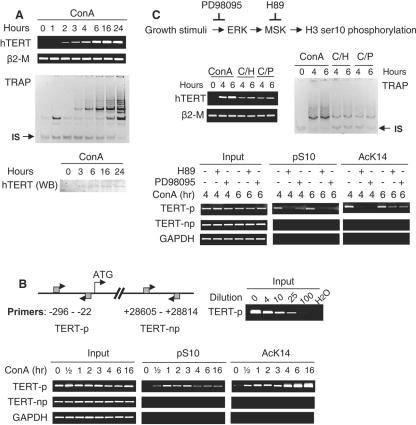
Association of H3 ser10 phosphorylation at the hTERT promoter with hTERT induction/telomerase activation in ConA-treated T cells. (A) Up-regulation of the hTERT expression and activation of telomerase in ConA-treated T cells. Upper panel, RT-PCR for hTERT mRNA analysis and TRAP assay for telomerase activity in the control and ConA-treated T cells. IS, internal standard. Lower panel, Western blot (WB) for hTERT protein detection. The specificity of the antibody against hTERT protein was demonstrated by introducing a Flag-hTERT expression vector into telomerase-negative human cells and obtaining appropriate signals with both hTERT and flag antibodies (data not shown). (B) Left upper panel, schematic presentation of the hTERT locus and PCR primer locations for the ChIP assay; right upper panel, validation of the semiquantitative PCR for the hTERT promoter sequence. The input DNA (corresponding to 2% of a chromatin sample) was diluted 0-, 4-, 10-, 25-, and 100-fold as indicated and was then subjected to PCR analyses using TERT-p primers. Lower panel, specific accumulation of histone H3 with ser10 phosphorylation and lys14 acetylation on the hTERT promoter in activated T cells, as determined using ChIP. (C) Attenuated induction of hTERT/telomerase expression and H3 phosphorylation/acetylation at the hTERT promoter by H89 and PD98095 in T cells treated with ConA. Top panel, the inhibitory mechanism of the MAPK signaling-mediated H3 phosphorylation by H89 (10 μM) and PD98095 (40 μM). Middle panel, diminished hTERT mRNA expression (left) and telomerase activity (right) induced by ConA treatment of T cells in the presence of H89 and PD98095. The cells with different treatments for various periods were analyzed for hTERT mRNA and telomerase activity by using RT-PCR and TRAP, respectively. C/H, ConA plus H89; C/P, ConA plus PD98095. IS, internal standard. Bottom panel, the ChIP assay for the occupancy of phosphorylated and acetylated histone H3 at the hTERT promoter in the ConA-treated T cells with and without H89 or PD98095.
Histone and nuclear protein extraction, Western blot, and immunofluorescence staining.
We isolated histone and nuclear proteins and performed Western blot and immunofluorescence according to the protocol described previously (15, 19). The specific primary antibodies against phospho-H3 (Upstate Biotechnology) and hTERT (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary antibodies conjugated with horseradish peroxidase or fluorescein isothiocyanate (Dako Cytomation, Glostrup, Denmark) were used for the analyses.
Telomerase activity assay.
Telomerase activity was determined by using a TRAPEZE kit (Chemicon International, Temecula, CA) according to the protocol provided by the manufacturer. One microgram of protein was used for each reaction, and 31 PCR cycles were carried out. The telomeric repeat amplification protocol (TRAP) result was visualized by staining the PCR product with SYBR Green I (Roche Diagnostics Scandinavia AB, Stockholm, Sweden) after polyacrylamide gel electrophoresis gel separation.
RESULTS
Rapid accumulation of ser10-phosphorylated histone H3 in T cells treated with ConA.
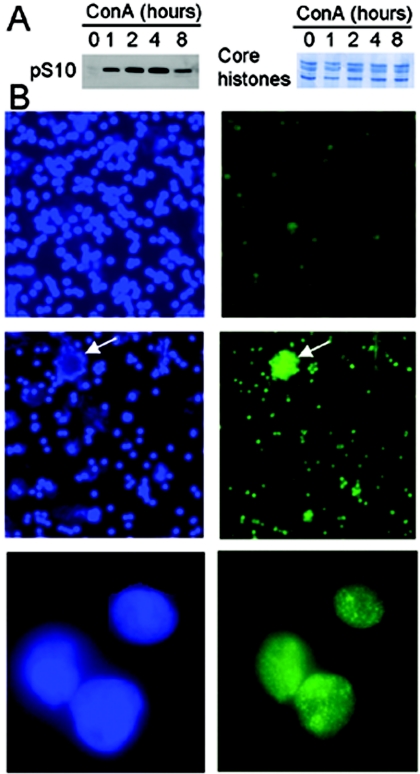
It is unclear whether ConA-stimulated T-cell proliferation through the ERK-MAPK cascade would lead to H3 phosphorylation, as seen in fibroblasts at G0/G1 transition. Thus, we first examined the H3 phosphorylation status in these cells by using both Western blotting and immunofluorescence staining. The amount of phosphorylated H3 was negligible in untreated resting T cells, whereas it accumulated promptly following exposure of the cells to ConA, as shown by Western blot (Fig. 1A). The peak level was observed approximately 4 h post-ConA stimulation, and a significant decrease occurred at 8 h. The immunofluorescence result exhibited a similar pattern, with a weak staining of the ser10-phosphorylated H3 signal in the control cells but bright signals in the ConA-treated cells (Fig. 1B). Moreover, in these activated T cells, the phosphorylated H3 was not distributed evenly; rather, in many foci scattered throughout the nuclei, there was a pattern in accordance with that observed in fibroblasts treated with growth factors (4) (Fig. 1B).
FIG. 1.
Rapid histone H3 ser10 phosphorylation in normal T cells stimulated with ConA. (A) Western blot analyses of ser10-phosphorylated H3 in the control and ConA-treated T cells (left). The equal loading of histones was verified by Coomassie blue staining of the duplicated gels (right). (B) Immunofluorescence staining of ser10-phosphorylated H3 in the control and ConA-treated T cells. Left and right: 4′,6′-diamidino-2-phenylindole (DAPI) and phosphorylated H3 (ser10) signals, respectively. Top panel, untreated cells (magnification, 20×). Middle (magnification, 20×) and bottom (magnification, 100×) panels, ConA-treated T cells for 1 h. Arrows indicate aggregated T cells with bright signals of phosphorylated H3 after activation.
Accumulation of histone H3 ser10 phosphorylation at the hTERT promoter preceding hTERT mRNA induction and telomerase activation in ConA-treated T cells.
Previous studies have shown that activated T cells undergo up-regulation of hTERT mRNA expression and of telomerase activity (3, 16, 19, 39). Consistent with earlier reports, we observed robust increases in hTERT expression in normal T cells treated with ConA (Fig. 2A). Enhanced levels of hTERT mRNA readily occurred 2 to 3 h after ConA exposure, and a maximal induction was seen at 16 to 24 h, which was concomitant with up-regulation of hTERT protein expression and of telomerase activity (Fig. 2A).
Having demonstrated the sequential onset of histone H3 ser10 phosphorylation and hTERT induction in activated T cells, we examined whether histone H3 at the hTERT locus was specifically targeted for ser10 phosphorylation. For this purpose, ChIP was performed and the primers (TERT-p) spanning the proximal promoter (Fig. 2B) were used for PCR amplification of DNA fragments precipitated by an antibody against ser10-phosphorylated H3. In resting T cells, there was no detectable histone H3 ser10 phosphorylation associated with the hTERT promoter, whereas ConA treatment within 30 min led to the accumulation of phosphorylated H3 at this promoter, and a maximal level was reached after 1 to 3 h, when hTERT mRNA was starting to appear (Fig. 2B). Thereafter, the H3 phosphorylation on the hTERT promoter decreased but persisted. To determine whether phosphorylation of H3 ser10 spreads throughout the hTERT locus, the same set of precipitated DNA was further analyzed for the presence of the downstream sequences of the hTERT gene by using another pair of PCR primers (TERT-np) indicated in Fig. 2B. These primers did not lead to detectable PCR signals (Fig. 2B), which indicate that H3 ser10 phosphorylation is likely limited to the hTERT promoter region. T lymphocytes from more than 10 healthy individuals were analyzed, and similar results were obtained. No-antibody controls were always performed to exclude nonspecific bindings (data not shown).
One of the important characteristics of H3 ser10 phosphorylation is that it is especially susceptible to hyperacetylation at lys14, because of a stronger preference of histone acetyltransferases (HATs) for ser10-phosphorylated H3 than for the unmodified form (6, 8). To see whether this was true for the hTERT chromatin in activated T cells, ChIP was performed with the antibody against lys14-acetylated histone H3. As shown in Fig. 2B, H3 lys14 acetylation concomitantly occurred at the same region of the hTERT promoter with slightly different kinetics, including persistent increases up to 16 h.
Defective induction of the hTERT expression by inhibiting H3 ser10 phosphorylation.
Having shown that the onset of H3 ser10 phosphorylation at the hTERT promoter was followed by transcriptional activation of this gene and induction of telomerase activity once T cells were entering the cell cycle in response to ConA stimulation, we sought to address whether these two events are causally linked by interfering with H3 ser10 phosphorylation in T cells. First, we incubated T cells with ConA in the presence of 10 μM H89, a compound that specifically inhibits MSK activities at this concentration, thereby abrogating histone H3 ser10 phosphorylation (35). ConA-mediated accumulation of H3 ser10 phosphorylation at the hTERT promoter and induction of hTERT mRNA/telomerase activity was significantly reduced by pretreatment of the cells with H89 (Fig. 2C). Furthermore, lys14 acetylation of H3 was also abrogated, likely secondary to diminished recruitment of HATs (Fig. 2C). Secondly, PD98095, a specific ERK inhibitor that blocks the MAPK signaling pathway (35), when incubated with T cells prior to the addition of ConA similarly led to a reduction in both hTERT promoter-associated H3 phosphorylation/acetylation and hTERT mRNA/telomerase expression (Fig. 2C). The above results support a mechanistic link between histone H3 phosphorylation and transcriptional hTERT activation in proliferating T cells.
Concomitant H3 ser10 phosphorylation at the hTERT promoter and up-regulation of hTERT expression in serum-starved quiescent malignant T cells at reentry into cell cycle.
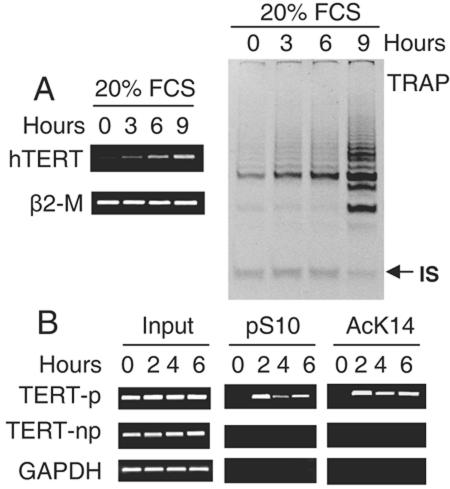
Holt et al. and others have demonstrated that immortalized and tumor cells exhibit growth-regulated telomerase/hTERT expression in a similar manner (13, 17). Serum withdrawal and contact inhibition induce a G0 quiescence of these cells coupled with transcriptional repression of hTERT and diminished telomerase activity; once the cells reenter the cell cycle pool, a rapid recovery of hTERT and telomerase expression occurs. To determine a potential association between H3 phosphorylation and reversible hTERT expression in malignant cells, T-cell lymphoma-derived Jurkat cells, after 72 h of serum depletion, were re-fed with 20% FCS and then analyzed for hTERT mRNA expression, telomerase activity, and H3 phosphorylation at the hTERT promoter. As depicted in Fig. 3, the result clearly showed a concomitant accumulation of the hTERT promoter-associated H3 phosphorylation and hTERT mRNA/telomerase induction in the treated Jurkat cells. Furthermore, significantly increased H3 lys14 acetylation was simultaneously identified at the hTERT promoter in these cells. This result is almost identical to that seen in ConA-treated T cells, which suggests that the H3 phosphorylation signaling governs the proliferation-regulated telomerase activity in both normal and malignant T cells.
FIG. 3.
Concomitant up-regulation of hTERT mRNA/telomerase expression and histone H3 phosphorylation/acetylation at the hTERT promoter in quiescent Jurkat cells upon reentry into cell cycle. (A) hTERT transcript (left) and telomerase activity (right) in Jurkat cells during transition from quiescence to G1 phase. Jurkat cells were first incubated in 0.5% FCS-containing medium for 72 h to induce quiescence and then re-fed with 20% of FCS. hTERT mRNA and telomerase activities were determined with the use of RT-PCR and TRAP assay, respectively. IS, internal standard. (B) Histone H3 phosphorylation/acetylation at the hTERT promoter in quiescent and FCS-treated Jurkat cells as determined using ChIP.
Transcriptional activation of the hTERT gene in normal fibroblasts by histone H3 ser10 phosphorylation modulators.
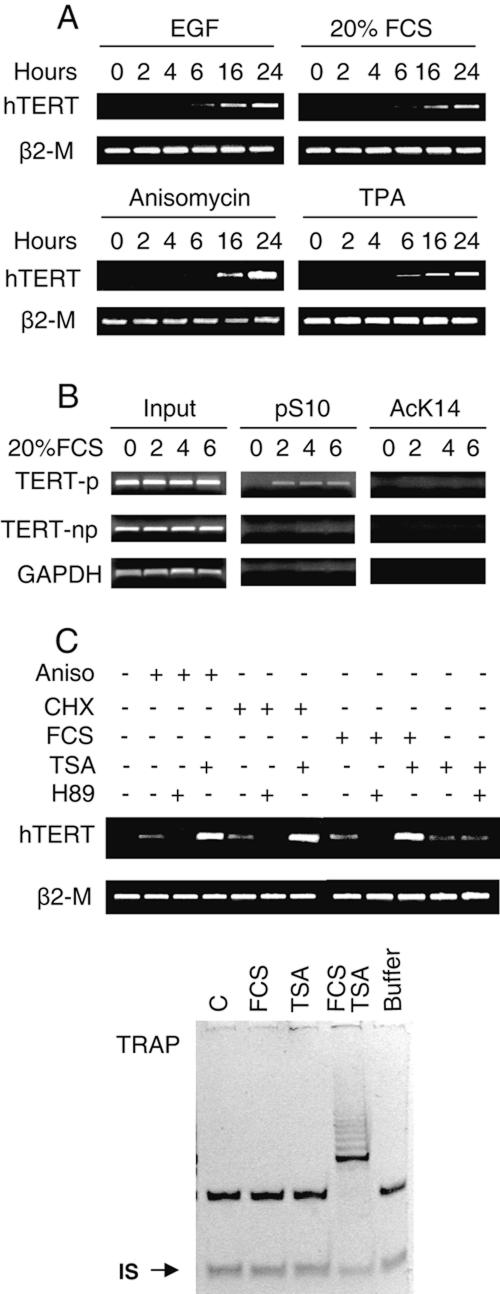
It has been well established that H3 ser10 phosphorylation is induced in normal fibroblasts through the MAPK cascade by either mitogen-stimulated ERK or stress-activated p38 signaling (10). Moreover, we previously observed induction of hTERT mRNA in HDFs treated with CHX (data not shown). Given a recent report that normal human fibroblasts transiently express hTERT mRNA in response to mitogen stimulation (31), we hypothesized that the H3 phosphorylation event is likely responsible for transcriptional regulation of the hTERT gene in this type of cell. To test this, dermal and lung HDFs under serum starvation for 48 to 72 h were exposed to different stimuli known to result in H3 phosphorylation and then analyzed for hTERT mRNA expression. As hypothesized, but still surprisingly, all the tested reagents, including EGF, 20% FCS, TPA, and the protein synthesis inhibitors anisomycin and CHX, induced detectable hTERT mRNA expression in two different strains of HDFs (Fig. 4A and data not shown). The induction of hTERT expression by these stimuli could be efficiently abolished in the presence of H89 (Fig. 4C). Compared to activated T cells, the abundance of hTERT transcripts in the treated HDFs was around 50-fold lower. Telomerase activity was undetectable in the FCS-treated cells using TRAP (Fig. 4C), probably explained by a technical limitation (31). The ChIP assay revealed the occupancy of phosphorylated ser10 at the hTERT promoter region in the treated but not control HDFs (Fig. 4B), which occurred prior to hTERT mRNA expression. However, H3 lys14 acetylation at the hTERT promoter was absent in both treated and untreated cells, as shown in Fig. 4B, which is in striking contrast to that seen in activated T cells. Taken together, histone H3 phosphorylation at ser10 via the MAPK cascade is a critical event that triggers transient hTERT transcription in HDFs.
FIG. 4.
Induction of hTERT mRNA and H3 phosphorylation at the hTERT promoter in normal human fibroblasts by growth and stress stimuli. (A) hTERT mRNA expression in fibroblasts treated with growth and stress stimuli. The fibroblasts were induced to quiescence by incubating them in 0.5% FCS-containing medium for 48 to 72 h and were then treated with EGF (200 ng/ml), 20% FCS, TPA (250 ng/ml), and anisomycin (Aniso; 5 μg/ml) for various periods as indicated. The cells were then analyzed for hTERT mRNA using RT-PCR. (B) Histone H3 phosphorylation on the hTERT promoter preceding hTERT mRNA expression in fibroblasts fed with FCS. (C) Synergistic effects of growth or stress stimuli and HDAC inhibition on induction of hTERT mRNA expression and telomerase activity in fibroblasts. Upper panel, quiescence-induced fibroblasts were treated with EGF, 20% FCS, or anisomycin or CHX (50 μg/ml) in the presence or absence of TSA (1 μM) or H89 (10 μM) and then were analyzed for hTERT mRNA expression. Lower panel, TRAP results of the fibroblasts treated with FCS or TSA and FCS plus TSA. IS, internal standard.
Synergistic trans-activation of the hTERT gene in normal human fibroblasts by histone H3 phosphorylation and acetylation.
We and others have previously shown that inhibition of HDACs by TSA leads to the transcriptional activation of the hTERT gene in normal and malignant cells (9, 11, 19, 25, 36, 39). In these studies, however, TSA-treated cells were exclusively maintained in 10% FCS, and it was unclear whether the observed hTERT induction was totally attributed to the HDAC inhibition. Moreover, we indeed documented a superinduction of hTERT mRNA in both T cells and HDFs treated with TSA in the presence of CHX (19). Given the present finding that CHX or serum alone was capable of stimulating hTERT expression through H3 phosphorylation, it is likely that histone H3 phosphorylation and acetylation cooperate to synergistically trans-activate the hTERT gene. To address this issue, we incubated 48-h serum-starved HDFs with either 20% FCS, anisomycin, TSA alone, or a combination of FCS or anisomycin with TSA for 16 h. Remarkably, TSA only induced a weak expression of hTERT mRNA in the absence of serum, with a level comparable to that seen in the cells treated with FCS or anisomycin alone. The combined treatment of the cells with TSA and serum or anisomycin led to much higher levels of hTERT mRNA than those in the cells incubated with any single reagent (Fig. 4C). Moreover, telomerase activity was only detectable in the cells treated with TSA plus FCS (Fig. 4C). Thus, both histone H3 phosphorylation and acetylation are required for the optimal induction of hTERT expression and telomerase activity. This set of data also suggest that TSA-induced high levels of hTERT mRNA observed in earlier studies virtually reflect a combined trans-activating effect of histone H3 phosphorylation and acetylation on the hTERT gene.
DISCUSSION
It has become clear during the past years that the transcriptional control of hTERT expression is a key that governs telomerase activation in both normal and malignant human cells (18, 21, 24). However, fundamental mechanisms for transcriptional regulation of this gene remain elusive, and one of the most poorly understood is the tightly proliferation-regulated hTERT expression. In the present study, we provide evidence that the MAPK cascade-mediated histone H3 phosphorylation at ser10 is crucial for transcriptional activation of the hTERT gene associated with proliferation stimulation. (i) Various growth and stress stimuli, known to lead to H3 ser10 phosphorylation, all induced hTERT expression and/or telomerase activity in normal human lymphocytes or fibroblasts. (ii) The hTERT promoter was specifically targeted for histone H3 ser10 phosphorylation that preceded the appearance of hTERT mRNA in these cells. (iii) Blockade of histone H3 ser10 phosphorylation resulted in significant reduction in both phosphorylated ser10 associated with the hTERT promoter and hTERT mRNA expression in stimulated cells.
Histone H3 ser10 phosphorylation occurs in two different cell cycle phases with opposite functions. During mitosis, H3 ser10 is globally phosphorylated, which is required for chromosome condensation and segregation, while in G0/G1 transition, a small fraction of transient H3 ser10 phosphorylation at specific loci leads to chromatin relaxation (10, 33). In Saccharomyces cerevisiae, a ser10-to-ala10 mutation or deletion of the H3 kinase Snf1 abrogates transcription of specific inducible genes (26). Knockout of the H3 kinases MSK1 and MSK2, the downstream effectors of the MAPK cascade, leads to defective H3 phosphorylation and subsequent impairments in induction of IE gene expression (35). These studies provide strong evidence that the ser10 phosphorylation event does not simply correlate with transcriptional activation but rather directly promotes transcriptional activation of specific genes. H3 ser10 phosphorylation was first shown to be associated with induction of the IE genes c-fos and c-jun in mammalian cells treated with growth factors, TPA, and protein synthesis inhibitors through the ERK or p38 MAPK pathway (28). Since then, more genes coding for proteins involved in cell proliferation, apoptosis, and differentiation have been identified to be transcriptionally targeted by H3 ser10 phosphorylation (33). We have now added the hTERT gene to this growing list. However, compared to the IE genes, hTERT mRNA is induced more slowly with its first appearance at 2 (T cells) to 6 (HDFs) h, and the most abundant levels are seen at around 16 to 24 h. This property makes the hTERT fall into a “late gene” category of H3 phosphorylation targets according to Hauser et al. (15).
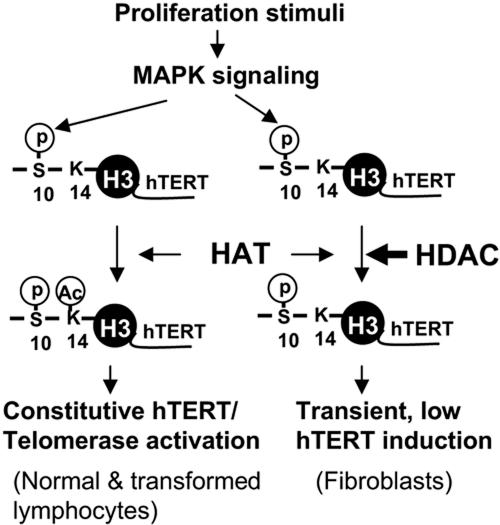
H3 ser10 phosphorylation acts as a critical integrator of signal-mediated chromatin reconfiguration. The event not only attenuates electronic interactions between DNA and histone tails by itself (20) but, more importantly, attracts HATs, thereby leading to acetylation of lys14 in the same histone tail at the target promoters (6, 8, 26, 27). The interplay between phosphorylation and acetylation results consequently in much more profound or synergistic effects on chromatin remodeling and transcriptional activation of the target genes. Consistent with this, we indeed found that histone H3 ser10 phosphorylation was coupled with lys14 acetylation at the hTERT promoter in both activated T cells and Jurkat cells, where constitutively high levels of hTERT mRNA and telomerase activity were induced. In serum-stimulated fibroblasts, however, H3 was only phosphorylated at ser10 without lys14 acetylation on the hTERT promoter, which was concomitant with transient, 50-fold lower hTERT expression compared to that in the T cells. The obtained results suggest that the role of HDACs on the hTERT promoter is predominant and cannot be overridden by an H3 phosphorylation event in fibroblasts. Either growth stimuli or inhibition of HDACs induced low levels of hTERT expression, whereas the combined treatment of fibroblasts led to dramatically enhanced expression of hTERT mRNA. This clearly indicates that both phosphorylation and acetylation of histone H3 are required to fully transactivate the hTERT gene. Based on the above observations, we propose a general model for the hTERT transcription and telomerase activity control in normal human cells and malignant lymphocytes (Fig. 5).
FIG. 5.
Working model for histone H3 phosphorylation and acetylation in controlling hTERT transcription and telomerase activation. The model is based on the present finding that both phosphorylation and acetylation of histone H3 are required to fully trans-activate the hTERT gene. First, growth stimuli trigger H3 ser10 phosphorylation at the hTERT promoter via the MAPK cascade in most, if not all, human cells with proliferation capacities. H3 ser10 phosphorylation then promotes HATs to acetylate lys14 in the same histone tail. Depending on the status of HDACs on the hTERT promoter, local H3 lys14 acetylation may occur, and synergistic effects of phosphorylation and acetylation lead to constitutively high hTERT expression and telomerase activation, as seen in normal and malignant T cells. On the other hand, if the role of HDACs on the hTERT promoter is predominant, no further lys14 is acetylated, and consequently ser10 phosphorylation alone induces transient, low levels of hTERT expression, as seen in fibroblasts. Thick arrow, the predominant role for HDACs at the hTERT promoter.
Although the presence of detectable telomerase activity does not necessarily mean stabilization of telomere length, constitutive hTERT expression and telomerase activation in immortalized and cancer cells are known to counteract telomere attrition and are required for sustained cellular proliferation (2, 34). Recent evidence has also emerged that inducible hTERT and telomerase expression plays physiological roles (31). Belair et al. observed that telomerase activation in cultured normal human uroepithelial cells was sufficient to prevent telomere shortening (1). Surprisingly, even transient induction of hTERT expression and trace amounts of telomerase activity in cycling fibroblasts were demonstrated to be essential for their intrinsic replicative potentials (30, 31). Given these observations, together with the fact that telomeric repetitive sequences are synthesized by telomerase in late S phase of the cell cycle, it is readily envisaged that integrating trans-activation of the hTERT gene with onset of the G0/G1 transition through the MAPK cascade-mediated H3 ser10 phosphorylation has important implications. First, in actively proliferating presenescent cells, the temporal profile of hTERT/telomerase induction is exactly compatible with its timing functional requirement, which may slow down the rate of telomere shortening or contribute to maintenance of telomere structure by preventing 3′-overhang loss, as observed in fibroblasts, thereby conferring a maximal physiological life span to the cells. Second, upon entry into senescence, permanent cell growth arrest leads to a persistent repression of hTERT transcription, which avoids inappropriate telomerase activation and thereby acts as a potent barrier to cellular immortalization. Finally, this regulatory mechanism might be crucial for telomerase-competent cells to maintain their stable telomere sizes, because these cells require a very efficient approach to prevent telomerase from elongating telomeres in the absence of DNA replication (17). Thus, the tightly proliferation-regulated hTERT and telomerase expression might be a novel mechanism through which cells are capable of avoiding telomerase action when in either physiological quiescence or unfavorable growth environments.
The present data are also relevant to telomerase activation during tumorigenesis. The oncogene-transformed mouse cells, where the Ras-MAPK pathway is constitutively active, display significantly higher levels of H3 phosphorylation and more relaxed chromatin structure compared to their parental cells (4). The constitutive activation of the MAPK cascade through Ras gene alteration and other mechanisms is widespread in human cancers, and, given a direct link between the MAPK signal, histone modification, and hTERT expression, the abnormally increased MAPK activity is likely one of the essential forces that target the hTERT chromatin and drive the transcriptional activation of the hTERT gene in transformed human cells. Indeed, Ras- and Raf-mediated transcriptional activation of the hTERT gene has recently been observed (12). Additionally, EGF-triggered Ras-ERK signaling is also implicated in a direct up-regulation of hTERT expression (29).
However, those two studies suggested the presence of multiple mechanisms for the MAPK cascade-induced telomerase activation (12, 29). Maida et al. (29) identified the functional ETS binding motif on the hTERT promoter through which Ras-ERK signaling activated hTERT transcription and up-regulated telomerase activity. Similarly, the oncogenic Ras was shown to stimulate the hTERT promoter activity via the ETS transcription factor ER81 and MAPK (12). Therefore, inhibition of the MAPK cascade may lead to the diminished effect of the ETS transcription factor on hTERT trans-activation in addition to a decrease in histone H3 phosphorylation-induced hTERT expression. It is also plausible that the ETS transcription factor and histone H3 phosphorylation may interact with each other and cooperate to trans-activate the hTERT gene.
It has been shown that MSKs are the H3 kinases responsible for ser10 phosphorylation upon G0/G1 transition or stress stimuli (35). Consistently, H89, an MSK inhibitor, significantly abrogated hTERT induction in normal T lymphocytes and fibroblasts. However, we failed to detect the presence of MSKs at the hTERT promoter (data not shown), and it is unclear whether this is due to a technical limitation of our ChIP assay. It remains to be delineated how exactly H3 ser10 associated with the hTERT chromatin is phosphorylated. Another intriguing issue is why ser10 phosphorylation is not followed by acetylation in HDFs, as seen in T cells and Jurkat cells. A recent report (23) provides some clues: certain tumor suppressors such as MAD1, known to repress target genes via recruiting corepressors and HDACs, are found closely associated with the hTERT promoter in HDFs and might contribute to persistent HDAC occupancy on the hTERT promoter region. Nevertheless, the present results reveal a fundamental role for histone H3 phosphorylation in the hTERT/telomerase induction mediated by proliferation signals and the novel cooperative interplay between histone H3 phosphorylation and acetylation in triggering constitutive expression of hTERT/telomerase, which provides new insights into the regulatory mechanisms of hTERT/telomerase expression in both normal and malignant human cells.
Acknowledgments
We thank J. Sedivy (Brown University) for LF1 human fibroblasts and Z. Yan (Karolinska Institutet) for normal human dermal fibroblasts.
This work was supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, the Swedish Research Council, Swedish Medical Society, and the Karolinska Institute Funds. D.X. was a research fellow of the Swedish Research Council.
REFERENCES
- 1.Belair, C. D., T. R. Yeager, P. M. Lopez, and C. A. Reznikoff. 1997. Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc. Natl. Acad. Sci. USA 94:13677-13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasco, M. A. 2003. Telomeres and cancer: a tale with many endings. Curr. Opin. Genet. Dev. 13:70-76. [DOI] [PubMed] [Google Scholar]
- 3.Buchkovich, K. J., and C. W. Greider. 1996. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol. Biol. Cell 7:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadee, D. N., M. J. Hendzel, C. P. Tylipski, C. D. Allis, D. P. Bazett-Jones, J. A. Wright, and J. R. Davie. 1999. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J. Biol. Chem. 274:24914-24920. [DOI] [PubMed] [Google Scholar]
- 5.Chadeneau, C., P. Siegel, C. B. Harley, W. J. Muller, and S. Bacchetti. 1995. Telomerase activity in normal and malignant murine tissues. Oncogene 11:893-898. [PubMed] [Google Scholar]
- 6.Cheung, P., K. G. Tanner, W. L. Cheung, P. Sassone-Corsi, J. M. Denu, and C. D. Allis. 2000. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell 5:905-915. [DOI] [PubMed] [Google Scholar]
- 7.Clayton, A. L., S. Rose, M. J. Barratt, and L. C. Mahadevan. 2000. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J. 19:3714-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements, A., A. N. Poux, W. S. Lo, L. Pillus, S. L. Berger, and R. Marmorstein. 2003. Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Mol. Cell 12:461-473. [DOI] [PubMed] [Google Scholar]
- 9.Cong, Y. S., and S. Bacchetti. 2000. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J. Biol. Chem. 275:35665-35668. [DOI] [PubMed] [Google Scholar]
- 10.Davie, J. R. 2003. MSK1 and MSK2 mediate mitogen- and stress-induced phosphorylation of histone H3: a controversy resolved. Science STKE 2003:PE33. [DOI] [PubMed] [Google Scholar]
- 11.Devereux, T. R., I. Horikawa, C. H. Anna, L. A. Annab, C. A. Afshari, and J. C. Barrett. 1999. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 59:6087-6090. [PubMed] [Google Scholar]
- 12.Goueli, B. S., and R. Janknecht. 2004. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol. Cell. Biol. 24:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greider, C. W. 1998. Telomerase activity, cell proliferation, and cancer. Proc. Natl. Acad. Sci. USA 95:90-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harle-Bachor, C., and P. Boukamp. 1996. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc. Natl. Acad. Sci. USA 93:6476-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser, C., B. Schuettengruber, S. Bartl, G. Lagger, and C. Seiser. 2002. Activation of the mouse histone deacetylase 1 gene by cooperative histone phosphorylation and acetylation. Mol. Cell. Biol. 22:7820-7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiyama, K., Y. Hirai, S. Kyoizumi, M. Akiyama, E. Hiyama, M. A. Piatyszek, J. W. Shay, S. Ishioka, and M. Yamakido. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155:3711-3715. [PubMed] [Google Scholar]
- 17.Holt, S. E., W. E. Wright, and J. W. Shay. 1996. Regulation of telomerase activity in immortal cell lines. Mol. Cell. Biol. 16:2932-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horikawa, I., and J. C. Barrett. 2003. Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms. Carcinogenesis 24:1167-1176. [DOI] [PubMed] [Google Scholar]
- 19.Hou, M., X. Wang, N. Popov, A. Zhang, X. Zhao, R. Zhou, A. Zetterberg, M. Bjorkholm, M. Henriksson, A. Gruber, and D. Xu. 2002. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp. Cell Res. 274:25-34. [DOI] [PubMed] [Google Scholar]
- 20.Khan, A. U., and S. Krishnamurthy. 2005. Histone modifications as key regulators of transcription. Front. Biosci. 10:866-872. [DOI] [PubMed] [Google Scholar]
- 21.Kyo, S., and M. Inoue. 2002. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene 21:688-697. [DOI] [PubMed] [Google Scholar]
- 22.Lai, S. R., S. M. Phipps, L. Liu, L. G. Andrews, and T. O. Tollefsbol. 2005. Epigenetic control of telomerase and modes of telomere maintenance in aging and abnormal systems. Front. Biosci. 10:1779-1796. [DOI] [PubMed] [Google Scholar]
- 23.Lin, S. Y., and S. J. Elledge. 2003. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 113:881-889. [DOI] [PubMed] [Google Scholar]
- 24.Liu, J. P. 1999. Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J. 13:2091-2104. [DOI] [PubMed] [Google Scholar]
- 25.Liu, L., S. N. Saldanha, M. S. Pate, L. G. Andrews, and T. O. Tollefsbol. 2004. Epigenetic regulation of human telomerase reverse transcriptase promoter activity during cellular differentiation. Genes Chromosomes Cancer 41:26-37. [DOI] [PubMed] [Google Scholar]
- 26.Lo, W. S., L. Duggan, N. C. Emre, R. Belotserkovskya, W. S. Lane, R. Shiekhattar, and S. L. Berger. 2001. Snf1-a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science 293:1142-1146. [DOI] [PubMed] [Google Scholar]
- 27.Lo, W. S., R. C. Trievel, J. R. Rojas, L. Duggan, J. Y. Hsu, C. D. Allis, R. Marmorstein, and S. L. Berger. 2000. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell 5:917-926. [DOI] [PubMed] [Google Scholar]
- 28.Mahadevan, L. C., A. C. Willis, and M. J. Barratt. 1991. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell 65:775-783. [DOI] [PubMed] [Google Scholar]
- 29.Maida, Y., S. Kyo, T. Kanaya, Z. Wang, N. Yatabe, M. Tanaka, M. Nakamura, M. Ohmichi, N. Gotoh, S. Murakami, and M. Inoue. 2002. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 21:4071-4079. [DOI] [PubMed] [Google Scholar]
- 30.Masutomi, K., R. Possemato, J. M. Wong, J. L. Currier, Z. Tothova, J. B. Manola, S. Ganesan, P. M. Lansdorp, K. Collins, and W. C. Hahn. 2005. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. USA 102:8222-8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masutomi, K., E. Y. Yu, S. Khurts, I. Ben-Porath, J. L. Currier, G. B. Metz, M. W. Brooks, S. Kaneko, S. Murakami, J. A. DeCaprio, R. A. Weinberg, S. A. Stewart, and W. C. Hahn. 2003. Telomerase maintains telomere structure in normal human cells. Cell 114:241-253. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277:955-959. [DOI] [PubMed] [Google Scholar]
- 33.Nowak, S. J., and V. G. Corces. 2004. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 20:214-220. [DOI] [PubMed] [Google Scholar]
- 34.Shay, J. W., and I. B. Roninson. 2004. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23:2919-2933. [DOI] [PubMed] [Google Scholar]
- 35.Soloaga, A., S. Thomson, G. R. Wiggin, N. Rampersaud, M. H. Dyson, C. A. Hazzalin, L. C. Mahadevan, and J. S. Arthur. 2003. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 22:2788-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takakura, M., S. Kyo, Y. Sowa, Z. Wang, N. Yatabe, Y. Maida, M. Tanaka, and M. Inoue. 2001. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 29:3006-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei, S., and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 38.Xu, D., A. Gruber, C. Peterson, and P. Pisa. 1998. Telomerase activity and the expression of telomerase components in acute myelogenous leukaemia. Br. J. Haematol. 102:1367-1375. [DOI] [PubMed] [Google Scholar]
- 39.Xu, D., N. Popov, M. Hou, Q. Wang, M. Bjorkholm, A. Gruber, A. R. Menkel, and M. Henriksson. 2001. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 98:3826-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]