Abstract
Coronaviruses are a family of enveloped, single-stranded, positive-strand RNA viruses classified within the Nidovirales order. This coronavirus family consists of pathogens of many animal species and of humans, including the recently isolated severe acute respiratory syndrome coronavirus (SARS-CoV). This review is divided into two main parts; the first concerns the animal coronaviruses and their pathogenesis, with an emphasis on the functions of individual viral genes, and the second discusses the newly described human emerging pathogen, SARS-CoV. The coronavirus part covers (i) a description of a group of coronaviruses and the diseases they cause, including the prototype coronavirus, murine hepatitis virus, which is one of the recognized animal models for multiple sclerosis, as well as viruses of veterinary importance that infect the pig, chicken, and cat and a summary of the human viruses; (ii) a short summary of the replication cycle of coronaviruses in cell culture; (iii) the development and application of reverse genetics systems; and (iv) the roles of individual coronavirus proteins in replication and pathogenesis. The SARS-CoV part covers the pathogenesis of SARS, the developing animal models for infection, and the progress in vaccine development and antiviral therapies. The data gathered on the animal coronaviruses continue to be helpful in understanding SARS-CoV.
INTRODUCTION
Coronaviruses infect many species of animals, including humans. Coronaviruses have been described for more than 50 years; the isolation of the prototype murine coronavirus strain JHM, for example, was reported in 1949 (7, 41). The molecular mechanisms of replication as well as the pathogenesis of several coronaviruses have been actively studied since the 1970s. Some of the animal viruses, such as porcine transmissible gastroenteritis virus (TGEV), bovine coronavirus (BCoV), and avian infectious bronchitis viruses (IBV), are of veterinary importance. The murine coronavirus mouse hepatitis virus (MHV) is studied as a model for human disease. This family of viruses remained relatively obscure, probably because there were no severe human diseases that could definitely be attributed to coronaviruses; human coronaviruses caused only the common cold. However, in the spring of 2003, when it became clear that a new human coronavirus was responsible for severe acute respiratory syndrome (SARS), coronaviruses became much more recognized. With the occurrence of the SARS epidemic, coronaviruses may now be considered “emerging pathogens.” The origin of the SARS coronavirus (SARS-CoV) poses interesting questions about coronavirus evolution and species specificity. Since the SARS epidemic, two new human respiratory coronaviruses have been described. In this review we discuss the pathogenesis of the previously known coronaviruses. We then discuss the newly isolated SARS-CoV. It has become evident that the body of information gathered over the last 30 years regarding coronavirus replication and pathogenesis has helped to begin understanding of the origin and the biology of SARS-CoV.
Taxonomy
The name “coronavirus,” coined in 1968, is derived from the “corona”-like or crown-like morphology observed for these viruses in the electron microscope (318). In 1975, the Coronaviridae family was established by the International Committee on the Taxonomy of Viruses. Recently, at the 10th International Nidovirus Symposium in Colorado Springs, Colo., in June 2005, it was proposed that the Coronaviridae family be divided into two subfamilies, the coronaviruses and the toroviruses, the latter of which cause enteric diseases in cattle and possibly in humans. The Coronaviridae family, along with the Arteviridae and Roniviridae families, form the Nidovirales order. The Arteviridae family includes swine and equine pathogens, and the Roniviridae family is composed of invertebrate viruses (64, 88).
Coronaviruses are divided into three genera (I to III), usually referred to as groups and based on serological cross-reactivity (218) (Table 1); more recent genome sequence analysis has confirmed this grouping (115). Group I coronaviruses include animal pathogens, such as TGEV of the pig, porcine epidemic diarrhea virus (PEDV), and feline infectious peritonitis virus (FIPV), as well as the human coronaviruses HCoV-229E and HKU1, which cause respiratory infections (see below). Group II also includes pathogens of veterinary relevance, such as BCoV, porcine hemagglutinating encephalomyelitis virus, and equine coronavirus, as well as human coronaviruses viruses OC43 and NL63, which, like HCoV-229E, also cause respiratory infections. Group II also includes viruses that infect both mice and rats. MHV is often studied as a prototype coronavirus; MHV is a group of highly related strains causing a variety of diseases, such as enteric disease, hepatitis, and respiratory disease, as well as encephalitis and chronic demyelination. Rat sialodacryoadenitis coronavirus also belongs to group II. There has been controversy about whether SARS-CoV defines a new group of coronaviruses or whether it is a distant member of group II (as discussed in “CORONAVIRUSES AS EMERGING PATHOGENS: SARS-CoV” below); given the data to date (113, 117), we have listed SARS-CoV in group II in Table 1. Group III thus far includes only avian coronaviruses, such as IBV, turkey coronavirus, and pheasant coronavirus (38). Recently, using reverse transcription-PCR (RT-PCR), coronavirus sequences were detected in the graylag goose (Anser anser), feral pigeon (Columbia livia), and mallard (Anas platyrhynchos) (147); phylogenetic analyses of the replicase and nucleocapsid (N) sequences suggest that these viruses are members of group III, but as yet they have not been isolated or characterized.
TABLE 1.
Coronaviruses, hosts, diseases, and receptors
| Group | Virus | Host | Disease(s) caused | Cellular receptor |
|---|---|---|---|---|
| I | 229E | Human | Respiratory infection | Human APN |
| TGEV | Pig | Respiratory and enteric infection | Porcine APN | |
| PRCoV | Pig | Respiratory infection | Porcine APN | |
| Canine coronavirus | Enteric infection | Canine APN | ||
| FeCoV | Enteric infection | Feline APN | ||
| FIPV | Cat | Respiratory, enteric, and neurologic infection, and hepatitis | Feline APN | |
| NL-63 | Human | Respiratory infection, croup | ACE2 | |
| II | OC43 | Human | Respiratory infection and possibly enteric infection | Neu5,9Ac2-containing moiety |
| MHV | Mouse | Enteric and neurologic infection and hepatitis | Murine CEACAM1 | |
| Sialodacryoadenitis coronavirus | Rat | Neurologic infection | NDa | |
| Hemagglutinating encephalomyocarditis virus | Pig | Respiratory, enteric, and neurologic infection | Neu5,9Ac2-containing moiety | |
| BCoV | Cow | Enteric infection | Neu5,9Ac2-containing moiety | |
| HKU1 | Human | Respiratory infection | ||
| SARS-CoV | Human | Severe acute respiratory syndrome | ACE2 | |
| III | IBV | Chicken | Respiratory infection, hepatitis, other | ND |
| Turkey coronavirus | Turkey | Respiratory and enteric infection | ND |
ND, not determined.
Coronavirus Diseases
Coronaviruses cause acute and chronic respiratory, enteric, and central nervous system (CNS) diseases in many species of animals, including humans (218). The pathogenesis of a few of these will be reviewed below.
Human coronavirus.
Previous to the emergence of SARS-CoV, there were two prototype human coronaviruses, OC43 and 229E, both etiologic agents of the common cold (218). There had long been speculation about the association of human coronaviruses with more serious human diseases such as multiple sclerosis (33), hepatitis (380), or enteric disease in newborns (262). However, none of these early associations had been substantiated. The recently identified SARS-CoV, which was shown to cause a severe acute respiratory syndrome was the first example of serious illness in humans caused by a coronavirus (267) and will be discussed in detail in below. Since the identification of SARS-CoV, there have been reports of two new human coronaviruses associated with respiratory disease. HKUI is a group II coronavirus isolated from an elderly patient with pneumonia (340). This virus has been difficult to propagate in cell culture, and there is little information available about the biology of this virus. HCoV-NL63 is a group I coronavirus isolated from a 7-month-old child in The Netherlands who was suffering from bronchiolitis and conjunctivitis (101, 320). It has subsequently been reported in other parts of the world, including Canada (12), Japan (86), Hong Kong (52), Australia (5), and Belgium (220). HCoV-NL63 is associated with serious respiratory symptoms, including upper respiratory infection, bronchiolitis, and pneumonia (86). The strong correlation of the presence of NL63 with croup in children with lower respiratory infections has suggested a causal relationship between the virus and croup (321). While primarily associated with infections of children, NL63 has been also been detected in immunocompromised adults with respiratory tract infections. This virus was independently isolated in New Haven, Connecticut, and called HCoV-NH (93). That group has suggested that this virus is associated with Kawasaki's disease in children (92); however, this has been disputed by two other reports (14, 87). While little is known about the pathogenesis of any of the human coronaviruses (229E, OC43, HKU1, NL63, and SARS-CoV), there have been detailed studies of the pathogenesis of some of the animal coronaviruses, which may contribute to the understanding of the human viruses. We summarize some of these data below.
Murine coronavirus.
There are many strains of murine coronavirus, or MHV, exhibiting different tropisms and levels of virulence. The commonly used laboratory strains infect primarily the liver and the brain and thus provide animal models for encephalitis and hepatitis as well as the immune-mediated demyelinating disease that develops late after infection, peaking at about 1 month postinfection (242). MHV infection of the mouse is regarded as one of the best animal models for the study of demyelinating diseases such as multiple sclerosis. Other strains cause enteric disease, are spread easily by an oral-fecal route in animal colonies, and are a particular danger to immunocompromised animals (10). The extensive studies of the pathogenesis of MHV and the resulting host immune response have been reviewed (206, 214, 242). It is clear that the level of virulence as well as the tropism of MHV strains results from the interplay of viral gene products and the host immune response. The contributions of individual viral genes to tropism and pathogenic phenotype are discussed later in this review.
The role of the immune response to MHV infection in viral clearance and pathogenesis in the CNS has been well characterized (157). Both antibody- and cell-mediated immune responses are required to protect against coronavirus infections. The CD8+ and CD4+ T cells are primarily responsible for clearance of the virus during acute infection (13-16, 42, 157, 187, 258, 259). Perforin-mediated mechanisms are necessary for clearance of virus from astrocytes and microglia, while gamma interferon (IFN-γ) has been implicated in clearance from oligodendrocytes (237) It is not clear how virus is cleared from neurons. In the case of MHV-A59 infection of the CNS, adoptive transfer of epitope-specific CD8+ T cells prior to infection reduces viral replication and the spread of viral antigen during the acute infection and significantly decreases the amount of demyelination developed by 4 weeks postinfection (151). These and other data (156) suggest that the development of demyelination depends on adequate spread of virus during the acute stage.
MHV T-cell epitopes have been mapped to several structural proteins; there may be additional epitopes, however, in the two-thirds of the genome that encodes the replicase proteins, a portion of the genome that has not yet been examined for epitopes. CD8+ T-cell epitopes have been identified in spike (S) and nucleocapsid proteins. The MHV spike has an immundominant CD8+ T-cell epitope (S510 to S518) and subdominant additional one (S598 to S605) in C57BL/6 mice, while in BALB/c mice there is only one identified CD8+ T-cell epitope in the nucleocapsid protein (N318 to N326) (15). CD4+ T-cell epitopes have been identified in the spike (322), M (346), and nucleocapsid (322) proteins of MHV (242). Neutralizing B-cell epitopes have been mapped primarily to the spike proteins, but nonneutralizing epitopes have been identified in the other viral structural proteins (68, 69, 108, 304). While MHV is cleared primarily by the cell-mediated immune response, in the absence of B cells, antibodies are essential to prevent reemergence of the virus in the CNS after initial T-cell mediated clearance. Interestingly, the requirement does not pertain to virus replication and clearance in the liver (189, 215).
The neurovirulent JHM infection is characterized by a strong and prolonged IFN-α/β response, along with elevated levels of macrophage chemoattractants such as CCL3 (MIP-1α), CCL4 (MIP-1β), and CXCL2 (MIP-2), as well as CXCL10 (IP-10) and CXCL5 (RANTES) (173). The increase in chemokines is associated with high levels of macrophages and neutrophils during acute infection and also in later demyelination stages (109). Recombinant virus studies suggest that the macrophage infiltration may be influenced by the S protein (260; K. T. Iacono and S. R. Weiss, unpublished data). The most neurovirulent isolate of JHM fails to induce a significant T-cell response; the resulting inability of the host to clear virus is likely responsible for the high mortality even at low doses of virus (201). Studies using knockout mice or antichemokine antisera have revealed the importance of CCL3, Mig, CXCL10, and CCR2 in the recruitment of T cells to the CNS during MHV infection (83, 129, 191, 192, 315). Sensitivity to IFN-α/β is strain specific for MHV. While the growth of low-virulent MHV-S and neurovirulent MHV-JHM was significantly suppressed in IFN-treated L cells compared with untreated cells, inhibition of the highly hepatovirulent MHV-2 stain was not observed in IFN-treated cells (302). These data suggest that MHV-2 may have a specific mechanism for evading the immune response.
Porcine coronavirus.
There are several porcine coronaviruses that have been studied (reviewed in references 89, 271, and 272). Transmissible gastroenteritis virus was recognized in 1946 (80). It is a major cause of viral enteritis and fetal diarrhea in swine; it is most severe in neonates, with mortality resulting in significant economic loss (89). In neonates, TGEV infects epithelial cells of the small intestines, leading to potentially fatal gastroenteritis. Infection also occurs in the upper respiratory tract and, less often, in the lungs (272). In adults, TGEV causes mild disease. Porcine respiratory virus (PRCoV) is an attenuated variant of TGEV. PRCoV infects lung epithelial cells, and antigen is found in type I and type II pneumocytes as well as alveolar macrophages; infection is followed by interstitial pneumonia. The genomes of TGEV and PRCoV are 96% identical except for the 5′ region of the spike gene, and the difference in pathogenic outcome between the two strains is associated with deletions of various lengths (nucleotides 45 to 752) within the 5′ end of the spike gene of PRCoV. Thus, emergence of PRCoV from TGEV resulted from deletions within the spike gene and is an example of evolution of a coronavirus with altered tissue tropism as well as reduced virulence (272).
Various types of vaccines have been evaluated for protection against TGEV (271, 272). Immunization of pregnant swine with attenuated TGEV is not sufficient to protect suckling pigs from infection. Inoculation of young pigs directly with attenuated virus is also unable to stimulate enough immunoglobulin A (IgA)-secreting cells in the intestines to protect against TGEV. However, sows recovering from virulent TGEV infection do produce enough milk IgA to protect suckling pigs from infection and diarrhea. Repeated infections with PRCoV, however, can protect against TGEV and may in fact do that in the field. Subunit vaccines using spike and nucleocapsid proteins have also been tested. The spike protein of TGEV has four major antigenic sites, two of which are neutralizing. The N protein has a functional CD4+ T-cell epitope. While these vaccines are unable to induce either passive or active protection against TGEV, they are able to boost responses in animals vaccinated with attenuated TGEV.
A relatively new group I porcine coronavirus is PEDV. This virus appeared in Europe in the late 1970s into the 1980s and spread to Asia, but it has not been reported in the United States (272). Interestingly, PEDV antibodies do not neutralize TGEV. PEDV shows some characteristics of human coronaviruses in that it is genetically more similar to HCoV-229E than other group I coronaviruses and, like SARS-CoV, replicates in Vero cells (272). Another porcine coronavirus, hemagglutinating enteric coronavirus, is a group II virus, antigenically unrelated to the other porcine viruses.
Avian coronavirus.
IBV causes a highly contagious disease in chickens; it is spread by aerosol and thus is of considerable economic importance to the poultry industry. IBV, which has also reported in pheasants and turkeys, replicates in upper respiratory tissues, with infection of bronchi and severe disease in young animals. Some strains of IBV cause more systemic infections, replicating in other tissues, including the kidney (causing nephritis), the oviduct (causing decreased egg production), and the gut (271, 272). While chickens of all ages are susceptible, very young chicks exhibit more severe respiratory signs and much higher mortality than older birds (59). While the mechanisms of protection against IBV-induced disease are not completely clear, high levels of antibodies are believed to prevent spread of virus from the respiratory tract to other organs. Maternal antibodies have also been shown to protect against IBV infection during the first 2 weeks of life. Adoptive transfer of CD8+ T cells has been shown to protect against IBV challenge (271).
Both live attenuated and inactivated vaccines have been developed and used to protect against IBV. Protection from live vaccines may be short lived, and serotype-specific and inactivated vaccines are unable to protect alone. However, inactivated vaccines may be used to boost birds that have been primed with live attenuated vaccine. Further difficulties in inducing protection by vaccination are due to the multiple serotypes of IBV, which are often not cross protective. Thus, subunit vaccines expressing the S1 subunit of spike protein, via baculovirus or from a fowlpox virus vector, induce protection in nearly all the animals vaccinated; however, differences of as small as 5% between among S1 sequences may result in poor cross protection (37).
Feline coronavirus.
The feline coronaviruses are composed of two biotypes. Feline enteric coronavirus (FeCoV), commonly found in multicat environments in an asymptomatic carrier state, causes seroconversion. FIPV, a less common variant of FeCoV, has the ability to replicate in macrophages, causing a severe and lethal disease. FIPV may be viewed as a virulent variant of FeCoV that is selected for during persistent infection (272). FIPV replicates initially in pharyngeal respiratory or intestinal epithelial cells. Infection of macrophages then leads to viremia and systemic spread of the virus, including inflammation of the abdominal and thoracic cavities and causing occasional ocular and neurological disorders (1, 71). A complication of FIPV infection involves immune-mediated pathology (138). This has presented a great challenge to vaccine development for FIPV. It has been shown that after vaccination against spike protein, cats challenged with FIPV develop an early-death syndrome caused by antibody-dependent enhancement of virus infection. A DNA vaccine approach, directed against the N and M proteins followed by the same to protein-expressed vial vaccinia virus, also has not been successful. Thus, the development of a vaccine against FIPV remains a challenge (271).
Bovine coronavirus.
BCoV is a ubiquitous virus worldwide as measured by serology. BCoV causes both respiratory and enteric disease, including calf diarrhea, winter dysentery in adults, and respiratory infections in cattle of all ages, including those with shipping fever. Viruses isolated from cattle with either respiratory or enteric disease are antigenically similar. Epidemiological studies suggest that serum antibody correlates with immunity. There are currently no vaccines available to prevent BCoV-associated disease (271, 272).
THE VIRION
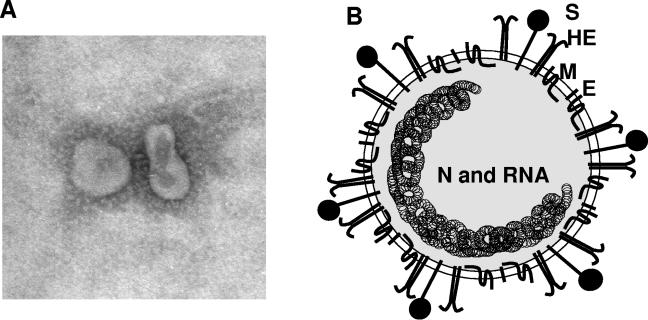
Coronaviruses are enveloped viruses with round and sometimes pleiomorphic virions of approximately 80 to 120 nm in diameter (Fig. 1). Coronaviruses contain positive-strand RNA, with the largest RNA genome (approximately 30 kb) reported to date (178, 196). The genome RNA is complexed with the basic nucleocapsid (N) protein to form a helical capsid found within the viral membrane. The membranes of all coronaviruses contain at least three viral proteins. These are spike (S), the type I glycoprotein that forms the peplomers on the virion surface, giving the virus its corona- or crown-like morphology in the electron microscope; the membrane (M) protein, a protein that spans the membrane three times and has a short N-terminal ectodomain and a cytoplasmic tail; and small membrane protein (E), a highly hydrophobic protein (18). The E protein of IBV has a short ectodomain, a transmembrane domain, and a cytoplasmic tail (63). The E protein of MHV is reported to span the membrane twice, such that both N and C termini are on the interior of the virion (202). Some group II coronaviruses have an additional membrane protein, hemagglutinin esterase (HE) (28). While the function of HE is not known, it is not an essential protein, and it has been speculated to aid in viral entry and/or pathogenesis in vivo and will be discussed below. HE is not encoded in the SARS-CoV genome. There is an additional group II virion protein called I for internal, as it is encoded within the nucleocapsid open reading frame (ORF). This is a nonessential protein of unknown function (97). It has recently been shown that the ORF 3a-encoded SARS protein is an additional structural protein (143). There may be other minor proteins, as yet undetected, included in virions.
FIG. 1.
Coronavirus virion. (A) Electron micrograph of MHV particles. (B) Schematic of virion. Viral particles contain an internal helical RNA-protein nucleocapsid surrounded by an envelope containing viral glycoproteins. Nucleocapsid (N) protein is a phosphoprotein that is complexed with genome RNA to form the nucleocapsid. Spike glycoprotein (S) forms the large glycosylated peplomers that are characteristic of coronaviruses. M, the transmembrane protein, is highly hydrophobic and spans the membrane three times. E, a membrane-spanning protein, is a minor component of the membrane. Some group II viruses express another glycoprotein, hemagglutinin-esterase (HE), which forms smaller spikes on virions.
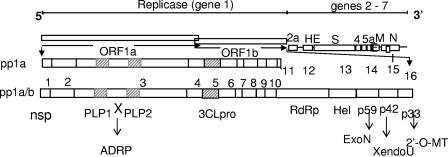
The genomes of all coronaviruses have a similar structure (Fig. 2). The 5′ approximately 20 to 22 kb carries the replicase gene, which encodes multiple enzymatic activities, which will be discussed below. The replicase gene products are encoded within two very large open reading frames, ORFs 1a and 1b, which are translated into two large polypeptides, pp1a and pp1ab, via a frameshifting mechanism involving a pseudoknot structure formed by the genomic RNA (25, 116, 178). The structural proteins are encoded within the 3′ one-third of the genome, for all coronaviruses, in the order S-E-M-N. (When the HE protein is expressed, it is encoded 5′ to S.) Each group of coronaviruses in addition encodes a group of unique small proteins; while these protein are nonessential and have been speculated to serve as accessory proteins and to interact or interfere with the host innate immune response, this has not been demonstrated for any of these proteins. There are untranslated regions (UTRs) on both the 5′ and 3′ ends of the genome, which are believed to interact with host and perhaps viral proteins to control RNA replication, which includes the synthesis of positive- and negative-strand genomic-length RNA. Likewise, there are conserved sequences at the beginning of the transcription sites for each of the multiple subgenomic mRNAs; these are called transcriptional regulatory sequences (previously known as intergenic sequences). Coronavirus transcription has been reviewed recently (27).
FIG. 2.
MHV genome organization and replicase proteins. The genome consists of seven genes. The first 22 kb contains the replicase gene, which is organized into two overlapping open reading frames, ORFs 1a and 1b. These ORFs are translated into the ∼400-kDa pp1a and the ∼800-kDa pp1ab replicase polyproteins. ORF 1b is translated via a translational frameshift encoded at the end of ORF 1a. The protein domains of the replicase polyprotein are indicated by nonstructural protein numbers (nsp1 to 16) and by confirmed or predicted functions: PLP1 and PLP2, papain-like proteases; X, domain encoding predicted adenosine diphosphate-ribose 1"-phosphatase activity (ADRP); 3CLpro, 3C-like protease; RdRp, putative RNA-dependent RNA polymerase; Hel, helicase; ExoN, putative exonuclease; XendoU, putative poly(U)-specific endoribonuclease; 2′-O-MT, methyltransferase. Genes 2 to 7 are translated from subgenomic mRNA species (not shown). Relative locations of coding regions for the structural proteins HE, S, E, M, N, and I are shown, as are the coding region for the group-specific ORF 2a (encoding a predicted cyclic phosphodiesterase), 4, and 5a proteins.
VIRAL LIFE CYCLE
We will briefly summarize the coronavirus life cycle (Fig. 3); this is not designed to be a comprehensive review, but rather to provide a context for discussion (below) of the functions of various viral proteins. Coronaviruses attach to specific cellular receptors via the spike protein (Table 1); this triggers a conformational change in spike which then mediates fusion between the viral and cell membranes which results in the release of the nucleocapsid into the cell (Fig. 3). Upon entry into the cell, the 5′ end of the genome RNA, ORFs 1a and 1b, are translated into pp1a and pp1ab; pp1ab is translated via a frameshift mechanism, which occurs at high frequency (25 to 30%) (25, 27). ORF 1a encodes one or two papain-like proteases (PLpro or PLP) and a picornavirus 3C-like protease (3CLpro), which function to process pp1a and pp1ab into the mature replicase proteins (178, 379; reviewed in reference 378). Also, encoded in the X domain of ORF 1a is a (putative) ADP-ribose 1"-phosphatase activity (287, 378). Encoded in ORF 1b and processed from pp1ab are an RNA-dependent RNA polymerase (RdRp) and a helicase (116), as well as other enzymatic activities, including a (putative) 3′-to-5′ exonuclease (ExoN), poly(U)-specific endoribonuclease (XendoU), and (putative) S-adenosylmethionine-dependent ribose 2′-O-methyltranferase (144, 287, 378). An additional putative enzymatic activity, cyclic phosphodiesterase, is encoded downstream in ORF 2a. These multiple enzymatic activities are speculated to play roles in metabolism of coronavirus RNA and/or in interfering with host cell processes (378).
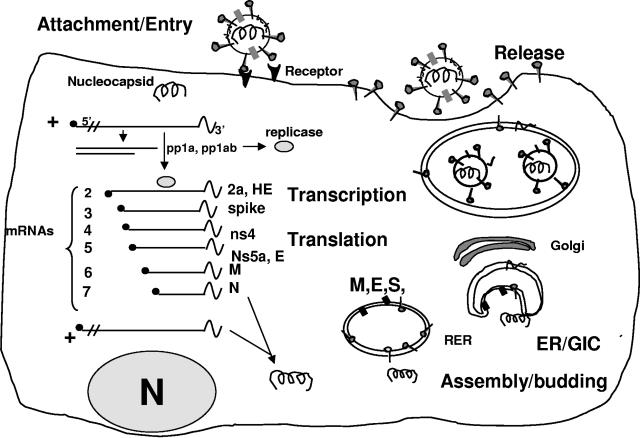
FIG. 3.
Model of coronavirus replication. After receptor interaction and fusion of viral and plasma membranes, virus-specific RNA and proteins are synthesized, probably entirely in the cytoplasm. Expression of coronaviruses starts with translation of two polyproteins, pp1a and pp1ab, which undergo cotranslational proteolytic processing into the proteins that form the replicase complex. This complex is used to transcribe a 3′-coterminal set of nested subgenomic mRNAs, as well as genomic RNA, that have a common 5′ “leader” sequence derived from the 5′ end of the genome. Proteins are translated from the 5′ end of each mRNA. New virions are assembled by budding into intracellular membranes and released through vesicles by the cell secretory mechanisms. RER, rough endoplasmic reticulum; ER/GIC, endoplasmic reticulum/Golgi intermediate compartment.
During infection with coronaviruses, as with all other RNA viruses, replication of genome and transcription of mRNAs must occur. Replication of the genome involves the synthesis of a full-length negative-strand RNA that is present at a low concentration and serves as template for full-length genomic RNA. Multiple (six in the case of MHV) overlapping 3′-coterminal subgenomic RNAs serve as mRNAs, as does full-length genomic RNA. Each mRNA has a common (75- to 78-nucleotide) leader sequence at its 5′ end; this leader is derived from the 5′ end of genome RNA (170, 283). In addition, negative-strand RNAs corresponding in length to each of the mRNAs as well as the full genomic length are present at low concentrations (26). The mechanism by which the group of positive- and negative-strand RNAs are synthesized involves a unique discontinuous transcription mechanism that is not completely understood. However, subgenomic mRNA synthesis is believed to be regulated by transcription-regulating sequences, present in the genome RNA, at the transcriptional start sites for each mRNA (171). The current model is that discontinuous transcription occurs during the synthesis of subgenomic negative-strand RNAs, with the antileader sequences being added onto the 3′ ends of negative-strand RNAs which then serve as templates for synthesis of mRNAs (90). Viral proteins are translated from individual mRNAs, generally from the 5′ ORF only (Fig. 3). The replicase, for example, is translated from the 5′ end of the genomic RNA. In some cases there may be two ORFs carried on and translated from one mRNA. An example of this is the E protein of MHV, which is translated from a downstream ORF (ORF 5b) on mRNA 5; it is believed that the translation of ORF 5b is mediated by an internal ribosome internal entry site (146). After translation, the M and E membrane proteins are localized to the Golgi intracellular membranes near, but just beyond, the endoplasmic reticulum Golgi intermediate compartment, which is believed to be the actual site of budding (154). Thus, in addition to M, other viral and/or cellular factors are probably required to determine the site of budding. M and E proteins, expressed in the absence of other viral proteins and viral RNA, are sufficient to produce virus-like particles (62, 63, 154, 160). The spike protein is distributed on intracellular membranes as well as the plasma membrane. The spike protein interacts with the transmembrane region of the M protein during assembly (74). For some viruses, spike-mediated cell-to-cell fusion occurs, thus promoting syncytium formation and viral spread. Nucleocapsid protein complexes with genome RNA, forming helical structures. The N protein interacts with the M protein (167), and budding into vesicles occurs. Virus is then transported to the cell surface, where it leaves the cell. Interestingly, TGEV and MHV appeared to exit epithelial cells from opposite sides. When the two viruses are used to experimentally infect the same cells, porcine epithelial cells (expressing MHV receptor), TGEV is released preferentially at the apical membrane, while MHV is released preferentially at the basolateral surface, suggesting that vesicles containing the two coronaviruses are targeted differently (266). This suggests that the two viruses are sorted at the Golgi into different transport vesicles carrying information directing them to different surfaces. Thus, the difference in site of release may contribute to the difference in virus spread found between TGEV and MHV. TGEV causes a localized enteric infection, while MHV spreads to multiple organs.
REVERSE GENETICS SYSTEM FOR CORONAVIRUSES
There are now several reverse genetics systems available for coronaviruses (Table 2). Full-length cDNA clones were initially difficult to develop, most likely due to the large size of the coronavirus genome. Thus, the first reverse genetics system available for coronaviruses was targeted recombination, which was developed for MHV (155, 166, 208) and then for TGEV (274) and FIPV (125). In the MHV system, recombination occurs between a murine coronavirus in which the ectodomain of the spike has been replaced with that of the feline coronavirus FIPV spike (called fMHV) and a synthetic RNA carrying the 3′ portion of the MHV genome from the HE gene through the 3′ end. Feline cells are infected with fMHV and then transfected with synthetic mRNA. Recombinants, expressing the MHV spike gene, are then selected on murine cells; parental virus and other viruses with the feline coronavirus spike cannot replicate. This system takes advantage of the high rate of recombination observed during coronavirus infection (204) and the strict host range specificity of these viruses.
TABLE 2.
Reverse genetics of coronaviruses
| Virus | Reference(s) for use of the following method:
|
|||
|---|---|---|---|---|
| Targeted recombination | Full-length cDNA
|
|||
| In vitro ligation | BACa vector | Vaccinia virus | ||
| TGEV | 274 | 362 | 2 | |
| 229E | 310 | |||
| FIPV | 125 | |||
| MHV | 155,166 | 364 | 58 | |
| SARS | 363 | |||
| IBV | 361 | 36 | ||
BAC, bacterial artificial chromosome.
Subsequently, reverse genetics systems utilizing full-length DNA copies were developed for several coronaviruses, including TGEV (2, 362), IBV (36, 361), HCoV-229E (310), MHV (364), and, most recently, SARS-CoV (363). Various strategies for generating infectious genome RNA have been developed, including cloning into and expression from recombinant vaccinia virus (36, 58, 310) or a bacterial artificial chromosome (2) and transcription from genomic-length DNA formed by ligation of multiple subclones (361-364). Targeted recombination and generation of recombinant viruses through the use of an infectious cDNA each have relative advantages (209). A clear limitation of the targeted recombination system is the inability to manipulate the very long replicase gene. This limitation is overcome by the infectious cDNA technology; indeed, this technology has been used to recover viruses with amino acid substitutions in ORFs 1a and 1b (144, 254, 290). These reverse genetics systems are extremely useful in defining the roles of the predicted RNA-processing enzymes encoded in the replicase gene, as discussed above. For example, a recent study used a full-length 229E cDNA clone to evaluate the effects of mutations within the uridylate-specific endoribonuclease (NendoU) and demonstrated that NendoU activity is necessary for viral replication and transcription (144). Targeted recombination involves the use of a vector only one-third the length of the full genome, which facilitates construction of site-directed mutants. Furthermore, the host range selection utilized in targeted recombination is so strong that it allows the selection of mutants that replicate inefficiently and the recovery of recombinants in which multiple crossovers have occurred to eliminate potentially lethal mutations. A parental MHV in which the genes are rearranged has been selected; the use of such a parental virus minimizes the possibility of double crossovers during targeted recombination, favoring the selection of recombinants that replicate inefficiently (112).
Reverse genetics technology has greatly advanced the understanding of coronaviruses. Both mutant and chimeric recombinant viruses have been used extensively in the investigation of the roles of spike and other proteins in coronavirus replication and pathogenesis, to investigate the structure/function relationship of the UTRs at the 5′ and 3′ ends of the genome, to begin to understand the roles of the enzymatic activities encoded in the replicase gene in coronavirus replication, to express foreign sequences in the place of a nonessential gene, and to select viruses with gene deletions or rearrangements that may serve as attenuated vaccines (21, 54, 76, 98, 112, 124, 200, 275). The roles of individual coronavirus genes in infection, particularly in pathogenesis, are discussed below.
ROLES OF CORONAVIRUS PROTEINS IN PATHOGENESIS
Spike Protein
Structure of the spike.
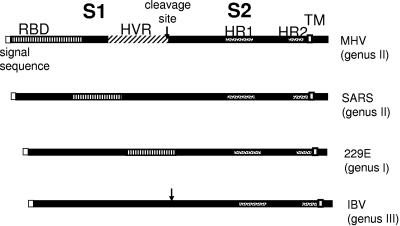
The coronavirus spike protein is a type I glycoprotein that forms the peplomers on coronavirus particles. (Figure 4 shows linear diagrams of several coronavirus spike proteins.) Some coronaviruses spikes (most from group II and III viruses) are cleaved into two subunits by a furin-like enzymatic activity during processing in the Golgi. The prototype MHV spike is 180 kDa; for most MHV strains, it is cleaved into two noncovalently associated subunits of about 90 kDa (294). The amino-terminal S1 subunit, which is believed to form the globular head of the mature protein, contains a receptor binding domain (RBD) within the first 330 amino acids (163). The RBDs of HCoV-229E (residues 417 and 547) and SARS-CoV (residues 318 to 510) spikes are also found in S1, although not at the amino termini (Fig. 4) (17, 339). S1 of MHV contains, downstream of the RBD, a “hypervariable domain” (HVR) that varies in length among strains. Comparison of sequences of various isolates of the JHM strains as well as one isolate of the A59 strain shows “in-frame” deletions of up to 450 nucleotides (relative to the MHV-4 isolate of JHM) in the HVR (236). The carboxy-terminal S2 subunit, which is conserved among all coronavirus spikes and is believed to form a stalk-like structure anchored in the membrane, contains two (or perhaps three [105]) heptad repeat (HR) domains as well as the putative fusion peptide (172, 198, 236, 299). A cysteine-rich domain that bridges the putative junction of the anchor and the cytoplasmic tail is necessary for fusion, as is the transmembrane domain (39).
FIG. 4.
Schematic of coronavirus spike proteins. Shown are spike proteins representative of those of all group I to III coronaviruses and of SARS-CoV. The coronavirus spike protein is synthesized as a precursor, cotranslationally glycosylated, and, in some cases, cleaved in the approximate middle into S1 and S2 subunits at a site with dibasic amino acids (BBXBB). S1 forms the external domain containing the receptor binding domain (RBD) at its 5′ end, followed by, in the case of MHV, a hypervariable domain (HVR). A short signal sequence in cleaved from the 5′ end of the mature protein. S2 is the transmembrane subunit containing two heptad repeats (HR1 and HR2) and the transmembrane (TM) domain.
Receptor interaction, fusion, and entry.
Coronaviruses attach to specific cellular receptors via the spike protein (Table 1). The first identified coronavirus receptor was CEACAM 1, utilized by MHV (141). Viral attachment triggers a conformational change in the spike protein that promotes the fusion of viral and cellular membranes (212, 369). While there are no crystal structures available for any coronavirus spike, it is believed that it may undergo changes similar to those of other type I fusion proteins, such as influenza virus hemagglutinin and human immunodeficiency virus gp120, in order to mediate fusion of viral and cellular membranes.
The coronavirus spike protein plays vital roles in viral entry, cell-to-cell spread, and determining tissue tropism. Coronavirus entry is, in general, not pH dependent, and thus it has been believed to occur directly at the plasma membrane and not via an endosomal route (Fig. 3). However, there are data suggesting that an endosomal route may be utilized by some viruses (156, 219). Entry of SARS-CoV is inhibited by lysosomotropic agents, suggesting an endosomal route of entry (285, 349). Furthermore, this inhibition may be overcome by protease treatment of virus that has attached to the cell. This, along with the observation that infection is blocked by inhibitors of the pH-sensitive endosomal protease cathepsin L, suggests that there is a requirement for cleavage of the SARS-CoV spike during entry through the endosomes (213, 284). Furthermore, entry at the plasma membrane following protease treatment is more efficient than entry by the endosomal route (213). Those authors suggested that SARS-CoV spike may be cleaved by the proteases produced by inflammatory cells present in the lungs of SARS patients and thus enter cells by the more efficient plasma membrane route (213). The highly hepatotropic MHV-2 strain may enter the cell by an endosomal route similar to that used by SARS-CoV. MHV-2, like SARS-CoV, encodes an uncleaved spike protein and is sensitive to lysosomotropic agents; however, trypsin treatment of cell-associated MHV-2 spike overcomes inhibition by lysosomotropic agents (Z. Qiu and S. R. Weiss, unpublished data). This suggests that entry at the cell surface may require a cleavage of spike in the viral membrane, while endosomal entry may provide for cleavage during entry. Finally, coronaviruses with cleaved spikes may also enter the cell by the endosomal route. For example, while wild-type MHV-JHM enters cells in culture by a pH-independent pathway, the OBLV60 mutant of JHM is inhibited by lysosomotropic agents and is believed to enter though a lysosomal pathway (221). Interestingly, OBLV60 is highly attenuated and exhibits restricted spread during infection of the murine central nervous system (239, 316).
In general, the host range of coronaviruses is extremely narrow. The ability of a coronavirus to replicate in a particular cell type depends solely on the ability to interact with its receptors (139). For example, murine coronavirus replicates in murine cells and not in human and hamster cells; however, once nonpermissive cells are transfected with the cDNA encoding MHV receptor, they become susceptible to MHV infection (85). Several coronavirus receptors have been identified. The group I coronaviruses human HCoV-229E, feline FIPV, and porcine TGEV all use aminopeptidase N (APN), a zinc-binding protease, of their respective host species as their receptors (352) (Table 1). There is some ability to recognize the corresponding APN receptor of another species; for example, HCoV-229E can utilize either human APN or feline APN as a receptor but cannot use porcine APN (334, 335). The receptor used by the murine coronavirus group is carcinoembryonic antigen-cell adhesion molecule (CEACAM) (CD66a) (43, 44, 84, 226). CEACAMs are glycoproteins possessing two or four immunoglobulin-like extracellular domains followed by a transmembrane domain and a cytoplasmic tail (226). They are involved in the intercellular adhesion and development of hepatocellular, colorectal, and epithelial tumors (13) and are expressed primarily on the epithelial and endothelial cells of the respiratory tract and intestines, as well as on other tissues (111, 265). The observation that transgenic mice with a knockout of the CEACAM1 gene are resistant to infection demonstrates that this is likely the only receptor for MHV (131). Interestingly, CEACAM1 is expressed at a low level in the brain, a major site of infection of some MHV strains, suggesting that low levels of receptor may be sufficient for mediating MHV entry. Expression of receptor has been demonstrated on only one central nervous system cell type, microglia; the receptor is downregulated on microglia during infection (257). MHV spread for the highly neurovirulent JHM strain may be enhanced by receptor-independent spread (103, 104) and/or by the expression of the hemagglutinin-esterase proteins (see below).
Other group II coronaviruses, such as BCoV, OC43, and porcine hemagglutinating encephalomyelitis virus, bind to 9-O-acetylated sialic acid-containing receptors (159, 253). It is not clear, however, what the specific receptor molecules are, and little is known about the entry process.
Soon after the identification of SARS-CoV, the receptor for this virus was identified as angiotensin-converting enzyme 2 (ACE2). ACE2, like APN, the group I coronavirus receptor, is a zinc metalloprotease (187). Human CD209L, a C-type lection (also called L-SIGN, DC-SIGNR, and DC-SIGN2), when expressed by transfected Chinese hamster ovary cells, renders the cells highly susceptible to SARS-CoV infection; however, it is significantly less efficient than ACE2 in mediating entry (145). SARS-CoV S protein is able to interact with the lectin DC-SIGN; while DC-SIGN binding enhances infection of ACE2-bearing cells, it cannot alone mediate entry in the absence of ACE2. Thus, the interaction of SARS-CoV with this lectin on dendritic cells (DCs), which are not permissive for infection, may augment transmission of SARS to its target cells (135). Surprisingly, it was recently shown that the newly identified group I human coronavirus NL63 also uses ACE2 as its receptor (136).
The spikes of some coronaviruses mediate cell-to-cell fusion of infected cells as well as virus/cell fusion during entry, presumably by a similar mechanism (369) (212). However, viral entry and cell-to-cell fusion do display some differences in requirements. For example some MHV-JHM spikes can mediate cell-to-cell fusion in the absence of CEACAM, while entry requires the CEACAM receptor. Furthermore spike proteins that have mutations that eliminate cleavage into S1 and S2 subunits carry out cell-to-cell fusion very inefficiently; however, they mediate entry into susceptible cells with similar efficiency as wild-type virus (75, 114, 181). Similarly, the MHV-2 strain encodes an uncleaved spike protein and does not carry out cell-to-cell fusion; this virus infects cells efficiently in vitro and causes severe hepatitis in vivo (70, 132, 150). The spike of MHV-A59, which is usually cleaved during replication in cell culture, is not cleaved when recovered from brains or livers of infected mice, suggesting that cleavage is not a prerequisite for infection for strains that express cleaved spike (133) and that entry of MHV into some types of cells in vivo may require an endosomal route of infection.
The heptad repeat domains and the putative fusion peptide are believed to play important roles in the fusion process (103). This has been explored most for the MHV spike. Substitution of charged amino acids for hydrophobic ones in HR1 (and within a candidate fusion peptide) eliminates the ability to induce cell-to-cell fusion (198). Mutations in the leucine zipper domain within HR2 inhibit the ability of spike to oligomerize and to inhibit cell-to-cell fusion (197). Amino acid substitutions at L1114 within the HR1 domain of the JHM spike (L1114R or L1114F) are particularly intriguing in that they have been reported in multiple studies, in association with several mutant phenotypes. An L1114R substitution is one of three mutations believed to contribute to the low pH dependence for viral entry of the OBLV60 variant of JHM as well as its neuroattenuation and restriction to olfactory bulbs during infection of mice (105). Furthermore, L1114R alone was sufficient to cause restriction of recombinant MHV to the olfactory bulbs during infection of mice (316). Substitutions at L1114 have been identified in the spike of an attenuated monoclonal-antibody-resistant mutant (327) and a soluble-receptor-resistant mutant (269, 270). Interestingly, L1114R and L1114F substitutions were identified as secondary mutations in several recombinant viruses expressing A59/JHM chimeric spike proteins (248, 316). The soluble-receptor-resistant mutant of JHM, srr7, (expressing a spike containing L1114F) demonstrated increased stability of the S1/S2 interaction, the loss of the ability to induce CEACAM-independent fusion (301), and altered interactions with the receptor CEACAM1b (an allele of CEACAM 1a expressed by resistant SJ/L mice) as well as resistance to neutralization by soluble CEACAM1a receptor (211, 212). Similarly, the L1114R mutation results in loss of receptor-independent fusion along with neuroattenuation. In support of the idea that the RBD interacts with S2, a mutation in the RBD could functionally suppress the effects of an L1114R mutation in HR1 of srr7 that affected the ability to use CEACAM1b (211). Thus, small changes within the HR domains (for example, a single amino acid substitution at L1114) may result in major alterations in spike/receptor interaction and hence in virus entry and finally pathogenesis in vivo.
Recent studies of the HR domains provide further evidence confirming that the coronavirus spike is indeed a class I fusion protein (23). Peptides representing HR1 and HR2 of MHV, when mixed together, assemble into an extremely stable oligomeric complex with both peptides in alpha-helical conformations and antiparallel to each other. In the native protein, such a conformation would be predicted to bring the N-terminal domain of HR1 and the transmembrane anchor into close proximity to facilitate the fusion process. Furthermore, the HR2 peptide was shown to be a potent inhibitor of virus entry, as well as of cell-to-cell fusion. Similar results were obtained for SARS HR domains. SARS-CoV HR1 and HR2 peptides, when mixed, assemble into a similar six-helix bundle; however, this complex was less stable than that of the corresponding MHV complex. The lack of stability may explain why HR2 peptides are less inhibitory for SARS than for MHV (22).
Role in pathogenesis.
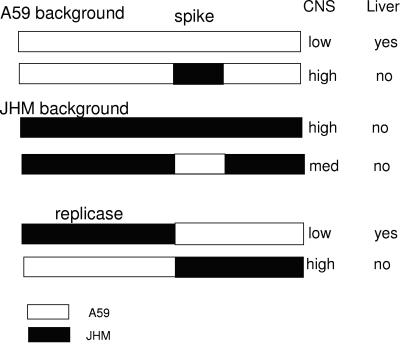
The use of recombinant coronaviruses, including MHV (223, 246), TGEV (274), and IBV (35, 134), has definitively demonstrated that the spike is a major determinant of tropism and pathogenicity. In the case of TGEV, the replacement of the spike gene of an attenuated respiratory strain of TGEV with the spike gene from a virulent enteric strain renders the virus enterotropic (274). Figure 5 summarizes the mapping of tropism and virulence with A59/JHM chimeric recombinant MHVs. The JHM strain is highly neurotropic, causing severe, usually fatal encephalitis and little if any hepatitis, while the A59 strain causes moderate hepatitis and is only weakly neurovirulent. The replacement of the spike gene in the genome of the A59 strain with the spike gene of the most highly neurotropic isolate of the JHM strain renders the resulting virus highly neurovirulent (223, 246). The high neurovirulence conferred by the JHM spike is associated with rapid spread through the CNS, which may occur, in part, independently of the CEACAM receptor and the large numbers of infected neurons (247). However, the resulting chimeric virus (JHM spike in the A59 background) is not as virulent as parental JHM, at least partially because it induces a much stronger CD8+ T-cell response. JHM fails to induce a strong enough CD8+ T-cell response to mediate clearance (201, 260; Iacono et al., unpublished data). The mechanisms that underlie the differences in the immune response in the brain to the closely related A59 and JHM strains are intriguing and not at all understood.
FIG. 5.
The molecular determinants of MHV pathogenesis. Chimeric A59/JHM recombinant viruses were selected by targeted recombination. These viruses were used to infect mice, and the abilities to infect the CNS and induce encephalitis and to infect the liver and cause hepatitis were assessed. The pathogenic phenotypes of the viruses are shown schematically and are discussed in the text.
The replacement of the spike protein of the moderately hepatotropic MHV-A59 with the spike of the nonhepatotropic JHM results in recombinant viruses with the ability to induce only minimal hepatitis (222). Similarly, a chimeric virus with the spike of MHV-2, a highly hepatotropic strain in the A59 background, is highly hepatotropic (223). Thus, for recombinant viruses with A59 background genes, the ability to induce hepatitis is dependent largely on the ability of the spike to mediate entry into cells of the liver. However, the outcome is somewhat different in chimeras in which background genes are derived from JHM. The replacement of the JHM spike with the A59 spike results in a chimeric virus that causes minimal infection of the liver and induces hepatitis very poorly; thus, the in the presence of JHM background genes, the spike of the A59 strain is unable to mediate efficient infection of the liver. The mechanism by which JHM background genes suppress infection of the liver is intriguing and merits further investigation.
In a similar spike exchange experiment performed with IBV, the ectodomain of the spike protein from the virulent M41-CK strain was used to replace the corresponding region within the apathogenic IBV Beaudette genome. The resulting chimeric virus displays the in vitro cellular tropism phenotype of M41-CK (35); however, the virus remains apathogenic. Thus, the M41-CK spike is not sufficient to render the chimeric virus virulent (134). The spike protein is therefore a major determinant of tropism and thus influences pathogenesis; however, the spike alone is not always the main determinant of pathogenesis, and as the data indicate, other genes also contribute to pathogenic phenotypes.
There are many strains of MHV and many isolates of the JHM strain, displaying different levels of neurovirulence. Among the JHM isolates, virulence is correlated with the presence of a long hypervariable domain (see above) within S1. The isolate referred to as MHV-4 (67) or MHVSD (231) has the longest MHV HVR among JHM spikes and is able to induce cell-to-cell fusion and viral spread in the absence of the CEACAM receptor (103, 104). It is likely that this ability is related to the less stable association of S1 and S2, such that the conformational changes in spike that lead to fusion are more easily triggered, and this in turn is at least partially responsible for its very high neurovirulence (103, 161). Similarly, deletions, as well as single-site mutations, within the HVR region have been shown to influence neurovirulence (67, 106, 201, 245).
Mutations within both the RBD of S1 and the heptad repeat domains within S2 have been show to influence pathogenesis. Mutations in the RBD are likely to affect the interaction between spike and the host cell and could thus affect viral entry and tropism, while mutations in the heptad repeats are likely to affect tropism by altering the fusion mechanism. Variation in the amino-terminal portions of the spike has also been noted in TGEV and IBV; the attenuated porcine coronavirus PRCoV has a deletion in the amino-terminal portion of S1 compared with the virulent TGEV (91). A single amino acid substitution within the RBD, S310G, is responsible for enhanced neurovirulence of a JHM isolate (231) Furthermore, a single Q159L amino acid substitution in this region eliminates the ability of MHV-A59 to infect the liver while having no effect on neurovirulence (180, 181). The observation that a one-amino-acid substitution in the RBD can confer a complete loss of tropism to the liver while not affecting infection of the brain, while using the same CEACAM receptor, suggests that other cell surface molecules may serve as cofactors or coreceptors in an organ-specific way. An E1035D substitution within HR1 may overcome the Q159L mutation, since a spike with both of these substitutions confers hepatotropism upon a recombinant MHV-A59 (224). In support of the idea that the RBD may interact with the HR domains, escape mutants selected by resistance to a monoclonal antibody mapping to the receptor binding domain of S1 had point mutations in the region of HR2, suggesting an interaction between these two physically distant portions of the spike (121). Furthermore, mutations within S1 may also affect host range; 21 amino acid substitutions and a 7-amino-acid insertion within the N-terminal region of spike, but downstream of the RBD, allow MHV infection of the usually resistant hamster, feline, and monkey cells (309).
SARS-CoV is believed to have jumped to humans from civets (see “CORONAVIRUSES AS EMERGING PATHOGENS: SARS-CoV” below). The adaptation of SARS-CoV to humans likely involved changes within the RBD. In comparison of the spike protein from civets and from humans, there are six amino acid differences within the RBD of the spike. The spike protein of civet SARS-CoV has low affinity for the human ACE2 SARS-CoV receptor. Substitution of two amino acids within the RBD of the human spike protein with those of the civet spike (N479K/T487S) almost abolishes the ability to infect (using the single-round infection assay) human cells expressing the SARS-CoV receptor. Conversely, substitution of the two residues within the civet spike with the human amino acids confers the ability to infect cells expressing the human receptor. Thus, it is likely that amino acids 479 and 487 are important for receptor interaction and hence species specificity and that selection of viruses with substitutions of these residues allowed the adaptation of SARS-CoV to humans (188, 256).
For MHV, most of the H-2b-restricted T-cell epitopes thus far identified are encoded in the spike gene. The MHV spike encodes an immundominant CD8+ T-cell epitope (S510 to S518), located within the HVR (and therefore absent from the many strains and variants with deletions in the HVR, such as A59) and an additional subdominant CD8+ epitope (S598 to S605) that is expressed by all MHVs. Mutation within the immunodominant epitope of the MHV spike has been reported as a mechanism to escape the immune response and achieve viral persistence; such epitope escape mutants selected in suckling mice were more virulent than wild-type virus (243). However when the same inactivating mutation was introduced into a recombinant virus, the resulting virus ranged from slightly to significantly attenuated in weanling mice, depending on the genetic background of the virus and the strain of the mouse infected (200). Under similar conditions, inactivating mutations within a foreign CD8 T-cell epitope (gp33 from lymphocytic choriomeningitis virus), introduced into recombinant MHVs in a nonessential gene, were readily selected in weanling mice previously immunized against this epitope (54). Thus, the likelihood of epitope escape occurring depends on multiple factors, such as the location of the epitope within an essential versus a nonessential protein and its effect on function of the protein, the background genes of the virus, and the age and strain of mouse (55, 152, 201). CD4 T-cell epitopes have been identified in the spike (127, 322) as well as the M (346) and N (322) proteins of MHV (242) and in the N proteins of porcine TGEV (4) and avian coronaviruses (20).
Studies with chimeric A59/JHM recombinant viruses demonstrated that genes other than the spike play a major role in determining tropism. In fact, JHM genes eliminate the ability of a virus expressing the A59 spike to cause hepatitis, and this is not due to the replicase but rather to genes in the 3′ end of the genome (223; S. Navas-Martin and S. R. Weiss, unpublished data) (see Fig. 5). Furthermore, the extent of T-cell response to recombinant MHVs, and thus the likelihood for viral clearance to occur, is not determined by the spike gene, but rather by background genes, again encoded in the 3′ end of genome (Iacono et al., unpublished data). Thus, other viral structural genes clearly influence pathogenic outcome dramatically, and these are discussed below.
SARS-CoV spike protein may play a role in pathogenesis by inducing interleukin-8 (IL-8) in the lungs via activations of MAPK and AP-1 (40). Such an activity was mapped to amino acids 324 to 688 of the SARS-CoV spike. This activity was detected in epithelial cells and fibroblasts by using baculovirus-expressed SARS-CoV spike; the location of the sequencing responsible for this activity overlaps with the RBD, suggesting that attachment to the ACE2 receptor may trigger this activation (40).
Hemagglutinin-Esterase Protein
The coronavirus HE glycoprotein forms a second type of spike, smaller (5 to 7 nm) than the spike protein peplomers, on the envelopes of some group II coronaviruses (151, 356). HE is synthesized as 42-kDa apoprotein, glycosylated to 65 kDa, and disulfide linked to form a homodimer; when expressed, the BCoV HE displays hemagglutinating and esterase activities (28, 151). The MHV HE displays 30% sequence homology to the HA1 subunit of the hemagglutinin-esterase fusion protein of influenza C virus (199). HE is thought to have been obtained via homologous RNA recombination involving a group II coronavirus before the split off of SARS-CoV, which does not encode an HE protein (287). Coronavirus HE proteins have not received much attention in the past, probably because they are nonessential for replication in tissue culture or, in the case of MHV, for virulence in mice.
Group II viruses that encode HE can be divided into two groups based on their sialic acid substrate specificity. BCoV, HCoV-OC43, and MHV-DVIM recognize and bind to Neu5,9Ac2 (278), and encode HEs with sialate-9-O-acetylesterase activity (286, 325, 326). MHV strains S and JHM bind to Neu4,5Ac2 and encode HEs with sialate-4-O-acetylesterase activity (259, 344). However, it is not clear whether it is HE or the spike that directly binds to sialic acid-containing receptors, and it has been argued that for BCoV, HCoV-OC43, and MHV, it is the spike protein rather than the HE that has the binding activity (165, 278, 344). However, HE proteins of some coronaviruses have been demonstrated to be sialic acid-specific lectins, as demonstrated by hemagglutination and/or hemadsorption assays, thus supporting the role of HE in receptor binding (244, 279, 359). For MHV-DVIM, hemagglutinating activity is associated with HE and not with spike (296; R. J. de Groot, personal communication). For BCoV, both HE and spike recognize the same receptor determinant of 9-O-acetylneuraminic acid on host cells (253). While HE is nonessential for replication in cell culture, spike is necessary and sufficient to mediate entry. Thus, the role of HE in coronavirus infection is still not clear and merits further investigation.
For MHV, the HE protein is expressed by a minority of strains; among these are the weakly pathogenic MHV-S, some isolates of the highly neurovirulent JHM strain (355), and enteropathogenic strains such as DVIM (344). While the highly tissue culture-adapted MHV-A59 genome encodes an HE protein (282), due to multiple mutations, the HE protein is not expressed and the gene is referred to as a pseudogene. Expression of the viral HE glycoprotein is not necessary for virulence in the animal as evidenced by the fact that MHV-A59 causes encephalitis and hepatitis, as well as demyelination, while it does not express HE. In tissue culture, viruses expressing HE have a relative growth disadvantage with respect to viruses that do not express HE. During serial passage in culture, there is a selection for variants in which mutations in the HE gene preclude insertion into the viral membrane (190).
The observation that HE expression is nonessential for the viral life cycle suggests that HE may play a role during infection of the animal (286). It has long been speculated that HE may play a role in acute and/or chronic disease induced by MHV, possibly as a determinant of cellular tropism (353, 354, 358), or may aid spread of the virus by augmenting attachment and/or exit from the cell (151). There are early data both supporting and arguing against this hypothesis. A higher level of mortality as well as increased infection of neurons was associated with a JHM variant that expressed high levels of HE compared with a variant that expressed less HE (353). Taguchi et al. (300) found that HE-expressing variants of MHV-JHM were selected for during propagation in cultured neural rat cells. Moreover, anti-HE monoclonal antibodies, when administered to mice, attenuated the acute encephalitis (354). However, in contrast to these studies, Lai and coworkers reported that in JHM-infected mice, viral variants defective for HE accumulate in the brain and spinal cord (172, 358). These studies were carried out before reverse genetics were available for MHV, and thus they were not able to distinguish between effects of HE and the influence of other genes in the comparison of various MHV isolates. A recent study compared the pathogeneses of isogenic recombinant viruses expressing a wild-type HE protein, expressing an HE protein in which the acetyl esterase activity has been eliminated by mutation, and not expressing the HE polypeptide. Surprisingly both viruses that expressed HE polypeptides (with or without a functional acetyl esterase activity) were more virulent when inoculated intracranially into mice (149). This result would be consistent with a model in which HE may enhance virus attachment and spread by binding to sialic acid-containing receptors and would suggest that the sialic acid binding domain is separate from the esterase domain. Similarly, in the case of influenza C virus hemagglutinin-esterase fusion protein, it has been proposed that there are two separate regions for binding to sialic acid, one for receptor binding and another for the catalytic (esterase) activity (130). We hypothesize that for MHV infection of the CNS, it is the binding activity of HE that augments spread of the virus in certain cell types and that at least in the CNS, the esterase activity is not important for enhanced spread. The esterase activity may be more important in other organs, such as the respiratory tract, where the virus may need to pass through mucus or have the ability to detach cells that may not be productively infected, both of which are believed to be functions of neuraminidases. In the case of influenza virus, it has recently been shown that the specificity of the neuraminidase (esterase) for a particular type of sialic acid determines the cell subtype infected within the respiratory track and hence the pathogenic outcome (210). Thus, by analogy, the HE of MHV may also play a role in tropism.
Membrane Protein
The M protein is the most abundant virion membrane protein. Aside from its role in viral assembly, the coronavirus M protein is believed to have functions in host interactions. It may be O glycosylated (groups I and III) or N glycosylated (group II). While glycosylation is not essential for viral assembly or infectivity (72), the glycosylation state of M protein is likely to play a role in virus-host interaction. For TGEV, the M protein has been shown to have interferogenic activity, and mutations in the M protein ectodomain that impair N glycosylation decrease this activity (175). For MHV, the selection of recombinant viruses with N, O, or no glycosylation demonstrated that while the glycosylation state of M protein does alter the ability to replicate in vitro, it may affect the ability to induce IFN-α in vitro and also to replicate in the liver in vivo (72).
Nucleocapsid Protein
In addition to its role as structural protein, N protein plays a role in transcription and also in pathogenesis. Expression of N protein is necessary for efficient recovery of virus from infectious cDNA clones (363, 364) and recently has been shown to enhance the replication of HCoV-229E genome RNA (277). The N protein of MHV has been implicated in fulminant hepatitis (230). Infection of mice with the highly hepatotropic MHV-3 strain stimulates expression of the fgl2 gene, which encodes a novel immune procoagulant molecule, fibrinogen-like protein 2, expressed in Kuppfer cells and endothelial cells of the liver. The ability to upregulate transcription of this gene maps to the nucleocapsid gene and correlates with the development of fulminant hepatitis (77, 230). While MHV proteins are generally restricted to the cytoplasm, the nucleocapisd proteins of coronaviruses representing groups I, II, and III were shown to localize to the nucleolus as well as to the cytoplasm (343). This report suggests that N protein induces a cell cycle delay or arrest, most likely in the G2/M phase, possibly by inhibition of cytokines.
Small Envelope Protein
The coronavirus E protein is an integral membrane protein (365). Along with the M protein, E plays an important role in viral assembly (324). E protein, when expressed alone or when expressed together with M, forms virus-like particles. Surprisingly, it was possible to select a recombinant MHV with a deletion of the E gene. Such a recombinant MHV has low infectivity and replicates poorly, indicating that while it is nonessential for MHV, E plays an important role in production of infectious virus (168). The E protein of TGEV, however, is essential; disruption of the E gene within TGEV proteins is lethal (65, 233). Apart from its role in virus assembly, E protein has additional functions during infection. It has recently been demonstrated that the E protein of SARS-CoV has cation-selective ion channel activity (337). While the role of this activity is as yet unknown, the E protein ion channel could function at the site of budding to enhance viral morphogenesis and assembly. E protein appears also to play a role in host-virus interaction, specifically in induction of apoptosis. E induces apoptosis in vitro in MHV-A59-infected 17Cl-1 cells via a caspase-dependent mechanism; such apoptosis is suppressed by a high level of Bcl-2 expression. Inhibition of MHV-induced apoptosis promotes virus production late in infection, suggesting that apoptosis may be a host response that limits the level of virus production (3). Whether this occurs in vivo during infection of the mouse has not yet been reported. Similarly, the E protein of SARS-CoV has been shown to induce apoptosis when expressed in Jurkat T cells, and this activity is inhibited by expression of the antiapoptotic protein Bcl-xL (348). Those authors suggest that T-cell apoptosis may contribute to the SARS-CoV-induced lymphopenia that is observed in most SARS patients.
Internal Protein
The genomes of several group II coronaviruses, including MHV, contain an internal ORF within the nucleocapsid gene (97, 174). This ORF, translated in the +1 reading frame with respect to the N protein, encodes a mostly hydrophobic 23-kDa polypeptide. The I gene product is expressed in MHV-infected cells and found within the virions as well. Selection and characterization of recombinant viruses in which the I gene is disrupted demonstrated that I protein is not essential either for the replication of MHV in tissue culture or for pathogenesis in the mouse (97). However, the I gene does confer a small-plaque morphology and may have an as-yet-unknown subtle role in pathogenesis.
Replicase Proteins
The replicase proteins could affect tropism and pathogenesis by determining the rate of viral replication, perhaps via interactions with noncoding 5′ and/or 3′ UTR sequences in the viral genome, with cell type-specific factors, or with elements of the immune response. The several enzymatic activities that have been predicted to be encoded in ORFs 1a and 1b, as described above, could be involved in subverting many aspects of host cell metabolism (378). With the availability of infectious cDNA clones, the replicase gene is now available for genetic analysis, and information concerning the role of the replicase proteins is likely to be forthcoming. For example, recently a one-amino-acid substitution in nsp14 (ExoN or p59), a protein with exonuclease activity, was shown to drastically reduce the virulence of a recombinant A59 without affecting the in vitro replication; the mechanism of this attenuation is not known (290).
The p28 protein of MHV, encoded at the extreme 5′ terminus of the genome and thus processed from the N terminus of pp1a, when expressed transiently in several different cultured cell types prevents cell cycle progression from G0/G1 to S phase (42).
Studies of A59/JHM chimeric MHVs, in which the A59 replicase gene is expressed with the JHM structural genes and vice versa, demonstrated that the replicase is not an important determinant of the difference in tropism and pathogenesis between the two strains (severe encephalitis versus hepatitis). It is rather the 3′ portion of the genome that is responsible for pathogenic properties (Navas-Martin and Weiss, unpublished data). This is consistent with the observation that the ability of MHV to induce fulminant hepatitis maps to the nucleocapsid gene (230).
Group-Specific Proteins
Like other RNA viruses, all coronaviruses encode, in addition to structural proteins and replicase proteins, small nonessential proteins of unknown function. There are many examples of such proteins, encoded by RNA viruses, which interact with and compromise the alpha/beta interferon response; among these are VP35 of Ebola virus (11), the V proteins of several paramyxoviruses (235, 360), and the NS1 and NS2 proteins of human and bovine respiratory syncytial viruses (289, 319). While there are not yet any specific examples of coronavirus proteins being involved in antihost defense by subverting the host innate immune response, it is quite plausible that one or more coronavirus nonessential proteins serve such an “accessory” function during infection in vivo.
The coronavirus genes encoding these proteins are sometimes referred to as “small ORFs” or “group-specific” genes, as they are conserved among each group of coronaviruses. The MHV genome contains ORFs 2a, 4, and 5a; the proteins encoded in these three ORFs appear to be nonessential for replication. In some strains of MHV, such as A59, ORF 4 is interrupted and becomes ORFs 4a and 4b (333), and there are reports of an MHV (JHM strain) isolate with a deletion of ORF 2a (280) as well as an MHV strain S isolate with deletions of most of ORFs 4 and 5a (357). The intriguing question of whether one or more of these ORFs encodes a protein with a role in pathogenesis remains unresolved. The use of reverse genetics makes it possible to address this question by the selection of isogenic viruses differing only in expression of a particular ORF. A recombinant MHV (JHM strain) lacking gene 4 has been shown to be as neurovirulent as the wild type in mice (232). In contrast, a recombinant MHV lacking ORFs 2a, 4, and 5a is avirulent in mice; however, because replication of this virus is inefficient in vitro, it is not possible to determine if the attenuation in mice is due to a specific function of a viral gene product or to a more general inability of the virus to replicate efficiently (73). There are a few examples of recombinant viruses in which elimination of expression of ORFs may confer changes in pathogenic phenotype. A recombinant virus with a deletion of ORF 2a (as well as the HE pseudogene) was attenuated despite its ability to replicate to similar titers as wild-type virus in vitro; this suggests that ORF 2a could play a role in virulence. In support of a role in pathogenesis for the ORF 2a-encoded 30-kDa protein is the report of a recombinant MHV in which a mutation within ORF 2a is associated with attenuation in animals (290). ORF 2a encodes a (putative) cyclic phosphodiesterase; it is intriguing to speculate that such an activity may participate in compromising the host response. It has been speculated that, in analogy to their cellular homologues, the cyclic phosphodiesterase along with the predicted ADP-ribose 1"-phosphatase activity, encoded in ORF 1a (discussed above), may mediate consecutive steps in processing of tRNA splicing products (58, 378).
In the case of the porcine coronavirus TGEV, expression of gene 7 is not essential for replication; however, a recombinant virus in which the expression of gene 7 was abrogated displays reduced virulence with less virus replication in the lung and gut, suggesting that the gene 7 product does influence in vivo replication and virulence (234). ORFs 3a and 3b of TGEV are also not essential for replication (217, 336). Various PRCoV isolates have deletions within ORFs 3a and 3b, suggesting that the loss of expression of these ORFs may be associated with attenuation; however, the attenuation in these strains is associated with deletions in the spike gene rather than in ORFs 3a and 3b. Furthermore, a recombinant with a deletion of these ORFs demonstrates wild-type tropism, replicates efficiently in animals, and displays only a very small reduction virulence (90, 274, 323).
Recombinant FIPVs with deletions of either ORFs 3a, 3b, and 3c or ORFs 7a and 7b multiply efficiently in cell culture but show an attenuated phenotype in the cat. This suggests that one or more of the proteins encoded in these ORFs may play a role in virulence (124). A recombinant IBV in which expression of gene 5 was eliminated replicates to a similar extent as wild-type virus in tissue culture, demonstrating that neither of the two proteins encoded in gene 5 is essential for replication and that these are candidate accessory proteins (34).
The human SARS-CoV genome encodes multiple small open reading fames (ORFs 3a, 3b, 6, 7a,7b, 8a, 8b, and 9b) that are presumed to encode eight group-specific accessory proteins (205, 287). There are no reports of proteins in the NCBI database with homology to any of these ORF-encoded protein. Like the group II coronavirus I protein gene, the SARS ORF 9b is within the nuclecocapsid gene of SARS-CoV and at a similar position (287); however, the predicted proteins has no homology with the I protein, and whether it is a minor virion protein is not known. It is possible that some of these ORFs, perhaps the smaller ones, may not encode authentic proteins; in fact, ORFs 3b, 6, and 8b appear to be in a poor context for translation (158). ORFs 8a and 8b may have been created by a deletion in ORF 8 of the animal SARS-CoV isolates; thus, ORF 8 may not be necessary for replication in humans (122) (see “CORONAVIRUSES AS EMERGING PATHOGENS: SARS-CoV” below).
Two of these ORFs have been shown to be expressed during infection, and the encoded proteins have been partially characterized. ORF 7a encodes a 122-amino-acid protein predicted to contain a cleaved signal sequence and a C-terminal transmembrane helix, indicating that it is likely to be a type I membrane protein. ORF 7a protein is expressed in Vero E6 cells and in the lungs of patients but is not present in virions (47, 96, 227, 306). ORF 7a is localized to the perinuclear region of both infected and transfected cells; it colocalizes with endoplasmic reticulum and intermediate compartment markers, consistent with the endoplasmic reticulum retrieval motif in the C-terminal tail domain (96). Confocal microscopy suggests that the short cytoplasmic tail and transmembrane domain function in trafficking of the ORF 7 protein within the endoplasmic reticulum and Golgi network (227). Analysis of the crystal structure of the N-terminal ectodomain of ORF 7a protein demonstrates a compact seven-stranded beta sandwich structure similar to that of members of the IgG superfamily (227).
The 274-residue protein encoded by ORF 3a is predicted to contain three transmembrane domains. It is found in virus particles, and in infected Vero E6 cells it is localized to perinuclear regions as well as the plasma membrane and with intracellular virus particles. The ORF 3a protein is present in two processed forms as well as the full-length protein. The ORF 3a protein can interact with the M, E, and S structural proteins, as well as with the ORF 7a protein (143, 306).
There are reports that ORF 7a (305), ORF 3a (177), and ORF 3b (366), in addition to E protein (see above) and N protein (298), each induce apoptosis. Since all of these studies involve overexpression of individual proteins, it is difficult to determine which if any play this role during infection; the analysis of recombinant viruses with specific mutations will be important to definitely demonstrate a role for any particular protein in apoptosis.
Recent data indicate that these small ORFs may vary among SARS isolates. For example, there are reports that in the genomes of SARS-CoV isolates from humans there are deletions within a single ORF 8, resulting in two ORFs, 8a and 8b (122, 287) (see “CORONAVIRUSES AS EMERGING PATHOGENS: SARS-CoV” below). In fact, other than differences in spike gene sequences, this was the most striking difference observed between the genomes of animal and human isolates of SARS-CoV. In another study, an “in-frame” deletion of 45 nucleotides was found to occur in ORF 7b after three passages of SARS-CoV in tissue culture (311). A deletion in ORFs 6 to 8 was observed in the adaptation of SARS-CoV to primate cell culture (251). While it is tempting to speculate that one or more of these ORFs participate in adaptation to the human host and/or subversion of the host innate immune response, there are no conclusive data on these issues.
CORONAVIRUSES AS EMERGING PATHOGENS: SARS-CoV
Severe Acute Respiratory Syndrome
In February 2003 the World Health Organization (WHO) received reports from China of a new respiratory illness outbreak in Guangdong Province (341). However, the first cases of “an atypical pneumonia” emerged in Guangdong Province (China) in late 2002 (238).
A novel virus was isolated from patients' lungs and sputa and cultivated on a monkey kidney cell line (Vero E6) (81, 82, 162, 240). The sequence information demonstrated that this was a previously unrecognized coronavirus (267, 287). Proof that this virus is the etiologic agent for SARS was provided by results of infections carried out in nonhuman primates, in which Koch's postulates were fulfilled (102). The SARS epidemic was officially controlled by July 2003 (6, 100). The epidemic was controlled only by strict isolation of patients. By the end of the epidemic, the CDC and WHO reported more than 8,000 cases, with more than 800 deaths worldwide (162). SARS cases were reported from 29 countries, mostly in Asia, although North America was also affected, most notably Toronto, Canada. A total of 156 suspected SARS cases were reported in the United States, although only 8 cases had confirmed serologic evidence of SARS-CoV infection (250).
SARS infection exhibits a wide clinical course, characterized mainly by fever, dyspnea, lymphopenia, and lower respiratory tract infection (229, 317). Concurrent gastrointestinal symptoms and diarrhea, with active replication of SARS-CoV in both the small and large intestines, are common (19, 179, 182, 240). Infected individuals have slightly decreased platelet counts, prolonged coagulation profiles, and mildly elevated serum hepatic enzymes. Although the route of transmission has not been clearly established, airborne droplets from infected patients may be the main route of transmission (375). Blood and fecal-oral transmission has been suggested to be the route of transmission for one index case (252).
Origin of SARS-CoV
Coronavirus biological vectors are not known. However, serological and genetic evidence from various studies supports a zoonotic origin of SARS-CoV (140). This hypothesis was first based on epidemiological reports demonstrating that early patients with SARS in Guangdong Province were exposed to live wild game animals held in markets serving the restaurant trade (375). In order to identify animals carrying SARS-CoV, a range of domestic and wild mammals in Guangdong Province were examined. Interestingly, SARS-like viruses genetically and antigenically related to the human SARS coronavirus were detected by RT-PCR in the nasal and fecal swabs of civet cats (Paguma larvata) and a raccoon dog (Nyctereutes procyonoides). Serological evidence of infection was found in these species and also in a Chinese ferret-badger (Melogale moschata) (122). Interestingly, animal traders working with live animals in these markets had high seroprevalence for both the human and animal SARS coronaviruses, although they did not have a history of SARS-like disease (122). SARS-like viruses isolated from animals had more than 99% homology with human SARS-CoV. However, compared with the animal viruses, the human SARS coronavirus isolated both from the early phase of epidemics and from May 2003 exhibited deletions in ORF 8 that differed in length (from 29 to 82 nucleotides at the early phase to a 415-nucleotide deletion resulting in the loss of the whole ORF 8 region at the late phase of the outbreak) (375). It is unknown whether deletions in ORF 8 confer an advantage to adaptation to humans or whether ORF 8 is dispensable in humans but not in animals. Poon et al. (250) speculated that the animal precursor of SARS coronavirus is thus likely to be inefficient at infecting humans, and repeated exposure to the precursor animal virus might lead to the abortive infection or antigenic stimulation that results in the observed serological response in the animal handlers. Thus, the live-animal markets were probably the site where the interspecies transfer of the animal virus to humans occurred. In this sense, molecular epidemiological studies have suggested several introductions of the animal coronavirus into humans. For example, phylogenetic analysis of the few human cases that occurred in December 2003 in Guandong Province revealed that this SARS-CoV is much closer to the palm civet CoV than to any SARS-CoV isolated from humans in the early epidemic (51, 288). Whether SARS-CoV has a reservoir in a wild animal species (142) remains to be further evaluated. In addition, ferrets and domestic cats are susceptible to SARS-CoV infection (207). Those authors reported that when noninfected ferrets and cats were housed with an infected animal, transmission occurred and viral titers increased gradually. Interestingly, efforts from several groups trying to identify a putative wild animal reservoir have led to the discovery of new animal coronaviruses in bats (249) and birds (147). The possibilities of interspecies transmission and wildlife reservoirs are not a new ideas for coronaviruses. Group I porcine, canine, and feline coronaviruses are antigenically related and may even be host range mutants derived from a common ancestor. In support of this idea, coronavirus infection may occur across species in pigs, dogs, and cats. Furthermore wild and domestic carnivores such as dogs and foxes may serve as reservoirs for TGEV (272).
The discovery of ACE2 as a receptor for SARS-CoV (187) is a major advance in our understanding of how SARS-CoV enters the cells and has allowed the elucidation, at the molecular level, of SARS-CoV cross-species transmission. Two groups have compared the spikes proteins derived from the 2002 to 2003 outbreak (TOR2), from the less severe 2003 to 2004 outbreak (GD), and from palm civets (SZ3) (122, 188, 256). A representative spike protein from the mild 2003 to 2004 outbreak and one from palm civets mediate more efficient infection of cells expressing palm civet ACE2 than of cells expressing human receptor. In contrast, spike protein from the severe 2002 to 2003 outbreak efficiently binds and utilizes both receptors. These data are consistent with the absence of human-to-human transmission during the 2003 to 2004 outbreak and with recent transmission of SARS-CoV from palm civets to humans (122, 288). This difference in the ability of civet and human SARS isolates to utilize human receptor is associated with amino acid substitutions in the RBD (see “Spike Protein” in “ROLE OF CORONAVIRUS PROTEINS IN PATHOGENESIS” above).
There has been some controversy about SARS-CoV classification. Early after its discovery, SARS-CoV was suggested to define a new group (IV) within the coronavirus groups (162, 205, 267). However, based on sequence comparisons and the observation that regions of ORF 1a of SARS-CoV contain domains that are unique to the group II coronaviruses, it has been suggested that it is more directly related to group II viruses, along with the bovine coronavirus, the human OC43 virus, and the murine coronavirus (MHV) (287). Thus, Gorbalenya et al. (117) have placed SARS within group II coronaviruses, in a subgroup IIb. Using bioinformatics methods, several groups have suggested possible recombination events for the origin of SARS (203, 261, 292, 371). Rest and Mindell (261) have suggested that the SARS-CoV polymerase may be a result of recombination. Stavrinides and Guttman (292) have reported a possible past recombination event between mammalian-like and avian-like parent viruses. Those authors suggested a mammalian-like origin for the replicase protein, an avian-like origin for the matrix and nucleocapsid proteins, and a mammalian-avian mosaic origin for the spike protein. However, the origin of SARS-CoV by recombination of mammalian and avian viruses seems unlikely. While recombination is one of the hallmarks of coronaviruses and there is evidence that may be it is a major force in coronavirus evolution, there is no evidence for recombination between members of different groups of coronaviruses. Consistent with this, Masters and colleagues have demonstrated, using chimeric recombinant viruses, that the 3′ UTR of SARS-CoV may substitute functionally for that of MHV, while 3′ UTRs from prototype group I or group II coronavirus cannot (113). Thus, the evidence to date suggests that SARS-CoV belongs within group II coronaviruses.
SARS Pathogenesis
The mechanism of injury caused by SARS-CoV infection remains unknown. A SARS disease model was proposed, consisting of three phases: viral replication, immune hyperactivity, and pulmonary destruction (317). SARS pathology of the lung has been associated with diffuse alveolar damage, epithelial cell proliferation, and an increase of macrophages. Multinucleate giant-cell infiltrates of macrophage or epithelial origin have been associated with putative syncytium-like formation that is characteristic of many coronavirus infections (228). The lymphopenia, hemophagocytosis in the lung, and white-pulp atrophy of the spleen observed in SARS patients are reminiscent of those reported for fatal influenza virus subtype H5N1 disease in 1997 (312). Strikingly, the presence of hemophagocytosis supports a cytokine deregulation (99). It is widely considered that SARS is a viral pneumonia. However, SARS patients may also exhibit gastrointestinal symptoms (313) and splenic atrophy and lymphadenopathy (79). Diarrhea is a very frequent finding in SARS patients (30 to 40% of patients). SARS-CoV replicates in enterocytes, with minimal disruption of the intestinal architecture. The absence of intestinal inflammation has been speculated to be a result of upregulation of transforming growth factor β (48) and an antiapoptotic host cellular response in the intestinal epithelial cells (241). Recent findings based on autopsies of SARS patients proposed that SARS is a systemic disease with widespread extrapulmonary dissemination, resulting in viral shedding in respiratory secretions, stools, urine, and even sweat (78, 95).
Proinflammatory cytokines released by stimulated macrophages in the alveoli may have a role in the pathogenesis of SARS. SARS-CoV infection of macrophages in vitro leads to the initiation of viral replication and viral protein synthesis, but replication is abortive and no virus particles are produced. In contrast to the case for influenza A virus and HCoV-229E, no IFN-α/β response is detected in macrophages, despite the induction of the expression of chemokines such as CXCL10/IFN-γ-inducible protein 10 and CCL2/monocyte chemotactic protein 1 (50).
Strikingly, it has been recently reported that SARS-CoV replicates in peripheral blood mononuclear cells (PBMCs) from SARS patients (184). Reghunathan et al. (258) have analyzed the gene expression profiles of PBMCs from 10 SARS patients compared with healthy controls. Analysis of gene expression of PBMCs of SARS patients by using a microarray platform that includes more than 8,000 gene sequences suggests that the response of SARS patients seems to be mainly an innate inflammatory response, rather than a specific immune response against a viral infection. Those authors did not find significant upregulation of major histocompatibility complex class I genes or major cytokines, including IFNs (IFN-α, IFN-β, and IFN-γ), or genes involved in complement-mediated cytolysis. They concluded that the immune response against the SARS-CoV may be different from that in other viral infections or that the virus may be using an unusual strategy to evade the host immune system and cause the pathogenesis and mortality.
Lymphopenia and increasing viral load in the first 10 days of SARS suggest immune evasion by SARS-CoV. The lack of an IFN-β response in SARS-CoV-infected cells has been reported in vitro, using human primary myeloid-derived dendritic cells (176) and the epithelial 293 cell line (291). Law et al. (176) proposed a mechanism of immune evasion by SARS-CoV in DCs, based on their findings of low expression of antiviral cytokines (IFN-α, IFN-β, IFN-γ, and IL-12p40), moderate upregulation of proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] and IL-6), and significant upregulation of proinflammatory cytokines (MIP-1α, RANTES, IP-10, and MCP-1). Spiegel et al. (291) demonstrated that SARS-CoV escapes interferon-mediated growth inhibition by preventing the induction of IFN-β through interfering with the activation of IFN regulatory factor 3. The mechanism of lymphopenia remains unclear. The rapid decrease in both CD4 and CD8 T cells may be associated with an adverse outcome (185, 338). Due to the absence of ACE2 expression in T- and B-cell lymphocytes (126), it seems unlikely that SARS-CoV-induced lymphopenia may be caused by direct viral infection. Rather it has been suggested that apoptosis of uninfected lymphocytes may lead to the acute lymphopenia observed in SARS patients. In this sense, various SARS-CoV proteins have been suggested to induce apoptosis in vitro. These include ORF 7a (305), ORF 3a (177), and ORF 3b (366), in addition to E protein (see above) and N protein (298) (see “Group-Specific Proteins” in “ROLES OF CORONAVIRUS PROTEINS IN PATHOGENESIS” above).
SARS ANIMAL MODELS
The development of animal models for SARS is a key to our understanding of SARS pathogenesis. Unfortunately, to date an animal model that reproduces the clinical symptoms and pathology observed in SARS-infected patients has not been reported. Nonhuman primates, domestic cats, ferrets, mice, and Golden Syrian hamsters have been experimentally infected with SARS-CoV. These animals support acute self-limited viral replication in the upper and lower respiratory tracts, although differences in outcomes exist. In contrast, pigs and chickens can be experimentally infected with SARS-CoV, but these species do not support efficient SARS-CoV replication (66). A summary of the animal model studies is given in Table 3.
TABLE 3.
SARS-CoV animal models
| Animal model and reference(s) | Species | Follow-up days p.i. | Major finding(s) |
|---|---|---|---|
| Nonhuman primates | |||
| 102, 164 | Cynomolgus macaques | 6 | SARS-CoV fulfills Koch's postulates; three of four macaques developed diffuse alveolar damage similar to that observed in SARS patients |
| 268 | Cynomolgus and rhesus macaques | 14 | Macaques developed a mild self-limited respiratory infection very different from that observed in humans |
| 216 | African green monkeys, cynomolgus and rhesus macaques | 2-4 | SARS-CoV replicated to a higher titer and for a longer time in the respiratory tracts of African green monkeys than in those of rhesus or cynomolgus macaques; virus was cleared by day 4 p.i. |
| 255 | Rhesus macaques | 5-60 | Interstitial pneumonia during the 60-day period; resolution observed starting at 30 days p.i.; some clinical signs |
| 119 | Marmosets | 2-7 | Clinical signs (diarrhea, dyspnea, fever) were observed; extrapulmonary disease |
| Rodents | |||
| 295 | BALB/c mice | 2-7 | SARS-CoV replicates in the upper and lower respiratory tracts, virus was cleared by day 7 p.i.; no clinical disease; mice developed neutralizing antibody immune response |
| 110 | C57B/L6 mice, beige, CD1−/−, RAG1−/− | 1-15 | In B6 mice, the virus was cleared by a mechanism independent of NK, NK-T, and B of T lymphocytes |
| 137 | 129SvEv, Stat1−/− | 1-22 | IFNs had a role in controlling SARS-CoV infection |
| 263 | Aged mice | Elevated levels of IFN-α, IFN-γ, and TNF-α, suggesting that a proinflammatory cytokine response may be responsible for SARS-associated events | |
| 264 | Golden Syrian hamsters | 2-14 | Longer duration of viral shedding from the upper respiratory tract (day 14 p.i.); a transient viremia; spread to extrapulmonary tissues (liver and spleen); most significantly, inflammation in the respiratory tract associated with viral replication |
| Ferrets | |||
| 207 | 2-14 | Multifocal lung pathology, milder than in macaques; lethargy and death (1/6 animals) | |
| 331 | 1-29 | Ferrets support SARS-CoV replication, but animals were asymptomatic; exarcerbated liver inflammation observed after vaccination with vaccinia virus-SARS-CoV spike | |
| Farmed civets | |||
| 342 | 1-33 | Farmed civets were susceptible to experimental infection with SARS-CoV and developed fever and lethargy |
Nonhuman Primate Models
The cynomolgus macaque (Macaca fascicularis) was the first animal subjected to experimental infection with SARS-CoV. Osterhaus and colleagues (102, 164), demonstrated that SARS-CoV fulfils Koch's postulates to a related host (cynomolgus macaque), leading to the development of a disease comparable to that observed in humans. Macaques were infected with 106 50% tissue culture infective doses (TCID50) of SARS-CoV intratracheally, intranasally, and on each conjunctiva. Some macaques became lethargic from 3 days postinfection (p.i.) onwards and developed a temporary skin rash and respiratory distress. Viral RNA was detected using RT-PCR in sputum and in nasal and pharyngeal swabs on days 2 to 6 p.i. Necropsies were performed at day 6 p.i. Macaques developed interstitial pneumonia of differing severity, some of them with necrosis of alveolar and bronchiolar epithelia. Occasional multinucleated cells of approximately 30 nuclei (syncytia), similar to those observed in SARS-infected human lungs, were present in the lumens of alveoli and bronchioles. SARS-CoV RNA was detected in lungs by RT-PCR, and virus was also isolated. SARS-CoV antigen was detected in type 2 pneumocytes in lungs. SARS-CoV RNA was occasionally detected in cerebrum, duodenum, kidney, nasal septum, skin, spleen, stomach, trachea, tracheobronchial lymph node, and urinary bladder.
Three other labs have attempted to develop nonhuman primate models. It is worth noting that the utility of the cynomolgus macaque model remains controversial. Rowe et al. (268) have demonstrated that cynomolgus as well as rhesus macaques develop a mild self-limited respiratory infection very different from that observed in humans. Clinical symptoms were mild and localized to the upper respiratory tract, consisting of mild cough, sneezing, and slightly decreased activity on days 2 to 3 after virus challenge. Animals did not demonstrate signs of respiratory distress and become asymptomatic by day 8 to 10 p.i. In contrast to the studies reported by Osterhaus and colleagues, necropsies were done at days 12 to 14 p.i. (instead of day 6 p.i.). Microscopic examination revealed patchy areas of mild interstitial edema and alveolar inflammation, with occasional areas of intra-alveolar edema, interspersed with normal lung histology. There was no evidence of syncytia or alveolar damage, as has been reported for humans. No viral antigen was detected in lungs by immunohistochemical analysis.
In addition to the studies described above, McAuliffe et al. (216) have evaluated the level of replication and serologic response to experimental infection with SARS-CoV in three species of Old World monkeys (African green, rhesus, and cynomolgus macaques). Although clinical illness was not present in any of these species, African green, rhesus, and cynomolgus monkeys were susceptible to infection with SARS-CoV. The level of replication in the respiratory tracts of African green monkeys was greater than that in cynomolgus monkeys, which was higher than that in rhesus macaques. The neutralizing antibody response correlated with the level of virus replication. Histopathologic examination of African green monkey lungs demonstrated interstitial pneumonitis in association with SARS-CoV replication on day 2 p.i., which was resolving by day 4 p.i. SARS-CoV antigen was present in bronchiolar epithelial cells and type I pneumocytes. The ability of primary infection to prevent reinfection was evaluated in African green monkeys by SARS-CoV challenge 2 months after the primary infection. These animals showed no evidence of viral replication or enhanced disease.
Qin et al. (255) investigated the susceptibility of rhesus macaques to SARS-CoV infection through nasal cavity inoculation. In contrast with previous studies, macaques were followed for a 60-day period postinfection. Viral RNA could be detected in all the infected monkeys up to 16 days after infection. Histopathological changes of interstitial pneumonia were found in the lungs during the 60 days after inoculation. Interstitial pneumonia was less prominent at later times, indicating resolution in these animals. The neutralizing antibody response persisted in the animals at day 60 p.i.
The differences in disease outcome observed in cynomolgus macaques after experimental infection with SARS-CoV are intriguing. Rowe at al (268). have proposed a model of disease to reconcile apparent differences in the macaque experiments reported to date. Taking all the data together, those authors believe that the clinical manifestations observed in macaques are not sufficiently robust to be useful in evaluating pathogenesis or assessing therapeutic efficacy. SARS-CoV replicates in the upper respiratory tract and lungs of macaques, although lung pathology and recovery of virus are more evident when animals are necropsied at early time points (i.e., days 2 to 6 p.i.) than at later time points (days 12 to 14 p.i.). In contrast to the previous studies (216, 268), Qin et al. (255) followed the macaques for a longer period (60 days p.i.) and reported interstitial pneumonia (with milder histopathology after 30 days p.i.) through the study. Interestingly, the lesions in the lung, although milder than the histopathological changes observed in SARS patients, were consistently observed in all animals, with hyaline membranes, lung edema, and desquamation of alveolar lining cells in some infected macaques. This is the first study that follows nonhuman primates for such a long period, and it suggests that SARS-CoV infection in macaques deserves further investigation.
The marmoset (Callithrix jacchus) is a New World nonhuman primate that supports SARS-CoV replication in the respiratory tract (119). All animals developed multifocal mononuclear cell interstitial pneumonitis, accompanied by multinucleated syncytial cells, edema, and bronchiolitis in most animals. SARS-CoV antigen localized primarily to infected alveolar macrophages and type 1 pneumocytes by immunohistochemistry. Viral RNA was detected in all animals from pulmonary tissue extracts obtained at necropsy. Interestingly, viral RNA was also detected in tracheobronchial lymph node and myocardium, together with inflammatory changes, in some animals.
Cat Model
Domestic cats (Felis domesticus) support SARS-CoV replication. Natural asymptomatic infection in cats was first documented during the community outbreak at Emory Gardens, Hong Kong (http://www.who.int/sars/en/whoconsensus.) Martina et al. (207) studied the susceptibility of cats to experimental SARS-CoV infection. Cats inoculated intratracheally with 106 TCID50 do not develop clinical signs, although cats shed virus from the pharynx starting at 2 days p.i. and continuing until day 10. The virus was isolated from nasal swabs on days 4 and 6 p.i., whereas rectal swabs were negative. SARS-CoV was isolated from the trachea and lungs, although titers were low (1 × 103 TCID50/ml), peaking at days 6 to 8 p.i. Some infected cats developed mild pulmonary lesions. Cats seroconvert by day 28 p.i.
Ferret Model
The ferret (Mustela furo) is been exploited as an animal model for other respiratory infections, such as influenza, because ferrets develop a clinical illness that resembles human influenza, with rhinorrhea, fever, and sneezing following intranasal inoculation. To date, there is evidence that ferrets support SARS-CoV replication and that lungs of infected ferrets show some pulmonary lesions milder than those observed in cynomolgus macaques (although no pathological analysis was reported in this study) (207). However, ferrets did not develop fever or respiratory signs, but they were lethargic. At the time this review was written, another group has reported results in ferrets that disagree with those of the above-mentioned study (331). In contrast to the first study (207), in the latter study ferrets were totally asymptomatic although they supported SARS-CoV replication. Ferret models are currently been studied by other groups, and future research will clarify their use as a model for SARS pathogenesis.
Rodent Models
The use of small animal models, in particular mice, to study the pathogenesis of SARS-CoV has obvious advantages over use of the nonhuman primate models. First, a mouse model, if successful, would be less expensive than a macaque model. Second, mice can be genetically engineered, allowing the study of specific components of the host immune response (or others) against viral infection. In addition, mouse models for other human infectious diseases have been successfully developed (31). In this respect, the identification of the human receptor for SARS-CoV made possible the generation of receptor-expressing transgenic mice.
BALB/c, C57BL/6, and 129SvEv mice have been evaluated by several labs as possible animal models for SARS. It seems that the route of inoculation was a key factor to demonstrate SARS-CoV replication in mice. Whereas Martina et al. (207) reported that SARS-CoV did not infect mice by intracranial inoculation, Subbarao et al. (295) demonstrated that SARS-CoV does infect mice (reported first for BALB/c mice [295] and subsequently for C57BL/6 mice [110]) by intranasal inoculation. The intranasal route was selected by those authors because SARS is a respiratory illness in humans. Intranasal inoculation of BALB/c mice resulted in virus recovery from the upper and lower respiratory tracts. However, mice did not develop clinical signs and even continued to gain weight. The virus replicated to higher titers in the lung than in the nasal turbinates, although it was cleared from the respiratory tract by day 7 p.i. The peak of viral replication was seen in the absence of disease at day 2 p.i., and viral antigen and SARS-CoV RNA were detected by immunohistochemistry and in situ hybridization in bronchiolar epithelial cells during the peak of viral replication. Mice exhibited mild and focal peribronchiolar mononuclear inflammatory infiltrates, with no significant pathology in other organs. Interestingly, primary infection conferred protection in the upper and lower respiratory tracts from subsequent SARS-CoV challenge, and antibody alone protected against viral replication.
C57BL/6 mice also support SARS-CoV replication, with a peak of viral replication on day 3 p.i. and clearance by day 9 p.i (110). Although C57BL/6 mice did not develop respiratory illness, similarly to BALB/c mice, viral RNA localized to bronchial and bronchiolar epithelia. SARS-CoV RNA was detected by RT-PCR at days 1, 3, 5, 7, and 9 p.i. in the lung, brain, heart, liver, and spleen (but not in the kidney). Brain was the only organ for which there was an increase in the number of positive samples with increasing time after infection. Remarkably, live virus was isolated from brain at days 9, 12, and 15 p.i., and SARS-CoV RNA was detected in the hippocampus by in situ hybridization.
Consistent with the above reports, Hogan et al. (137) demonstrated replication of SARS-CoV following intranasal inoculation in both 129SvEv and Stat1−/− mice (which have impaired IFN responses). Both strains of mice developed bronchiolitis and patchy interstitial pneumonia. However, whereas 129SvEv immunocompetent mice resolved the infection, Stat1-deficient mice progressed to develop diffuse interstitial pneumonia and systemic spread of the virus to the liver and spleen. These results suggested that IFNs may have a role in controlling SARS-CoV infection. In agreement with these data, IFNs inhibit the replication of SARS-CoV in vitro (reviewed in reference 56) and in animal studies (123). In contrast, Glass et al. (110) have demonstrated that SARS-CoV is rapidly cleared in beige, CD1−/−, and RAG1−/− mice, suggesting that, at least in B6 mice, the virus is cleared by a mechanism independent of NK, NK-T, and B and T lymphocytes.
In contrast to young BALB/c and B6 mice, aged SARS-infected BALB/c mice showed elevated levels of IFN-α and -γ and TNF-α, suggesting that the proinflammatory cytokine response may be responsible for SARS-associated events (263).
Golden Syrian hamsters are susceptible to SARS-CoV infection after intranasal inoculation (264). Similarly to the mouse model, hamsters did not exhibit clinical disease; the peak of replication virus occurred on day 2 p.i., and virus was cleared by day 7 p.i. In contrast to the BALB/c mouse model, in which the virus is detected only in the respiratory tract and is cleared by day 5, hamsters demonstrate a longer duration of viral shedding from the upper respiratory tract (day 14 p.i), a transient viremia, spread to extrapulmonary tissues (liver and spleen), and most significantly, inflammation in the respiratory tract, which is associated with viral replication. The humoral immune response, as measured by mean serum neutralizing antibody titers 28 days after virus administration, is more robust in hamsters than in mice or African green monkeys.
Taking all the data together, it can be argued that none of the above-mentioned animals (mice, hamsters, nonhuman primates, or ferrets) reproduce the SARS-induced disease observed in humans. Respiratory tracts of these animals can be experimentally infected with SARS-CoV; however, infections remain subclinical, and pathological findings are not consistent with the human disease. For instance, intraalveolar edema, pneumocyte necrosis, or hyaline membranes were not observed in mice (not even in the Stat1−/− mice). Therefore, animal models for SARS pathogenesis need to be further developed. One of the avenues that require more investigation is to further adapt SARS-CoV to various animal species. This adaptation will likely lead to more pathogenic SARS variants in a particular host. Variants of influenza virus and Ebola virus, which are pathogenic for mice, have been selected by serial passage in the mouse (24, 29, 330). Experience with other coronaviruses such as MHV suggests that adaptation will occur by serial passage (8, 46, 224, 309). It should be emphasized that these animals (in particular mice and macaques) are currently being exploited for vaccine studies. However, our understanding of SARS pathogenesis will be hampered until animal models in which clinical disease and pathological findings mimic SARS human disease are developed.
VACCINE STRATEGIES AGAINST SARS
Previous experiences with coronavirus vaccines (recently reviewed in references 225, 271, and 272) are relevant for SARS vaccine development. Several studies aimed at passive and active immunization have exploited the animal models described above for SARS replication. (As mentioned above, an authentic animal model for SARS pathogenesis has not yet been developed.) Subbarao et al. (295) demonstrated that passive transfer of immune serum protects naive BALB/c mice from SARS-CoV infection. Various studies have reported that human monoclonal antibodies confer some protection against SARS. Traggiai et al. (314) have developed an improved method for Epstein-Barr virus transformation of human B cells. This method was used to analyze the memory repertoire of a patient who recovered from SARS-CoV infection and to isolate monoclonal antibodies specific for different viral proteins. Although some of these monoclonal antibodies exhibited in vitro neutralizing activity, only one of such antibodies conferred protection in vivo in a mouse model of SARS-CoV infection. This study highlights the possibility of evaluating the memory repertoire of immune donors to efficiently isolate neutralizing antibodies that have been selected in the course of natural infection in SARS-infected patients. Human IgG monoclonal antibodies against SARS with in vitro neutralizing activity and protection in a ferret model have been developed using phage display libraries (308). In both mouse and ferret models, administration of human monoclonal antibody with in vitro neutralization activity reduced SARS titers in the lungs (3- to 6-log10-unit decrease), also protecting from lung pathology in ferrets.
Several studies were directed towards the development of active immunization strategies. Inactivated virions, recombinant antigen, DNA vaccines, adenoviral vectors, vaccinia virus Ankara and recombinant parainfluenza virus type 3 vectors, and rhabdovirus-based vectors are being investigated. Inactivated SARS vaccines have been reported to elicit systemic humoral immunity in mice and high titers of spike-specific antibodies that block receptor binding and virus entry in cell culture (94, 128, 303, 307, 345). In addition, UV-inactivated virion induced regional lymph node T-cell proliferation and significant levels of cytokine (IL-2, IL-4, IL-5, IFN-γ, and TNF-α) production upon restimulation with inactivated SARS-CoV virions in vitro. However, none of these studies have addressed whether inactivated whole SARS-CoV virions confer protection from virus challenge. Recently, Zhou et al. (376) have reported that inactivated SARS-CoV induces humoral and mucosal immunity against challenge with SARS-CoV in rhesus monkeys.
SARS-CoV spike glycoprotein (329, 350, 370), M (329), and nucleocapsid (153, 329, 372, 377) have been evaluated as candidate vaccines, using DNA immunization in mice. Interestingly, DNA vaccination can induce humoral and cellular immunity against SARS-CoV in the mouse model. Yang et al. (350) demonstrated that a DNA vaccine encoding the codon-optimized SARS spike glycoprotein induces neutralizing antibody as well as T-cell responses. Protection from SARS-CoV challenge was mediated by a humoral immune response but not by a T-cell-dependent mechanism. Zeng et al. (370) have reported that mice immunized by plasmids encoding fragments of S1 developed a Th-1 antibody isotype switching. A DNA vaccine encoding calreticulin (which has been shown to enhance major histocompatibility complex class I presentation to CD8 T cells) linked to the nucleocapsid generates strong N-specific humoral and cellular immunity and protects mice against a vaccinia virus expressing nucleocapsid. A prime-boost combination of DNA (SARS spike under control of the cytomegalovirus promoter and intron A) and whole killed SARS-CoV vaccines elicited higher antibody responses than DNA or whole killed virus vaccines alone (367).
Adenovirus-based vaccination strategies against SARS-CoV, using replication-defective adenovirus type 5 vectors expressing structural SARS proteins (S1, M, and N), have also been reported. Vaccinated rhesus macaques developed antibody responses against fragment S1 of spike, virus-neutralizing antibody responses, and T-cell responses against the nucleocapsid (107). Similarly, Zakhartchouk et al. (368) demonstrated that vaccination of C57B/L6 mice with adenovirus type 5-expressing nucleocapsid elicited SARS-CoV-specific humoral and T-cell-mediated immune responses in C57B/L6 mice.
The highly attenuated modified vaccinia virus Ankara (MVA) has been used to express the spike glycoprotein of SARS-CoV in vaccination experiments using the mouse (16) and the ferret (332) models, with different results. Intranasal and intramuscular administration of MVA encoding the SARS-CoV spike protein led to the induction of a humoral immune response in BALB/c mice, as well as reduced viral titers in the respiratory tract. In ferrets, vaccination with MVA encoding the spike or nucleocapsid induced a vigorous antibody response; however, it did not prevent virus infection and spreading. Liver inflammation (in the absence of viral antigen) was found in all MVA-spike-vaccinated ferrets and in only one MVA-nucleocapsid-vaccinated animal after challenge with SARS-CoV (331). Inflammation in the livers of ferrets vaccinated with MVA-nucleocapsid was similar to that in the MVA control group. It is important to note that this study did not find any clinical disease in ferrets after infection with SARS-CoV (in contrast to others [207]). These authors pointed out that although their results need to be further investigated, they may suggest antibody-dependent enhancement, similar to the case for FIPV. For this feline coronavirus, antibodies acquired either through a passive transfer of immune serum against the spike protein of FIPV or by immunization with a recombinant vaccinia virus expressing the spike protein lead to accelerated infection by the antibody-dependent enhancement mechanism (60, 61).
Recombinant bovine-human parainfluenza virus type 3 vector (BHPIV3) is a version of bovine parainfluenza virus type 3 in which the genes encoding the bovine parainfluenza virus type 3 major protective antigens, the fusion (F) and hemagglutinin-neuraminidase (HN) glycoproteins were replaced with their counterparts from human parainfluenza virus type 3. BHPIV3 is being developed as a live attenuated, intranasal pediatric vaccine against human parainfluenza virus type 3. Immunization of African green monkeys with a single dose of BHPIV3 expressing SARS-CoV spike protein administered via the respiratory tract induced the production of SARS-CoV-neutralizing antibodies (32). A recombinant BHPIV3 expressing SARS-CoV structural protein (S, M, and N) individually or in combination has been evaluated for immunogenicity and protective efficacy in hamsters, which support both SARS-CoV and BHPIV3 replication in lungs (30). A single intranasal administration of BHPIV3 expressing the SARS-CoV spike protein induced a high titer of SARS-CoV-neutralizing antibodies, only twofold less than that induced by SARS-CoV infection. In the absence of spike, expression of M, N, or E did not induce a detectable serum SARS-CoV-neutralizing antibody response. Immunization with BHPIV3 expressing spike provided complete protection against SARS-CoV challenge in the lower respiratory tract and partial protection in the upper respiratory tract.
Faber et al. (94) have generated recombinant rabies virus expressing the spike or the nucleocapsid protein of SARS-CoV. These vectors induced a neutralizing antibody response in mice. Those authors concluded that the use of rabies virus vectors as vaccines may be promising for vaccination in animals against SARS.
Kapadia et al. (148) have developed an attenuated vesicular stomatitis virus vector that encodes the SARS-CoV spike. Mice vaccinated with vesicular stomatitis virus S developed SARS-CoV-neutralizing antibody and were able to control a challenge with SARS-CoV performed at 1 month or 4 months after a single vaccination. In addition, by passive antibody transfer experiments, those authors demonstrated that the antibody response induced by the vaccine was sufficient for controlling SARS-CoV infection.
THERAPY
Based on a cytokine deregulation hypothesis, the first treatment protocols for SARS patients included the administration of steroids, which was aimed at modulating the exacerbated cytokine response, similarly to the treatment of nonviral acute respiratory distress syndrome (169). However, treatments of SARS infection have been ineffective (157, 179, 317). Treatments have been based on the administration of antibacterials (to prevent secondary bacterial infections) and steroids (to modulate cytokine deregulation) in combination with ribavirin (a nucleoside analog with broad antiviral activity). Currently, there is no antiviral therapy for SARS disease. Attempts have been made to study in vitro susceptibility to various compounds with potential anti-SARS activity. However, many contradictory findings have been reported from different labs, making it difficult to achieve an international agreement about anti-SARS strategies. The use of antiviral antibodies (discussed above and below), entry inhibitors (22, 193), proteinase inhibitors (194, 195, 347), calpain inhibitors (9), human immunodeficiency virus type 1 protease inhibitors (53), nucleoside analogues (such as ribavirin), interferons, and short interfering RNAs has been reported (reviewed in reference 120).
Plasma donated from patients who had recovered from SARS has been administered as immunotherapy to SARS patients. Human convalescent-phase plasma apparently had a beneficial effect if administered early in the course of SARS infection (49). These studies suggested that SARS hyperimmune globulin containing high titers of SARS-CoV-neutralizing antibodies could be used in the case of possible future outbreaks. The protective efficacy of several human monoclonal SARS-CoV-neutralizing antibodies has been recently demonstrated using various animal models (mice and ferrets) (118, 297, 308, 314). It should be noted that although the use of SARS-CoV-neutralizing antibodies may be promising, SARS-CoV entry could be enhanced by antibodies (351). Interestingly, human antibodies that neutralized pseudotyped lentiviruses expressing the spike glycoprotein derived from most human SARS-CoV isolates enhanced entry of lentivirus pseudotyped with the palm civet spike glycoproteins.
The effect of ribavirin in cell culture, using various cell lines, has been studied by several groups and remains controversial (56). Overall, it seems that ribavirin may inhibit SARS-CoV replication, depending on the cell line, but usually at concentrations that are above the mean plasma levels in treated individuals (157).
There is limited experience with IFN treatment in SARS patients. Treatment with IFN-alphacon-1 (a nonnaturally occurring synthetic recombinant IFN-α) resulted in a more rapid resolution. The activity of IFN-α/β against SARS-CoV in animals has been explored using cynomolgous macaques. Pegylated IFN-α-2b treatment prior to SARS-CoV infection substantially protected macaques from SARS challenge. It remains to be elucidated whether direct antiviral activity or immunomodulatory effects determined the IFN protection observed in macaques. Many studies have reported effects of IFNs on SARS-CoV replication in vitro (45, 293, 373). The antiviral potential of IFN-α, -β, and -γ has been assessed in cell culture, with IFN-β being the most potent inhibitor of SARS-CoV (57). After this first report, others have reported the efficacy of different IFN-α subtypes and human leukocyte IFN-α against SARS replication (for a review, see reference 56). IFN-γ has little activity against SARS-CoV in vitro (57). However, IFN-β and-γ may act synergistically against SARS-CoV infection in vitro (273, 276).
Short interfering RNAs that inhibit the expression of SARS-CoV genes have been demonstrated by several groups using various cell lines (183, 186, 281, 328, 342, 374), as well as in the macaque model with promising results (183).
Although potential anti-SARS agents are being identified using cell lines as well as SARS animal models (with the considerations discussed above), the development of therapies for SARS that could be rapidly and safely administered to humans in the event of an outbreak needs to be based in a better understanding of SARS pathogenesis. Further investigations are needed to define the role of the immune response in SARS disease, as there is evidence suggesting that lung damage could be more immune mediated rather than directly virus induced. In addition, recent data from various laboratories suggest that SARS may be a systemic disease with widespread extrapulmonary dissemination.
CONCLUDING REMARKS AND PROSPECTS
Coronaviruses are a fascinating group of viruses, providing animal models of pathogenesis, unusual molecular mechanisms of transcription and recombination, and new emerging pathogens. The emergence of SARS and the identification of a coronavirus as the etiologic agent of the disease was a surprise to the coronavirus community, as it was the first definitive association of a coronavirus with severe disease in humans. While it is not clear whether SARS-CoV will again emerge into the human population, it has spurred on the awareness to consider coronaviruses as the cause of human respiratory and perhaps other types of disease. The identification of NL63 and HKU1 provides examples of other newly described human coronaviruses.
The data gathered during the many years of research on the animal coronaviruses enabled the very rapid identification of SARS-CoV as well as the sequencing of the genome. The very quick development of a reverse genetics system for SARS-CoV was based on previous systems for other coronaviruses; this system will allow the dissection of the roles of individual genes in infection. The knowledge that multiple viral genes contribute to pathogenesis and in particular to the type of immune response tells us that small changes in sequence can have larger effects on pathogenic phenotype. The observations that coronavirus tropism variants may be readily selected during replication in tissue culture and/or animals and that variants with changes and increased host range are also readily selected in tissue culture are all helpful in the understanding of the emergence of SARS into the human population. The identification and characterization of the proteases and the replicase as well as the identification of several putative enzymatic activities encoded within ORFs 1a and 1b of other coronaviruses have provided possible targets for which to evaluate potential drug therapies. The experience with development of coronavirus vaccines will aid the developments of vaccines for SARS as well. Future directions for SARS-CoV research include further understanding of the mechanisms of replication; elucidation of the molecular determinants of virulence and tropism and the immune response, with attention to the possible roles of group-specific proteins; development of vaccine strategies and antiviral therapies for animal and human viruses; and very likely the isolation and characterization of new pathogenic human coronaviruses. The knowledge we have about other coronaviruses, as summarized above, will certainly hasten the understanding of SARS-CoV.
Acknowledgments
We are indebted to many colleagues for helpful discussions and thoughts. We apologize to any investigators whose work we have inadvertently omitted.
We acknowledge NIH grants AI17418, AI60021 (formerly NS21954), NS30606, and AI47800, as well as grant RG2585B5 from the National Multiple Sclerosis Society, to S.R.W. S.N.-M. is funded by Drexel College of Medicine.
REFERENCES
- 1.Addie, D. D. 2004. Feline coronavirus—that enigmatic little critter. Vet. J. 167:5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almazan, F., J. M. Gonzalez, Z. Penzes, A. Izeta, E. Calvo, J. Plana-Duran, and L. Enjuanes. 2000. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 97:5516-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An, S., C. J. Chen, X. Yu, J. L. Leibowitz, and S. Makino. 1999. Induction of apoptosis in murine coronavirus-infected cultured cells and demonstration of E protein as an apoptosis inducer. J. Virol. 73:7853-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton, I. M., C. Sune, R. H. Meloen, F. Borras-Cuesta, and L. Enjuanes. 1995. A transmissible gastroenteritis coronavirus nucleoprotein epitope elicits T helper cells that collaborate in the in vitro antibody synthesis to the three major structural viral proteins. Virology 212:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arden, K. E., M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2005. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J. Med. Virol. 75:455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf, H. 2003. WHO declares Beijing to be free of SARS. Lancet 361:2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey, O. T., A. M. Pappenheimer, F. Sargent, M. D. Cheever, and J. B. Daniels. 1949. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. II. Pathology. J. Exp. Med. 90:195-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baric, R. S., E. Sullivan, L. Hensley, B. Yount, and W. Chen. 1999. Persistent infection promotes cross-species transmissibility of mouse hepatitis virus. J. Virol. 73:638-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnard, D. L., V. D. Hubbard, J. Burton, D. F. Smee, J. D. Morrey, M. J. Otto, and R. W. Sidwell. 2004. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-d-N4-hydroxycytidine. Antiviral Chem. Chemother. 15:15-22. [DOI] [PubMed] [Google Scholar]
- 10.Barthold, S. W., D. S. Beck, and A. L. Smith. 1993. Enterotropic coronavirus (mouse hepatitis virus) in mice: influence of host age and strain on infection and disease. Lab. Anim. Sci. 43:276-284. [PubMed] [Google Scholar]
- 11.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastien, N., K. Anderson, L. Hart, P. Van Caeseele, K. Brandt, D. Milley, T. Hatchette, E. C. Weiss, and Y. Li. 2005. Human coronavirus NL63 infection in Canada. J. Infect. Dis. 191:503-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243-249. [DOI] [PubMed] [Google Scholar]
- 14.Belay, E. D., D. D. Erdman, L. J. Anderson, T. C. Peret, S. J. Schrag, B. S. Fields, J. C. Burns, and L. B. Schonberger. 2005. Kawasaki disease and human coronavirus. J. Infect. Dis. 192:352-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann, C., M. McMillan, and S. Stohlman. 1993. Characterization of the Ld-restricted cytotoxic T-lymphocyte epitope in the mouse hepatitis virus nucleocapsid protein. J. Virol. 67:7041-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisht, H., A. Roberts, L. Vogel, A. Bukreyev, P. L. Collins, B. R. Murphy, K. Subbarao, and B. Moss. 2004. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA 101:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonavia, A., B. D. Zelus, D. E. Wentworth, P. J. Talbot, and K. V. Holmes. 2003. Identification of a receptor-binding domain of the spike glycoprotein of human coronavirus HCoV-229E. J. Virol. 77:2530-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond, C. W., J. L. Leibowitz, and J. A. Robb. 1979. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology 94:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth, C. M., L. M. Matukas, G. A. Tomlinson, A. R. Rachlis, D. B. Rose, H. A. Dwosh, S. L. Walmsley, T. Mazzulli, M. Avendano, P. Derkach, I. E. Ephtimios, I. Kitai, B. D. Mederski, S. B. Shadowitz, W. L. Gold, L. A. Hawryluck, E. Rea, J. S. Chenkin, D. W. Cescon, S. M. Poutanen, and A. S. Detsky. 2003. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801-2809. [DOI] [PubMed] [Google Scholar]
- 20.Boots, A. M., J. G. Kusters, J. M. van Noort, K. A. Zwaagstra, E. Rijke, B. A. van der Zeijst, and E. J. Hensen. 1991. Localization of a T-cell epitope within the nucleocapsid protein of avian coronavirus. Immunology 74:8-13. [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch, B. J., C. A. de Haan, and P. J. Rottier. 2004. Coronavirus spike glycoprotein, extended at the carboxy terminus with green fluorescent protein, is assembly competent. J. Virol. 78:7369-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch, B. J., B. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. Osterhaus, and P. J. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 101:8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch, B. J., R. van der Zee, C. A. de Haan, and P. J. Rottier. 2003. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 77:8801-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray, M., K. Davis, T. Geisbert, C. Schmaljohn, and J. Huggins. 1998. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 178:651-661. [DOI] [PubMed] [Google Scholar]
- 25.Bredenbeek, P. J., C. J. Pachuk, A. F. Noten, J. Charite, W. Luytjes, S. R. Weiss, and W. J. Spaan. 1990. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59; a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucleic Acids Res. 18:1825-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brian, D. A. 2001. Nidovirus genome replication and subgenomic mRNA synthesis. Pathways followed and cis-acting elements required. Adv. Exp. Med. Biol. 494:415-428. [DOI] [PubMed] [Google Scholar]
- 27.Brian, D. A., and R. S. Baric. 2005. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 287:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brian, D. A., B. G. Hogue, and T. E. Kienzle. 1995. The coronavirus hemagglutinin esterase glycoprotein, p. 165-179. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
- 29.Brown, E. G., H. Liu, L. C. Kit, S. Baird, and M. Nesrallah. 2001. Pattern of mutation in the genome of influenza A virus on adaptation to increased virulence in the mouse lung: identification of functional themes. Proc. Natl. Acad. Sci. USA 98:6883-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buchholz, U. J., A. Bukreyev, L. Yang, E. W. Lamirande, B. R. Murphy, K. Subbarao, and P. L. Collins. 2004. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA 101:9804-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buer, J., and R. Balling. 2003. Mice, microbes and models of infection. Nat Rev. Genet. 4:195-205. [DOI] [PubMed] [Google Scholar]
- 32.Bukreyev, A., E. W. Lamirande, U. J. Buchholz, L. N. Vogel, W. R. Elkins, M. St Claire, B. R. Murphy, K. Subbarao, and P. L. Collins. 2004. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet 363:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burks, J. S., B. L. DeVald, L. D. Jankovsky, and J. Gerdes. 1980. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science 209:933-934. [DOI] [PubMed] [Google Scholar]
- 34.Casais, R., M. Davies, D. Cavanagh, and P. Britton. 2005. Gene 5 of the avian coronavirus infectious bronchitis virus is not essential for replication. J. Virol. 79:8065-8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casais, R., B. Dove, D. Cavanagh, and P. Britton. 2003. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 77:9084-9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casais, R., V. Thiel, S. G. Siddell, D. Cavanagh, and P. Britton. 2001. Reverse genetics system for the avian coronavirus infectious bronchitis virus. J. Virol. 75:12359-12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavanagh, D. 2003. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 32:567-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavanagh, D., K. Mawditt, B. Welchman Dde, P. Britton, and R. E. Gough. 2002. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 31:81-93. [DOI] [PubMed] [Google Scholar]
- 39.Chang, K. W., Y. Sheng, and J. L. Gombold. 2000. Coronavirus-induced membrane fusion requires the cysteine-rich domain in the spike protein. Virology 269:212-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang, Y. J., C. Y. Liu, B. L. Chiang, Y. C. Chao, and C. C. Chen. 2004. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 173:7602-7614. [DOI] [PubMed] [Google Scholar]
- 41.Cheever, F. S., J. B. Daniels, A. M. Pappenheimer, and O. T. Baily. 1949. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. I. Isolation and biological properties of the virus. J. Exp. Med. 90:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen, C. J., K. Sugiyama, H. Kubo, C. Huang, and S. Makino. 2004. Murine coronavirus nonstructural protein p28 arrests cell cycle in G0/G1 phase. J. Virol. 78:10410-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, D. S., M. Asanaka, F. S. Chen, J. E. Shively, and M. M. Lai. 1997. Human carcinoembryonic antigen and biliary glycoprotein can serve as mouse hepatitis virus receptors. J. Virol. 71:1688-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, D. S., M. Asanaka, K. Yokomori, F.-I. Wang, S. B. Hwang, H.-P. Li, and M. M. C. Lai. 1995. Pregnancy-specific glycoprotein is expressed in the brain and serves as receptor for mouse hepatitis virus. Proc. Natl. Acad. Sci. USA 92:12095-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, F., K. H. Chan, Y. Jiang, R. Y. Kao, H. T. Lu, K. W. Fan, V. C. Cheng, W. H. Tsui, I. F. Hung, T. S. Lee, Y. Guan, J. S. Peiris, and K. Y. Yuen. 2004. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 31:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen, W., B. Yount, L. Hensley, and R. S. Baric. 1998. Receptor homologue scanning functions in the maintenance of MHV-A59 persistence in vitro. Adv. Exp. Med. Biol. 440:743-750. [DOI] [PubMed] [Google Scholar]
- 47.Chen, Y. Y., B. Shuang, Y. X. Tan, M. J. Meng, P. Han, X. N. Mo, Q. S. Song, X. Y. Qiu, X. Luo, Q. N. Gan, X. Zhang, Y. Zheng, S. A. Liu, X. N. Wang, N. S. Zhong, and D. L. Ma. 2005. The protein X4 of severe acute respiratory syndrome-associated coronavirus is expressed on both virus-infected cells and lung tissue of severe acute respiratory syndrome patients and inhibits growth of Balb/c 3T3 cell line. Chinese Med. J. (Engl.) 118:267-274. [PubMed] [Google Scholar]
- 48.Cheng, V. C., I. F. Hung, B. S. Tang, C. M. Chu, M. M. Wong, K. H. Chan, A. K. Wu, D. M. Tse, K. S. Chan, B. J. Zheng, J. S. Peiris, J. J. Sung, and K. Y. Yuen. 2004. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 38:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng, Y., R. Wong, Y. O. Soo, W. S. Wong, C. K. Lee, M. H. Ng, P. Chan, K. C. Wong, C. B. Leung, and G. Cheng. 2005. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 24:44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung, C. Y., L. L. Poon, I. H. Ng, W. Luk, S. F. Sia, M. H. Wu, K. H. Chan, K. Y. Yuen, S. Gordon, Y. Guan, and J. S. Peiris. 2005. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79:7819-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chinese SARS Molecular Epidemiology Consortium. 2004. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 303:1666-1669. [DOI] [PubMed] [Google Scholar]
- 52.Chiu, S. S., K. H. Chan, K. W. Chu, S. W. Kwan, Y. Guan, L. L. Poon, and J. S. Peiris. 2005. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 40:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu, C. M., V. C. Cheng, I. F. Hung, M. M. Wong, K. H. Chan, K. S. Chan, R. Y. Kao, L. L. Poon, C. L. Wong, Y. Guan, J. S. Peiris, and K. Y. Yuen. 2004. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chua, M. M., K. C. MacNamara, L. San Mateo, H. Shen, and S. R. Weiss. 2004. Effects of an epitope-specific CD8+ T-cell response on murine coronavirus central nervous system disease: protection from virus replication and antigen spread and selection of epitope escape mutants. J. Virol. 78:1150-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chua, M. M., J. J. Phillips, S. H. Seo, E. Lavi, and S. R. Weiss. 2001. Mutation of the immunodominant CD8+ epitope in the MHV-4 spike protein. Adv. Exp. Med. Biol. 494:121-125. [DOI] [PubMed] [Google Scholar]
- 56.Cinatl, J., Jr., M. Michaelis, G. Hoever, W. Preiser, and H. W. Doerr. 2005. Development of antiviral therapy for severe acute respiratory syndrome. Antiviral Res. 66:81-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, H. Rabenau, and H. W. Doerr. 2003. Treatment of SARS with human interferons. Lancet 362:293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coley, S. E., E. Lavi, S. G. Sawicki, L. Fu, B. Schelle, N. Karl, S. G. Siddell, and V. Thiel. 2005. Recombinant mouse hepatitis virus strain A59 from cloned, full-length cDNA replicates to high titers in vitro and is fully pathogenic in vivo. J. Virol. 79:3097-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook, J. K. A., and A. P. Mockett. 1995. Epidemiology of infectious bronchitis virus, p. 317-336. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
- 60.Corapi, W. V., R. J. Darteil, J. C. Audonnet, and G. E. Chappuis. 1995. Localization of antigenic sites of the S glycoprotein of feline infectious peritonitis virus involved in neutralization and antibody-dependent enhancement. J. Virol. 69:2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corapi, W. V., C. W. Olsen, and F. W. Scott. 1992. Monoclonal antibody analysis of neutralization and antibody-dependent enhancement of feline infectious peritonitis virus. J. Virol. 66:6695-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corse, E., and C. E. Machamer. 2003. The cytoplasmic tails of infectious bronchitis virus E and M proteins mediate their interaction. Virology 312:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corse, E., and C. E. Machamer. 2000. Infectious bronchitis virus E protein is targeted to the Golgi complex and directs release of virus-like particles. J. Virol. 74:4319-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowley, J. A., C. M. Dimmock, K. M. Spann, and P. J. Walker. 2000. Gill-associated virus of Penaeus monodon prawns: an invertebrate virus with ORF1a and ORF1b genes related to arteri- and coronaviruses. J. Gen. Virol. 81:1473-1484. [DOI] [PubMed] [Google Scholar]
- 65.Curtis, K. M., B. Yount, and R. S. Baric. 2002. Heterologous gene expression from transmissible gastroenteritis virus replicon particles. J. Virol. 76:1422-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Czub, M., H. Weingartl, S. Czub, R. He, and J. Cao. 2005. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine 23:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalziel, R. G., P. W. Lampert, P. J. Talbot, and M. J. Buchmeier. 1986. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J. Virol. 59:463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniel, C., R. Anderson, M. J. Buchmeier, J. O. Fleming, W. J. Spaan, H. Wege, and P. J. Talbot. 1993. Identification of an immunodominant linear neutralization domain on the S2 portion of the murine coronavirus spike glycoprotein and evidence that it forms part of complex tridimensional structure. J. Virol. 67:1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniel, C., M. Lacroix, and P. J. Talbot. 1994. Mapping of linear antigenic sites on the S glycoprotein of a neurotropic murine coronavirus with synthetic peptides: a combination of nine prediction algorithms fails to identify relevant epitopes and peptide immunogenicity is drastically influenced by the nature of the protein carrier. Virology 202:540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das Sarma, J., L. Fu, J. C. Tsai, S. R. Weiss, and E. Lavi. 2000. Demyelination determinants map to the spike glycoprotein gene of coronavirus mouse hepatitis virus. J. Virol. 74:9206-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Groot, R. J., and M. C. Horzinek. 1995. Feline infectious peritonitis, p. 293-315. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
- 72.de Haan, C. A., M. de Wit, L. Kuo, C. Montalto-Morrison, B. L. Haagmans, S. R. Weiss, P. S. Masters, and P. J. Rottier. 2003. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferogenic capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology 312:395-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Haan, C. A., P. S. Masters, X. Shen, S. Weiss, and P. J. Rottier. 2002. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology 296:177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Haan, C. A., M. Smeets, F. Vernooij, H. Vennema, and P. J. Rottier. 1999. Mapping of the coronavirus membrane protein domains involved in interaction with the spike protein. J. Virol. 73:7441-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Haan, C. A., K. Stadler, G. J. Godeke, B. J. Bosch, and P. J. Rottier. 2004. Cleavage inhibition of the murine coronavirus spike protein by a furin-like enzyme affects cell-cell but not virus-cell fusion. J. Virol. 78:6048-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Haan, C. A., L. van Genne, J. N. Stoop, H. Volders, and P. J. Rottier. 2003. Coronaviruses as vectors: position dependence of foreign gene expression. J. Virol. 77:11312-11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding, J. W., Q. Ning, M. F. Liu, A. Lai, J. Leibowitz, K. M. Peltekian, E. H. Cole, L. S. Fung, C. Holloway, P. A. Marsden, H. Yeger, M. J. Phillips, and G. A. Levy. 1997. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J. Virol. 71:9223-9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding, Y., L. He, Q. Zhang, Z. Huang, X. Che, J. Hou, H. Wang, H. Shen, L. Qiu, Z. Li, J. Geng, J. Cai, H. Han, X. Li, W. Kang, D. Weng, P. Liang, and S. Jiang. 2004. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 203:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ding, Y., H. Wang, H. Shen, Z. Li, J. Geng, H. Han, J. Cai, X. Li, W. Kang, D. Weng, Y. Lu, D. Wu, L. He, and K. Yao. 2003. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 200:282-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doyle, L. P., and L. M. Hutchins. 1995. Transmissible gastroenteritis in pigs. J. Am. Vet. Assoc. 108:257. [PubMed] [Google Scholar]
- 81.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 82.Drosten, C., W. Preiser, S. Gunther, H. Schmitz, and H. W. Doerr. 2003. Severe acute respiratory syndrome: identification of the etiological agent. Trends Mol. Med. 9:325-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195-3204. [DOI] [PubMed] [Google Scholar]
- 84.Dveksler, G. S., C. W. Dieffenbach, C. B. Cardellichio, K. McCuaig, M. N. Pensiero, G. S. Jiang, N. Beauchemin, and K. V. Holmes. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dveksler, G. S., S. E. Gagneten, C. A. Scanga, C. B. Cardellichio, and K. V. Holmes. 1996. Expression of the recombinant anchorless N-terminal domain of mouse hepatitis virus (MHV) receptor makes hamster of human cells susceptible to MHV infection. J. Virol. 70:4142-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebihara, T., R. Endo, X. Ma, N. Ishiguro, and H. Kikuta. 2005. Detection of human coronavirus NL63 in young children with bronchiolitis. J. Med. Virol. 75:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ebihara, T., R. Endo, X. Ma, N. Ishiguro, and H. Kikuta. 2005. Lack of association between New Haven coronavirus and Kawasaki disease. J. Infect. Dis. 192:351-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enjuanes, L., D. Cavanagh, K. Holmes, M. M. C. Lai, H. Laude, P. Masters, P. Rottier, S. G. Sidell, W. J. M. Spaan, F. Taguchi, and P. Talbot. 2000. Coronaviridae, p. 835-849. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Classification and nomemclature of viruses. Accademic Press, San Diego, Calif.
- 89.Enjuanes, L., C. Smerdou, J. Castilla, I. M. Anton, J. M. Torres, I. Sola, J. Golvano, J. M. Sanchez, and B. Pintado. 1995. Development of protection against coronavirus induced diseases. Adv. Exp. Med. Biol. 380:197-211. [DOI] [PubMed] [Google Scholar]
- 90.Enjuanes, L., I. Sola, S. Alonso, D. Escors, and S. Zuniga. 2005. Coronavirus reverse genetics and development of vectors for gene expression. Curr. Top. Microbiol. Immunol. 287:161-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Enjuanes, L., and B. A. M. van der Zeijst. 1995. Molecular basis of transmissible gastroenterititis virus, p. 337-376. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
- 92.Esper, F., E. D. Shapiro, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2005. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 191:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Esper, F., C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2005. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J. Infect. Dis. 191:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Faber, M., E. W. Lamirande, A. Roberts, A. B. Rice, H. Koprowski, B. Dietzschold, and M. J. Schnell. 2005. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J. Gen. Virol. 86:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farcas, G. A., S. M. Poutanen, T. Mazzulli, B. M. Willey, J. Butany, S. L. Asa, P. Faure, P. Akhavan, D. E. Low, and K. C. Kain. 2005. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J. Infect. Dis. 191:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fielding, B. C., Y. J. Tan, S. Shuo, T. H. Tan, E. E. Ooi, S. G. Lim, W. Hong, and P. Y. Goh. 2004. Characterization of a unique group-specific protein (U122) of the severe acute respiratory syndrome coronavirus. J. Virol. 78:7311-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischer, F., D. Peng, S. T. Hingley, S. R. Weiss, and P. S. Masters. 1997. The internal open reading frame within the nucleocapsid gene of mouse hepatitis virus encodes a structural protein that is not essential for viral replication. J. Virol. 71:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fischer, F., C. F. Stegen, C. A. Koetzner, and P. S. Masters. 1997. Analysis of a recombinant mouse hepatitis virus expressing a foreign gene reveals a novel aspect of coronavirus transcription. J. Virol. 71:5148-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisman, D. N. 2000. Hemophagocytic syndromes and infection. Emerg. Infect. Dis. 6:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fleck, F. 2003. WHO says SARS outbreak is over, but fight should go on. Br. Med. J. 327:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fouchier, R. A., N. G. Hartwig, T. M. Bestebroer, B. Niemeyer, J. C. de Jong, J. H. Simon, and A. D. Osterhaus. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. USA 101:6212-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fouchier, R. A., T. Kuiken, M. Schutten, G. Van Amerongen, G. J. Van Doornum, B. G. Van Den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gallagher, T. M., and M. J. Buchmeier. 2001. Coronavirus spike proteins in viral entry and pathogenesis. Virology 279:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gallagher, T. M., M. J. Buchmeier, and S. Perlman. 1992. Cell receptor-independent infection by a neurotropic murine coronavirus. Virology 191:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gallagher, T. M., C. Escarmis, and M. J. Buchmeier. 1991. Alteration of the pH dependence of coronavirus-induced cell fusion: effect of mutations in the spike glycoprotein. J. Virol. 65:1916-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallagher, T. M., S. E. Parker, and M. J. Buchmeier. 1990. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J. Virol. 64:731-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao, W., A. Tamin, A. Soloff, L. D'Aiuto, E. Nwanegbo, P. D. Robbins, W. J. Bellini, S. Barratt-Boyes, and A. Gambotto. 2003. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 362:1895-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilmore, W., J. O. Fleming, S. A. Stohlman, and L. P. Weiner. 1987. Characterization of the structural proteins of the murine coronavirus strain A59 using monoclonal antibodies. Proc. Soc. Exp. Biol. Med. 185:177-186. [DOI] [PubMed] [Google Scholar]
- 109.Glass, W. G., B. P. Chen, M. T. Liu, and T. E. Lane. 2002. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 15:261-272. [DOI] [PubMed] [Google Scholar]
- 110.Glass, W. G., K. Subbarao, B. Murphy, and P. M. Murphy. 2004. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 173:4030-4039. [DOI] [PubMed] [Google Scholar]
- 111.Godfraind, C., S. G. Langreth, C. B. Cardellichio, R. Knobler, J. P. Coutelier, M. Dubois-Dalcq, and K. V. Holmes. 1995. Tissue and cellular distribution of an adhesion molecule in the carcinoembryonic antigen family that serves as a receptor for mouse hepatitis virus. Lab. Invest. 73:615-627. [PubMed] [Google Scholar]
- 112.Goebel, S. J., B. Hsue, T. F. Dombrowski, and P. S. Masters. 2004. Characterization of the RNA components of a putative molecular switch in the 3′ untranslated region of the murine coronavirus genome. J. Virol. 78:669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goebel, S. J., J. Taylor, and P. S. Masters. 2004. The 3′ cis-acting genomic replication element of the severe acute respiratory syndrome coronavirus can function in the murine coronavirus genome. J. Virol. 78:7846-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gombold, J. L., S. T. Hingley, and S. R. Weiss. 1993. Fusion-defective mutants of mouse hepatitis virus A59 contain a mutation in the spike protein cleavage signal. J. Virol. 67:4504-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez, J. M., P. Gomez-Puertas, D. Cavanagh, A. E. Gorbalenya, and L. Enjuanes. 2003. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch. Virol. 148:2207-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gorbalenya, A. E. 2001. Big nidovirus genome. When count and order of domains matter. Adv. Exp. Med. Biol. 494:1-17. [PubMed] [Google Scholar]
- 117.Gorbalenya, A. E., E. J. Snijder, and W. J. Spaan. 2004. Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78:7863-7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greenough, T. C., G. J. Babcock, A. Roberts, H. J. Hernandez, W. D. Thomas, Jr., J. A. Coccia, R. F. Graziano, M. Srinivasan, I. Lowy, R. W. Finberg, K. Subbarao, L. Vogel, M. Somasundaran, K. Luzuriaga, J. L. Sullivan, and D. M. Ambrosino. 2005. Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J. Infect. Dis. 191:507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Greenough, T. C., A. Carville, J. Coderre, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, and K. Mansfield. 2005. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 167:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Groneberg, D. A., S. M. Poutanen, D. E. Low, H. Lode, T. Welte, and P. Zabel. 2005. Treatment and vaccines for severe acute respiratory syndrome. Lancet Infect. Dis. 5:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grosse, B., and S. G. Siddell. 1994. Single amino acid changes in the S2 subunit of the MHV surface glycoprotien confer resistance to neutralization by S1 subunit-specific monoclonal antibody. Virology 202:814-824. [DOI] [PubMed] [Google Scholar]
- 122.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. [DOI] [PubMed] [Google Scholar]
- 123.Haagmans, B. L., T. Kuiken, B. E. Martina, R. A. Fouchier, G. F. Rimmelzwaan, G. van Amerongen, D. van Riel, T. de Jong, S. Itamura, K. H. Chan, M. Tashiro, and A. D. Osterhaus. 2004. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 10:290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haijema, B. J., H. Volders, and P. J. Rottier. 2004. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 78:3863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haijema, B. J., H. Volders, and P. J. Rottier. 2003. Switching species tropism: an effective way to manipulate the feline coronavirus genome. J. Virol. 77:4528-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamming, I., W. Timens, M. L. Bulthuis, A. T. Lely, G. J. Navis, and H. van Goor. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haring, J. S., L. L. Pewe, and S. Perlman. 2001. High-magnitude, virus-specific CD4 T-cell response in the central nervous system of coronavirus-infected mice. J. Virol. 75:3043-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He, R., F. Dobie, M. Ballantine, A. Leeson, Y. Li, N. Bastien, T. Cutts, A. Andonov, J. Cao, T. F. Booth, F. A. Plummer, S. Tyler, L. Baker, and X. Li. 2004. Analysis of multimerization of the SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun. 316:476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Held, K. S., B. P. Chen, W. A. Kuziel, B. J. Rollins, and T. E. Lane. 2004. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology 329:251-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hellebo, A., U. Vilas, K. Falk, and R. Vlasak. 2004. Infectious salmon anemia virus specifically binds to and hydrolyzes 4-O-acetylated sialic acids. J. Virol. 78:3055-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hemmila, E., C. Turbide, M. Olson, S. Jothy, K. V. Holmes, and N. Beauchemin. 2004. Ceacam1a−/− mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J. Virol. 78:10156-10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hingley, S. T., I. Leparc-Goffart, S. H. Seo, J. C. Tsai, and S. R. Weiss. 2002. The virulence of mouse hepatitis virus strain A59 is not dependent on efficient spike protein cleavage and cell-to-cell fusion. J. Neurovirol. 8:400-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hingley, S. T., I. Leparc-Goffart, and S. R. Weiss. 1998. The spike protein of murine coronavirus mouse hepatitis virus strain A59 is not cleaved in primary glial cells and primary hepatocytes. J. Virol. 72:1606-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hodgson, T., R. Casais, B. Dove, P. Britton, and D. Cavanagh. 2004. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J. Virol. 78:13804-13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hofmann, H., and S. Pohlmann. 2004. Cellular entry of the SARS coronavirus. Trends Microbiol. 12:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hofmann, H., K. Pyrc, L. van der Hoek, M. Geier, B. Berkhout, and S. Pohlmann. 2005. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 102:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hogan, R. J., G. Gao, T. Rowe, P. Bell, D. Flieder, J. Paragas, G. P. Kobinger, N. A. Wivel, R. G. Crystal, J. Boyer, H. Feldmann, T. G. Voss, and J. M. Wilson. 2004. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires Stat1. J. Virol. 78:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hohdatsu, T., M. Yamada, R. Tominaga, K. Makino, K. Kida, and H. Koyama. 1998. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 60:49-55. [DOI] [PubMed] [Google Scholar]
- 139.Holmes, K. V. 1996. Coronaviridae: the viruses and their replication, p. 1075-1103. In D. M. Knipe, P. M. Howley, and B. N. Fields (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 140.Holmes, K. V. 2003. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Investig. 111:1605-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holmes, K. V., and S. R. Compton. 1995. Coronavirus receptors, p. 5571. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
- 142.Holmes, K. V., and L. Enjuanes. 2003. Virology. The SARS coronavirus: a postgenomic era. Science 300:1377-1378. [DOI] [PubMed] [Google Scholar]
- 143.Ito, N., E. C. Mossel, K. Narayanan, V. L. Popov, C. Huang, T. Inoue, C. J. Peters, and S. Makino. 2005. Severe acute respiratory syndrome coronavirus 3a protein is a viral structural protein. J. Virol. 79:3182-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ivanov, K. A., T. Hertzig, M. Rozanov, S. Bayer, V. Thiel, A. E. Gorbalenya, and J. Ziebuhr. 2004. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc. Natl. Acad. Sci. USA 101:12694-12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jeffers, S. A., S. M. Tusell, L. Gillim-Ross, E. M. Hemmila, J. E. Achenbach, G. J. Babcock, W. D. Thomas, Jr., L. B. Thackray, M. D. Young, R. J. Mason, D. M. Ambrosino, D. E. Wentworth, J. C. Demartini, and K. V. Holmes. 2004. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 101:15748-15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jendrach, M., V. Thiel, and S. Siddell. 1999. Characterization of an internal ribosome entry site within mRNA 5 of murine hepatitis virus. Arch. Virol. 144:921-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jonassen, C. M., T. Kofstad, I. L. Larsen, A. Lovland, K. Handeland, A. Follestad, and A. Lillehaug. 2005. Molecular identification and characterization of novel coronaviruses infecting graylag geese (Anser anser), feral pigeons (Columbia livia) and mallards (Anas platyrhynchos). J. Gen. Virol. 86:1597-1607. [DOI] [PubMed] [Google Scholar]
- 148.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kazi, L., A. Lissenburg, R. Watson, R. J. deGroot, and S. Weiss. Expression of hemagglutinin esterase protein from recombinant mouse hepatitis virus enhances neurovirulence. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 150.Keck, J. G., L. H. Soe, S. Makino, S. A. Stohlman, and M. M. Lai. 1988. RNA recombination of murine coronaviruses: recombination between fusion-positive mouse hepatitis virus A59 and fusion-negative mouse hepatitis virus 2. J. Virol. 62:1989-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kienzle, T. E., S. Abraham, B. G. Hogue, and D. A. Brian. 1990. Structure and orientation of expressed bovine coronavirus hemagglutinin-esterase protein. J. Virol. 64:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim, T. S., and S. Perlman. 2003. Protection against CTL escape and clinical disease in a murine model of virus persistence. J. Immunol. 171:2006-2013. [DOI] [PubMed] [Google Scholar]
- 153.Kim, T. W., J. H. Lee, C. F. Hung, S. Peng, R. Roden, M. C. Wang, R. Viscidi, Y. C. Tsai, L. He, P. J. Chen, D. A. Boyd, and T. C. Wu. 2004. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 78:4638-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klumperman, J., J. K. Locker, A. Meijer, M. C. Horzinek, H. J. Geuze, and P. J. Rottier. 1994. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 68:6523-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koetzner, C. A., M. M. Parker, C. S. Ricard, L. S. Sturman, and P. S. Masters. 1992. Repair and mutagenesis of the genome of a deletion mutant of the coronavirus mouse hepatitis virus by targeted RNA recombination. J. Virol. 66:1841-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kooi, C., M. Cervin, and R. Anderson. 1991. Differentiation of acid-pH-dependent and -nondependent entry pathways for mouse hepatitis virus. Virology 180:108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koren, G., S. King, S. Knowles, and E. Phillips. 2003. Ribavirin in the treatment of SARS: a new trick for an old drug? Can. Med. Assoc. J. 168:1289-1292. [PMC free article] [PubMed] [Google Scholar]
- 158.Kozak, M. 1984. Compilation and anlaysis of sequences upstream from the translational start site in eukaryotic mRNA. Nucleic Acids Res. 12:857-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krempl, C., B. Schultze, and G. Herrler. 1995. Analysis of cellular receptors for human coronavirus OC43. Adv. Exp. Med. Biol. 380:371-374. [DOI] [PubMed] [Google Scholar]
- 160.Krijnse-Locker, J., M. Ericsson, P. J. Rottier, and G. Griffiths. 1994. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 124:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krueger, D. K., S. M. Kelly, D. N. Lewicki, R. Ruffolo, and T. M. Gallagher. 2001. Variations in disparate regions of the murine coronavirus spike protein impact the initiation of membrane fusion. J. Virol. 75:2792-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, and L. J. Anderson. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 163.Kubo, H., Y. K. Yamada, and F. Taguchi. 1994. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J. Virol. 68:5403-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kuiken, T., R. A. Fouchier, M. Schutten, G. F. Rimmelzwaan, G. van Amerongen, D. van Riel, J. D. Laman, T. de Jong, G. van Doornum, W. Lim, A. E. Ling, P. K. Chan, J. S. Tam, M. C. Zambon, R. Gopal, C. Drosten, S. van der Werf, N. Escriou, J. C. Manuguerra, K. Stohr, J. S. Peiris, and A. D. Osterhaus. 2003. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Künkel, F., and G. Herrler. 1993. Structural and functional analysis of the surface protein of human coronavirus OC43. Virology 195:195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuo, L., G. J. Godeke, M. J. Raamsman, P. S. Masters, and P. J. Rottier. 2000. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J. Virol. 74:1393-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuo, L., and P. S. Masters. 2002. Genetic evidence for a structural interaction between the carboxy termini of the membrane and nucleocapsid proteins of mouse hepatitis virus. J. Virol. 76:4987-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kuo, L., and P. S. Masters. 2003. The small envelope protein E is not essential for murine coronavirus replication. J. Virol. 77:4597-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lai, K. N., J. C. Leung, C. N. Metz, F. M. Lai, R. Bucala, and H. Y. Lan. 2003. Role for macrophage migration inhibitory factor in acute respiratory distress syndrome. J. Pathol. 199:496-508. [DOI] [PubMed] [Google Scholar]
- 170.Lai, M. M., R. S. Baric, P. R. Brayton, and S. A. Stohlman. 1984. Characterization of leader RNA sequences on the virion and mRNAs of mouse hepatitis virus, a cytoplasmic RNA virus. Proc. Natl. Acad. Sci. USA 81:3626-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.La Monica, N., K. Yokomori, and M. M. Lai. 1992. Coronavirus mRNA synthesis: identification of novel transcription initiation signals which are differentially regulated by different leader sequences. Virology 188:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.LaMonica, N., L. R. Banner, V. L. Morris, and M. M. C. Lai. 1991. Localization of extensive deletions in the structural genes of two neurotropic variants of murine coronavirus JHM. Virology 182:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lane, T. E., V. C. Asensio, N. Yu, A. D. Paoletti, I. L. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 174.Lapps, W., B. G. Hogue, and D. A. Brian. 1987. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein genes. Virology 157:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Laude, H., J. Gelfi, L. Lavenant, and B. Charley. 1992. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 66:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Law, H. K., C. Y. Cheung, H. Y. Ng, S. F. Sia, Y. O. Chan, W. Luk, J. M. Nicholls, J. S. Peiris, and Y. L. Lau. 2005. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood 106:2366-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Law, P. T., C. H. Wong, T. C. Au, C. P. Chuck, S. K. Kong, P. K. Chan, K. F. To, A. W. Lo, J. Y. Chan, Y. K. Suen, H. Y. Chan, K. P. Fung, M. M. Waye, J. J. Sung, Y. M. Lo, and S. K. Tsui. 2005. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J. Gen. Virol. 86:1921-1930. [DOI] [PubMed] [Google Scholar]
- 178.Lee, H. J., C. K. Shieh, A. E. Gorbalenya, E. V. Koonin, N. La Monica, J. Tuler, A. Bagdzhadzhyan, and M. M. Lai. 1991. The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. Virology 180:567-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee, N., D. Hui, A. Wu, P. Chan, P. Cameron, G. M. Joynt, A. Ahuja, M. Y. Yung, C. B. Leung, K. F. To, S. F. Lui, C. C. Szeto, S. Chung, and J. J. Sung. 2003. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348:1986-1994. [DOI] [PubMed] [Google Scholar]
- 180.Leparc-Goffart, I., S. T. Hingley, M. M. Chua, X. Jiang, E. Lavi, and S. R. Weiss. 1997. Altered pathogenesis of a mutant of the murine coronavirus MHV-A59 is associated with a Q159L amino acid substitution in the spike protein. Virology 239:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leparc-Goffart, I., S. T. Hingley, M. M. Chua, J. Phillips, E. Lavi, and S. R. Weiss. 1998. Targeted recombination within the spike gene of murine coronavirus mouse hepatitis virus-A59: Q159 is a determinant of hepatotropism. J. Virol. 72:9628-9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leung, W. K., K. F. To, P. K. Chan, H. L. Chan, A. K. Wu, N. Lee, K. Y. Yuen, and J. J. Sung. 2003. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125:1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li, B. J., Q. Tang, D. Cheng, C. Qin, F. Y. Xie, Q. Wei, J. Xu, Y. Liu, B. J. Zheng, M. C. Woodle, N. Zhong, and P. Y. Lu. 2005. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in rhesus macaque. Nat. Med. 11:944-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li, L., J. Wo, J. Shao, H. Zhu, N. Wu, M. Li, H. Yao, M. Hu, and R. H. Dennin. 2003. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J. Clin. Virol. 28:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li, T., Z. Qiu, L. Zhang, Y. Han, W. He, Z. Liu, X. Ma, H. Fan, W. Lu, J. Xie, H. Wang, G. Deng, and A. Wang. 2004. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 189:648-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li, T., Y. Zhang, L. Fu, C. Yu, X. Li, Y. Li, X. Zhang, Z. Rong, Y. Wang, H. Ning, R. Liang, W. Chen, L. A. Babiuk, and Z. Chang. 2005. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. 12:751-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li, W., M. J. Moore, N. Vasilieva, J. Sui, S. K. Wong, M. A. Berne, M. Somasundaran, J. L. Sullivan, K. Luzuriaga, T. C. Greenough, H. Choe, and M. Farzan. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li, W., C. Zhang, J. Sui, J. H. Kuhn, M. J. Moore, S. Luo, S. K. Wong, I. C. Huang, K. Xu, N. Vasilieva, A. Murakami, Y. He, W. A. Marasco, Y. Guan, H. Choe, and M. Farzan. 2005. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 24:1634-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin, M. T., D. R. Hinton, N. W. Marten, C. C. Bergmann, and S. A. Stohlman. 1999. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 162:7358-7368. [PubMed] [Google Scholar]
- 190.Lissenburg, A., M. Vrolijk, A. van Vliet, M. Langereis, J. de Groot-Mijnes, P. Rottier, and R. J. de Groot. Luxury at a cost? Recombinant mouse hepatitis viruses expressing the accessory hemagglutinin esterase protein display reduced fitness in vitro. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 191.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 192.Liu, M. T., and T. E. Lane. 2001. Chemokine expression and viral infection of the central nervous system: regulation of host defense and neuropathology. Immunol. Res. 24:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu, S., G. Xiao, Y. Chen, Y. He, J. Niu, C. R. Escalante, H. Xiong, J. Farmar, A. K. Debnath, P. Tien, and S. Jiang. 2004. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 363:938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu, Y. C., V. Huang, T. C. Chao, C. D. Hsiao, A. Lin, M. F. Chang, and L. P. Chow. 2005. Screening of drugs by FRET analysis identifies inhibitors of SARS-CoV 3CL protease. Biochem. Biophys. Res. Commun. 333:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu, Z., C. Huang, K. Fan, P. Wei, H. Chen, S. Liu, J. Pei, L. Shi, B. Li, K. Yang, Y. Liu, and L. Lai. 2005. Virtual screening of novel noncovalent inhibitors for SARS-CoV 3C-like proteinase. J. Chem. Inf Model. 45:10-17. [DOI] [PubMed] [Google Scholar]
- 196.Lomniczi, B. J. 1977. Biological properties of avian coronavirus RNA. Gen. Virol. 36:531-533. [DOI] [PubMed] [Google Scholar]
- 197.Luo, Z., A. M. Matthews, and S. R. Weiss. 1999. Amino acid substitutions within the leucine zipper domain of the murine coronavirus spike protein cause defects in oligomerization and the ability to induce cell-to-cell fusion. J. Virol. 73:8152-8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Luo, Z., and S. R. Weiss. 1998. Roles in cell-to-cell fusion of two conserved hydrophobic regions in the murine coronavirus spike protein. Virology 244:483-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Luytjes, W., P. J. Bredenbeek, A. F. Noten, M. C. Horzinek, and W. J. Spaan. 1988. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology 166:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.MacNamara, K. C., M. M. Chua, P. T. Nelson, H. Shen, and S. R. Weiss. 2005. Increased epitope-specific CD8+ T cells prevent murine coronavirus spread to the spinal cord and subsequent demyelination. J. Virol. 79:3370-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.MacNamara, K. C., M. M. Chua, J. J. Phillips, and S. R. Weiss. 2005. Contributions of the viral genetic background and a single amino acid substitution in an immunodominant CD8+ T-cell epitope to murine coronavirus neurovirulence. J. Virol. 79:9108-9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maeda, J., J. F. Repass, A. Maeda, and S. Makino. 2001. Membrane topology of coronavirus E protein. Virology 281:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Magiorkinis, G., E. Magiorkinis, D. Paraskevis, A. M. Vandamme, M. Van Ranst, V. Moulton, and A. Hatzakis. 2004. Phylogenetic analysis of the full-length SARS-CoV sequences: evidence for phylogenetic discordance in three genomic regions. J. Med. Virol. 74:369-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Makino, S., J. G. Keck, S. A. Stohlman, and M. M. Lai. 1986. High-frequency RNA recombination of murine coronaviruses. J. Virol. 57:729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. [DOI] [PubMed] [Google Scholar]
- 206.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 207.Martina, B. E., B. L. Haagmans, T. Kuiken, R. A. Fouchier, G. F. Rimmelzwaan, G. Van Amerongen, J. S. Peiris, W. Lim, and A. D. Osterhaus. 2003. SARS virus infection of cats and ferrets. Nature 425:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Masters, P. S. 1999. Reverse genetics of the largest RNA viruses. Adv. Virus Res. 53:245-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Masters, P. S., and P. J. Rottier. 2005. Coronavirus reverse genetics by targeted RNA recombination. Curr. Top. Microbiol. Immunol. 287:133-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Matsuyama, S., and F. Taguchi. 2002. Communication between S1N330 and a region in S2 of murine coronavirus spike protein is important for virus entry into cells expressing CEACAM1b receptor. Virology 295:160-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Matsuyama, S., and F. Taguchi. 2002. Receptor-induced conformational changes of murine coronavirus spike protein. J. Virol. 76:11819-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Matsuyama, S., M. Ujike, S. Morikawa, M. Tashiro, and F. Taguchi. 2005. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. USA 102:12543-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Matthews, A. E., S. R. Weiss, and Y. Paterson. 2002. Murine hepatitis virus—a model for virus-induced CNS demyelination. J. Neurovirol. 8:76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Matthews, A. E., S. R. Weiss, M. J. Shlomchik, L. G. Hannum, J. L. Gombold, and Y. Paterson. 2001. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J. Immunol. 167:5254-5263. [DOI] [PubMed] [Google Scholar]
- 216.McAuliffe, J., L. Vogel, A. Roberts, G. Fahle, S. Fischer, W. J. Shieh, E. Butler, S. Zaki, M. St Claire, B. Murphy, and K. Subbarao. 2004. Replication of SARS coronavirus administered into the respiratory tract of African green, rhesus and cynomolgus monkeys. Virology 330:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McGoldrick, A., J. P. Lowings, and D. J. Paton. 1999. Characterisation of a recent virulent transmissible gastroenteritis virus from Britain with a deleted ORF 3a. Arch. Virol. 144:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McIntosh, K. 1974. Coronaviruses: a comparative review. Curr. Top. Microbiol. Immunol. 63:85-129. [Google Scholar]
- 219.Mizzen, L., A. Hilton, S. Cheley, and R. Anderson. 1985. Attenuation of murine coronavirus infection by ammonium chloride. Virology 142:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Moes, E., L. Vijgen, E. Keyaerts, K. Zlateva, S. Li, P. Maes, K. Pyrc, B. Berkhout, L. van der Hoek, and M. Van Ranst. 2005. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect. Dis. 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nash, T. C., and M. J. Buchmeier. 1997. Entry of mouse hepatitis virus into cells by endosomal and nonendosomal pathways. Virology 233:1-8. [DOI] [PubMed] [Google Scholar]
- 222.Navas, S., S. H. Seo, M. M. Chua, J. D. Sarma, E. Lavi, S. T. Hingley, and S. R. Weiss. 2001. Murine coronavirus spike protein determines the ability of the virus to replicate in the liver and cause hepatitis. J. Virol. 75:2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Navas, S., and S. R. Weiss. 2003. Murine coronavirus-induced hepatitis: JHM genetic background eliminates A59 spike-determined hepatotropism. J. Virol. 77:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Navas-Martin, S., S. T. Hingley, and S. R. Weiss. 2005. Murine coronavirus evolution in vivo: functional compensation of a detrimental amino acid substitution in the receptor binding domain of the spike glycoprotein. J. Virol. 79:7629-7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Navas-Martin, S. R., and S. Weiss. 2004. Coronavirus replication and pathogenesis: implications for the recent outbreak of severe acute respiratory syndrome (SARS), and the challenge for vaccine development. J. Neurovirol. 10:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nedellec, P., G. S. Dveksler, E. Daniels, C. Turbide, B. Chow, A. A. Basile, K. V. Holmes, and N. Beauchemin. 1994. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J. Virol. 68:4525-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nelson, C. A., A. Pekosz, C. A. Lee, M. S. Diamond, and D. H. Fremont. 2005. Structure and intracellular targeting of the SARS-coronavirus Orf7a accessory protein. Structure (Cambridge) 13:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nicholls, J. M., L. L. Poon, K. C. Lee, W. F. Ng, S. T. Lai, C. Y. Leung, C. M. Chu, P. K. Hui, K. L. Mak, W. Lim, K. W. Yan, K. H. Chan, N. C. Tsang, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2003. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361:1773-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nie, Q. H., X. D. Luo, J. Z. Zhang, and Q. Su. 2003. Current status of severe acute respiratory syndrome in China. World J. Gastroenterol. 9:1635-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ning, Q., M. Liu, P. Kongkham, M. M. Lai, P. A. Marsden, J. Tseng, B. Pereira, M. Belyavskyi, J. Leibowitz, M. J. Phillips, and G. Levy. 1999. The nucleocapsid protein of murine hepatitis virus type 3 induces transcription of the novel fgl2 prothrombinase gene. J. Biol. Chem. 274:9930-9936. [DOI] [PubMed] [Google Scholar]
- 231.Ontiveros, E., T. S. Kim, T. M. Gallagher, and S. Perlman. 2003. Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein. J. Virol. 77:10260-10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ontiveros, E., L. Kuo, P. S. Masters, and S. Perlman. 2001. Inactivation of expression of gene 4 of mouse hepatitis virus strain JHM does not affect virulence in the murine CNS. Virology 289:230-238. [DOI] [PubMed] [Google Scholar]
- 233.Ortego, J., D. Escors, H. Laude, and L. Enjuanes. 2002. Generation of a replication-competent, propagation-deficient virus vector based on the transmissible gastroenteritis coronavirus genome. J. Virol. 76:11518-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ortego, J., I. Sola, F. Almazan, J. E. Ceriani, C. Riquelme, M. Balasch, J. Plana, and L. Enjuanes. 2003. Transmissible gastroenteritis coronavirus gene 7 is not essential but influences in vivo virus replication and virulence. Virology 308:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Parisien, J. P., J. F. Lau, J. J. Rodriguez, B. M. Sullivan, A. Moscona, G. D. Parks, R. A. Lamb, and C. M. Horvath. 2001. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283:230-239. [DOI] [PubMed] [Google Scholar]
- 236.Parker, S. E., T. M. Gallagher, and M. J. Buchmeier. 1989. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology 173:664-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 238.Parry, J. 2003. WHO investigates China's fall in SARS cases. Br. Med. J. 326:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pearce, B. D., M. V. Hobbs, T. S. McGraw, and M. J. Buchmeier. 1994. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68:5483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Peiris, J. S., C. M. Chu, V. C. Cheng, K. S. Chan, I. F. Hung, L. L. Poon, K. I. Law, B. S. Tang, T. Y. Hon, C. S. Chan, K. H. Chan, J. S. Ng, B. J. Zheng, W. L. Ng, R. W. Lai, Y. Guan, and K. Y. Yuen. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Peiris, J. S., Y. Guan, and K. Y. Yuen. 2004. Severe acute respiratory syndrome. Nat. Med. 10:S88-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Perlman, S. 1998. Pathogenesis of coronavirus-induced infections. Review of pathological and immunological aspects. Adv. Exp. Med. Biol. 440:503-513. [PubMed] [Google Scholar]
- 243.Pewe, L., G. F. Wu, E. M. Barnett, R. F. Castro, and S. Perlman. 1996. Cytotoxic T cell-resistant variants are selected in a virus-induced demyelinating disease. Immunity 5:253-262. [DOI] [PubMed] [Google Scholar]
- 244.Pfleiderer, M., E. Routledge, G. Herrler, and S. G. Siddell. 1991. High level transient expression of the murine coronavirus haemagglutinin-esterase. J. Gen. Virol. 72:1309-1315. [DOI] [PubMed] [Google Scholar]
- 245.Phillips, J. J., M. Chua, S. H. Seo, and S. R. Weiss. 2001. Multiple regions of the murine coronavirus spike glycoprotein influence neurovirulence. J. Neurovirol. 7:421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Phillips, J. J., M. M. Chua, E. Lavi, and S. R. Weiss. 1999. Pathogenesis of chimeric MHV4/MHV-A59 recombinant viruses: the murine coronavirus spike protein is a major determinant of neurovirulence. J. Virol. 73:7752-7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Phillips, J. J., M. M. Chua, G. F. Rall, and S. R. Weiss. 2002. Murine coronavirus spike glycoprotein mediates degree of viral spread, inflammation, and virus-induced immunopathology in the central nervous system. Virology 301:109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Phillips, J. J., and S. R. Weiss. 2001. MHV neuropathogenesis: the study of chimeric S genes and mutations in the hypervariable region. Adv. Exp. Med. Biol. 494:115-119. [DOI] [PubMed] [Google Scholar]
- 249.Poon, L. L., D. K. Chu, K. H. Chan, O. K. Wong, T. M. Ellis, Y. H. Leung, S. K. Lau, P. C. Woo, K. Y. Suen, K. Y. Yuen, Y. Guan, and J. S. Peiris. 2005. Identification of a novel coronavirus in bats. J. Virol. 79:2001-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Poon, L. L., Y. Guan, J. M. Nicholls, K. Y. Yuen, and J. S. Peiris. 2004. The aetiology, origins, and diagnosis of severe acute respiratory syndrome. Lancet Infect. Dis. 4:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Poon, L. L., C. S. Leung, K. H. Chan, K. Y. Yuen, Y. Guan, and J. S. Peiris. 2005. Recurrent mutations associated with isolation and passage of SARS coronavirus in cells from non-human primates. J. Med. Virol. 76:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Poon, L. L., O. K. Wong, K. H. Chan, W. Luk, K. Y. Yuen, J. S. Peiris, and Y. Guan. 2003. Rapid diagnosis of a coronavirus associated with severe acute respiratory syndrome (SARS). Clin. Chem. 49:953-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Popova, R., and X. Zhang. 2002. The spike but not the hemagglutinin/esterase protein of bovine coronavirus is necessary and sufficient for viral infection. Virology 294:222-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Prentice, E., J. McAuliffe, X. Lu, K. Subbarao, and M. R. Denison. 2004. Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J. Virol. 78:9977-9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Qin, C., J. Wang, Q. Wei, M. She, W. A. Marasco, H. Jiang, X. Tu, H. Zhu, L. Ren, H. Gao, L. Guo, L. Huang, R. Yang, Z. Cong, Y. Wang, Y. Liu, Y. Sun, S. Duan, J. Qu, L. Chen, W. Tong, L. Ruan, P. Liu, H. Zhang, J. Zhang, D. Liu, Q. Liu, T. Hong, and W. He. 2005. An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. J. Pathol. 206:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Qu, X. X., P. Hao, X. J. Song, S. M. Jiang, Y. X. Liu, P. G. Wang, X. Rao, H. D. Song, S. Y. Wang, Y. Zuo, A. H. Zheng, M. Luo, H. L. Wang, F. Deng, H. Z. Wang, Z. H. Hu, M. X. Ding, G. P. Zhao, and H. Deng. 2005. Identification of two critical amino acid residues of the SARS coronavirus spike protein for its variation in zoonotic tropism transition via a double-substitution strategy. J. Biol. Chem. 280:29588-29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ramakrishna, C., C. C. Bergmann, K. V. Holmes, and S. A. Stohlman. 2004. Expression of the mouse hepatitis virus receptor by central nervous system microglia. J. Virol. 78:7828-7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Reghunathan, R., M. Jayapal, L. Y. Hsu, H. H. Chng, D. Tai, B. P. Leung, and A. J. Melendez. 2005. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Regl, G., A. Kaser, M. Iwersen, H. Schmid, G. Kohla, B. Strobl, U. Vilas, R. Schauer, and R. Vlasak. 1999. The hemagglutinin-esterase of mouse hepatitis virus strain S is a sialate-4-O-acetylesterase. J. Virol. 73:4721-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rempel, J. D., S. J. Murray, J. Meisner, and M. J. Buchmeier. 2004. Mouse hepatitis virus neurovirulence: evidence of a linkage between S glycoprotein expression and immunopathology. Virology 318:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rest, J. S., and D. P. Mindell. 2003. SARS associated coronavirus has a recombinant polymerase and coronaviruses have a history of host-shifting. Infect. Genet. Evol. 3:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Resta, S., J. P. Luby, C. R. Rosenfiled, and J. D. Siegel. 1985. Isolation and propagation of a human enteric coronavirus. Science 229:978-981. [DOI] [PubMed] [Google Scholar]
- 263.Roberts, A., C. Paddock, L. Vogel, E. Butler, S. Zaki, and K. Subbarao. 2005. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 79:5833-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Roberts, A., L. Vogel, J. Guarner, N. Hayes, B. Murphy, S. Zaki, and K. Subbarao. 2005. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J. Virol. 79:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Robitaille, J., L. Izzi, E. Daniels, B. Zelus, K. V. Holmes, and N. Beauchemin. 1999. Comparison of expression patterns and cell adhesion properties of the mouse biliary glycoproteins Bbgp1 and Bbgp2. Eur. J. Biochem. 264:534-544. [DOI] [PubMed] [Google Scholar]
- 266.Rossen, J. W., C. P. Bekker, G. J. Strous, M. C. Horzinek, G. S. Dveksler, K. V. Holmes, and P. J. Rottier. 1996. A murine and a porcine coronavirus are released from opposite surfaces of the same epithelial cells. Virology 224:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300:1394-1399. [DOI] [PubMed] [Google Scholar]
- 268.Rowe, T., G. Gao, R. J. Hogan, R. G. Crystal, T. G. Voss, R. L. Grant, P. Bell, G. P. Kobinger, N. A. Wivel, and J. M. Wilson. 2004. Macaque model for severe acute respiratory syndrome. J. Virol. 78:11401-11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Saeki, K., N. Ohtsuka, and F. Taguchi. 1997. Identification of spike protein residues of murine coronavirus responsible for receptor-binding activity by use of soluble receptor-resistant mutants. J. Virol. 71:9024-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Saeki, K., N. Ohtsuka, and F. Taguchi. 1998. Isolation and characterization of murine coronavirus mutants resistant to neutralization by soluble receptors. Adv. Exp. Med. Biol. 440:11-16. [DOI] [PubMed] [Google Scholar]
- 271.Saif, L. J. 2004. Animal coronavirus vaccines: lessons for SARS. Dev. Biol. 119:129-140. [PubMed] [Google Scholar]
- 272.Saif, L. J. 2004. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev. Sci. Technol. 23:643-660. [DOI] [PubMed] [Google Scholar]
- 273.Sainz, B., Jr., E. C. Mossel, C. J. Peters, and R. F. Garry. 2004. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology 329:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sanchez, C. M., A. Izeta, J. M. Sanchez-Morgado, S. Alonso, I. Sola, M. Balasch, J. Plana-Duran, and L. Enjuanes. 1999. Targeted recombination demonstrates that the spike gene of transmissible gastroenteritis coronavirus is a determinant of its enteric tropism and virulence. J. Virol. 73:7607-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sarma, J. D., E. Scheen, S. H. Seo, M. Koval, and S. R. Weiss. 2002. Enhanced green fluorescent protein expression may be used to monitor murine coronavirus spread in vitro and in the mouse central nervous system. J. Neurovirol. 8:381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Scagnolari, C., E. Vicenzi, F. Bellomi, M. G. Stillitano, D. Pinna, G. Poli, M. Clementi, F. Dianzani, and G. Antonelli. 2004. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antiviral Ther. 9:1003-1011. [PubMed] [Google Scholar]
- 277.Schelle, B., N. Karl, B. Ludewig, S. G. Siddell, and V. Thiel. 2005. Selective replication of coronavirus genomes that express nucleocapsid protein. J. Virol. 79:6620-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schultze, B., H. J. Gross, R. Brossmer, and G. Herrler. 1991. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 65:6232-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schultze, B., K. Wahn, H. D. Klenk, and G. Herrler. 1991. Isolated HE-protein from hemagglutinating encephalomyelitis virus and bovine coronavirus has receptor-destroying and receptor-binding activity. Virology 180:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Schwarz, B., E. Routledge, and S. G. Siddell. 1990. Murine coronavirus nonstructural protein ns2 is not essential for virus replication in transformed cells. J. Virol. 64:4784-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shi, Y., H. Yang de, J. Xiong, J. Jia, B. Huang, and Y. X. Jin. 2005. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. 15:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shieh, C. K., H. J. Lee, K. Yokomori, N. La Monica, S. Makino, and M. M. Lai. 1989. Identification of a new transcriptional initiation site and the corresponding functional gene 2b in the murine coronavirus RNA genome. J. Virol. 63:3729-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shieh, C. K., L. Soe, S. Makino, M. F. Chang, S. A. Stohlman, and M. M. C. Lai. 1987. The 5′ end sequence of the murine coronavirus genome: implications for multiple fusion sites in leader primed transcription. Virology 156:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Simmons, G., D. N. Gosalia, A. J. Rennekamp, J. D. Reeves, S. L. Diamond, and P. Bates. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 102:11876-11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Simmons, G., J. D. Reeves, A. J. Rennekamp, S. M. Amberg, A. J. Piefer, and P. Bates. 2004. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. USA 101:4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Smits, S. L., G. J. Gerwig, A. L. van Vliet, A. Lissenberg, P. Briza, J. P. Kamerling, R. Vlasak, and R. J. de Groot. 2005. Nidovirus sialate-O-acetylesterases: evolution and substrate specificity of corona- and toroviral receptor-destroying enzymes. J. Biol. Chem. 280:6933-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Snijder, E. J., P. J. Bredenbeek, J. C. Dobbe, V. Thiel, J. Ziebuhr, L. L. Poon, Y. Guan, M. Rozanov, W. J. Spaan, and A. E. Gorbalenya. 2003. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 331:991-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Song, H. D., C. C. Tu, G. W. Zhang, S. Y. Wang, K. Zheng, L. C. Lei, Q. X. Chen, Y. W. Gao, H. Q. Zhou, H. Xiang, H. J. Zheng, S. W. Chern, F. Cheng, C. M. Pan, H. Xuan, S. J. Chen, H. M. Luo, D. H. Zhou, Y. F. Liu, J. F. He, P. Z. Qin, L. H. Li, Y. Q. Ren, W. J. Liang, Y. D. Yu, L. Anderson, M. Wang, R. H. Xu, X. W. Wu, H. Y. Zheng, J. D. Chen, G. Liang, Y. Gao, M. Liao, L. Fang, L. Y. Jiang, H. Li, F. Chen, B. Di, L. J. He, J. Y. Lin, S. Tong, X. Kong, L. Du, P. Hao, H. Tang, A. Bernini, X. J. Yu, O. Spiga, Z. M. Guo, H. Y. Pan, W. Z. He, J. C. Manuguerra, A. Fontanet, A. Danchin, N. Niccolai, Y. X. Li, C. I. Wu, and G. P. Zhao. 2005. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. USA 102:2430-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and gamma interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sperry, S., L. Kazi, R. Graham, R. Baric, S. Weiss, and M. Denison. 2005. Single amino acid substitutions in nonstructural ORF1b-nsp14 and ORF2a 30kDa proteins of the murine coronavirus MHV-A59 are attenuating in mice. J. Virol. 79:3391-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Spiegel, M., A. Pichlmair, L. Martinez-Sobrido, J. Cros, A. Garcia-Sastre, O. Haller, and F. Weber. 2005. Inhibition of beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 79:2079-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Stavrinides, J., and D. S. Guttman. 2004. Mosaic evolution of the severe acute respiratory syndrome coronavirus. J. Virol. 78:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Stroher, U., A. DiCaro, Y. Li, J. E. Strong, F. Aoki, F. Plummer, S. M. Jones, and H. Feldmann. 2004. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-alpha. J. Infect. Dis. 189:1164-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sturman, L. S., and K. V. Holmes. 1977. Characterization of coronavirus. II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology 77:650-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Subbarao, K., J. McAuliffe, L. Vogel, G. Fahle, S. Fischer, K. Tatti, M. Packard, W. J. Shieh, S. Zaki, and B. Murphy. 2004. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 78:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sugiyama, K., M. Kasai, S. Kato, H. Kasai, and K. Hatakeyama. 1998. Haemagglutinin-esterase protein (HE) of murine corona virus: DVIM (diarrhea virus of infant mice). Arch. Virol. 143:1523-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sui, J., W. Li, A. Roberts, L. J. Matthews, A. Murakami, L. Vogel, S. K. Wong, K. Subbarao, M. Farzan, and W. A. Marasco. 2005. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 79:5900-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Surjit, M., B. Liu, S. Jameel, V. T. Chow, and S. K. Lal. 2004. The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J. 383:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Taguchi, F. 1995. The S2 subunit of the murine coronavirus spike protein is not involved in receptor binding. J. Virol. 69:7260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Taguchi, F., P. T. Massa, and V. ter Meulen. 1986. Characterization of a variant virus isolated from neural cell culture after infection of mouse coronavirus JHMV. Virology 155:267-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Taguchi, F., and S. Matsuyama. 2002. Soluble receptor potentiates receptor-independent infection by murine coronavirus. J. Virol. 76:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Taguchi, F., and S. G. Siddell. 1985. Difference in sensitivity to interferon among mouse hepatitis viruses with high and low virulence for mice. Virology 147:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Takasuka, N., H. Fujii, Y. Takahashi, M. Kasai, S. Morikawa, S. Itamura, K. Ishii, M. Sakaguchi, K. Ohnishi, M. Ohshima, S. Hashimoto, T. Odagiri, M. Tashiro, H. Yoshikura, T. Takemori, and Y. Tsunetsugu-Yokota. 2004. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int. Immunol. 16:1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Talbot, P. J., and M. J. Buchmeier. 1985. Antigenic variation among murine coronaviruses: evidence for polymorphism on the pepolmer glycoprotein, E2. Virus Res. 2:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Tan, Y. J., B. C. Fielding, P. Y. Goh, S. Shen, T. H. Tan, S. G. Lim, and W. Hong. 2004. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J. Virol. 78:14043-14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tan, Y. J., E. Teng, S. Shen, T. H. Tan, P. Y. Goh, B. C. Fielding, E. E. Ooi, H. C. Tan, S. G. Lim, and W. Hong. 2004. A novel severe acute respiratory syndrome coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis. J. Virol. 78:6723-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tang, L., Q. Zhu, E. Qin, M. Yu, Z. Ding, H. Shi, X. Cheng, C. Wang, G. Chang, F. Fang, H. Chang, S. Li, X. Zhang, X. Chen, J. Yu, J. Wang, and Z. Chen. 2004. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 23:391-394. [DOI] [PubMed] [Google Scholar]
- 308.ter Meulen, J., A. B. Bakker, E. N. van den Brink, G. J. Weverling, B. E. Martina, B. L. Haagmans, T. Kuiken, J. de Kruif, W. Preiser, W. Spaan, H. R. Gelderblom, J. Goudsmit, and A. D. Osterhaus. 2004. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363:2139-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Thackray, L. B., and K. V. Holmes. 2004. Amino acid substitutions and an insertion in the spike glycoprotein extend the host range of the murine coronavirus MHV-A59. Virology 324:510-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Thiel, V., J. Herold, B. Schelle, and S. G. Siddell. 2001. Infectious RNA transcribed in vitro from a cDNA copy of the human coronavirus genome cloned in vaccinia virus. J. Gen. Virol. 82:1273-1281. [DOI] [PubMed] [Google Scholar]
- 311.Thiel, V., K. A. Ivanov, A. Putics, T. Hertzig, B. Schelle, S. Bayer, B. Weissbrich, E. J. Snijder, H. Rabenau, H. W. Doerr, A. E. Gorbalenya, and J. Ziebuhr. 2003. Mechanisms and enzymes involved in SARS coronavirus genome expression. J. Gen. Virol. 84:2305-2315. [DOI] [PubMed] [Google Scholar]
- 312.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63:242-246. [DOI] [PubMed] [Google Scholar]
- 313.To, K. F., J. H. Tong, P. K. Chan, F. W. Au, S. S. Chim, K. C. Chan, J. L. Cheung, E. Y. Liu, G. M. Tse, A. W. Lo, Y. M. Lo, and H. K. Ng. 2004. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J. Pathol. 202:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Traggiai, E., S. Becker, K. Subbarao, L. Kolesnikova, Y. Uematsu, M. R. Gismondo, B. R. Murphy, R. Rappuoli, and A. Lanzavecchia. 2004. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10:871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Trifilo, M. J., C. C. Bergmann, W. A. Kuziel, and T. E. Lane. 2003. CC chemokine ligand 3 (CCL3) regulates CD8+-T-cell effector function and migration following viral infection. J. Virol. 77:4004-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Tsai, J. C., L. de Groot, J. D. Pinon, K. T. Iacono, J. J. Phillips, S. H. Seo, E. Lavi, and S. R. Weiss. 2003. Amino acid substitutions within the heptad repeat domain 1 of murine coronavirus spike protein restrict viral antigen spread in the central nervous system. Virology 312:369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tsui, P. T., M. L. Kwok, H. Yuen, and S. T. Lai. 2003. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 9:1064-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tyrrel, D. A. J., J. D. Almedia, D. M. Berry, C. H. Cunningham, D. Hamre, M. S. Hofstad, L. Malluci, and K. McIntosh. 1968. Coronavirus. Nature 220:650. [Google Scholar]
- 319.Valarcher, J. F., J. Furze, S. Wyld, R. Cook, K. K. Conzelmann, and G. Taylor. 2004. Role of alpha/beta inerferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 77:8426-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.van der Hoek, L., K. Pyrc, M. F. Jebbink, W. Vermeulen-Oost, R. J. Berkhout, K. C. Wolthers, P. M. Wertheim-van Dillen, J. Kaandorp, J. Spaargaren, and B. Berkhout. 2004. Identification of a new human coronavirus. Nat. Med. 10:368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.van der Hoek, L., K. Sure, G. Ihorst, A. Stang, K. Pyrc, M. F. Jebbink, G. Petersen, J. Forster, B. Berkhout, and K. Uberla. 2005. Croup is associated with the novel coronavirus NL63. PLoS Med. 2:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.van der Veen, R. C. 1996. Immunogenicity of JHM virus proteins: characterization of a CD4+ T cell epitope on nucleocapsid protein which induces different T-helper cell subsets. Virology 225:339-346. [DOI] [PubMed] [Google Scholar]
- 323.Vaughn, E. M., P. G. Halbur, and P. S. Paul. 1995. Sequence comparison of porcine respiratory coronavirus isolates reveals heterogeneity in the S, 3, and 3-1 genes. J. Virol. 69:3176-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vennema, H., G. J. Godeke, J. W. Rossen, W. F. Voorhout, M. C. Horzinek, D. J. Opstelten, and P. J. Rottier. 1996. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 15:2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vlasak, R., W. Luytjes, J. Leider, W. Spaan, and P. Palese. 1988. The E3 protein of bovine coronavirus is a receptor-destroying enzyme with acetylesterase activity. J. Virol. 62:4686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Vlasak, R., W. Luytjes, W. Spaan, and P. Palese. 1988. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl. Acad. Sci. USA 85:4526-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wang, F. I., J. O. Fleming, and M. M. Lai. 1992. Sequence analysis of the spike protein gene of murine coronavirus variants: study of genetic sites affecting neuropathogenicity. Virology 186:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang, Z., L. Ren, X. Zhao, T. Hung, A. Meng, J. Wang, and Y. G. Chen. 2004. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J. Virol. 78:7523-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wang, Z., Z. Yuan, M. Matsumoto, U. R. Hengge, and Y. F. Chang. 2005. Immune responses with DNA vaccines encoded different gene fragments of severe acute respiratory syndrome coronavirus in BALB/c mice. Biochem. Biophys. Res. Commun. 327:130-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ward, A. C. 1996. Neurovirulence of influenza A virus. J. Neurovirol. 2:139-151. [DOI] [PubMed] [Google Scholar]
- 331.Weingartl, H., M. Czub, S. Czub, J. Neufeld, P. Marszal, J. Gren, G. Smith, S. Jones, R. Proulx, Y. Deschambault, E. Grudeski, A. Andonov, R. He, Y. Li, J. Copps, A. Grolla, D. Dick, J. Berry, S. Ganske, L. Manning, and J. Cao. 2004. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 78:12672-12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weingartl, H. M., J. Copps, M. A. Drebot, P. Marszal, G. Smith, J. Gren, M. Andova, J. Pasick, P. Kitching, and M. Czub. 2004. Susceptibility of pigs and chickens to SARS coronavirus. Emerg. Infect. Dis. 10:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Weiss, S. R., P. W. Zoltick, and J. L. Leibowitz. 1993. The ns 4 gene of mouse hepatitis virus (MHV), strain A 59 contains two ORFs and thus differs from ns 4 of the JHM and S strains. Arch. Virol. 129:301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wentworth, D. E., and K. V. Holmes. 2001. Addition of a single glycosylation site to hAPN blocks human coronavirus-229E receptor activity. Adv. Exp. Med. Biol. 494:199-204. [DOI] [PubMed] [Google Scholar]
- 335.Wentworth, D. E., and K. V. Holmes. 2001. Molecular determinants of species specificity in the coronavirus receptor aminopeptidase N (CD13): influence of N-linked glycosylation. J. Virol. 75:9741-9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wesley, R. D., R. D. Woods, and A. K. Cheung. 1991. Genetic analysis of porcine respiratory coronavirus, an attenuated variant of transmissible gastroenteritis virus. J. Virol. 65:3369-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wilson, L., C. McKinlay, P. Gage, and G. Ewart. 2004. SARS coronavirus E protein forms cation-selective ion channels. Virology 330:322-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wong, R. S., A. Wu, K. F. To, N. Lee, C. W. Lam, C. K. Wong, P. K. Chan, M. H. Ng, L. M. Yu, D. S. Hui, J. S. Tam, G. Cheng, and J. J. Sung. 2003. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Br. Med. J. 326:1358-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wong, S. K., W. Li, M. J. Moore, H. Choe, and M. Farzan. 2004. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 279:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Woo, P. C., S. K. Lau, C. M. Chu, K. H. Chan, H. W. Tsoi, Y. Huang, B. H. Wong, R. W. Poon, J. J. Cai, W. K. Luk, L. L. Poon, S. S. Wong, Y. Guan, J. S. Peiris, and K. Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.World Health Organization. 2003. Severe acute respiratory syndrome (SARS): multi-country outbreak. World Health Organization, Geneva, Switzerland.
- 342.Wu, C. J., H. W. Huang, C. Y. Liu, C. F. Hong, and Y. L. Chan. 2005. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. 65:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wurm, T., H. Chen, T. Hodgson, P. Britton, G. Brooks, and J. A. Hiscox. 2001. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J. Virol. 75:9345-9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wurzer, W. J., K. Obojes, and R. Vlasak. 2002. The sialate-4-O-acetylesterases of coronaviruses related to mouse hepatitis virus: a proposal to reorganize group 2 Coronaviridae. J. Gen. Virol. 83:395-402. [DOI] [PubMed] [Google Scholar]
- 345.Xiong, S., Y. F. Wang, M. Y. Zhang, X. J. Liu, C. H. Zhang, S. S. Liu, C. W. Qian, J. X. Li, J. H. Lu, Z. Y. Wan, H. Y. Zheng, X. G. Yan, M. J. Meng, and J. L. Fan. 2004. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol. Lett. 95:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xue, S., A. Jaszewski, and S. Perlman. 1995. Identification of a CD4+ T cell epitope within the M protein of a neurotropic coronavirus. Virology 208:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yang, H., M. Yang, Y. Ding, Y. Liu, Z. Lou, Z. Zhou, L. Sun, L. Mo, S. Ye, H. Pang, G. F. Gao, K. Anand, M. Bartlam, R. Hilgenfeld, and Z. Rao. 2003. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA 100:13190-13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yang, Y., Z. Xiong, S. Zhang, Y. Yan, J. Nguyen, B. Ng, H. Lu, J. Brendese, F. Yang, H. Wang, and X. F. Yang. 2005. Bcl-xL inhibits T cell apotosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem. J. 392:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Yang, Z. Y., Y. Huang, L. Ganesh, K. Leung, W. P. Kong, O. Schwartz, K. Subbarao, and G. J. Nabel. 2004. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 78:5642-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yang, Z. Y., W. P. Kong, Y. Huang, A. Roberts, B. R. Murphy, K. Subbarao, and G. J. Nabel. 2004. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 428:561-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yang, Z. Y., H. C. Werner, W. P. Kong, K. Leung, E. Traggiai, A. Lanzavecchia, and G. J. Nabel. 2005. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. USA 102:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yeager, C. L., R. A. Ashmun, R. K. Williams, C. B. Cardellichio, L. H. Shapiro, A. T. Look, and K. V. Holmes. 1992. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yokomori, K., M. Asanaka, S. A. Stohlman, S. Makino, R. A. Shubin, W. Gilmore, L. P. Weiner, F. I. Wang, and M. M. Lai. 1995. Neuropathogenicity of mouse hepatitis virus JHM isolates differing in hemagglutinin-esterase protein expression. J. Neurovirol. 1:330-339. [DOI] [PubMed] [Google Scholar]
- 354.Yokomori, K., S. C. Baker, S. A. Stohlman, and M. M. Lai. 1992. Hemagglutinin-esterase-specific monoclonal antibodies alter the neuropathogenicity of mouse hepatitis virus. J. Virol. 66:2865-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yokomori, K., L. R. Banner, and M. M. Lai. 1991. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology 183:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yokomori, K., N. La Monica, S. Makino, C. K. Shieh, and M. M. Lai. 1989. Biosynthesis, structure, and biological activities of envelope protein gp65 of murine coronavirus. Virology 173:683-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yokomori, K., and M. M. Lai. 1991. Mouse hepatitis virus S RNA sequence reveals that nonstructural proteins ns4 and ns5a are not essential for murine coronavirus replication. J. Virol. 65:5605-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Yokomori, K., S. A. Stohlman, and M. M. Lai. 1993. The detection and characterization of multiple hemagglutinin-esterase (HE)-defective viruses in the mouse brain during subacute demyelination induced by mouse hepatitis virus. Virology 192:170-178. [DOI] [PubMed] [Google Scholar]
- 359.Yoo, D., F. L. Graham, L. Prevec, M. D. Parker, M. Benko, T. Zamb, and L. A. Babiuk. 1992. Synthesis and processing of the haemagglutinin-esterase glycoprotein of bovine coronavirus encoded in the E3 region of adenovirus. J. Gen. Virol. 73:2591-2600. [DOI] [PubMed] [Google Scholar]
- 360.Yoshikura, H., and F. Taguchi. 1978. Mouse hepatitis virus strain MHV-S: formation of pseudotypes with a murine leukemia virus envelope. Intervirology 10:132-136. [DOI] [PubMed] [Google Scholar]
- 361.Youn, S., J. L. Leibowitz, and E. W. Collisson. 2005. In vitro assembled, recombinant infectious bronchitis viruses demonstrate that the 5a open reading frame is not essential for replication. Virology 332:206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yount, B., K. M. Curtis, and R. S. Baric. 2000. Strategy for systematic assembly of large RNA and DNA genomes: transmissible gastroenteritis virus model. J. Virol. 74:10600-10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yount, B., K. M. Curtis, E. A. Fritz, L. E. Hensley, P. B. Jahrling, E. Prentice, M. R. Denison, T. W. Geisbert, and R. S. Baric. 2003. Reverse genetics with a full-length infectious cDNA of severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 100:12995-13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yount, B., M. R. Denison, S. R. Weiss, and R. S. Baric. 2002. Systematic assembly of a full-length infectious cDNA of mouse hepatitis virus strain A59. J. Virol. 76:11065-11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Yu, X., W. Bi, S. R. Weiss, and J. L. Leibowitz. 1994. Mouse hepatitis virus gene 5b protein is a new virion envelope protein. Virology 202:1018-1023. [DOI] [PubMed] [Google Scholar]
- 366.Yuan, X., Y. Shan, Z. Zhao, J. Chen, and Y. Cong. 2005. G0/G1 arrest and apoptosis induced by SARS-CoV 3b protein in transfected cells. Virol. J. 2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zakhartchouk, A. N., Q. Liu, M. Petric, and L. A. Babiuk. 2005. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine 23:4385-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zakhartchouk, A. N., S. Viswanathan, J. B. Mahony, J. Gauldie, and L. A. Babiuk. 2005. Severe acute respiratory syndrome coronavirus nucleocapsid protein expressed by an adenovirus vector is phosphorylated and immunogenic in mice. J. Gen. Virol. 86:211-215. [DOI] [PubMed] [Google Scholar]
- 369.Zelus, B. D., J. H. Schickli, D. M. Blau, S. R. Weiss, and K. V. Holmes. 2003. Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37°C either by soluble murine CEACAM1 receptors or by pH 8. J. Virol. 77:830-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zeng, F., K. Y. Chow, C. C. Hon, K. M. Law, C. W. Yip, K. H. Chan, J. S. Peiris, and F. C. Leung. 2004. Characterization of humoral responses in mice immunized with plasmid DNAs encoding SARS-CoV spike gene fragments. Biochem. Biophys. Res. Commun. 315:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang, X. W., Y. L. Yap, and A. Danchin. 2005. Testing the hypothesis of a recombinant origin of the SARS-associated coronavirus. Arch. Virol. 150:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhao, P., J. Cao, L. J. Zhao, Z. L. Qin, J. S. Ke, W. Pan, H. Ren, J. G. Yu, and Z. T. Qi. 2005. Immune responses against SARS-coronavirus nucleocapsid protein induced by DNA vaccine. Virology 331:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zheng, B., M. L. He, K. L. Wong, C. T. Lum, L. L. Poon, Y. Peng, Y. Guan, M. C. Lin, and H. F. Kung. 2004. Potent inhibition of SARS-associated coronavirus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN-gamma). J. Interferon Cytokine Res. 24:388-390. [DOI] [PubMed] [Google Scholar]
- 374.Zheng, B. J., Y. Guan, Q. Tang, C. Du, F. Y. Xie, M. L. He, K. W. Chan, K. L. Wong, E. Lader, M. C. Woodle, P. Y. Lu, B. Li, and N. Zhong. 2004. Prophylactic and therapeutic effects of small interfering RNA targeting SARS-coronavirus. Antiviral Ther. 9:365-374. [PubMed] [Google Scholar]
- 375.Zhong, N. S., B. J. Zheng, Y. M. Li, Poon, Z. H. Xie, K. H. Chan, P. H. Li, S. Y. Tan, Q. Chang, J. P. Xie, X. Q. Liu, J. Xu, D. X. Li, K. Y. Yuen, Peiris, and Y. Guan. 2003. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 362:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhou, J., W. Wang, Q. Zhong, W. Hou, Z. Yang, S. Y. Xiao, R. Zhu, Z. Tang, Y. Wang, Q. Xian, H. Tang, and L. Wen. 2005. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine 23:3202-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhu, M. S., Y. Pan, H. Q. Chen, Y. Shen, X. C. Wang, Y. J. Sun, and K. H. Tao. 2004. Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine. Immunol. Lett. 92:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ziebuhr, J. 2005. The coronavirus replicase. Curr. Top. Microbiol. Immunol. 287:57-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ziebuhr, J., V. Thiel, and A. E. Gorbalenya. 2001. The autocatalytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogous papain-like proteases that cleave the same peptide bond. J. Biol. Chem. 276:33220-33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zuckerman, A. J., P. E. Taylor, and D. Almeida. 1970. Presence of particles other than the Australia-SH antigen in a case of active hepatitis with cirrhosis. Br. Med. J. 1:262-264. [DOI] [PMC free article] [PubMed] [Google Scholar]