New physical, pharmacological, biological, and surgical treatments offer the possibility of tailor made therapy
Chronic wounds represent a major health burden and drain on resources. Recent advances in our understanding of chronic wound biology have led to the development of several new treatments that offer renewed hope to patients with ulcers and other chronic wounds. Defining the role of these new treatment, in the context of the increasing number of patients with chronic wounds, represents the next challenge. This review describes our view of the current and future developments in chronic wound management.
Summary points
Greater interest in wound healing is needed to ensure higher standards of basic care
Precise identification of the systemic, local, and molecular factors underlying the wound healing problem in individual patients should allow better tailored treatment.
Allogeneic skin grafting and bioengineered skin equivalents are being used successfully in patients with venous leg ulcers and diabetic patients with foot ulcers
Methods
The material presented in this article has been compiled from the published literature (located by searching Medline, PubMed, Embase, and Zetoc with the search terms “wound healing,” “treatment and venous leg,” “treatment and diabetic foot,” and “treatment and pressure ulcer”) presentations at international meetings, and our own 30 years of clinical experience managing acute and chronic wounds.
The clinical burden of wounds
The management of chronic wounds places an enormous drain on healthcare resources; studies have calculated the cost of wounds to the NHS to be about £1bn a year.1 In the United Kingdom around 24 000 admissions a year are for patients with diabetic foot ulceration, thereby costing the NHS some £17m.2 Foot ulceration is the commonest complication of diabetes that requires hospitalisation, and in the United States management of this problem is estimated to cost $150m a year.3 Venous leg ulceration costs an estimated £400m annually in the United Kingdom,4 most of this cost being for dressings and community nurse visits. The cost of treating pressure ulceration, some of which may have been prevented from arising, was estimated to be £300m over 10 years ago.5 These sums show only the direct costs and do not reflect the frustration, economic loss, and impaired quality of life experienced by patients with chronic ulcers.
Biology of skin healing
Wound healing involves a complex interaction between epidermal and dermal cells, the extracellular matrix, controlled angiogenesis, and plasma derived proteins—all coordinated by an array of cytokines and growth factors. This dynamic process is classically, but somewhat artificially, divided into three overlapping phases—inflammation, proliferation, and remodelling. Thrombus formation—which requires interaction between endothelial cells, platelets, and coagulation factors—achieves haemostasis after tissue injury. Trapped cells within the clot, predominantly platelets, trigger an inflammatory response by the release of vasodilators and chemoattractants and activation of the complement cascade.6
Inflammation—In the early phase of inflammation neutrophils predominate, removing bacteria and other foreign material from the wound by releasing enzymes and by phagocytosis. Later in the inflammatory phase neutrophils reduce in number and are replaced by macrophages. Interest has focused on the role of macrophages in coordinating the transition from inflammation to proliferation through the release of soluble mediators, which include platelet derived growth factor, tumour necrosis factor α, transforming growth factor β, and insulin growth factor 1.7
Proliferation—Fibroblasts are the key cells involved in the production of the extracellular matrix. In addition to producing collagen, they produce tenascin, fibronectin, and proteoglycans such as hyaluronic acid. Production of extracellular matrix is seen clinically as formation of granulation tissue. The combination of new tissue and contraction of surrounding tissues is essential for the healing of ulcers.8 While new matrix is synthesised, existing matrix in and around the wound margin is degraded by several enzyme systems such as matrix metalloproteinases and plasminogen activators. Research interest has centred on the role of the metalloproteinases, a family of over 14 enzymes with proteolytic activity. The effect of these enzymes is regulated by tissue inhibitors, which are believed to be important in healing by preventing excessive matrix degradation.9 While some keratinocytes at the wound edge proliferate, others undergo a marked transformation to enable them to phagocytose debris and migrate across the wound bed. Keratinocyte migration coupled with wound contraction results in re-epithelialisation and wound closure.
Remodelling—Once closure of the wound has been achieved, remodelling of the resulting scar takes places over months or years, with a reduction of both cell content and blood flow in the scar tissue.
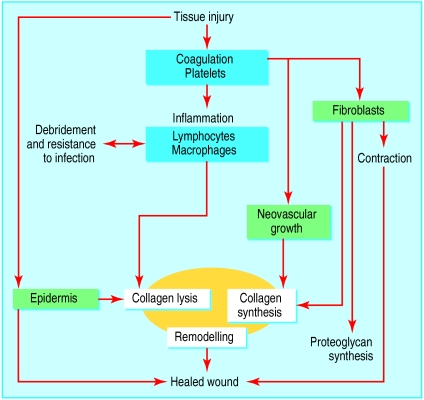
This superficial description of wound healing conveys some of the complexity of this biological process and shows the potential for modification in patients' with healing difficulties (fig 1).10
Figure 1.
Fundamental interrelation of the wound healing phases—inflammation (blue), proliferation (green), and tissue remodelling (yellow). The inflammatory phase is thought to coordinate wound healing, but we do not understand the true complexities of the process10
Why do some wounds fail to heal?
Many factors can impair healing. Local factors include the presence of foreign bodies, tissue maceration, ischaemia, and infection. Systemic factors as diverse as advanced age, malnutrition, diabetes, and renal disease may be important. It is important to quantify the impact of disease states on healing and to target specific treatments to correct the abnormality in individual patients. Although good clinical practice should aim to remove or reduce the impact of these factors, it is not always possible to do so.
In addition to local and systemic factors that impair healing, reduction in tissue growth factors, an imbalance between proteolytic enzymes and their inhibitors, and the presence of senescent cells seem to be particularly important in chronic wounds.
Reduced levels of active growth factors in the wound environment may partly explain why certain wounds fail to heal. Chronic ulcers are known to have reduced levels of platelet derived growth factor, basic fibroblast growth factor, epidermal growth factor, and transforming growth factor β compared with acute wounds.11 It has been suggested that growth factors may become trapped by extracellular matrix molecules12 or may be degraded by proteases to an excessive degree,13 resulting in non-healing.
Imbalance between proteinases and their inhibitors—Excessive proteinase activity in chronic wounds, probably from overexpression of matrix metalloproteins, results in abnormal degradation of the extracellular matrix. Many new treatment strategies are directed at modifying the imbalance—by the topical application of proteinase inhibitors, by inducing the expression of endogenous inhibitors, or by combining proteinase inhibitors with growth factors.13
Senescent cells—Dermal fibroblasts have an age related decrease in proliferation potential, called senescence.14 Fibroblasts in chronic wounds have impaired responsiveness to growth hormone, which may be due to an increased number of senescent cells.15,16
New approaches
Basic care
There is a role for pressure relief by good nursing care and the use of mattresses and cushions in patients with pressure ulceration.17 Similarly, regular debridement of callus, nail care, and pressure offloading footwear are fundamental to the care of foot disease from diabetic neuropathy.18 Use of compression bandages and stockings is an essential and effective component to managing venous ulceration.19 These basic tenets of wound care should underpin all new treatments.
Dressings
A major focus of chronic wound care in recent years has been the development of dressings that promote a moist environment to assist healing. Winter showed in an animal model that re-epithelialisation of a partial thickness acute wound proceeded 1.5 times more rapidly if the wound was occluded.20 Occlusive dressings have not shown such dramatic effects in clinical studies on patients with chronic wounds, but they may still benefit patients by reducing pain and by improving convenience of use and cost effectiveness. Advances in dressing technology have not yet resulted in the development of materials that correct abnormalities in the healing cascade, with the exception of dressings containing hyaluronic acid, which specifically promotes healing (L Miller et al, 5th European conference on advances in wound management, Harrogate, 1995).
Topical growth factors
The normal function of growth factors is to attract various cell types into the wound, stimulate cellular proliferation, promote angiogenesis, and regulate synthesis and degradation of extracellular matrix (table 1). Clinical results from topical application of growth factors to chronic wounds have not been as dramatic as first hoped. This is unsurprising when one considers the complexity of the wound healing process.
Table 1.
Examples of growth factors involved in healing
| Growth factor or cytokine | Effect on wound | Current status |
|---|---|---|
| Transforming growth factor β | Re-epithelialisation | Initial studies in venous ulcers encouraging |
| Neovascularisation | ||
| Increased granulation tissue and collagen | ||
| Reduced scar formation | ||
| Platelet derived growth factor | Re-epithelialisation | Licensed for the treatment of neuropathic diabetic foot ulcers |
| Neovascularisation | ||
| Increased granulation tissue and collagen | ||
| Fibroblast growth factor | Re-epithelialisation | Biological effects in pressure ulcers |
| Neovascularisation of a provisional matrix | No effect on diabetic or venous ulcers demonstrated to date | |
| Interleukin 1 β | Healing of infected wounds | Currently under trial for pressure ulcers |
| Granulocyte macrophage-colony stimulating factor | Improved healing in acute wounds | Pilot studies in infected diabetic foot ulcers encouraging |
To date, only platelet derived growth factor has been licensed for use, for treating non-infected foot ulcers up to 5 cm2 in diabetic patients (becaplermin, Regranex). Research studies have shown that it also has some value in treating pressure ulcers.21 Although not yet licensed, granulocyte colony stimulating factor has been evaluated for treating infected foot ulcers in diabetic patients and was associated with more rapid resolution of cellulitis and decreased antibiotic requirements.22 Furthermore, fibroblast growth factor has been assessed for treating pressure ulcers,23 and epidermal growth factor used for venous leg ulceration.24
In the future growth factors may be administered sequentially, in combination, or at timed intervals to more closely mimic the normal healing process. The diversity of growth factors and types of chronic wound suggest that these factors have potential as new treatments if patients' individual requirements can be identified.
Autologous skin grafts
Venous leg ulcers have been successfully treated by autologous skin grafting (using a patient's own skin), using split thickness and pinch skin grafting techniques. For successful management of pressure ulcers, both cutaneous and subcutaneous tissues need to be grafted, particularly over bony prominences. This is best achieved by fasciocutaneous or musculocutaneous flap surgery.
Bioengineered skin equivalents
Twenty years ago cultured epidermal autografts, in which a small skin biopsy is cultured to produce large epidermal sheets, were developed to treat patients with extensive burns. Unfortunately, these have had only limited success, because of the time taken to produce sufficient amounts of skin for grafting, graft fragility, difficulty in application, poor rate of uptake, and the frequent occurrence of infection.25
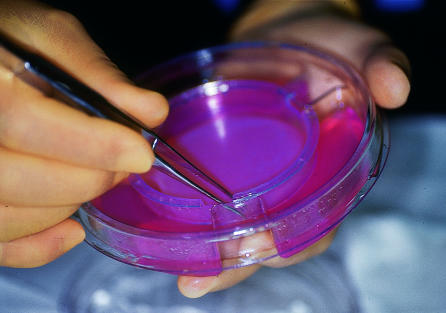
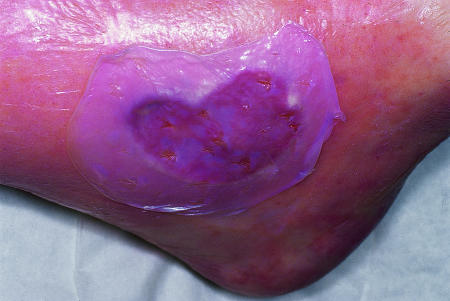
However, the potential benefit of this technology was evident and led to the development of skin equivalents, in which donor tissue with limited immunogenicity is used. There are now several commercially available products. Alloderm is a dermal matrix without immunogenic cells, whereas Integra is a combination of dermal fibroblasts and bovine collagen. Dermagraft consists of non-immunogenic neonatal fibroblast cultured on a polyglactin mesh and has been used to treat burns26 and diabetic foot ulcers27 (fig 2). Apligraf contains both epidermal and dermal components and has been used to treat diabetic foot ulcers28 and venous leg ulcers29 (fig 3). All of these products have been used to treat burns.
Figure 2.
Dermagraft—non-immunogenic neonatal fibroblasts cultured on a polyglactin mesh—is being investigated for treating diabetic foot ulcers. The dressing is stored frozen, and, after warming in a saline bath, the edges are trimmed (top) so that when it is applied to the wound it lies within the ulcer boundary (bottom)
Figure 3.
Apligraf—a bilayered skin equivalent containing newborn dermal fibroblasts in a bovine collagen matrix with overlying epidermal cells—is currently licensed to treat venous leg ulcers and diabetic foot ulcers. It comes frozen lying on a culture gel (top). Small slits are made in it, after which it is trimmed and laid onto the wound surface so that the edges overlie the ulcer edge (bottom)
Bioengineered skin replacements are absorbed into the wound bed and are thought to exert their effect on chronic ulcers, at least in part, by altering the profile of cytokines within the chronic wound, though the exact mode of action is unknown. Table 2 shows currently available skin replacements.
Table 2.
Currently available skin substitutes
| Type of skin substitute | Composition | Current status |
|---|---|---|
| Epidermal | Cultured autologous epidermal cells | Used for severe burn injuries |
| Cultured allogeneic epidermal cells | Used for severe burn injuries | |
| Dermal: | ||
| Alloderm | Acellular allogeneic human skin | Approved for treating burns |
| Integra | Bovine collagen with chondroitin 6-sulfate | Approved for treating burns |
| Dermagraft-TC | Fibroblasts on nylon mesh | In clinical trials for treating diabetic foot ulceration |
| Combined epidermal and dermal: | ||
| Apligraf | Bovine collagen, allogeneic fibroblasts, and epidermal cells | Licensed for treating diabetic foot ulcers and venous leg ulcers |
| Composite Cultured Skin | Collagen matrix substrate with fibroblasts and epidermal cells | In trials |
Future developments
There is a strong theoretical basis to justify the further development and clinical evaluation of proteinase inhibitors as a means of improving healing of chronic ulcers. In the near future gene therapy may allow genes important in healing to be delivered directly into a wound. Interest is presently focused on vascular endothelial growth factor, a key component in the promotion of angiogenesis.30 Another potential treatment lies in the development of skin equivalents from embryonic stem cells. However, the ethical implications of using such technology will first have to be addressed.
Future developments will depend very much on public and professional support for further research. One of the major barriers to effective wound care is the lack of interest and enthusiasm shown by many clinicians for this subject. Provision of an effective service for patients with wound problems requires the skills of many specialties. It is unimportant who is in charge of such patients, but that person must have an interest in the subject and have access to a wide range of interventions, including physical, pharmacological, biological, and surgical options. The real challenge for the future is to select appropriate interventions for each patient. This can be achieved only by developing appropriate clinical services and undertaking more high quality basic and clinical research in this important but neglected subject.
Additional educational resources
European Pressure Ulcer Advisory Panel www.epuap.org
Wound Healing Society www.woundheal.org
European Tissue Repair Society www.etrs.org
Australian Wound Management Association www.awma.com.au
European Wound Management Association www.ewma.com
UK Wound Care Society www.woundcaresociety.org
UK Tissue Viability Society www.tvs.org.uk
Leaper DJ, Harding KG, eds. Wounds: biology and management. Oxford: Oxford University Press, 1998
Boulton AJM, Connor H, Cavanagh PR. The foot in diabetes. Chichester: Wiley, 2000
Clark RAF. Molecular and cellular biology of wounds. 2nd ed. London: Plenum Press, 1996
Footnotes
Competing interests: The Wound Healing Research Unit is an independently funded unit within the University of Wales College of Medicine. It receives income from academic grants, research charities and several commercial concerns. KGH has received reimbursements for attending symposia and speaking and for consultancy work from companies supporting the unit.
References
- 1.Harding KG. The future of wound healing. In: Leaper DJ, Harding KJ, editors. Wounds: biology and management. Oxford: Oxford University Press; 1998. p. 191. [Google Scholar]
- 2.Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy and ulceration in diabetes. Diabetes Care. 1998;21:42–48. doi: 10.2337/diacare.21.1.42. [DOI] [PubMed] [Google Scholar]
- 3.Reiber GE. Diabetic foot care: financial implications and practical guidelines. Diabetes Care. 1992;15(suppl 1):29–31. doi: 10.2337/diacare.15.1.s29. [DOI] [PubMed] [Google Scholar]
- 4.Ruckley CV. Socio-economic impact of chronic venous insufficiency and leg ulcers. Angiology. 1997;48:67–69. doi: 10.1177/000331979704800111. [DOI] [PubMed] [Google Scholar]
- 5.Waterlow J. Prevention is better than cure. Nurs Times. 1988;84:69–70. [PubMed] [Google Scholar]
- 6.Yamada KM, Clark RAF. Provisional matrix. In: Clark RAF, editor. The molecular and cellular biology of wound repair. 2nd ed. London: Plenum Press; 1996. pp. 51–94. [Google Scholar]
- 7.Cooper DM, Yu EZ, Hennessey P, Ko F, Robson MC. Determination of endogenous cytokines in chronic wounds. Ann Surg. 1994;219:688–692. doi: 10.1097/00000658-199406000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark RAF. Biology of dermal wound repair. Dermatol Clin. 1993;11:647–666. [PubMed] [Google Scholar]
- 9.Mauch C, Krieg T, Bauer EA. Role of the extracellular matrix in the degradation of connective tissue. Arch Dermatol Res. 1994;287:107–114. doi: 10.1007/BF00370728. [DOI] [PubMed] [Google Scholar]
- 10.Hunt TK. Basic principles of wound healing. J Trauma. 1990;30:122–18s. doi: 10.1097/00005373-199012001-00025. [DOI] [PubMed] [Google Scholar]
- 11.Higley HR, Ksander GA, Gerhardt CO, Falanga V. Extravasation of macromolecules and possible trapping of transforming growth factor-beta in venous ulceration. Br J Dermatol. 1995;132:79–85. doi: 10.1111/j.1365-2133.1995.tb08629.x. [DOI] [PubMed] [Google Scholar]
- 12.Bucalo B, Eaglstein WH, Falanga V. Inhibition of cell proliferation by chronic wound fluid. Wound Repair Regen. 1993;1:181–186. doi: 10.1046/j.1524-475X.1993.10308.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathological processes. Wound Repair Regen. 1999;7:410–422. doi: 10.1046/j.1524-475x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 14.Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependant. J Invest Dermatol. 1999;112:463–469. doi: 10.1046/j.1523-1747.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Hasan A, Murata H, Falabella A, Ochoa S, Zhou L, Badiavas E, et al. Dermal fibroblasts from venous ulcers are unresponsive to the action of transforming growth factor-beta 1. J Dermatol Sci. 1997;16:59–66. doi: 10.1016/s0923-1811(97)00622-1. [DOI] [PubMed] [Google Scholar]
- 16.Stanley AC, Park HY, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be eliminated with growth factors. J Vasc Surg. 1997;26:994–999. doi: 10.1016/s0741-5214(97)70012-0. [DOI] [PubMed] [Google Scholar]
- 17.Colin D, Loyant R, Abraham P, Saumet JL. Changes in sacral transcutaneous oxygen tension in evaluation of different mattresses in the prevention of pressure ulcers. Adv Wound Care. 1996;9:25–28. [PubMed] [Google Scholar]
- 18.Boulton AJ, Bowker JH, Gadia M, Lemerman R, Caswell K, Skyler JS, et al. Use of plaster casts in the management of diabetic neuropathic foot ulcers. Diabetes Care. 1986;9:149–152. doi: 10.2337/diacare.9.2.149. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher A, Cullum N, Sheldon TA. A systematic review of compression treatment for venous leg ulcers. BMJ. 1997;316:576–580. doi: 10.1136/bmj.315.7108.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter GD. Formation of a scab and the rate of epithelialisation of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 21.Rees RS, Robson MC, Smiell JM, Perry BH Pressure Ulcer Study Group. Becaplermin gel in the treatment of pressure ulcers. Wound Repair Regen. 1997;7:141–147. doi: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Gough A, Clapperton M, Rolando N, Foster AVM, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:833–859. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 23.Robson MC, Phillips LG, Lawrence WT, Bishop JB, Youngerman JS, Hayward PG, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on healing of chronic pressure sores. Ann Surg. 1992;216:401–408. doi: 10.1097/00000658-199210000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falanga V, Eaglstein WH, Bucalo B, Katz MH, Harris B, Carson P. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers. J Dermatol Surg Oncol. 1992;18:604–606. doi: 10.1111/j.1524-4725.1992.tb03514.x. [DOI] [PubMed] [Google Scholar]
- 25.Eldad A, Burt A, Clarke JA. Cultured epithelium as a skin substitute. Burns. 1987;13(3):173–180. doi: 10.1016/0305-4179(87)90161-6. [DOI] [PubMed] [Google Scholar]
- 26.Hansbrough JF. Dermagraft-TC for partial thickness burns: a clinical evaluation. J Burn Care Rehabil. 1997;18s:25–28. doi: 10.1097/00004630-199701001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, et al. Use of Dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–354. doi: 10.2337/diacare.19.4.350. [DOI] [PubMed] [Google Scholar]
- 28.Veves A, Falanga V, Armstrong DG, Sabolinski ML. Graftskin, a human skin equivalent is effective in the management of non infected neuropathic diabetic foot ulcers. Diabetes Care. 2001;24:290–295. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 29.Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M et al, and the Human Skin Equivalent Investigators Group. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- 30.Taub PJ, Silver L, Weinberg H. Plastic surgical perspectives on vascular endothelial growth factor as gene therapy for angiogenesis. Plast Reconstruct Surg. 2000;105:1034–1042. doi: 10.1097/00006534-200003000-00031. [DOI] [PubMed] [Google Scholar]