Abstract
Heat shock proteins (HSPs) are recognized as significant participants in cancer immunity. We previously reported that HSP70 expression following hyperthermia using magnetic nanoparticles induces antitumor immunity. In the present study, we examine whether the antitumor immunity induced by hyperthermia is enhanced by administration of recombinant HSP70 protein into the tumor in situ. Hyperthermia was conducted using our original magnetite cationic liposomes (MCLs), which have a positive surface charge and generate heat in an alternating magnetic field (AMF) due to hysteresis loss. MCLs and recombinant mouse HSP70 (rmHSP70) were injected into melanoma nodules in C57BL/6 mice, which were subjected to AMF for 30 min. Temperature within the tumor reached 43°C and was maintained by controlling the magnetic field intensity. The combined treatment strongly inhibited tumor growth over a 30-day period and complete regression of tumors was observed in 20% (2/10) of mice. It was also found that systemic antitumor immunity was induced in the cured mice. This study suggests that novel combined therapy using exogenous HSP70 and hyperthermia has great potential in cancer treatment.
Keywords: Heat shock protein, Cancer immunotherapy, Hyperthermia, Magnetite, Cationic liposome
Introduction
Hyperthermia is a promising approach for cancer therapy [21, 34]. The inevitable technical problem with hyperthermia is the difficulty in heating only the local tumor region and avoiding damage to healthy tissue. Magnetic nanoparticles have been applied to hyperthermia in an attempt to overcome these disadvantages [11, 18]. Magnetic nanoparticles generate heat in an alternating magnetic field (AMF) due to hysteresis loss [26]. We have developed magnetite cationic liposomes (MCLs) for intracellular hyperthermia [27, 37]. The hyperthermic effects of these MCLs were investigated in an in vivo study, and complete tumor regression was observed [38]. Because several researchers reported that heat treatment itself can enhance the immunogenicity of cancer cells [12, 19, 20, 23], we previously studied the antitumor immunity induced by hyperthermia of T-9 rat glioma cells in vivo [39]. This induced-immunity continued for an extended period of time, and rats treated with hyperthermia completely rejected rechallenge with T-9 cells. Furthermore, we examined the mechanism of antitumor immunity induced by hyperthermia with regard to the role of heat shock proteins (HSPs) [8, 9].
HSPs are families of highly conserved proteins that are induced in cells by a large variety of stresses, including heat shock [25]. Because expression of the HSP70 protects cells from heat-induced apoptosis and the cells acquire thermotolerance [22], HSP70 expression has been considered to be a complicating factor in hyperthermia. On the other hand, recent reports have shown the importance of HSPs, such as HSP70, HSP90, and glucose-regulated protein 96 (gp96), in immune reactions [15, 28]. Three immunological roles for HSPs have been proposed: (1) HSPs are chaperones that physiologically associate with antigenic peptides [7]; (2) HSP-peptide complexes encounter antigen-presenting cells (APCs), and are then presented by MHC class I molecules [5, 13]; and (3) HSPs activate APCs to stimulate secretion of proinflammatory cytokines and up-regulation of costimulatory molecules, resulting in response of the innate immune system [2, 3]. It has been demonstrated that HSP-peptide complexes purified from tumors can elicit cancer immunity via the innate and adaptive immune response, and thus they have been used as cancer vaccines [24, 30, 32, 33]. HSP-mediated antitumor immunity is also caused by HSP-peptide complexes released from dying tumor cells [4, 14, 31]. We previously demonstrated that HSP70 expression following hyperthermia induced antitumor immunity in T-9 rat glioma [8, 9]. Our hyperthermia system using MCLs overcame thermotolerance and induced necrotic cell death that was correlated with HSP70 expression. We purified the HSP70-peptide complexes from the tumor after hyperthermia, and found that rats immunized with T-9–derived HSP70 strongly suppressed T-9 tumor growth, suggesting that HSP70 chaperones some antigenic peptides after hyperthermia [9]. Tumor rejection assay after hyperthermia treatment of implanted T-9 cells with incorporated MCLs was performed, and tumor growth was strongly suppressed in rats subjected to hyperthermia, and 50% of these rats were protected from challenge with T-9 cells. Immunogenicity was also enhanced when HSP70-overexpressing T-9 cells, which constitutively expressed HSP70 by gene transfer ex vivo, were killed via hyperthermia-induced necrosis in rats, after which all rats were completely protected from challenge with T-9 cells [9]. These results suggest that via HSP70-peptide complexes released during necrotic tumor cell death in vivo, our hyperthermia system confers antitumor immunity. This phenomenon, which may be called in situ vaccination, has important implications in the development of novel antitumor therapies.
When applying hyperthermia as a tumor vaccine, administration of an immunostimulant may supplement the vaccine-induced response against tumor cells. In the present study, we used a recombinant mouse HSP70 protein (rmHSP70) as an immune adjuvant. We studied the combined effects of rmHSP70 protein administration into tumors in situ and hyperthermia using MCLs on melanoma, and investigated the feasibility of this novel strategy in vivo. To the best of our knowledge, this is the first time that the combination of recombinant HSP70 and hyperthermia has been shown to exhibit an obvious antitumor effect against malignant melanoma.
Materials and methods
Cell line and animals
Mouse B16 melanoma cells (Riken Cell Bank, Tsukuba, Japan) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin G and 0.1 mg/ml streptomycin). Cells were grown at 37°C in an atmosphere containing 5% CO2. Female C57BL/6 mice (age 4 weeks) were purchased from Charles River Japan (Yokohama). Animal experiments were performed according to the principles laid down in the “Guide for the Care and Use of Laboratory Animals” prepared under the direction of the office of the prime minister of Japan.
Preparation of magnetite cationic liposomes
Magnetite (Fe3O4; average particle size 10 nm), used as the core of the MCLs, was kindly provided by Toda Kogyo (Hiroshima, Japan). MCLs were prepared with colloidal magnetite and a lipid mixture consisting of N-(α-trimethylammonioacetyl)-didodecyl-d-glutamate chloride, dilauroylphosphatidyl-choline, and dioleoylphosphatidyl-ethanolamine in a 1:2:2 molar ratio as previously described [27]. MCL concentrations were expressed as the net magnetite concentration.
Establishment of tumors in mice
Cell suspensions including approximately 1×106 melanoma cells in 0.1-ml phosphate buffered saline (PBS) were injected subcutaneously into C57BL/6 mice, which were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight). Melanoma nodules growing to 6 mm in diameter were used for the experiments. Tumor diameter was measured every 3 days. Size was determined by the following formula,
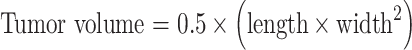 |
1 |
where, length and width are measured in millimeters.
The therapeutic effects of rmHSP70 injection with hyperthermia in vivo
Two types of heat shock protein 70, an inducible type and a constitutive type (HSP70 and HSC70, respectively), were used in this study. Recombinant mouse heat shock protein 70 (rmHSP70) and recombinant mouse heat shock cognate protein 70 (rmHSC70) were purchased from BioDynamics Laboratory (Tokyo). The endotoxin content of rmHSP70 and rmHSC70 was routinely measured by Limulus ambocyte lysate assay kit (BioWhittaker, Walkersville, MD) [2].
The rmHSP70 (20 or 80 μg) and rmHSC70 (80 μg) were rehydrated in 20 μl of PBS. MCL solution (0.1 ml; net magnetite weight 2 mg) was mixed with each concentration of rmHSP70 or rmHSC70 solution. The mixture was injected into the center of the tumor using a 26-gauge needle and infusion pump (SP100i; World Precision Instruments, Sarasota, FL) for 30 min. Mice in the nontreated group and in the hyperthermia group were injected with MCL solution only. Mice were then subjected to hyperthermia treatment. A magnetic field was created by using a horizontal coil (inner diameter 7 cm, length 7 cm) with a transistor inverter (Dai-ichi High Frequency, Tokyo) [38]. Anesthetized mice were laid inside the coil such that the tumor region was at the center. The magnetic field frequency and intensity were 118 kHz and 30.6 kA/m (384 Oe), respectively. Tumor and rectal temperatures were measured by optical fiber probe (Anritsu Meter, Tokyo).
In vitro cytotoxicity assay
Splenic lymphocytes derived from cured mice, which were treated with rmHSP70 (80 μg) plus hyperthermia, at 14 days after hyperthermic treatment were isolated using the Medimachine System (DAKO, Glostrup, Denmark). Naive mice that had been born at almost the same time were used as controls. Contaminant erythrocytes were lysed in 0.75% ammonium chloride. Cytotoxic activity was determined by lactate dehydrogenase (LDH) release assay [6] according to manufacturer’s instructions (Promega, Madison, WI). Briefly, target cells (B16 cells, 5×104 cells) were mixed with different ratios of effector cells and incubated for 4 h at 37°C in an atmosphere containing 5% CO2. Release of LDH was then evaluated by fluorescence value. The percent cytotoxicity was calculated as follows:
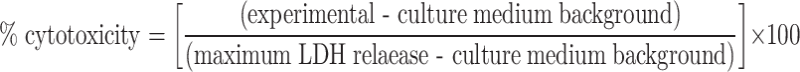 |
2 |
Statistical analysis
All experiments were repeated at least twice. Levels of statistical significance in tumor growth experiments were evaluated using the Mann-Whitney rank sum test. For survival analysis, differences in survival rates were analyzed by a log-rank test. Differences were considered statistically significant when the P value was less than 0.05.
Results
MCL-induced hyperthermia
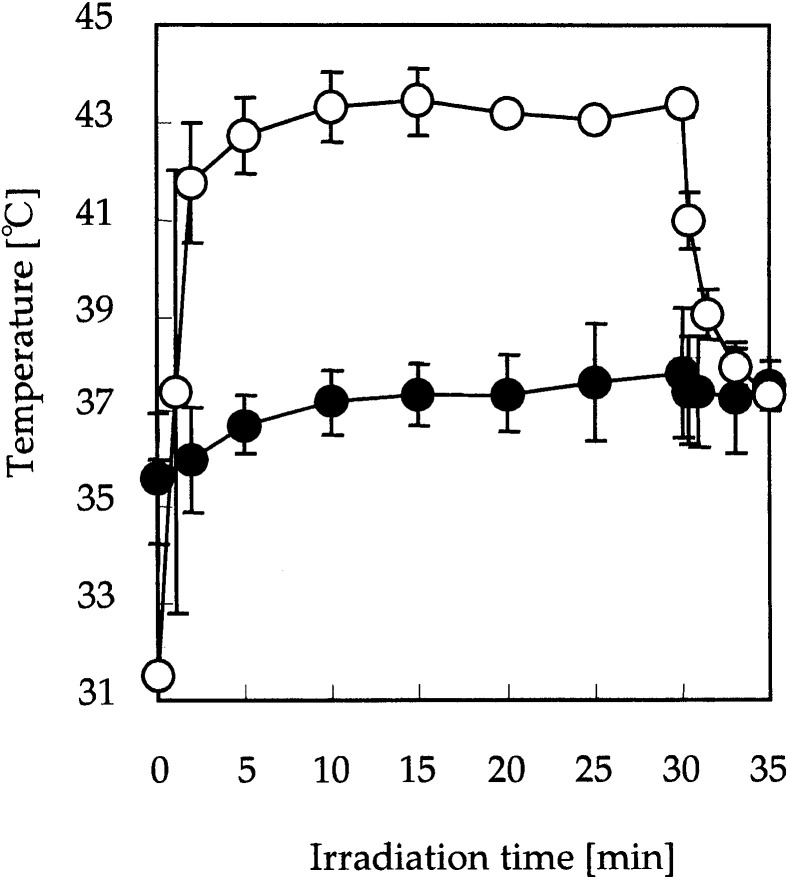
MCLs were injected into the tumor, and AMF was applied to the whole body of the mouse. Figure 1 shows the temperature at the tumor surface and in the rectum during AMF application. Tumor temperature increased rapidly to 43°C and was then maintained for 30 min by careful control of AMF intensity. When AMF was discontinued, tumor temperature decreased to 37°C within 5 min. In contrast, the temperature in the rectum hardly increased during the treatment. This suggests that the MCLs injected into the tumor were specifically heated by AMF, and that heat generation was controllable by adjusting the power of the AMF generator.
Fig. 1.
MCL-induced hyperthermia. MCLs were injected directly into subcutaneous tumors in mice. Mice were subjected to an alternating magnetic field for 30 min. Temperatures at the tumor surface (open circles) and in the rectum (solid circles) were measured by optical fiber probes. Temperature of the tumor was maintained at 43°C. Data points and bars represent mean and SD of five mice
Therapeutic effects of MCL-induced hyperthermia with recombinant HSP70
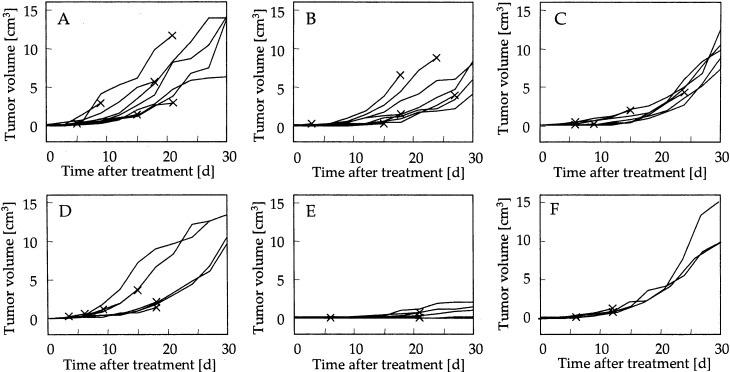
Figure 2 shows the time courses of tumor growth in each group. A control group of mice was injected with MCLs, but AMF was not applied. Tumor volume increased over 30 days in the control group, as shown in Fig. 2A. In the hyperthermia group (Fig. 2B), which was injected with MCLs and subjected to AMF treatment, and in the rmHSP70 (80 μg) group (Fig. 2C), which was injected with both rmHSP70 (80 μg) and MCLs but not irradiated, tumor volume at 30 days posttreatment was not significantly reduced when compared with the control group (P=0.12 and P=0.06, respectively). For the rmHSP70 (20 μg) plus hyperthermia group (Fig. 2D), which was injected with both rmHSP70 (20 μg) and MCLs, and was then subjected to AMF treatment, tumor volume at 30 days was similar to that observed for the control group (P=0.28). For the higher dose of rmHSP70 (80 μg) plus hyperthermia group (Fig. 2E), which was injected with rmHSP70 (80 μg) and MCLs, and was subjected to AMF treatment, tumor growth was strongly inhibited for 30 days and average tumor volume on day 30 was 0.7±0.9 cm3, which was significantly smaller than that of the control group (12.1±3.8 cm3, P<0.01), the hyperthermia group (6.7±2.0 cm3, P<0.01), and the rmHSP70 group (9.8±1.9 cm3, P<0.005). In addition to these significant differences in tumor volume between the rmHSP70 (80 μg) plus hyperthermia group and the other groups, a significant difference was also observed with tumor size in the rmHSP70 (20 μg) plus hyperthermia group (11.2±1.9 cm3, P<0.05), suggesting that the effects of rmHSP70 was dose dependent. Complete tumor regression in mice undergoing combined therapy was observed within 14 days in 2 of 10 mice. Interestingly, no significant antitumor effects were observed in the rmHSC70 plus hyperthermia groups, either with administration of 20 μg (data not shown) or 80 μg of rmHSC70 (Fig. 2F). The endotoxin content of both HSP70 and HSC70 was less than 50 EU/mg.
Fig. 2A–F.
Antitumor effects of administration of rmHSP70 combined with hyperthermia. Time courses of tumor growth after MCL injection in nontreatment (A, n=10), hyperthermia treatment alone (B, n=10), rmHSP70 (80 μg) injection alone (C, n=10), rmHSP70 (20 μg) injection plus hyperthermia treatment (D, n=10), rmHSP70 (80 μg) injection plus hyperthermia treatment (E, n=10), and rmHSC70 (80 μg) plus hyperthermia treatment (F, n=6) groups. Each line represents tumor growth in a single mouse. Crosses indicate when each mouse died
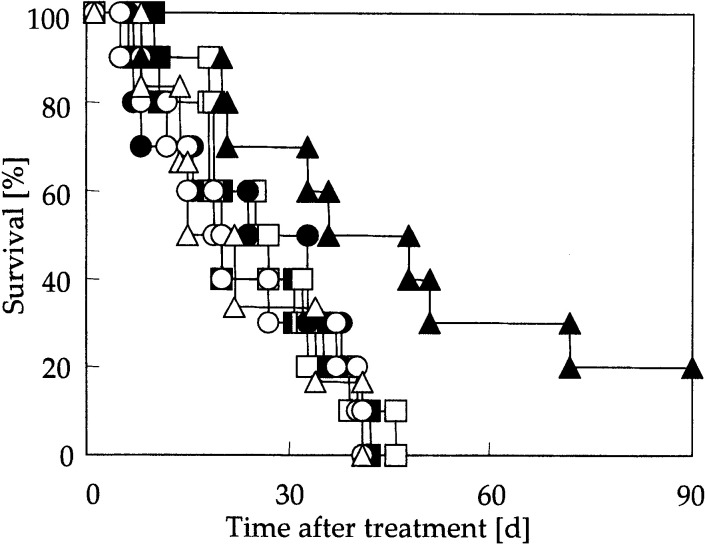
The percentage survival of tumor-bearing mice over a period of 90 days following hyperthermia is shown in Fig. 3. In the control group, the hyperthermia group, the rmHSP70 group, the rmHSP70 (20 μg) plus hyperthermia group, and the rmHSC70 (80 μg) plus hyperthermia group, all mice were dead due to pulmonary metastases and/or enlarged tumor at the inoculated site within 46 days. On the other hand, 2 of 10 mice in the rmHSP70 (80 μg) plus hyperthermia group exhibited complete cure and survived for 90 days after hyperthermia. Even though all mice in this group were not cured completely, survival was significantly extended when compared with the other groups (P<0.05, versus that of all the other groups).
Fig. 3.
Percentage survival of tumor-bearing mice over period of 90 days after MCL injection. Solid squares nontreatment (n=10), open squares hyperthermia treatment (n=10), solid circles rmHSP70 (80 μg)injection (n=10), open circles rmHSP70 (20 μg) injection plus hyperthermia treatment (n=10), solid triangles rmHSP70 (80 μg) injection plus hyperthermia treatment (n=10), open triangles rmHSC70 (80 μg) injection plus hyperthermia treatment (n=6)
Antitumor immunity induction by combination therapy of recombinant HSP70 with hyperthermia using MCLs
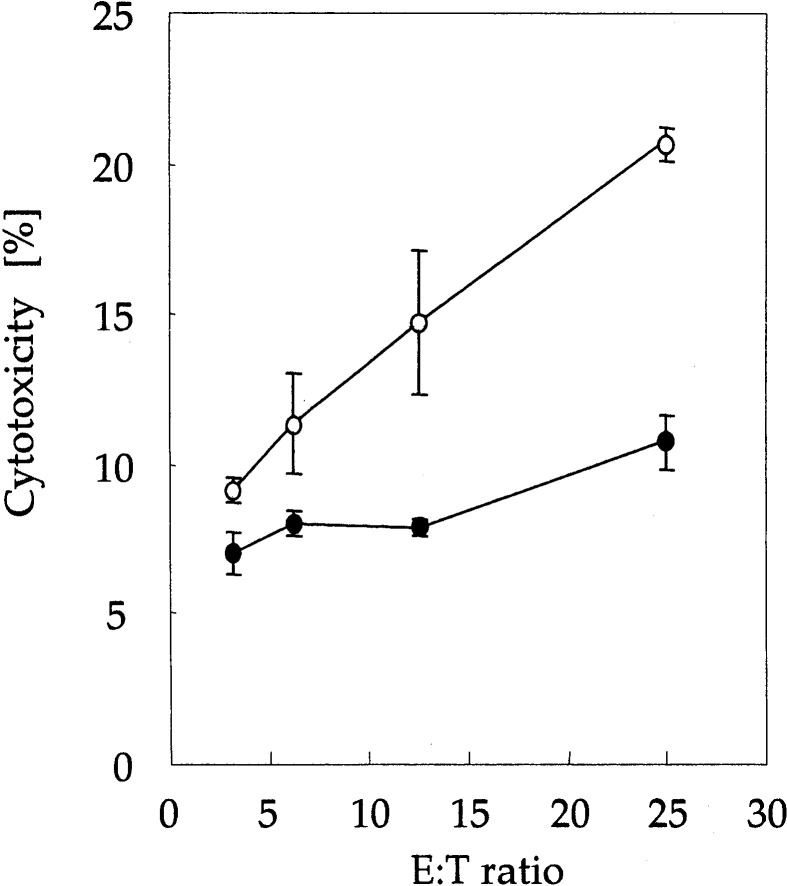
In order to determine whether combined therapy conferred antitumor immunity against melanoma cells, in vitro cytotoxicity assay was performed. Splenic lymphocytes were derived from cured mice (in the rmHSP70 (80 μg) plus hyperthermia group) and naive mice. As shown in Fig. 4, splenic lymphocytes from cured mice showed greater cytotoxic activity against B16 melanoma cells than did spleen cells from naive mice.
Fig. 4.
Cytotoxic activity of splenocytes against B16 melanoma cells. Spleen cells were derived from mice in the combined therapy group (treated with rmHSP70 (80 μg) injection plus hyperthermia treatment) at 14 days after hyperthermia treatment (open circles) and naive mice (solid circles). Effector cell to target cell (E:T) ratios ranged from 3.125 to 25:1. Data points and bars represent mean and SD of three independent experiments
Discussion
Hyperthermia has been used for many years to treat a wide variety of tumors in both experimental animals and patients. Almost all recent hyperthermia methods, including capacitive heating using a radiofrequency (RF) electric field [1], heat not only the tumor but also surrounding healthy tissue, which is damaged by nonspecific heating. Therefore, new methods that heat only the target area are needed. Our method of inducing intracellular hyperthermia using MCLs is able to heat the tumor specifically, as shown in Fig. 1. Moreover, the degree of heat generation in the tumor can be controlled by altering the magnetic field intensity, which allows the operator to induce the tumor cell necrosis without damaging the surrounding normal tissue [38]. In the present study, we set the tumor temperature at 43°C in order to investigate the effects of combining hyperthermia with administration of HSP70, but this temperature was insufficiently high to destroy the malignant melanoma. Our hyperthermia system can be performed at higher temperatures and can be conducted repeatedly without damaging healthy tissue. For example, complete regression of B16 melanoma was observed in 90% of mice using our hyperthermia system at 46°C applied once daily for 2 days [29].
The most important finding from our studies was that recombinant HSP70 administration in situ proved to be a powerful immunostimulant for hyperthermia. Immunization with HSP70-peptide complexes isolated from cancer cells elicits T-cell responses [32]. The immunogenicity of HSP70 protein results from its capacity to bind peptides within cells. In the present study, recombinant HSP70, which has not chaperoned any peptides, was used. One possible mechanism of the antitumor activity induced by hyperthermia with rmHSP70 is the association of rmHSP70 with tumor antigens produced in response to hyperthermic treatment. We previously reported that necrotic cells subjected to hyperthermia released endogenous HSP70 proteins [9], suggesting that endogenous proteins and peptides, which were antigenic but not associated with HSP70, may also be released from necrotic cells. These endogenous antigens may form complexes with exogenous recombinant HSP70, and newly produced HSP70-peptide complexes may induce antitumor immunity. Interestingly, no significant antitumor effects were observed in the rmHSC70 plus hyperthermia group (Fig. 2F). Ménoret et al. [16, 17] demonstrated that expression of inducible HSP70, but not constitutive HSC70, was involved in tumor immunogenicity, suggesting that interaction of inducible HSP70 with polypeptide substrates may preferentially occur in oxidized microenvironment because the stresses that induce HSP70 expression also give rise to cellular oxidation. Because hyperthermia is known to induce intracellular oxidation [35], hyperthermia induced by MCLs may promote an adaptive immune response specific for newly chaperoned peptides.
Alternatively, HSP70 functions as a direct activator of antigen-presenting cells (APCs), stimulating cytokine secretion from monocytes and inducing the maturation of dendritic cells (DCs) through CD14 [2] and/or CD91 [5] receptors. This cytokine-like ability of HSP70 to stimulate the innate immune system is independent of the peptides they chaperone [2], suggesting that HSP70 is a natural adjuvant. We previously reported that APCs, such as macrophages and DCs, infiltrate into tumors following hyperthermia with MCLs [9, 10]. The APCs recruited into tumor tissue after hyperthermia may be stimulated by administration of rmHSP70 to secrete inflammatory cytokines and mediate maturation of DCs, resulting in induction of immune responses.
We believe that these two functions of exogenous rmHSP70 in the innate and adaptive immune responses induce antitumor activity when administration is combined with hyperthermia. However, further investigation is needed to fully elucidate the mechanism.
B16 mouse melanoma cells are known to be poorly immunogenic because they express a lower amount of H-2 antigen, and are widely utilized in immunological experiments to assess the effect of immunotherapy [10, 29, 36]. We previously studied the H-2Kb amount on B16 cells by flow cytometry [10]. Flow cytometric analysis using the antimouse H-2Kb antibody revealed that B16 cells expressed no or a lower amount of MHC class I antigen compared with that of EL4 cells. Efficient recognition of tumor cells by cytolytic T lymphocytes (CTL) is often dependent on the presentation of cytosolic peptides in the context of MHC class I molecules. This process may be influenced by various molecular chaperones such as HSPs. Wells et al. reported that stably transfected clones of B16 which constitutively expressed the human HSP70 exhibited significantly increased levels of MHC class I antigens on their surface [36]. This HSP70-mediated up-regulation of surface MHC class I antigen represented an increase in the amount of functional MHC-peptide complexes as measured by conformation-dependent antibodies and recognition by MHC class I–restricted CTL. We previously showed the augmentation of MHC class I antigen presentation via HSP70 expression by hyperthermia [8]. The T-9 rat glioma cell surface presentation of MHC class I antigen increased in tandem with increased HSP70 expression and the immunogenicity of the cells was enhanced by hyperthermia. We previously demonstrated the augmentation of H-2Kb presentation in B16 melanoma cells by hyperthermia [10], and that the hyperthermia induced an antitumor immunity specific to B16 cells [29]. In the present study, although we have not elucidated the roles of HSP70 in combination with hyperthermia, we suppose that the antitumor immunity specific to B16 melanoma induced by hyperthermia was enhanced by the treatment of HSP70. Since the H-2Kb expression on nonheated B16 cells is low, the role of other effector cells such as the natural killer cells remains to be investigated. In addition to the low immunogenicity, we used large established tumors (0.6 cm in diameter) to examine the preclinical feasibility of the combined therapy used in the present study.
In the present study, we showed that combined therapy using rmHSP70 and MCL-induced hyperthermia resulted in systemic antitumor immunity. Cured mice were only obtained from the combined therapy group, and antitumor immunity was induced in these animals (Fig. 4). Although eight mice died from pulmonary metastases and/or enlarged tumor after subcutaneous injection of B16 cells, survival was prolonged, as shown in Fig. 3. These results indicate that this combined therapy is able to kill tumor cells, not only by direct heating but also by strongly inducing the antitumor immune response. We therefore conclude that this novel approach (combined therapy of exogenous recombinant HSP70 and hyperthermia using MCLs) should be applied to patients with advanced malignancies.
Acknowledgements
The authors would like to thank Toda Kogyo Co. for supplying the magnetite. This work was partially supported by a Grant-in-Aid for Scientific Research (No. 13853005 and 15760587), the University Start-Up Creation Support System and the 21st Century COE Program “Nature-Guided Materials Processing” from the Ministry of Education, Science, Sports and Culture of Japan.
References
- 1.Abe Cancer. 1986;58:1589. doi: 10.1002/1097-0142(19861015)58:8<1589::aid-cncr2820580802>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Asea Nat Med. 2000;6:435. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 3.Basu Cell Stress Chaperones. 2000;5:443. doi: 10.1379/1466-1268(2000)005<0443:hsptfo>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu Int Immunol. 2000;12:1539. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 5.Basu Immunity. 2001;14:303. [Google Scholar]
- 6.Chen Cancer Res. 2000;60:1035. [Google Scholar]
- 7.Ishii J Immunol. 1999;162:1303. [PubMed] [Google Scholar]
- 8.Ito Cancer Immunol Immunother. 2001;50:515. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito Cancer Immunol Immunother. 2003;52:80. doi: 10.1007/s00262-002-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Cancer Sci. 2003;94:308. doi: 10.1111/j.1349-7006.2003.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan Int J Hyperthermia. 1993;9:51. [Google Scholar]
- 12.Kubista Anticancer Res. 2002;22:789. [PubMed] [Google Scholar]
- 13.Li Curr Opin Immunol. 2002;14:45. doi: 10.1016/S0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 14.Melcher Nat Med. 1998;4:581. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 15.Ménoret Sem Immunol. 1998;25:654. [PubMed] [Google Scholar]
- 16.Ménoret J Immunol. 1995;155:740. [PubMed] [Google Scholar]
- 17.Ménoret Int J Hyperthermia. 2002;18:490. doi: 10.1080/02656730210146926. [DOI] [PubMed] [Google Scholar]
- 18.Minamimura Int J Oncol. 2000;16:1153. doi: 10.3892/ijo.16.6.1153. [DOI] [PubMed] [Google Scholar]
- 19.Mise Cancer Res. 1990;50:6199. [PubMed] [Google Scholar]
- 20.Mondovi Cancer. 1972;30:885. doi: 10.1002/1097-0142(197210)30:4<885::aid-cncr2820300404>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 21.Moroz Int J Hyperthermia. 2002;18:267. doi: 10.1080/02656730110108785. [DOI] [PubMed] [Google Scholar]
- 22.Mosser Mol Cell Biol. 2000;20:7146. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozdemirli J Immunol. 1991;147:4027. [PubMed] [Google Scholar]
- 24.Parmiani J Natl Cancer Inst. 2002;94:805. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger J Biol Chem. 1990;265:12111. [PubMed] [Google Scholar]
- 26.Shinkai Jpn J Hyperthermic Oncol. 1994;10:168. [Google Scholar]
- 27.Shinkai Jpn J Cancer Res. 1996;87:1179. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava Immunity. 1998;8:657. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Melanoma Res. 2003;13:129. doi: 10.1097/00008390-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Tamura Science. 1997;278:117. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 31.Todryk J Immunol. 1999;163:1398. [PubMed] [Google Scholar]
- 32.Udono J Exp Med. 1993;178:1391. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udono J Immunol. 1994;152:5398. [PubMed] [Google Scholar]
- 34.Van Annu Oncol. 2002;13:1173. doi: 10.1093/annonc/mdf280. [DOI] [Google Scholar]
- 35.Wallen Int J Hyperthermia. 1997;13:517. doi: 10.3109/02656739709023550. [DOI] [PubMed] [Google Scholar]
- 36.Wells Int Immunol. 1998;10:609. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 37.Yanase Jpn J Cancer Res. 1997;88:630. doi: 10.1111/j.1349-7006.1997.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanase Jpn J Cancer Res. 1998;89:463. doi: 10.1111/j.1349-7006.1998.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanase Jpn J Cancer Res. 1998;89:775. doi: 10.1111/j.1349-7006.1998.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]