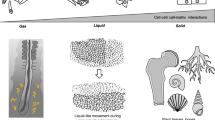
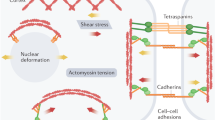
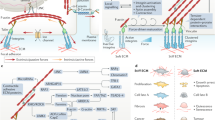
Abstract
From embryonic development, postnatal growth and adult homeostasis to reparative and disease states, cells and tissues undergo constant changes in genome activity, cell fate, proliferation, movement, metabolism and growth. Importantly, these biological state transitions are coupled to changes in the mechanical and material properties of cells and tissues, termed mechanical state transitions. These mechanical states share features with physical states of matter, liquids and solids. Tissues can switch between mechanical states by changing behavioural dynamics or connectivity between cells. Conversely, these changes in tissue mechanical properties are known to control cell and tissue function, most importantly the ability of cells to move or tissues to deform. Thus, tissue mechanical state transitions are implicated in transmitting information across biological length and time scales, especially during processes of early development, wound healing and diseases such as cancer. This Review will focus on the biological basis of tissue-scale mechanical state transitions, how they emerge from molecular and cellular interactions, and their roles in organismal development, homeostasis, regeneration and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kim, S., Pochitaloff, M., Stooke-Vaughan, G. A. & Campas, O. Embryonic tissues as active foams. Nat. Phys. 17, 859–866 (2021).
Guillot, C. & Lecuit, T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 340, 1185–1189 (2013).
Tetley, R. J. & Mao, Y. The same but different: cell intercalation as a driver of tissue deformation and fluidity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170328 (2018).
Founounou, N. et al. Tissue fluidity mediated by adherens junction dynamics promotes planar cell polarity-driven ommatidial rotation. Nat. Commun. 12, 6974 (2021).
Chen, T., Saw, T. B., Mege, R. M. & Ladoux, B. Mechanical forces in cell monolayers. J. Cell Sci. 131, jcs218156 (2018).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012).
Miroshnikova, Y. A. et al. Adhesion forces and cortical tension couple cell proliferation and differentiation to drive epidermal stratification. Nat. Cell Biol. 20, 69–80 (2018).
Rossen, N. S., Tarp, J. M., Mathiesen, J., Jensen, M. H. & Oddershede, L. B. Long-range ordered vorticity patterns in living tissue induced by cell division. Nat. Commun. 5, 5720 (2014).
Özkaya, N., Nordin, M., Goldsheyder, D. & Leger, D. (eds) Fundamentals of Biomechanics: Equilibrium, Motion, and Deformation 221–236 (Springer International Publishing, 2012).
Snoeijer, J. H., Pandey, A., Herrada, M. A. & Eggers, J. The relationship between viscoelasticity and elasticity. Proc. Math. Phys. Eng. Sci. 476, 20200419 (2020).
Cacopardo, L. & Ahluwalia, A. Engineering and monitoring 3D cell constructs with time-evolving viscoelasticity for the study of liver fibrosis in vitro. Bioengineering 8, 106 (2021).
Clement, R., Dehapiot, B., Collinet, C., Lecuit, T. & Lenne, P. F. Viscoelastic dissipation stabilizes cell shape changes during tissue morphogenesis. Curr. Biol. 27, 3132–3142.e4 (2017).
Duda, M. et al. Polarization of myosin II refines tissue material properties to buffer mechanical stress. Dev. Cell 48, 245–260.e7 (2019).
Liu, A. S. et al. Matrix viscoplasticity and its shielding by active mechanics in microtissue models: experiments and mathematical modeling. Sci. Rep. 6, 33919 (2016).
Teranishi, A. et al. Epithelial folding irreversibility is controlled by elastoplastic transition via mechanosensitive actin bracket formation. Preprint at bioRxiv https://doi.org/10.1101/2023.12.19.572470 (2024).
Zhijie, W., Mark, J. G. & Naomi, C. C. In Viscoelastic and Viscoplastic Materials (ed. Mohamed Fathy, E.-A.) (IntechOpen, 2016).
Bi, D., Lopez, J. H., Schwarz, J. M. & Manning, M. L. A density-independent rigidity transition in biological tissues. Nat. Phys. 11, 1074–1079 (2015).
Lawson-Keister, E. & Manning, M. L. Jamming and arrest of cell motion in biological tissues. Curr. Opin. Cell Biol. 72, 146–155 (2021).
Atia, L., Fredberg, J. J., Gov, N. S. & Pegoraro, A. F. Are cell jamming and unjamming essential in tissue development? Cell Dev. 168, 203727 (2021).
Bocanegra-Moreno, L., Singh, A., Hannezo, E., Zagorski, M. & Kicheva, A. Cell cycle dynamics control fluidity of the developing mouse neuroepithelium. Nat. Phys. 19, 1050–1058 (2023).
Garcia, S. et al. Physics of active jamming during collective cellular motion in a monolayer. Proc. Natl Acad. Sci. USA 112, 15314–15319 (2015).
Mongera, A. et al. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 561, 401–405 (2018).
Park, J. A. et al. Unjamming and cell shape in the asthmatic airway epithelium. Nat. Mater. 14, 1040–1048 (2015).
Tetley, R. J. et al. Tissue fluidity promotes epithelial wound healing. Nat. Phys. 15, 1195–1203 (2019).
Campàs, O., Noordstra, I. & Yap, A. S. Adherens junctions as molecular regulators of emergent tissue mechanics. Nat. Rev. Mol. Cell Biol. 25, 252–269 (2023).
Fletcher, D. A. & Mullins, R. D. Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010).
Kasza, K. E. et al. The cell as a material. Curr. Opin. Cell Biol. 19, 101–107 (2007).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22, 536–545 (2012).
Lappalainen, P., Kotila, T., Jegou, A. & Romet-Lemonne, G. Biochemical and mechanical regulation of actin dynamics. Nat. Rev. Mol. Cell Biol. 23, 836–852 (2022).
Curran, S. et al. Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev. Cell 43, 480–492.e6 (2017).
Yamamoto, T., Sussman, D. M., Shibata, T. & Manning, M. L. Non-monotonic fluidization generated by fluctuating edge tensions in confluent tissues. Soft Matter 18, 2168–2175 (2022).
Matis, M. The mechanical role of microtubules in tissue remodeling. Bioessays 42, e1900244 (2020).
Takeda, M., Sami, M. M. & Wang, Y. C. A homeostatic apical microtubule network shortens cells for epithelial folding via a basal polarity shift. Nat. Cell Biol. 20, 36–45 (2018).
Booth, A. J. R., Blanchard, G. B., Adams, R. J. & Roper, K. A dynamic microtubule cytoskeleton directs medial actomyosin function during tube formation. Dev. Cell 29, 562–576 (2014).
Enomoto, T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct. Funct. 21, 317–326 (1996).
Liu, B. P., Chrzanowska-Wodnicka, M. & Burridge, K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes. Commun. 5, 249–255 (1998).
Roper, K. Microtubules enter centre stage for morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190557 (2020).
Colin, L. et al. Cortical tension overrides geometrical cues to orient microtubules in confined protoplasts. Proc. Natl Acad. Sci. USA 117, 32731–32738 (2020).
Durand-Smet, P., Spelman, T. A., Meyerowitz, E. M. & Jonsson, H. Cytoskeletal organization in isolated plant cells under geometry control. Proc. Natl Acad. Sci. USA 117, 17399–17408 (2020).
Brangwynne, C. P. et al. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J. Cell Biol. 173, 733–741 (2006).
Janson, M. E., de Dood, M. E. & Dogterom, M. Dynamic instability of microtubules is regulated by force. J. Cell Biol. 161, 1029–1034 (2003).
van der Vaart, B., Akhmanova, A. & Straube, A. Regulation of microtubule dynamic instability. Biochem. Soc. Trans. 37, 1007–1013 (2009).
D’Angelo, A., Dierkes, K., Carolis, C., Salbreux, G. & Solon, J. In vivo force application reveals a fast tissue softening and external friction increase during early embryogenesis. Curr. Biol. 29, 1564–1571.e6 (2019).
Kechagia, Z. et al. The laminin-keratin link shields the nucleus from mechanical deformation and signalling. Nat. Mater. 22, 1409–1420 (2023).
Seltmann, K., Fritsch, A. W., Kas, J. A. & Magin, T. M. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc. Natl Acad. Sci. USA 110, 18507–18512 (2013).
Bergert, M. et al. Cell surface mechanics gate embryonic stem cell differentiation. Cell Stem Cell 28, 209–216.e4 (2021).
De Belly, H. et al. Membrane tension gates ERK-mediated regulation of pluripotent cell fate. Cell Stem Cell 28, 273–284.e6 (2021).
Yanagida, A. et al. Cell surface fluctuations regulate early embryonic lineage sorting. Cell 185, 777–793.e20 (2022).
Hurst, S., Vos, B. E., Brandt, M. & Betz, T. Intracellular softening and fluidification reveals a mechanical switch of cytoskeletal material contributions during division. Nat. Phys. 17, 1270–1276 (2021).
Molines, A. T. et al. Physical properties of the cytoplasm modulate the rates of microtubule polymerization and depolymerization. Dev. Cell 57, 466–479.e6 (2022).
Najafi, J., Dmitrieff, S. & Minc, N. Size- and position-dependent cytoplasm viscoelasticity through hydrodynamic interactions with the cell surface. Proc. Natl Acad. Sci. USA 120, e2216839120 (2023).
Grosser, S. et al. Cell and nucleus shape as an indicator of tissue fluidity in carcinoma. Phys. Rev. X 11, 011033 (2021).
Kim, S., Amini, R. & Campàs, O. A nuclear jamming transition in vertebrate organogenesis. Preprint at bioRxiv https://doi.org/10.1101/2022.07.31.502244 (2022).
Baye, L. M. & Link, B. A. Nuclear migration during retinal development. Brain Res. 1192, 29–36 (2008).
Garcia, M. A., Nelson, W. J. & Chavez, N. Cell-cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 10, a029181 (2018).
Ladoux, B., Nelson, W. J., Yan, J. & Mege, R. M. The mechanotransduction machinery at work at adherens junctions. Integr. Biol. 7, 1109–1119 (2015).
Lecuit, T. & Yap, A. S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539 (2015).
Maitre, J. L. & Heisenberg, C. P. Three functions of cadherins in cell adhesion. Curr. Biol. 23, R626–R633 (2013).
Schotz, E. M. et al. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. HFSP J. 2, 42–56 (2008).
Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436 (2008).
Maitre, J. L. et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256 (2012).
Sahu, P. et al. Small-scale demixing in confluent biological tissues. Soft Matter 16, 3325–3337 (2020).
Rubsam, M. et al. Adherens junctions and desmosomes coordinate mechanics and signaling to orchestrate tissue morphogenesis and function: an evolutionary perspective. Cold Spring Harb. Perspect. Biol. 10, a029207 (2018).
Heisenberg, C. P. & Bellaiche, Y. Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013).
Tsai, T. Y., Garner, R. M. & Megason, S. G. Adhesion-based self-organization in tissue patterning. Annu. Rev. Cell Dev. Biol. 38, 349–374 (2022).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Wickstrom, S. A., Radovanac, K. & Fassler, R. Genetic analyses of integrin signaling. Cold Spring Harb. Perspect. Biol. 3, a005116 (2011).
Legate, K. R., Wickstrom, S. A. & Fassler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23, 397–418 (2009).
Walma, D. A. C. & Yamada, K. M. The extracellular matrix in development. Development 147, dev175596 (2020).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Keeley, D. P. & Sherwood, D. R. Tissue linkage through adjoining basement membranes: the long and the short term of it. Matrix Biol. 75-76, 58–71 (2019).
Lawson, C. D. & Burridge, K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 5, e27958 (2014).
Tlili, S. et al. Shaping the zebrafish myotome by intertissue friction and active stress. Proc. Natl Acad. Sci. USA 116, 25430–25439 (2019).
Di Talia, S. & Vergassola, M. Waves in embryonic development. Annu. Rev. Biophys. 51, 327–353 (2022).
Ng, M. R., Besser, A., Brugge, J. S. & Danuser, G. Mapping the dynamics of force transduction at cell-cell junctions of epithelial clusters. eLife 3, e03282 (2014).
Peyret, G. et al. Sustained oscillations of epithelial cell sheets. Biophys. J. 117, 464–478 (2019).
Ruppel, A. et al. Force propagation between epithelial cells depends on active coupling and mechano-structural polarization. eLife 12, e83588 (2023).
Serra-Picamal, X. et al. Mechanical waves during tissue expansion. Nat. Phys. 8, 628–634 (2012).
Abreu-Blanco, M. T., Verboon, J. M., Liu, R., Watts, J. J. & Parkhurst, S. M. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J. Cell Sci. 125, 5984–5997 (2012).
Brock, J., Midwinter, K., Lewis, J. & Martin, P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J. Cell Biol. 135, 1097–1107 (1996).
Davidson, L. A., Hoffstrom, B. G., Keller, R. & DeSimone, D. W. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin α5β1, fibronectin, and tissue geometry. Dev. Biol. 242, 109–129 (2002).
Fernandez-Gonzalez, R. & Zallen, J. A. Wounded cells drive rapid epidermal repair in the early Drosophila embryo. Mol. Biol. Cell 24, 3227–3237 (2013).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000).
Martin, P. & Lewis, J. Actin cables and epidermal movement in embryonic wound healing. Nature 360, 179–183 (1992).
Peralta, X. G. et al. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophys. J. 92, 2583–2596 (2007).
Wood, W. et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell Biol. 4, 907–912 (2002).
Zhang, S., Teng, X., Toyama, Y. & Saunders, T. E. Periodic oscillations of myosin-II mechanically proofread cell-cell connections to ensure robust formation of the cardiac vessel. Curr. Biol. 30, 3364–3377.e4 (2020).
Nishimura, T. & Takeichi, M. Remodeling of the adherens junctions during morphogenesis. Curr. Top. Dev. Biol. 89, 33–54 (2009).
Ilina, O. et al. Cell-cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 22, 1103–1115 (2020).
Iyer, K. V., Piscitello-Gomez, R., Paijmans, J., Julicher, F. & Eaton, S. Epithelial viscoelasticity is regulated by mechanosensitive E-cadherin turnover. Curr. Biol. 29, 578–591.e575 (2019).
Farhadifar, R., Roper, J. C., Aigouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Chen, D. Y., Crest, J., Streichan, S. J. & Bilder, D. Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation. Nat. Commun. 10, 3339 (2019).
Harunaga, J. S., Doyle, A. D. & Yamada, K. M. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 394, 197–205 (2014).
Sui, L. et al. Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms. Nat. Commun. 9, 4620 (2018).
Vahey, M. D. & Fletcher, D. A. The biology of boundary conditions: cellular reconstitution in one, two, and three dimensions. Curr. Opin. Cell Biol. 26, 60–68 (2014).
Thery, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 123, 4201–4213 (2010).
Amack, J. D. & Manning, M. L. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science 338, 212–215 (2012).
Atia, L. et al. Geometric constraints during epithelial jamming. Nat. Phys. 14, 613–620 (2018).
Bi, D., Lopez, J. H., Schwarz, J. M. & Manning, M. L. Energy barriers and cell migration in densely packed tissues. Soft Matter 10, 1885–1890 (2014).
Keys, A. S., Abate, A. R., Glotzer, S. C. & Durian, D. J. Measurement of growing dynamical length scales and prediction of the jamming transition in a granular material. Nat. Phys. 3, 260–264 (2007).
Petridou, N. I., Corominas-Murtra, B., Heisenberg, C. P. & Hannezo, E. Rigidity percolation uncovers a structural basis for embryonic tissue phase transitions. Cell 184, 1914–1928.e19 (2021).
Ranft, J. et al. Fluidization of tissues by cell division and apoptosis. Proc. Natl Acad. Sci. USA 107, 20863–20868 (2010).
Brandstatter, T. et al. Curvature induces active velocity waves in rotating spherical tissues. Nat. Commun. 14, 1643 (2023).
Glentis, A. et al. The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces. Sci. Adv. 8, eabn5406 (2022).
Marzio, M., Das, A., Fredberg, J. J. & Bi, D. Epithelial layer fluidization by curvature-induced unjamming. Preprint at https://doi.org/10.48550/arXiv.2305.12667 (2023).
Werner, M., Kurniawan, N. A., Korus, G., Bouten, C. V. C. & Petersen, A. Mesoscale substrate curvature overrules nanoscale contact guidance to direct bone marrow stromal cell migration. J. R. Soc. Interface 15, 20180162 (2018).
Pinheiro, D., Kardos, R., Hannezo, É. & Heisenberg, C.-P. Morphogen gradient orchestrates pattern-preserving tissue morphogenesis via motility-driven unjamming. Nat. Phys. 18, 1482–1493 (2022).
Saadaoui, M., Rocancourt, D., Roussel, J., Corson, F. & Gros, J. A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science 367, 453–458 (2020).
Petridou, N. I., Grigolon, S., Salbreux, G., Hannezo, E. & Heisenberg, C. P. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat. Cell Biol. 21, 169–178 (2019).
Barriga, E. H., Franze, K., Charras, G. & Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527 (2018).
Jain, A. et al. Regionalized tissue fluidization is required for epithelial gap closure during insect gastrulation. Nat. Commun. 11, 5604 (2020).
Banavar, S. P. et al. Mechanical control of tissue shape and morphogenetic flows during vertebrate body axis elongation. Sci. Rep. 11, 8591 (2021).
Collinet, C. & Lecuit, T. Programmed and self-organized flow of information during morphogenesis. Nat. Rev. Mol. Cell Biol. 22, 245–265 (2021).
Nelson, C. M. Choreographing tissue morphogenesis. Semin. Cell Dev. Biol. 55, 79 (2016).
Durel, J. F. & Nerurkar, N. L. Mechanobiology of vertebrate gut morphogenesis. Curr. Opin. Genet. Dev. 63, 45–52 (2020).
Miller, S. A. et al. Domains of differential cell proliferation suggest hinged folding in avian gut endoderm. Dev. Dyn. 216, 398–410 (1999).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011).
Hozumi, S. et al. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature 440, 798–802 (2006).
Shyer, A. E. et al. Villification: how the gut gets its villi. Science 342, 212–218 (2013).
Goriely, A. & Vandiver, R. On the mechanical stability of growing arteries. IMA J. Appl. Math. 75, 549–570 (2010).
Kücken, M. & Newell, A. C. Fingerprint formation. J. Theor. Biol. 235, 71–83 (2005).
Lambert, R. K., Codd, S. L., Alley, M. R. & Pack, R. J. Physical determinants of bronchial mucosal folding. J. Appl. Physiol. 77, 1206–1216 (1994).
Richman, D. P., Stewart, R. M., Hutchinson, J. W. & Caviness, V. S. Jr. Mechanical model of brain convolutional development. Science 189, 18–21 (1975).
Menshykau, D. et al. Image-based modeling of kidney branching morphogenesis reveals GDNF-RET based Turing-type mechanism and pattern-modulating WNT11 feedback. Nat. Commun. 10, 239 (2019).
Walton, K. D. et al. Villification in the mouse: Bmp signals control intestinal villus patterning. Development 143, 427–436 (2016).
Landge, A. N., Jordan, B. M., Diego, X. & Muller, P. Pattern formation mechanisms of self-organizing reaction-diffusion systems. Dev. Biol. 460, 2–11 (2020).
Lawton, A. K. et al. Cerebellar folding is initiated by mechanical constraints on a fluid-like layer without a cellular pre-pattern. eLife 8, e45019 (2019).
Engstrom, T. A., Zhang, T., Lawton, A. K., Joyner, A. L. & Schwarz, J. M. Buckling without bending: a new paradigm in morphogenesis. Phys. Rev. X 8, 041053 (2018).
Spurlin, J. W. et al. Mesenchymal proteases and tissue fluidity remodel the extracellular matrix during airway epithelial branching in the embryonic avian lung. Development 146, dev175257 (2019).
Green, J. B. & Sharpe, J. Positional information and reaction-diffusion: two big ideas in developmental biology combine. Development 142, 1203–1211 (2015).
Schweisguth, F. & Corson, F. Self-organization in pattern formation. Dev. Cell 49, 659–677 (2019).
Tozluoglu, M. et al. Planar differential growth rates initiate precise fold positions in complex epithelia. Dev. Cell 51, 299–312.e4 (2019).
Glover, J. D. et al. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS Biol. 15, e2002117 (2017).
Ho, W. K. W. et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 17, e3000132 (2019).
Shyer, A. E. et al. Emergent cellular self-organization and mechanosensation initiate follicle pattern in the avian skin. Science 357, 811–815 (2017).
Villeneuve, C. et al. Mechanical forces across compartments coordinate cell shape and fate transitions to generate tissue architecture. Nat. Cell Biol. 26, 207–218 (2024).
O’Brien, L. E. Tissue homeostasis and non-homeostasis: from cell life cycles to organ states. Annu. Rev. Cell Dev. Biol. 38, 395–418 (2022).
Tai, K., Cockburn, K. & Greco, V. Flexibility sustains epithelial tissue homeostasis. Curr. Opin. Cell Biol. 60, 84–91 (2019).
Classen, A. K., Anderson, K. I., Marois, E. & Eaton, S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell 9, 805–817 (2005).
Gibson, M. C., Patel, A. B., Nagpal, R. & Perrimon, N. The emergence of geometric order in proliferating metazoan epithelia. Nature 442, 1038–1041 (2006).
Takeichi, M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15, 397–410 (2014).
De, R., Zemel, A. & Safran, S. A. Do cells sense stress or strain? Measurement of cellular orientation can provide a clue. Biophys. J. 94, L29–L31 (2008).
Obbink-Huizer, C. et al. Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech. Model. Mechanobiol. 13, 227–236 (2014).
Blanchard, G. B. et al. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nat. Methods 6, 458–464 (2009).
Chen, K. et al. Role of boundary conditions in determining cell alignment in response to stretch. Proc. Natl Acad. Sci. USA 115, 986–991 (2018).
Nava, M. M. et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800–817.e22 (2020).
Riveline, D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001).
Yonemura, S., Wada, Y., Watanabe, T., Nagafuchi, A. & Shibata, M. α-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533–542 (2010).
Loza, A. J. et al. Cell density and actomyosin contractility control the organization of migrating collectives within an epithelium. Mol. Biol. Cell 27, 3459–3470 (2016).
Özkaya, N., Leger, D., Goldsheyder, D. & Nordin, M. (eds) Fundamentals of Biomechanics: Equilibrium, Motion, and Deformation 361–387 (Springer International Publishing, 2017).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Cheng, G., Tse, J., Jain, R. K. & Munn, L. L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One 4, e4632 (2009).
Ernest, N. J., Habela, C. W. & Sontheimer, H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J. Cell Sci. 121, 290–297 (2008).
Matoz-Fernandez, D. A., Agoritsas, E., Barrat, J.-L., Bertin, E. & Martens, K. Nonlinear rheology in a model biological tissue. Phys. Rev. Lett. 118, 158105 (2017).
Shraiman, B. I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005).
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Angelini, T. E. et al. Glass-like dynamics of collective cell migration. Proc. Natl Acad. Sci. USA 108, 4714–4719 (2011).
Steinberg, M. S. Reconstruction of tissues by dissociated cells. Science 141, 401–408 (1963).
Steinberg, M. S. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 173, 395–434 (1970).
Arboleda-Estudillo, Y. et al. Movement directionality in collective migration of germ layer progenitors. Curr. Biol. 20, 161–169 (2010).
Ninomiya, H. et al. Cadherin-dependent differential cell adhesion in Xenopus causes cell sorting in vitro but not in the embryo. J. Cell Sci. 125, 1877–1883 (2012).
Hervieux, N. et al. Mechanical shielding of rapidly growing cells buffers growth heterogeneity and contributes to organ shape reproducibility. Curr. Biol. 27, 3468–3479.e4 (2017).
Perez-Gonzalez, C. et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 23, 745–757 (2021).
Krndija, D. et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365, 705–710 (2019).
Guiu, J. et al. Tracing the origin of adult intestinal stem cells. Nature 570, 107–111 (2019).
Sumigray, K. D., Terwilliger, M. & Lechler, T. Morphogenesis and compartmentalization of the intestinal crypt. Dev. Cell 45, 183–197.e5 (2018).
Blanpain, C. & Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 22, 339–373 (2006).
Biggs, L. C., Kim, C. S., Miroshnikova, Y. A. & Wickström, S. A. Mechanical forces in the skin: roles in tissue architecture, stability, and function. J. Invest. Dermatol. 140, 284–290 (2020).
Devany, J., Sussman, D. M., Yamamoto, T., Manning, M. L. & Gardel, M. L. Cell cycle-dependent active stress drives epithelia remodeling. Proc. Natl Acad. Sci. USA 118, e1917853118 (2021).
Li, H., Zheng, Y., Han, Y. L., Cai, S. & Guo, M. Nonlinear elasticity of biological basement membrane revealed by rapid inflation and deflation. Proc. Natl Acad. Sci. USA 118, e2022422118 (2021).
Bhattacharya, S. et al. The biophysical property of the limbal niche maintains stemness through YAP. Cell Death Differ. 30, 1601–1614 (2023).
Eberwein, P., Nohava, J., Schlunck, G. & Swain, M. Nanoindentation derived mechanical properties of the corneoscleral rim of the human eye. Key Eng. Mater. 606, 117–120 (2014).
Driscoll, T. P., Cosgrove, B. D., Heo, S. J., Shurden, Z. E. & Mauck, R. L. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 108, 2783–2793 (2015).
Dupont, S. & Wickstrom, S. A. Mechanical regulation of chromatin and transcription. Nat. Rev. Genet. 23, 624–643 (2022).
Eliazer, S. et al. Wnt4 from the niche controls the mechano-properties and quiescent state of muscle stem cells. Cell Stem Cell 25, 654–665.e4 (2019).
Gilbert, P. M. et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J. Clin. Invest. 120, 1535–1550 (2010).
Huerta-López, C. et al. Cell response to extracellular matrix energy dissipation outweighs rigidity sensing. Preprint at bioRxiv https://doi.org/10.1101/2022.11.16.516826 (2022).
Ladoux, B. & Mege, R. M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 18, 743–757 (2017).
Mosaffa, P., Tetley, R. J., Rodriguez-Ferran, A., Mao, Y. & Munoz, J. J. Junctional and cytoplasmic contributions in wound healing. J. R. Soc. Interface 17, 20200264 (2020).
Hosseini, M., Brown, J., Khosrotehrani, K., Bayat, A. & Shafiee, A. Skin biomechanics: a potential therapeutic intervention target to reduce scarring. Burn. Trauma 10, tkac036 (2022).
Gurtner, G. C. et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann. Surg. 254, 217–225 (2011).
Erickson, J. R. & Echeverri, K. Learning from regeneration research organisms: the circuitous road to scar free wound healing. Dev. Biol. 433, 144–154 (2018).
Guzman-Herrera, A. & Mao, Y. Polarity during tissue repair, a multiscale problem. Curr. Opin. Cell Biol. 62, 31–36 (2020).
Aragona, M. et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 8, 14684 (2017).
Lisse, T. S., King, B. L. & Rieger, S. Comparative transcriptomic profiling of hydrogen peroxide signaling networks in zebrafish and human keratinocytes: implications toward conservation, migration and wound healing. Sci. Rep. 6, 20328 (2016).
Park, S. et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 19, 155–163 (2017).
Richardson, R. & Hammerschmidt, M. The role of Rho kinase (Rock) in re-epithelialization of adult zebrafish skin wounds. Small GTPases 9, 230–236 (2018).
Richardson, R. et al. Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals. Development 143, 2077–2088 (2016).
Rezvani, O. et al. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manag. 55, 22–28 (2009).
Contreras, E. G., Gaete, M., Sanchez, N., Carrasco, H. & Larrain, J. Early requirement of hyaluronan for tail regeneration in Xenopus tadpoles. Development 136, 2987–2996 (2009).
Fukazawa, T., Naora, Y., Kunieda, T. & Kubo, T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development 136, 2323–2327 (2009).
Chen, L. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218 (2018).
Anon, E. et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl Acad. Sci. USA 109, 10891–10896 (2012).
Brugues, A. et al. Forces driving epithelial wound healing. Nat. Phys. 10, 683–690 (2014).
Kamran, Z. et al. In vivo imaging of epithelial wound healing in the cnidarian Clytia hemisphaerica demonstrates early evolution of purse string and cell crawling closure mechanisms. BMC Dev. Biol. 17, 17 (2017).
Bement, W. M., Forscher, P. & Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 121, 565–578 (1993).
Danjo, Y. & Gipson, I. K. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J. Cell Sci. 111, 3323–3332 (1998).
Schultz, G. S., Davidson, J. M., Kirsner, R. S., Bornstein, P. & Herman, I. M. Dynamic reciprocity in the wound microenvironment. Wound Repair. Regen. 19, 134–148 (2011).
Shellard, A. & Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 600, 690–694 (2021).
Ng, M. R., Besser, A., Danuser, G. & Brugge, J. S. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J. Cell Biol. 199, 545–563 (2012).
Sonam, S. et al. Mechanical stress driven by rigidity sensing governs epithelial stability. Nat. Phys. 19, 132–141 (2023).
Yun, M. H. Changes in regenerative capacity through lifespan. Int. J. Mol. Sci. 16, 25392–25432 (2015).
Larson, B. J., Longaker, M. T. & Lorenz, H. P. Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 126, 1172–1180 (2010).
Moore, A. L. et al. Scarless wound healing: transitioning from fetal research to regenerative healing. Wiley Interdiscip. Rev. Dev. Biol. 7, https://doi.org/10.1002/wdev.309 (2018).
Leung, A., Crombleholme, T. M. & Keswani, S. G. Fetal wound healing: implications for minimal scar formation. Curr. Opin. Pediatr. 24, 371–378 (2012).
Fan, C. et al. Age-related alterations of hyaluronan and collagen in extracellular matrix of the muscle spindles. J. Clin. Med. 11, 86 (2021).
Ge, Y. et al. The aging skin microenvironment dictates stem cell behavior. Proc. Natl Acad. Sci. USA 117, 5339–5350 (2020).
Koester, J. et al. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat. Cell Biol. 23, 771–781 (2021).
Li, M. et al. Time-resolved extracellular matrix atlas of the developing human skin dermis. Front. Cell Dev. Biol. 9, 783456 (2021).
Segel, M. et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 (2019).
Martin, P. & Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 173, 370–378 (2015).
Karsdal, M. A. et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G807–G830 (2015).
Liu, M., Tolg, C. & Turley, E. Dissecting the dual nature of hyaluronan in the tumor microenvironment. Front. Immunol. 10, 947 (2019).
Pickup, M. W., Mouw, J. K. & Weaver, V. M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014).
Talbott, H. E., Mascharak, S., Griffin, M., Wan, D. C. & Longaker, M. T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 29, 1161–1180 (2022).
Heidelbaugh, J. J. & Bruderly, M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am. Fam. Physician 74, 756–762 (2006).
Arriazu, E. et al. Extracellular matrix and liver disease. Antioxid. Redox Signal. 21, 1078–1097 (2014).
Pinter, M., Trauner, M., Peck-Radosavljevic, M. & Sieghart, W. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open 1, e000042 (2016).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Metcalf, K. J., Alazzeh, A., Werb, Z. & Weaver, V. M. Leveraging microenvironmental synthetic lethalities to treat cancer. J. Clin. Invest. 131, e143765 (2021).
Pfeifer, C. R., Alvey, C. M., Irianto, J. & Discher, D. E. Genome variation across cancers scales with tissue stiffness — an invasion-mutation mechanism and implications for immune cell infiltration. Curr. Opin. Syst. Biol. 2, 103–114 (2017).
Wullkopf, L. et al. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 29, 2378–2385 (2018).
Piersma, B., Hayward, M. K. & Weaver, V. M. Fibrosis and cancer: a strained relationship. Biochim. Biophys. Acta Rev. Cancer 1873, 188356 (2020).
Rice, A. J. et al. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 (2017).
Swaminathan, V. et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 71, 5075–5080 (2011).
Glentis, A. et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 8, 924 (2017).
Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Wolf, K. et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069–1084 (2013).
Matthews, H. K. et al. Oncogenic signaling alters cell shape and mechanics to facilitate cell division under confinement. Dev. Cell 52, 563–573.e3 (2020).
Nyga, A., Ganguli, S., Matthews, H. K. & Baum, B. The role of RAS oncogenes in controlling epithelial mechanics. Trends Cell Biol. 33, 60–69 (2023).
Palamidessi, A. et al. Publisher correction: unjamming overcomes kinetic and proliferation arrest in terminally differentiated cells and promotes collective motility of carcinoma. Nat. Mater. 21, 1448 (2022).
Mitchel, J. A. et al. In primary airway epithelial cells, the unjamming transition is distinct from the epithelial-to-mesenchymal transition. Nat. Commun. 11, 5053 (2020).
Stancil, I. T. et al. Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 12, 4566 (2021).
Ito, J. T. et al. Extracellular matrix component remodeling in respiratory diseases: what has been found in clinical and experimental studies? Cells 8, 342 (2019).
Martin, E. et al. Arp2/3-dependent mechanical control of morphogenetic robustness in an inherently challenging environment. Dev. Cell 56, 687–701.e7 (2021).
Villars, A., Letort, G., Valon, L. & Levayer, R. DeXtrusion: automatic recognition of epithelial cell extrusion through machine learning in vivo. Development 150, dev201747 (2023).
Tsinman, T. K. et al. Lack of skeletal muscle contraction disrupts fibrous tissue morphogenesis in the developing murine knee. J. Orthop. Res. 41, 2305–2314 (2023).
Haase, K. & Pelling, A. E. Investigating cell mechanics with atomic force microscopy. J. R. Soc. Interface 12, 20140970 (2015).
Prevedel, R., Diz-Munoz, A., Ruocco, G. & Antonacci, G. Brillouin microscopy: an emerging tool for mechanobiology. Nat. Methods 16, 969–977 (2019).
Campas, O. et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11, 183–189 (2014).
Serwane, F. et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat. Methods 14, 181–186 (2017).
Bush, J. & Maruthamuthu, V. In situ determination of exerted forces in magnetic pulling cytometry. AIP Adv. 9, 035221 (2019).
Hochmuth, R. M. Micropipette aspiration of living cells. J. Biomech. 33, 15–22 (2000).
Bufi, N., Durand-Smet, P. & Asnacios, A. Single-cell mechanics: the parallel plates technique. Methods Cell Biol. 125, 187–209 (2015).
Kong, W. et al. Experimental validation of force inference in epithelia from cell to tissue scale. Sci. Rep. 9, 14647 (2019).
Catala-Castro, F., Schaffer, E. & Krieg, M. Exploring cell and tissue mechanics with optical tweezers. J. Cell Sci. 135, jcs259355 (2022).
Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833 (2014).
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Mammoto, A., Mammoto, T. & Ingber, D. E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 (2012).
Kefauver, J. M., Ward, A. B. & Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576 (2020).
Hannezo, E. & Heisenberg, C. P. Mechanochemical feedback loops in development and disease. Cell 178, 12–25 (2019).
Aoki, K. et al. Propagating wave of ERK activation orients collective cell migration. Dev. Cell 43, 305–317.e5 (2017).
Boocock, D., Hirashima, T. & Hannezo, E. Interplay between mechanochemical patterning and glassy dynamics in cellular monolayers. PRX Life 1, 013001 (2023).
Hino, N. et al. ERK-mediated mechanochemical waves direct collective cell polarization. Dev. Cell 53, 646–660.e8 (2020).
Guilak, F., Butler, D. L., Goldstein, S. A. & Baaijens, F. P. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 47, 1933–1940 (2014).
Humphrey, J. D. & Schwartz, M. A. Vascular mechanobiology: homeostasis, adaptation, and disease. Annu. Rev. Biomed. Eng. 23, 1–27 (2021).
Tschumperlin, D. J., Boudreault, F. & Liu, F. Recent advances and new opportunities in lung mechanobiology. J. Biomech. 43, 99–107 (2010).
Chugh, M., Munjal, A. & Megason, S. G. Hydrostatic pressure as a driver of cell and tissue morphogenesis. Semin. Cell Dev. Biol. 131, 134–145 (2022).
Acknowledgements
The authors are indebted to Romain Levayer, Rashmi Priya and Yekaterina Miroshnikova for providing thoughtful advice on the manuscript. They apologize to colleagues whose work they have inadvertently failed to cite. Y.M. was supported by a Medical Research Council award MR/W027437/1, a Lister Institute Research Prize and EMBO Young Investigator Programme, and would like to thank Lin Jing Ying Lin Quan for discussions prior to writing this manuscript. The Wickström lab is supported by the Academy of Finland and Max Planck Society.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Timothy Saunders and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Blastocyst
-
A fluid-filled sphere of cells that forms during the first 5–9 days of mammalian embryonic development and generates all embryonic and extra-embryonic tissues.
- Blastoderm
-
The single layer of embryonic epithelial tissue that makes up the blastula, the early embryonic stage characterized by a hollow, spherical structure, with a fluid-filled cavity called the blastocoel.
- Cell extrusion
-
This term describes the controlled elimination or removal of cells from an epithelium while maintaining epithelial barrier integrity.
- Cortical tension
-
This describes the sustained contraction of the cortical cytoskeleton. It is largely but not exclusively based on actomyosin contraction and depends on the density of the cortex as well as on its structure and composition.
- Emergent properties
-
New property or behaviour of a system that results from the combination of or interaction between two or more different components or processes, none of which displayed the behaviour individually.
- Friction
-
A force that resists motion when the surface of one object (such as a cell) comes into contact with the surface of another object (for example, a cell or extracellular matrix). In cells, this force is typically generated by adhesion molecules.
- Interfacial tension
-
The tension at the boundary between two objects such as a junctional interface between two cells.
- Presomitic mesoderm
-
This is a region of the embryo also known as paraxial or somitic mesoderm that flanks the neural tube and gives rise to somites.
- Shear stress
-
A stress that is applied parallel or tangential to the surface of a material, as opposed to stress that is applied perpendicularly.
- Tensile forces
-
A force that has two components — tensile stress and tensile strain — that act on a material to stretch it while it is under tension.
- Ventral furrow
-
This is an invagination generated by the first large-scale morphogenetic movement in the Drosophila melanogaster embryo, where the morphogenetic movement transforms a single layer of columnar epithelial cells into a multi-layered structure by triggering internalization of the most ventrally positioned cells of the embryonic epithelium.
- Vertex models
-
A type of statistical mechanics model used to model the behaviour of adherent cell collectives, mostly epithelia. In vertex models, cell shape is represented by a set of vertices that mark the common point of three or more neighbouring cells and on which forces from within cells and in between cells act. These models can be two-dimensional or three-dimensional.
- Wetting force
-
An adhesive force between a liquid and a solid, resulting from intermolecular interactions between the two and keeping the surfaces of both materials in contact with each other.
- Yield strength
-
The stress at which a material ceases elastic deformation and undergoes plastic, permanent deformation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, Y., Wickström, S.A. Mechanical state transitions in the regulation of tissue form and function. Nat Rev Mol Cell Biol 25, 654–670 (2024). https://doi.org/10.1038/s41580-024-00719-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-024-00719-x
This article is cited by
-
Tissues pushing on
Nature Materials (2024)