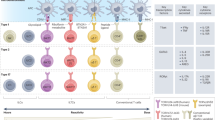
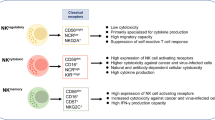
Abstract
Immuno-oncology is an emerging field that has revolutionized cancer treatment. Most immunomodulatory strategies focus on enhancing T cell responses, but there has been a recent surge of interest in harnessing the relatively underexplored natural killer (NK) cell compartment for therapeutic interventions. NK cells show cytotoxic activity against diverse tumour cell types, and some of the clinical approaches originally developed to increase T cell cytotoxicity may also activate NK cells. Moreover, increasing numbers of studies have identified novel methods for increasing NK cell antitumour immunity and expanding NK cell populations ex vivo, thereby paving the way for a new generation of anticancer immunotherapies. The role of other innate lymphoid cells (group 1 innate lymphoid cell (ILC1), ILC2 and ILC3 subsets) in tumours is also being actively explored. This Review provides an overview of the field and summarizes current immunotherapeutic approaches for solid tumours and haematological malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bottcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037 (2018).
Pallmer, K. & Oxenius, A. Recognition and regulation of T cells by NK cells. Front. Immunol. 7, 251 (2016).
Ferlazzo, G. & Moretta, L. Dendritic cell editing by natural killer cells. Crit. Rev. Oncog. 19, 67–75 (2014).
Morandi, B. et al. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLOS One 7, e39170 (2012).
Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018).
Mirchandani, A. S. et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 192, 2442–2448 (2014).
Saranchova, I. et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci. Rep. 8, 2924 (2018).
Robinette, M. L. & Colonna, M. Innate lymphoid cells and the MHC. HLA 87, 5–11 (2016).
Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000). This publication demonstrates that perforin-mediated tumour cell lysis controls in vivo tumour growth.
Street, S. E. et al. Host perforin reduces tumor number but does not increase survival in oncogene-driven mammary adenocarcinoma. Cancer Res. 67, 5454–5460 (2007).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Smyth, M. J., Crowe, N. Y. & Godfrey, D. I. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 13, 459–463 (2001).
Smyth, M. J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Glasner, A. et al. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J. Immunol. 188, 2509–2515 (2012).
Halfteck, G. G. et al. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J. Immunol. 182, 2221–2230 (2009).
Glasner, A. et al. NKp46 receptor-mediated interferon-γ production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity 48, 107–119 (2018).
Finnberg, N., Klein-Szanto, A. J. & El-Deiry, W. S. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J. Clin. Invest. 118, 111–123 (2008).
Ebbo, M. et al. Low circulating natural killer cell counts are associated with severe disease in patients with common variable immunodeficiency. EBioMedicine 6, 222–230 (2016).
Brittenden, J., Heys, S. D., Ross, J. & Eremin, O. Natural killer cells and cancer. Cancer 77, 1226–1243 (1996).
Schantz, S. P., Campbell, B. H. & Guillamondegui, O. M. Pharyngeal carcinoma and natural killer cell activity. Am. J. Surg. 152, 467–474 (1986).
Schantz, S. P. & Ordonez, N. G. Quantitation of natural killer cell function and risk of metastatic poorly differentiated head and neck cancer. Nat. Immun. Cell Growth Regul. 10, 278–288 (1991).
Schantz, S. P., Savage, H. E., Racz, T., Taylor, D. L. & Sacks, P. G. Natural killer cells and metastases from pharyngeal carcinoma. Am. J. Surg. 158, 361–366 (1989).
Pross, H. F. & Lotzova, E. Role of natural killer cells in cancer. Nat. Immun. 12, 279–292 (1993).
Imai, K., Matsuyama, S., Miyake, S., Suga, K. & Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799 (2000).
Tartter, P. I., Steinberg, B., Barron, D. M. & Martinelli, G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch. Surg. 122, 1264–1268 (1987).
Garcia-Iglesias, T. et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 9, 186 (2009).
Eckl, J. et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med. 90, 55–66 (2012).
Platonova, S. et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 71, 5412–5422 (2011).
Carrega, P. et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875 (2008).
Schleypen, J. S. et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int. J. Cancer 106, 905–912 (2003).
Schleypen, J. S. et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 12, 718–725 (2006).
Faveeuw, C., Di Mauro, M. E., Price, A. A. & Ager, A. Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int. Immunol. 12, 241–251 (2000).
Halama, N. et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin. Cancer Res. 17, 678–689 (2011).
Molgora, M. et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114 (2017).
Delconte, R. B. et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol. 17, 816–824 (2016).
Paolino, M. et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512 (2014).
Ruggeri, L. et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 94, 333–339 (1999).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002). References 37 and 38 describe the occurrence of NK cell alloreactivity against AML blasts after HLA-mismatched transplantation, which occurred without exacerbating graft-versus-host disease.
Hsu, K. C. et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 105, 4878–4884 (2005).
Cooley, S. et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116, 2411–2419 (2010).
Stringaris, K. et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 99, 836–847 (2014).
Sarkar, S. et al. Optimal selection of natural killer cells to kill myeloma: the role of HLA-E and NKG2A. Cancer Immunol. Immunother. 64, 951–963 (2015).
Hejazi, M. et al. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica 100, 643–652 (2015).
Coles, S. J. et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 25, 792–799 (2011).
Benson, D. M. Jr et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010).
Vari, F. et al. Immune evasion via PD-1/PD-L1 on NK-cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 131, 1809–1819 (2018).
Cifaldi, L. et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 67, 3037–3046 (2015).
Ponzetta, A. et al. Multiple myeloma impairs bone marrow localization of effector natural killer cells by altering the chemokine microenvironment. Cancer Res. 75, 4766–4777 (2015).
Costello, R. T. et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 99, 3661–3667 (2002).
Hughes, A. et al. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 129, 1166–1176 (2017).
Fauriat, C. et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109, 323–330 (2007).
Chretien, A. S. et al. NKp46 expression on NK cells as a prognostic and predictive biomarker for response to allo-SCT in patients with AML. Oncoimmunology 6, e1307491 (2017).
Chretien, A. S. et al. NKp30 expression is a prognostic immune biomarker for stratification of patients with intermediate-risk acute myeloid leukemia. Oncotarget 8, 49548–49563 (2017).
Sanchez-Correa, B. et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 90, 109–115 (2012).
Carlsten, M. et al. Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia 24, 1607–1616 (2010).
Torelli, G. F. et al. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica 99, 1248–1254 (2014).
Szczepanski, M. J., Szajnik, M., Welsh, A., Whiteside, T. L. & Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 96, 1302–1309 (2011).
Hilpert, J. et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J. Immunol. 189, 1360–1371 (2012).
Epling-Burnette, P. K. et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 109, 4816–4824 (2007).
Jinushi, M. et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl Acad. Sci. USA 105, 1285–1290 (2008).
Zocchi, M. R. et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood 119, 1479–1489 (2012).
Ferrari de Andrade, L. et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 359, 1537–1542 (2018).
Reiners, K. S. et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 121, 3658–3665 (2013).
Baessler, T. et al. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood 115, 3058–3069 (2010).
Nuebling, T. et al. The immune checkpoint modulator OX40 and its ligand OX40L in NK-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol. Res. 6, 209–221 (2018).
Riether, C. et al. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 214, 359–380 (2017).
Simoni, Y. et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46, 148–161 (2017).
Carrega, P. et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 6, 8280 (2015).
Dieu-Nosjean, M. C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26, 4410–4417 (2008).
Kirchberger, S. et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931 (2013).
Irshad, S. et al. RORγt(+) innate lymphoid cells promote lymph node metastasis of breast cancers. Cancer Res. 77, 1083–1096 (2017).
Munneke, J. M. et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 124, 812–821 (2014). This article suggests that activated ILCs following HSCT can protect against graft-versus-host disease.
Trabanelli, S. et al. CD127+ innate lymphoid cells are dysregulated in treatment naive acute myeloid leukemia patients at diagnosis. Haematologica 100, e257–e260 (2015).
de Weerdt, I. et al. Innate lymphoid cells are expanded and functionally altered in chronic lymphocytic leukemia. Haematologica 101, e461–e464 (2016).
Kini Bailur, J. et al. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: target for IMiD therapy. Blood Adv. 1, 2343–2347 (2017).
Romano, M. et al. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology 6, e1345402 (2017).
Trabanelli, S. et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 8, 593 (2017). This article deciphers a new tolerogenic pathway involving interactions between PGD 2 and B7-H6 on tumour cells and CRTH2 and NKp30 on ILC2s, which in turn activate MDSCs via IL-13 secretion, inducing an immunosuppressive microenvironment that facilitates tumour immunoescape.
Dadi, S. et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377 (2016).
Mlecnik, B. et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl Med. 6, 228ra37 (2014).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Cortez, V. S. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 18, 995–1003 (2017). Publications 80 and 81 describe a novel mechanism of TGFβ-driven tumour immune escape.
Stanietsky, N. et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl Acad. Sci. USA 106, 17858–17863 (2009).
Otegbeye, F. et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLOS One 13, e0191358 (2018).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity 38, 769–781 (2013).
Karvellas, C. J., Fedorak, R. N., Hanson, J. & Wong, C. K. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can. J. Gastroenterol. 21, 443–446 (2007).
Levin, A. D., Wildenberg, M. E. & van den Brink, G. R. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J. Crohns Colitis 10, 989–997 (2016).
Popivanova, B. K. et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 118, 560–570 (2008).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Goldszmid, R. S. et al. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36, 1047–1059 (2012).
Bie, Q. et al. Polarization of ILC2s in peripheral blood might contribute to immunosuppressive microenvironment in patients with gastric cancer. J. Immunol. Res. 2014, 923135 (2014).
Di Stefano, A. B. et al. Survivin is regulated by interleukin-4 in colon cancer stem cells. J. Cell. Physiol. 225, 555–561 (2010).
Zhou, R., Qian, S., Gu, X., Chen, Z. & Xiang, J. Interleukin-13 and its receptors in colorectal cancer (Review). Biomed. Rep. 1, 687–690 (2013).
Jovanovic, I. P. et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 134, 1669–1682 (2014).
Tomas, A., Futter, C. E. & Eden, E. R. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 24, 26–34 (2014).
Hams, E. et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl Acad. Sci. USA 111, 367–372 (2014).
McHedlidze, T. et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371 (2013).
Lim, A. I. et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med. 213, 569–583 (2016).
Ikutani, M. et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 188, 703–713 (2012).
Eisenring, M., vom Berg, J., Kristiansen, G., Saller, E. & Becher, B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat. Immunol. 11, 1030–1038 (2010). This article provides evidence that a non-NK ILC population can control tumour growth.
Ebbo, M., Crinier, A., Vely, F. & Vivier, E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 17, 665–678 (2017).
Chan, I. H. et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 7, 842–856 (2014).
Jiang, R. et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 13, 59 (2013).
Roy, S. & Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285 (2017).
Wu, T. et al. Elevated serum IL-22 levels correlate with chemoresistant condition of colorectal cancer. Clin. Immunol. 147, 38–39 (2013).
Hernandez, P., Gronke, K. & Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 48, 15–31 (2018).
Crome, S. Q. et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat. Med. 23, 368–375 (2017).
Romagne, F. et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 (2009).
Kohrt, H. E. et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 123, 678–686 (2014).
Benson, D. M. Jr et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 118, 6387–6391 (2011).
Fernandez, N. C. et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105, 4416–4423 (2005).
Kim, S. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005).
Anfossi, N. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006). This article indicates that NK cells lacking inhibitory KIRs for self MHC class I are hyporesponsive against MHC class I-deficient cells, suggesting that KIR–MHC class I interactions are crucial for NK cell education in humans.
Carlsten, M. et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin. Cancer Res. 22, 5211–5222 (2016).
Mamessier, E. et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 121, 3609–3622 (2011).
Collins, S. M. et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 62, 1841–1849 (2013).
Bezman, N. A. et al. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv. 1, 753–765 (2017).
Hagner, P. R. et al. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br. J. Haematol. 179, 399–409 (2017).
Zitvogel, L., Rusakiewicz, S., Routy, B., Ayyoub, M. & Kroemer, G. Immunological off-target effects of imatinib. Nat. Rev. Clin. Oncol. 13, 431–446 (2016).
Kreutzman, A. et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 116, 772–782 (2010).
Vergoulidou, M. More than a decade of tyrosine kinase inhibitors in the treatment of solid tumors: what we have learned and what the future holds. Biomark. Insights 10 (Suppl. 3), 33–40 (2015).
Beatty, G. L. et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin. Cancer Res. 23, 3269–3276 (2017).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 484, 529–533 (2012).
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22, 680–690 (2016).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).
Mao, C. et al. Interleukin-2 as maintenance therapy for children and adults with acute myeloid leukaemia in first complete remission. Cochrane Database Syst. Rev. 11, CD010248 (2015).
Szczepanski, M. J. et al. Interleukin-15 enhances natural killer cell cytotoxicity in patients with acute myeloid leukemia by upregulating the activating NK cell receptors. Cancer Immunol. Immunother. 59, 73–79 (2010).
Wagner, J. A. et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Invest. 127, 4042–4058 (2017).
Felices, M. et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3, e96219 (2018).
Gleason, M. K. et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 123, 3016–3026 (2014).
Vallera, D. A. et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res. 22, 3440–3450 (2016).
Geller, M. A. et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 13, 98–107 (2011).
Granzin, M. et al. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front. Immunol. 8, 458 (2017).
Chu, J. et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 28, 917–927 (2014).
Jiang, H. et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 8, 297–310 (2014).
Kloss, S. et al. Optimization of human NK cell manufacturing: fully automated separation, improved ex vivo expansion using IL-21 with autologous feeder cells, and generation of anti-CD123-CAR-expressing effector cells. Hum. Gene Ther. 28, 897–913 (2017).
Chang, Y. H. et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 73, 1777–1786 (2013).
O’Sullivan, T. et al. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 7, 989–998 (2014).
Tang, H. et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 (2016).
Putz, E. M. et al. NK cell heparanase controls tumor invasion and immune surveillance. J. Clin. Invest. 127, 2777–2788 (2017).
Makkouk, A. et al. Characterizing CD137 upregulation on NK cells in patients receiving monoclonal antibody therapy. Ann. Oncol. 28, 415–420 (2017).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293 (2015).
Moretta, L. et al. Surface NK receptors and their ligands on tumor cells. Semin. Immunol. 18, 151–158 (2006).
Moretta, A. et al. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 14, 619–648 (1996).
Ortaldo, J.R. & Young, H.A. Mouse Ly49 NK receptors: balancing activation and inhibition. Molecular Immunology 42, 445–450 (2005).
Tomasello, E. et al. Mapping of NKp46+ cells in healthy human lymphoid and non-lymphoid tissues. Front. Immunol. 3, 344 (2012).
Narni-Mancinelli, E. et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl Acad. Sci. 108, 18324–18329 (2011).
Glatzer, T. et al. RORγt+ innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity 38, 1223–1235 (2013).
Killig, M., Glatzer, T. & Romagnani, C. Recognition strategies of group 3 innate lymphoid cells. Front. Immunol. 5, 142 (2014).
Salimi, M. et al. Group 2 innate lymphoid cells express functional NKp30 receptor inducing type 2 cytokine production. J. Immunol. 196, 45–54 (2016).
Vely, F. et al. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 17, 1291–1299 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Lee, J. C., Lee, K. M., Kim, D. W. & Heo, D. S. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J. Immunol. 172, 7335–7340 (2004).
Cortez, V. S. et al. Transforming growth factor-β signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity 44, 1127–1139 (2016).
Vitale, M., Cantoni, C., Pietra, G., Mingari, M. C. & Moretta, L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 44, 1582–1592 (2014).
Zingoni, A. et al. Targeting NKG2D and NKp30 ligands shedding to improve NK cell-based immunotherapy. Crit. Rev. Immunol. 36, 445–460 (2016).
Wang, W. et al. Tumor-released Galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J. Biol. Chem. 289, 33311–33319 (2014).
Xiao, Q. et al. DKK2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med. 24, 262–270 (2018).
Chiossone, L., Vienne, M., Kerdiles, Y. M. & Vivier, E. Natural killer cell immunotherapies against cancer: checkpoint inhibitors and more. Semin. Immunol. 31, 55–63 (2017).
Krzywinska, E. et al. Loss of HIF-1alpha in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat. Commun. 8, 1597 (2017).
Spranger, S. & Gajewski, T. F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 18, 139–147 (2018).
Lopez-Soto, A. et al. Epithelial-mesenchymal transition induces an antitumor immune response mediated by NKG2D receptor. J. Immunol. 190, 4408–4419 (2013).
Sathe, P. et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat. Commun. 5, 4539 (2014).
Ishigami, S. et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 88, 577–583 (2000).
Coca, S. et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79, 2320–2328 (1997).
Donskov, F. & von der Maase, H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 24, 1997–2005 (2006).
Pasero, C. et al. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 76, 2153–2165 (2016).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540, 588–592 (2016).
Lopez-Soto, A., Gonzalez, S., Smyth, M. J. & Galluzzi, L. Control of metastasis by NK cells. Cancer Cell 32, 135–154 (2017).
Maurer, S. et al. Platelet-mediated shedding of NKG2D ligands impairs NK cell immune-surveillance of tumor cells. Oncoimmunology 7, e1364827 (2018).
Bastid, J. et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol. Res. 3, 254–265 (2015).
Gao, Z. W., Dong, K. & Zhang, H. Z. The roles of CD73 in cancer. Biomed. Res. Int. 2014, 460654 (2014).
Lee, H. et al. A novel pro-angiogenic function for interferon-γ-secreting natural killer cells. Invest. Ophthalmol. Vis. Sci. 55, 2885–2892 (2014).
Taylor, S. et al. PD-1 regulates KLRG1+ group 2 innate lymphoid cells. J. Exp. Med. 214, 1663–1678 (2017).
Maazi, H. et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42, 538–551 (2015).
Barrow, A. D. et al. Natural killer cells control tumor growth by sensing a growth factor. Cell 172, 534–548 (2018).
Pende, D. et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 105, 2066–2073 (2005).
Mastaglio, S. et al. Natural killer receptor ligand expression on acute myeloid leukemia impacts survival and relapse after chemotherapy. Blood Adv. 2, 335–346 (2018).
Salih, H. R. et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 102, 1389–1396 (2003).
Chretien, A. S. et al. Natural killer defective maturation is associated with adverse clinical outcome in patients with acute myeloid leukemia. Front. Immunol. 8, 573 (2017).
Chretien, A. S. et al. Increased NK cell maturation in patients with acute myeloid leukemia. Front. Immunol. 6, 564 (2015).
Rea, D. et al. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica 102, 1368–1377 (2017).
Hara, R. et al. NKG2D gene polymorphisms are associated with disease control of chronic myeloid leukemia by dasatinib. Int. J. Hematol. 106, 666–674 (2017).
Boissel, N. et al. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J. Immunol. 176, 5108–5116 (2006).
El-Sherbiny, Y. M. et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 67, 8444–8449 (2007).
Costello, R. T. et al. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology 139, 338–341 (2013).
Acknowledgements
The laboratory of E.V. is supported by funding from the European Research Council (ERC) under the European Union Horizon 2020 research and innovation programme (Targeting innate lymphoid cells (TILC), grant agreement number 694502); the Agence Nationale de la Recherche, Equipe Labellisée “La Ligue”, Ligue Nationale contre le Cancer, MSDAvenir, Innate Pharma and institutional grants to the CIML (Institut National Français de Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS) and Aix-Marseille University) and to Marseille Immunopôle. P.-Y.D. is a fellow of the Fondation de France. M.V. is a recipient of an individual PhD grant from the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation. The authors thank M. Blery, Y. Morel and S. Cornen for helpful comments.
Reviewer information
Nature Reviews Immunology thanks M. Smyth and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
E.V., L.C., P.-Y.D. and M.V. contributed to researching data, discussion of content and the writing of this article. E.V., L.C. and P.-Y.D. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.C. and E.V. are employees of Innate Pharma. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Innate Pharma: https://innate-pharma.com/fr/portefeuille/technologie-anticorps-bispecifiques-engageant-cellules-nk
Supplementary information
Glossary
- Asialo-GM1
-
The cell surface glycolipid GM1 with its sialic acid groups removed. Asiolo-GM1 is expressed by natural killer cells and also by a subset of T cells and myeloid cells.
- NK1.1
-
An activating C-type lectin receptor that is expressed by natural killer cells and natural killer T cells in C57Bl/6 mice.
- Natural killer cell p46-related protein
-
(NKp46). An activating receptor that is expressed by human and mouse natural killer cells and subsets of group 1 innate lymphoid cells (ILC1s) and ILC3s.
- Killer cell immunoglobulin-like receptors
-
(KIRs). A family of receptors (including CD158) for MHC class I molecules that are expressed on natural killer (NK) cells and a subset of T cells. They regulate NK cell activation and tolerance.
- Leukocyte immunoglobulin-like receptor subfamily B member 1
-
(LIR1). A receptor for a broad range of MHC class I molecules that is expressed by monocytes, natural killer cells, T cells and B cells. Engagement of LIR1 results in inhibitory immune signalling.
- The ‘missing ligand’ model
-
A model that postulates that a natural killer (NK) cell-mediated graft-versus-leukaemia effect will also occur when the donor NK cells express an inhibitory killer cell immunoglobulin-like receptor (KIR) for which neither donor nor recipient expresses a relevant MHC class I ligand.
- The ‘donor haplotype’ model
-
A model suggesting that assessing donor killer cell immunoglobulin-like receptor (KIR) haplotypes is important for determining the efficacy of haematopoietic stem cell transplantation; KIR gene clusters include haplotype A, which contains predominantly inhibitory KIRs, and haplotype B, whose members are more diverse.
- CD57
-
A suggested marker for replicative senescence. CD57 is absent on fetal natural killer (NK) cells, increases with age and defines a subpopulation of highly differentiated circulating NK cells.
- Natural cytotoxicity receptors
-
(NCRs). A family of activating receptors (including natural killer cell p30-related protein (NKp30), NKp44 and NKp46) that is selectively expressed by innate lymphoid cells.
- Pomalidomide
-
An anti-angiogenic and immunomodulatory drug that is a derivative of thalidomide.
- Myeloid-derived suppressor cells
-
(MDSCs). A heterogeneous population of immature myeloid cells that rapidly expand during inflammation and are able to downregulate immune responses.
- Amphiregulin
-
A member of the epidermal growth factor (EGF) family that signals through the EGF receptor (EGFR).
- Bispecific killer cell engagers
-
(BiKEs). Bispecific monoclonal antibodies that bind activating natural killer cell receptors at one end and tumour antigens at the other.
- Trispecific killer cell engagers
-
(TriKEs). Molecules containing two antibody fragments, which are directed against an activating natural killer cell receptor and a tumour antigen, and an immune stimulatory cytokine crosslinker.
- Rituximab
-
A cytotoxic antibody directed against CD20 that destroys both normal and malignant B cells and is therefore used to treat diseases characterized by having too many B cells.
- Lenalidomide
-
A derivative of thalidomide that inhibits tumour angiogenesis and tumour cell proliferation.
- Durvalumab
-
An antibody that blocks programmed cell death 1 ligand 1 (PDL1).
- Cetuximab
-
An antibody that blocks epidermal growth factor receptor (EGFR).
- ALT803
-
An IL-15–IL-15 receptor subunit-α (IL-15Rα) complex fused to an IgG1 crystallizable fragment (Fc) domain; in this construct, IL-15 is additionally mutated to increase its biological activity.
- Melphalan
-
A chemotherapy drug that acts as an alkylating agent.
Rights and permissions
About this article
Cite this article
Chiossone, L., Dumas, PY., Vienne, M. et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18, 671–688 (2018). https://doi.org/10.1038/s41577-018-0061-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-018-0061-z