Key Points
-
The term dysbiosis has been used to describe disruptions of microbial communities that lead to a shift in steady-state composition that is distinct from that induced by infections. Recent work has suggested that, in addition to bacterial dysbiosis, fungal dysbiosis might contribute to the pathology of several immune-mediated conditions of non-infectious origin.
-
Fungal dysbiosis is observed in human diseases affecting different barrier surfaces, including the mouth, vagina, skin, lungs and gut.
-
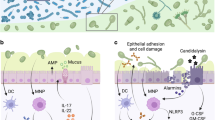
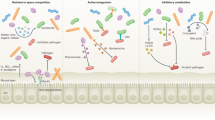
The mucosal immune system can respond to changes in fungal communities; several antifungal immunity pathways such as C-type lectin receptors, and the IL-1β and inflammasome pathways, might have a role in sensing these fluctuations.
-
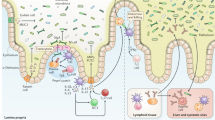
The interaction between fungi and the host immune system has mainly been studied in the context of infection. However, fungal communities reside on the barrier surfaces of various mammals and are dynamic and responsive to environmental and pathophysiological changes.
-
The mammalian gut is a unique site in which fungal infections are rare but fungal dysbiosis occurs frequently.
-
Dysbiosis probably affects all communities of the microbiota, including bacterial and fungal species.
Abstract
Fungi and mammals share a co-evolutionary history and are involved in a complex web of interactions. Studies focused on commensal bacteria suggest that pathological changes in the microbiota, historically known as dysbiosis, are at the root of many inflammatory diseases of non-infectious origin. However, the importance of dysbiosis in the fungal community — the mycobiota — was only recently acknowledged to have a pathological role, as novel findings have suggested that mycobiota disruption can have detrimental effects on host immunity. Fungal dysbiosis and homeostasis are dynamic processes that are probably more common than actual fungal infections, and therefore constantly shape the immune response. In this Review, we summarize specific mycobiota patterns that are associated with fungal dysbiosis, and discuss how mucosal immunity has evolved to distinguish fungal infections from dysbiosis and how it responds to these different conditions. We propose that gut microbiota dysbiosis is a collective feature of complex interactions between prokaryotic and eukaryotic microbial communities that can affect immunity and that can influence health and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Peay, K. G., Kennedy, P. G. & Talbot, J. M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 14, 434–447 (2016).
Remy, W., Taylor, T. N., Hass, H. & Kerp, H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl Acad. Sci. USA 91, 11841–11843 (1994).
Wainright, P. O., Hinkle, G., Sogin, M. L. & Stickel, S. K. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science 260, 340–342 (1993).
Casadevall, A. Fungi and the rise of mammals. PLoS Pathog. 8, e1002808 (2012).
Brown, G. D. & Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 19, 311–315 (2003).
Fisher, M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 (2012).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl Med. 4, 165rv13 (2012).
Lanternier, F. et al. Deep dermatophytosis and inherited CARD9 deficiency. N. Engl. J. Med. 369, 1704–1714 (2013).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013). This paper describes the presence and diversity of Malassezia species in the human skin and is by far the most comprehensive culture-independent study of the skin mycobiome.
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
Solomon, K. V. et al. Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science 351, 1192–1195 (2016).
Belkaid, Y. & Harrison, O. J. Homeostatic immunity and the microbiota. Immunity 46, 562–576 (2017).
Dollive, S. et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS ONE 8, e71806 (2013).
Hoffmann, C. et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE 8, e66019 (2013).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014). This study shows that the human gut microbiota, including fungi, bacteria and viruses, changes rapidly in response to a diet consisting entirely of either animal products or plant products.
Iliev, I. D. et al. Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science 336, 1314–1317 (2012).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2016). This study used next-generation sequencing to simultaneously characterize the faecal fungal and bacterial microbiota in patients with ulcerative colitis and Crohn's disease.
Metchnikoff, E. The Prolongation of Life: Optimistic Studies 161–183 (G. P. Putnam's Sons, 1908).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
Marsland, B. J. & Gollwitzer, E. S. Host-microorganism interactions in lung diseases. Nat. Rev. Immunol. 14, 827–835 (2014).
Lewis, J. D. et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn's disease. Cell Host Microbe 18, 489–500 (2015). This article shows that inflammation, antibiotics and diet independently affect the gut microbiota in patients with Crohn's disease, and identifies an association between antibiotic use and increased fungal load.
Liguori, G. et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn's disease patients. J. Crohns Colitis 10, 296–305 (2016).
Hoarau, G. et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn's disease. mBio 7, e01250-16 (2016).
Wheeler, M. L. et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19, 865–873 (2016). This study shows that targeted fungal community dysbiosis in the mouse gut can have local and systemic effects on immunity and inflammation.
Ghannoum, M. A. et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6, e1000713 (2010).
Bittinger, K. et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol. 15, 487 (2014).
Lindahl, B. D. et al. Fungal community analysis by high-throughput sequencing of amplified markers — a user's guide. New Phytol. 199, 288–299 (2013).
Tang, J., Iliev, I. D., Brown, J., Underhill, D. M. & Funari, V. A. Mycobiome: approaches to analysis of intestinal fungi. J. Immunol. Methods 421, 112–121 (2015).
Underhill, D. M. & Iliev, I. D. The mycobiota: interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 14, 405–416 (2014).
Motooka, D. et al. Fungal ITS1 deep-sequencing strategies to reconstruct the composition of a 26-species community and evaluation of the gut mycobiota of healthy Japanese individuals. Front. Microbiol. 8, 238 (2017).
Krasner, R. I., Young, G. & Yudkofsky, P. L. Interactions of oral strains of Candida albicans and lactobacilli. J. Bacteriol. 72, 525–529 (1956).
Mukherjee, P. K. et al. Oral mycobiome analysis of HIV-infected patients: identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 10, e1003996 (2014).
Hallen-Adams, H. E. & Suhr, M. J. Fungi in the healthy human gastrointestinal tract. Virulence 8, 352–358 (2017).
Suhr, M. J., Banjara, N. & Hallen-Adams, H. E. Sequence-based methods for detecting and evaluating the human gut mycobiome. Lett. Appl. Microbiol. 62, 209–215 (2016).
Cutler, J. E., Deepe, G. S. Jr & Klein, B. S. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5, 13–28 (2007).
Luan, C. et al. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci. Rep. 5, 7980 (2015).
Li, Q. et al. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn's disease. J. Clin. Gastroenterol. 48, 513–523 (2014).
Kirsner, J. B. Historical origins of current IBD concepts. World J. Gastroenterol. 7, 175–184 (2001).
Tamboli, C. P., Neut, C., Desreumaux, P. & Colombel, J. F. Dysbiosis in inflammatory bowel disease. Gut 53, 1–4 (2004).
Ott, S. J. et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand. J. Gastroenterol. 43, 831–841 (2008).
Qiu, X. et al. Changes in the composition of intestinal fungi and their role in mice with dextran sulfate sodium-induced colitis. Sci. Rep. 5, 10416 (2015).
Chehoud, C. et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1948–1956 (2015).
Israeli, E. et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 54, 1232–1236 (2005).
Standaert-Vitse, A. et al. Candida albicans colonization and ASCA in familial Crohn's disease. Am. J. Gastroenterol. 104, 1745–1753 (2009).
Schaffer, T. et al. Anti-Saccharomyces cerevisiae mannan antibodies (ASCA) of Crohn's patients crossreact with mannan from other yeast strains, and murine ASCA IgM can be experimentally induced with Candida albicans. Inflamm. Bowel Dis. 13, 1339–1346 (2007).
Sharon, G., Sampson, T. R., Geschwind, D. H. & Mazmanian, S. K. The central nervous system and the gut microbiome. Cell 167, 915–932 (2016).
Strati, F. et al. Altered gut microbiota in Rett syndrome. Microbiome 4, 41 (2016).
Strati, F. et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 24 (2017).
Charlson, E. S. et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am. J. Respir. Crit. Care Med. 186, 536–545 (2012).
Delhaes, L. et al. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community — implications for therapeutic management. PLoS ONE 7, e36313 (2012).
Kim, S. H. et al. Global analysis of the fungal microbiome in cystic fibrosis patients reveals loss of function of the transcriptional repressor Nrg1 as a mechanism of pathogen adaptation. PLoS Pathog. 11, e1005308 (2015). This paper shows the presence of stable fungal communities in the lungs of patients with cystic fibrosis and identifies mutations in the transcriptional repressor NRG1 in C. albicans that seem to be a common mechanism of adaptation.
Willger, S. D. et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2, 40 (2014).
van Woerden, H. C. et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect. Dis. 13, 69 (2013).
Noverr, M. C., Noggle, R. M., Toews, G. B. & Huffnagle, G. B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72, 4996–5003 (2004).
Kim, Y. G. et al. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe 15, 95–102 (2014).
Farr, A. et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet. Gynecol. Scand. 94, 989–996 (2015).
Drell, T. et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 8, e54379 (2013).
Merenstein, D. et al. Colonization by Candida species of the oral and vaginal mucosa in HIV-infected and noninfected women. AIDS Res. Hum. Retroviruses 29, 30–34 (2013).
Guo, R. et al. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb. Ecol. 64, 918–927 (2012).
Oh, J. et al. Temporal stability of the human skin microbiome. Cell 165, 854–866 (2016).
Oh, J. et al. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res. 23, 2103–2114 (2013).
Smeekens, S. P. et al. Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J. Innate Immun. 6, 253–262 (2014).
Kalan, L. et al. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio 7, e01058-16 (2016). This study shows that fungal communities in diabetic foot ulcers are associated with poor outcomes and longer healing times.
Strati, F. et al. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front. Microbiol. 7, 1227 (2016).
Jo, J. H. et al. Diverse human skin fungal communities in children converge in adulthood. J. Invest. Dermatol. 136, 2356–2363 (2016).
Conti, H. R. et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311 (2009).
Hise, A. G. et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009).
De Luca, A. et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 9, e1003486 (2013).
Kashem, S. W. et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 42, 356–366 (2015).
Ferwerda, B. et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 (2009).
Glocker, E. O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009).
Gessner, M. A. et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect. Immun. 80, 410–417 (2012).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Jhingran, A. et al. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 11, e1004589 (2015).
Rivera, A. et al. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 208, 369–381 (2011).
Trautwein-Weidner, K. et al. Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis. PLoS Pathog. 11, e1005164 (2015).
Lionakis, M. S. et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J. Clin. Invest. 123, 5035–5051 (2013).
Conti, H. R. et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20, 606–617 (2016).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Netea, M. G., Joosten, L. A., van der Meer, J. W., Kullberg, B. J. & van de Veerdonk, F. L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 15, 630–642 (2015).
Plato, A., Hardison, S. E. & Brown, G. D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 37, 97–106 (2015).
Taylor, P. R. et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 (2007).
Marakalala, M. J. et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 9, e1003315 (2013).
Tang, C. et al. Inhibition of dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 18, 183–197 (2015).
Moyes, D. L. et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532, 64–68 (2016). This study identifies a C. albicans toxin that directly disrupts the epithelial layer, triggers a danger response and leads to epithelial activation.
Pierce, J. V. & Kumamoto, C. A. Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. mBio 3, e00117-12 (2012).
de Vries, H. S. et al. Genetic association analysis of the functional c.714T>G polymorphism and mucosal expression of dectin-1 in inflammatory bowel disease. PLoS ONE 4, e7818 (2009).
Plantinga, T. S. et al. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 49, 724–732 (2009).
van der Velden, W. J. et al. Role of the mycobiome in human acute graft-versus-host disease. Biol. Blood Marrow Transplant. 19, 329–332 (2013).
Saijo, S. et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010).
Wuthrich, M. et al. Fonsecaea pedrosoi-induced Th17-cell differentiation in mice is fostered by dectin-2 and suppressed by mincle recognition. Eur. J. Immunol. 45, 2542–2552 (2015).
Ishikawa, T. et al. Identification of distinct ligands for the C-type lectin receptors mincle and dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 13, 477–488 (2013).
Barrett, N. A., Maekawa, A., Rahman, O. M., Austen, K. F. & Kanaoka, Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182, 1119–1128 (2009).
Nakamura, Y. et al. Dectin-2 deficiency promotes Th2 response and mucin production in the lungs after pulmonary infection with Cryptococcus neoformans. Infect. Immun. 83, 671–681 (2015).
Hu, X. P. et al. Dectin-2 polymorphism associated with pulmonary cryptococcosis in HIV-uninfected Chinese patients. Med. Mycol. 53, 810–816 (2015).
Zhao, X. Q. et al. C-Type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-kappaB activation. J. Biol. Chem. 289, 30052–30062 (2014).
Zhu, L. L. et al. C-Type lectin receptors dectin-3 and dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39, 324–334 (2013).
Wang, T. et al. Dectin-3 deficiency promotes colitis development due to impaired antifungal innate immune responses in the gut. PLoS Pathog. 12, e1005662 (2016).
LeibundGut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638 (2007).
Bishu, S. et al. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect. Immun. 82, 1173–1180 (2014).
Drummond, R. A. et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 11, e1005293 (2015).
Wang, X. et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J. Allergy Clin. Immunol. 133, 905–908.e3 (2014).
Lanternier, F. et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J. Allergy Clin. Immunol. 135, 1558–1568.e2 (2015).
International HapMap Consortium et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Frazer, K. A. et al. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Sokol, H. et al. Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology 145, 591–601.e3 (2013).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Drummond, R. A. & Lionakis, M. S. Mechanistic insights into the role of C-type lectin receptor/CARD9 signaling in human antifungal immunity. Front. Cell. Infect. Microbiol. 6, 39 (2016).
Whibley, N. et al. Delinking CARD9 and IL-17: CARD9 protects against Candida tropicalis infection through a TNF-α-dependent, IL-17-independent mechanism. J. Immunol. 195, 3781–3792 (2015).
Conti, H. R. & Gaffen, S. L. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J. Immunol. 195, 780–788 (2015).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Borghi, M. et al. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe 18, 198–209 (2015).
Tomalka, J. et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 7, e1002379 (2011).
Lev-Sagie, A. et al. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J. Obstet. Gynecol. 200, 303.e1–303.e6 (2009).
Jaeger, M. et al. Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC. Eur. J. Clin. Microbiol. Infect. Dis. 35, 797–801 (2016).
Zhao, X. et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat. Med. 23, 337–346 (2017).
Wirnsberger, G. et al. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nat. Med. 22, 915–923 (2016).
Xiao, Y. et al. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat. Med. 22, 906–914 (2016).
Friedman, J. & Alm, E. J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 8, e1002687 (2012).
Wolfson, S. A. Black hairy tongue associated with penicillin therapy. J. Am. Med. Assoc. 140, 1206–1208 (1949).
Boris, S. & Barbes, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2, 543–546 (2000).
Noverr, M. C. & Huffnagle, G. B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 72, 6206–6210 (2004).
Lynch, A. S. & Robertson, G. T. Bacterial and fungal biofilm infections. Annu. Rev. Med. 59, 415–428 (2008).
Pande, K., Chen, C. & Noble, S. M. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat. Genet. 45, 1088–1091 (2013). This study shows that in the gut, C. albicans adopts a commensal form induced through downregulation of virulence factors and induction of metabolic functions.
Fan, D. et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 21, 808–814 (2015). This work shows that commensal bacteria can confer resistance to C. albicans by promoting the HIF1α-mediated expression of the antimicrobial peptide LL-37.
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
de Bekker, C. et al. Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evol. Biol. 14, 166 (2014).
Lin, C. H. et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat. Med. 22, 994–1001 (2016).
Sterkel, A. K. et al. Fungal mimicry of a mammalian aminopeptidase disables innate immunity and promotes pathogenicity. Cell Host Microbe 19, 361–374 (2016). This study shows that the pathogenic yeast Blastomyces dermatitidis produces a close mimic of a mammalian ectopeptidase, which interferes with innate antifungal responses in the lung.
Poulsen, M. & Boomsma, J. J. Mutualistic fungi control crop diversity in fungus-growing ants. Science 307, 741–744 (2005).
Nelson, K. E. & Williams, C. M. Infectious Disease Epidemiology: Theory and Practice 3rd edn (Jones & Bartlett Learning, 2014).
Lane, N. The unseen world: reflections on Leeuwenhoek (1677) 'Concerning little animals'. Phil. Trans. R. Soc. B Biol. Sci. 370, 20140344 (2015).
Pasteur, L. & Illo, J. Pasteur and rabies: an interview of 1882. Med. Hist. 40, 373–377 (1996).
Henle, J. On Miasmata and Contagia (Johns Hopkins Press, 1938).
Koch, R. An address on bacteriological research. Br. Med. J. 2, 380–383 (1890).
Evans, A. S. Causation and disease: the Henle–Koch postulates revisited. Yale J. Biol. Med. 49, 175–195 (1976).
Fredricks, D. N. & Relman, D. A. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin. Microbiol. Rev. 9, 18–33 (1996).
Byrd, A. L. & Segre, J. A. Infectious disease. Adapting Koch's postulates. Science 351, 224–226 (2016).
Olesen, S. W. & Alm, E. J. Dysbiosis is not an answer. Nat. Microbiol. 1, 16228 (2016).
Petersen, C. & Round, J. L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 16, 1024–1033 (2014).
Acknowledgements
The authors would like to thank members of the Iliev laboratory and the New York Host-Mycobiota Group for helpful suggestions related to the manuscript. This work was funded by the US National Institutes of Health (grants DK098310 and AI123819 to I.D.I.), Kenneth Rainin Foundation (Innovator and Breakthrough awards to I.D.I), Swiss National Science Foundation (fellowship P2ZHP3_164850 to I.L.) and support from the Jill Roberts Institute for Research in IBD. The authors apologize to all the contributors to this field whose work could not be cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Symbiosis
-
An ecological relationship between different species that persistently live in close contact. It includes relationships such as mutualism, parasitism and commensalism.
- Dysbiosis
-
A generalized term indicating changes in the composition of the microbiota that is caused by multiple factors. It can be either a cause or a consequence of disease. The term is undergoing revision in light of recent advances in microbiome science.
- Internal transcribed spacer
-
(ITS). A sequence in the fungal genome positioned between the 18S and 5.8S (ITS1) or the 5.8S and 28S (ITS2) fungal rDNA that is widely used in mycobiome next-generation sequencing. The variability of the ITS regions enables them to be used to classify fungal genera and species.
- Operational taxonomic units
-
(OTUs). Clusters of marker gene sequences (for example, 16S rRNA or internal transcribed spacer) based on sequence similarity used for taxonomy-independent community analysis.
- Richness
-
The number of different species represented in an ecological community.
- Commensalism
-
A relationship between two species through which one organism benefits from the other without affecting it.
- Inflammatory bowel disease
-
(IBD). A relapsing and remitting condition of complex aetiology. It is characterized by inflammation of the lower digestive tract with possible extra-intestinal manifestations. The most common types of IBD are Crohn's disease and ulcerative colitis.
- Alpha diversity
-
A biodiversity measure of the mean species diversity within an individual environmental habitat.
- Onychomycosis
-
A fungal infection of the fingernails or toenails.
- Chromoblastomycosis
-
A chronic localized infection of the skin and subcutaneous tissue caused by pigmented fungi that contain sclerotic bodies.
- Phaeohyphomycosis
-
A heterogeneous group of fungal infections that are characterized by the presence of pigmented fungal cells.
- Deep-seated dermatophytosis
-
A fungal infection of deep keratinized tissue (including skin, hair and claws).
- Secukinumab
-
A human monoclonal antibody that binds to IL-17A and inhibits its functions.
Rights and permissions
About this article
Cite this article
Iliev, I., Leonardi, I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol 17, 635–646 (2017). https://doi.org/10.1038/nri.2017.55
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2017.55
This article is cited by
-
Fungal coexistence in the skin mycobiome: a study involving Malassezia, Candida, and Rhodotorula
AMB Express (2024)
-
Regulation of intestinal epithelial homeostasis by mesenchymal cells
Inflammation and Regeneration (2024)
-
Enhanced visualization of nuclear staining and cell cycle analysis for the human commensal Malassezia
Scientific Reports (2024)
-
Dysbiosis of the intestinal fungal microbiota increases lung resident group 2 innate lymphoid cells and is associated with enhanced asthma severity in mice and humans
Respiratory Research (2023)
-
Candida makes a lasting impression in COVID-19
Nature Immunology (2023)