Abstract
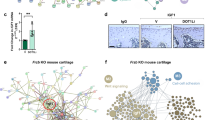
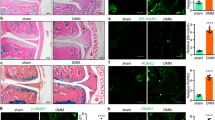
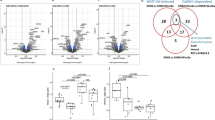
Degenerative and inflammatory joint diseases lead to a destruction of the joint architecture. Whereas degenerative osteoarthritis results in the formation of new bone, rheumatoid arthritis leads to bone resorption. The molecular basis of these different patterns of joint disease is unknown. By inhibiting Dickkopf-1 (DKK-1), a regulatory molecule of the Wnt pathway, we were able to reverse the bone-destructive pattern of a mouse model of rheumatoid arthritis to the bone-forming pattern of osteoarthritis. In this way, no overall bone erosion resulted, although bony nodules, so-called osteophytes, did form. We identified tumor necrosis factor-α (TNF) as a key inducer of DKK-1 in the mouse inflammatory arthritis model and in human rheumatoid arthritis. These results suggest that the Wnt pathway is a key regulator of joint remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Walsh, N.C., Crotti, T.N., Goldring, S.R. & Gravallese, E.M. Rheumatic diseases: the effects of inflammation on bone. Immunol. Rev. 208, 228–251 (2005).
Klippel, J.H. Primer on the Rheumatic Diseases 12th edn. (Arthritis Foundation, Atlanta, 2001).
Smolen, J.S. & Steiner, G. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2, 473–488 (2003).
Feldmann, M. et al. Anti-TNF therapy: where have we got to in 2005? Autoimmunity 25, 26–28 (2005).
Lam, J. et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488 (2000).
Bertolini, D.R., Nedwin, G.E., Bringman, T.S., Smith, D.D. & Mundy, G.R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319, 516–518 (1986).
Keffer, J. et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 10, 4025–4031 (1991).
Gravallese, E.M. et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am. J. Pathol. 152, 943–951 (1998).
Kong, Y.Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 397, 315–323 (1999).
Pettit, A.R. et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 159, 1689–1699 (2001).
Redlich, K. et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J. Clin. Invest. 110, 1419–1427 (2002).
Miller, J.R. The Wnts. Genome Biol. [online] 3, 3001 (2002).
Mao, B. et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417, 664–667 (2002).
Holmen, S.L. et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J. Bone Miner. Res. 19, 2033–2040 (2004).
Glinka, A. et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391, 357–362 (1998).
Bafico, A., Liu, G., Yaniv, A., Gazit, A. & Aaronson, S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6. Nat. Cell Biol. 3, 683–686 (2001).
Morvan, F. et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 21, 934–945 (2006).
Tian, E. et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494 (2003).
Van Beuningen, H.M. et al. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage 8, 25–33 (2000).
Scharstuhl, A. et al. Adenoviral overexpression of Smad-7 and Smad-6 differentially regulates TGF-beta-mediated chondrocyte proliferation and proteoglycan synthesis. Arthritis Rheum. 48, 3442–3451 (2003).
Peifer, M. & Polakis, P. Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science 287, 1606–1609 (2000).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Glass, D.A. II et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005).
Gong, Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001).
Zwerina, J. et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 50, 277–290 (2004).
Takeshita, F. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. USA 102, 12177–12182 (2005).
Arnett, F.C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324 (1988).
Van der Linden, S., Valkenburg, H.A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Van Gestel, A.M. et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 39, 34–40 (1996).
Garrett, S. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 21, 2286–2291 (1994).
Gortz, B. et al. Arthritis induces lymphocytic bone marrow inflammation and endosteal bone formation. J. Bone Miner. Res. 19, 990–998 (2004).
Acknowledgements
We thank E. Wagner for scientific discussions and B. Tuerk and M. Tryniecki for technical assistance. We also thank G. Kollias (Alexander Fleming Biomedical Research Center) for providing hTNFtg mice, J. Behrens (University of Erlangen-Nuremberg) for providing an antibody against conductin/axin-2, A.H. Wanivenhaus (Medical University of Vienna) for human synovial tissue samples and C. Hartmann (Institute of Molecular Pathology) for the osteocalcin probe. This study was supported by the START prize of the Austrian Science Fund (G.S.) and the Deutsche Forschungsgemeinschaft (DFG; Interdisziplinäres Zentrum für Klinische Forschung Erlangen).
Author information
Authors and Affiliations
Contributions
D.Diarra conducted the in vivo analyses of hTNFtg mice and contributed to manuscript preparation. M.S. performed the analyses of collagen-induced arthritis and contributed to manuscript preparation. K.P. and J.Z. worked on the in vitro analysis of hTNFtg mice and human samples. M.S.O. performed microcomputed tomography. D. Dwyer conducted the analyses on collagen-induced arthritis. A.K. collected human samples and worked on in vitro analysis of hTNFtg mice. J.S. conducted data analyses on murine and human samples. M.H. and C.S. analyzed the GPI-induced arthritis model. D.v.d.H. and R.L. analyzed samples from spondylarthropathy patients. D.L., W.G.R. and G.S. supervised the project and contributed to manuscript preparation.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
DKK-1 affects joint remodeling in collagen- and GPI- induced arthritis. (PDF 180 kb)
Supplementary Fig. 2
Regulation of DKK-1 expression and its involvement in bone metabolism. (PDF 202 kb)
Supplementary Fig. 3
The influence of OPG on the effects of DKK-1 inhibition. (PDF 295 kb)
Supplementary Fig. 4
Functional Wnt- signalling in joints after systemic blockade of DKK-1. (PDF 240 kb)
Rights and permissions
About this article
Cite this article
Diarra, D., Stolina, M., Polzer, K. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13, 156–163 (2007). https://doi.org/10.1038/nm1538
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1538
This article is cited by
-
Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints
Nature Communications (2024)
-
The interplay of rheumatoid arthritis and osteoporosis: exploring the pathogenesis and pharmacological approaches
Clinical Rheumatology (2024)
-
DKK-1 and Its Influences on Bone Destruction: A Comparative Study in Collagen-Induced Arthritis Mice and Rheumatoid Arthritis Patients
Inflammation (2024)
-
The Role of Early Treatment in the Management of Axial Spondyloarthritis: Challenges and Opportunities
Rheumatology and Therapy (2024)
-
Increased synovial immunohistochemistry reactivity of TGF-β1 in erosive peripheral psoriatic arthritis
BMC Musculoskeletal Disorders (2023)