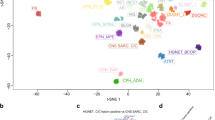
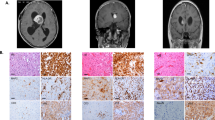
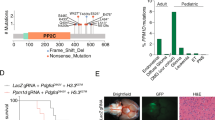
Abstract
The most common pediatric brain tumors are low-grade gliomas (LGGs). We used whole-genome sequencing to identify multiple new genetic alterations involving BRAF, RAF1, FGFR1, MYB, MYBL1 and genes with histone-related functions, including H3F3A and ATRX, in 39 LGGs and low-grade glioneuronal tumors (LGGNTs). Only a single non-silent somatic alteration was detected in 24 of 39 (62%) tumors. Intragenic duplications of the portion of FGFR1 encoding the tyrosine kinase domain (TKD) and rearrangements of MYB were recurrent and mutually exclusive in 53% of grade II diffuse LGGs. Transplantation of Trp53-null neonatal astrocytes expressing FGFR1 with the duplication involving the TKD into the brains of nude mice generated high-grade astrocytomas with short latency and 100% penetrance. FGFR1 with the duplication induced FGFR1 autophosphorylation and upregulation of the MAPK/ERK and PI3K pathways, which could be blocked by specific inhibitors. Focusing on the therapeutically challenging diffuse LGGs, our study of 151 tumors has discovered genetic alterations and potential therapeutic targets across the entire range of pediatric LGGs and LGGNTs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Armstrong, G.T. et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro-oncol. 13, 223–234 (2011).
Arora, R.S. et al. Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro-oncol. 11, 403–413 (2009).
Qaddoumi, I., Sultan, I. & Gajjar, A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer 115, 5761–5770 (2009).
Bouffet, E. et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J. Clin. Oncol. 30, 1358–1363 (2012).
Duffner, P.K., Cohen, M.E., Myers, M.H. & Heise, H.W. Survival of children with brain tumors: SEER Program, 1973–1980. Neurology 36, 597–601 (1986).
Fisher, P.G. et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr. Blood Cancer 51, 245–250 (2008).
Gajjar, A. et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J. Clin. Oncol. 15, 2792–2799 (1997).
Gnekow, A.K. et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-oncol. 14, 1265–1284 (2012).
Merchant, T.E., Conklin, H.M., Wu, S., Lustig, R.H. & Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 27, 3691–3697 (2009).
Stokland, T. et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro-oncol. 12, 1257–1268 (2010).
Forshew, T. et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J. Pathol. 218, 172–181 (2009).
Jones, D.T. et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68, 8673–8677 (2008).
Pfister, S. et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Invest. 118, 1739–1749 (2008).
Sievert, A.J. et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism–based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 19, 449–458 (2009).
Listernick, R., Charrow, J. & Gutmann, D.H. Intracranial gliomas in neurofibromatosis type 1. Am. J. Med. Genet. 89, 38–44 (1999).
Listernick, R., Ferner, R.E., Liu, G.T. & Gutmann, D.H. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann. Neurol. 61, 189–198 (2007).
DeClue, J.E. et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69, 265–273 (1992).
Dias-Santagata, D. et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS ONE 6, e17948 (2011).
Lin, A. et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J. Neuropathol. Exp. Neurol. 71, 66–72 (2012).
Schindler, G. et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 121, 397–405 (2011).
Tatevossian, R.G. et al. MAPK pathway activation and the origins of pediatric low-grade astrocytomas. J. Cell Physiol. 222, 509–514 (2010).
Tatevossian, R.G. et al. MYB upregulation and genetic aberrations in a subset of pediatric low-grade gliomas. Acta Neuropathol. 120, 731–743 (2010).
Stephens, P.J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Louis, D.N. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007).
Pollack, I.F. The role of surgery in pediatric gliomas. J. Neurooncol. 42, 271–288 (1999).
Wisoff, J.H. et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery 68, 1548–1554, discussion 1554–1555 (2011).
Louis, D.N. Molecular pathology of malignant gliomas. Annu. Rev. Pathol. 1, 97–117 (2006).
Ohgaki, H. & Kleihues, P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 28, 177–183 (2011).
Riemenschneider, M.J., Jeuken, J.W., Wesseling, P. & Reifenberger, G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 120, 567–584 (2010).
Cin, H. et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 121, 763–774 (2011).
Jones, D.T. et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 28, 2119–2123 (2009).
Ciesielski, M.J. & Fenstermaker, R.A. Oncogenic epidermal growth factor receptor mutants with tandem duplication: gene structure and effects on receptor function. Oncogene 19, 810–820 (2000).
Lin, W.M. et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 68, 664–673 (2008).
Rand, V. et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc. Natl. Acad. Sci. USA 102, 14344–14349 (2005).
Singh, D. et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 337, 1231–1235 (2012).
Li, F., Zhai, Y.P., Tang, Y.M., Wang, L.P. & Wan, P.J. Identification of a novel partner gene, TPR, fused to FGFR1 in 8p11 myeloproliferative syndrome. Genes Chromosom. Cancer 51, 890–897 (2012).
Turner, N. et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 70, 2085–2094 (2010).
Turner, N. & Grose, R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer 10, 116–129 (2010).
Wang, M. et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J. Neuropathol. Exp. Neurol. 64, 875–881 (2005).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Sturm, D. et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22, 425–437 (2012).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Müller, W., Afra, D. & Schroder, R. Supratentorial recurrences of gliomas. Morphological studies in relation to time intervals with astrocytomas. Acta Neurochir. (Wien) 37, 75–91 (1977).
Okamoto, Y. et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 108, 49–56 (2004).
Peraud, A., Kreth, F.W., Wiestler, O.D., Kleihues, P. & Reulen, H.J. Prognostic impact of TP53 mutations and P53 protein overexpression in supratentorial WHO grade II astrocytomas and oligoastrocytomas. Clin. Cancer Res. 8, 1117–1124 (2002).
Pollack, I.F., Claassen, D., al-Shboul, Q., Janosky, J.E. & Deutsch, M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J. Neurosurg. 82, 536–547 (1995).
Jones, D.T. et al. Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol. 121, 753–761 (2011).
Korshunov, A. et al. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol. 118, 401–405 (2009).
Parsons, D.W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
von Deimling, A., Korshunov, A. & Hartmann, C. The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol. 21, 74–87 (2011).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Ohgaki, H. et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 64, 6892–6899 (2004).
Ohgaki, H. & Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 170, 1445–1453 (2007).
Bettegowda, C. et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333, 1453–1455 (2011).
Yip, S. et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J. Pathol. 226, 7–16 (2012).
Flaherty, K.T. et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 367, 107–114 (2012).
Katoh, M. Genetic alterations of FGF receptors: an emerging field in clinical cancer diagnostics and therapeutics. Expert Rev. Anticancer Ther. 10, 1375–1379 (2010).
Ren, M., Qin, H., Ren, R. & Cowell, J.K. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia 27, 32–40 (2013).
Zhang, J. et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481, 329–334 (2012).
Zhang, J. et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481, 157–163 (2012).
Wang, J. et al. CREST maps somatic structural variation in cancer genomes with base-pair resolution. Nat. Methods 8, 652–654 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McPherson, A. et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput. Biol. 7, e1001138 (2011).
Mullighan, C.G. et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471, 235–239 (2011).
Zhang, J. et al. SNPdetector: a software tool for sensitive and accurate SNP detection. PLoS Comput. Biol. 1, e53 (2005).
Downing, J.R. et al. The Pediatric Cancer Genome Project. Nat. Genet. 44, 619–622 (2012).
Dees, N.D. et al. MuSiC: identifying mutational significance in cancer genomes. Genome Res. 22, 1589–1598 (2012).
Edmonson, M.N. et al. Bambino: a variant detector and alignment viewer for next-generation sequencing data in the SAM/BAM format. Bioinformatics 27, 865–866 (2011).
Mullighan, C.G. Single nucleotide polymorphism microarray analysis of genetic alterations in cancer. Methods Mol. Biol. 730, 235–258 (2011).
Persons, D.A. et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood 93, 488–499 (1999).
Bajenaru, M.L. et al. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol. Cell Biol. 22, 5100–5113 (2002).
Endersby, R., Zhu, X., Hay, N., Ellison, D.W. & Baker, S.J. Nonredundant functions for Akt isoforms in astrocyte growth and gliomagenesis in an orthotopic transplantation model. Cancer Res. 71, 4106–4116 (2011).
Jonkers, J. et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 (2001).
Ellison, D.W. et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J. Clin. Oncol. 29, 1400–1407 (2011).
Tang, B. et al. Characterization of signal transduction through the TCR-ζ chain following T cell stimulation with analogue peptides of type II collagen 260–267. J. Immunol. 160, 3135–3142 (1998).
Acknowledgements
We thank W. Evans for advice and support and P. Nagahawatte for submitting the genomic data to EBI. We are grateful for support from Anatomic Pathology and the Hartwell Center of Biotechnology and Bioinformatics at St. Jude Children's Research Hospital and from Beckman Coulter Genomics. We acknowledge the St. Jude Children's Research Hospital tissue resource facility, from which tissue samples were obtained in accordance with institutional review board approval for the Pediatric Cancer Genome Project. This work was supported by the St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project and the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital and by a grant from the US National Institutes of Health (NIH; CA096832).
Author information
Authors and Affiliations
Consortia
Contributions
D.W.E., J.Z., R.G.T., B.T., L.D., R.K., D.S., R.J.G., E.R.M., R.K.W., J.R.D. and S.J.B. designed experiments or supervised research. I.Q., F.A.B., S.S. and A.G. provided samples or clinical data. D.W.E. undertook all pathological evaluations. J.Z., G.W., R.G.T., J.D.D., B.T., W.O., C.P., C.P.M., C.L., C.K., L.D., M.P., R.L., R.H., X.C., E.H., P.N., M.R., K.B., J.C., J.B., J.M., G.S., Y.L., L.W., J.W., J.E., D.Z., R.S.F., L.L.F., B.V., H.L.M., C.T., C.G.M., R.K., D.S., S.J.B. and D.W.E. performed experiments, analyzed data or prepared tables and figures. D.J.D. and K.O. contributed reagents, materials or analysis tools. D.W.E. and J.Z. wrote the manuscript, with contributions from G.W., R.G.T. and S.J.B.
Corresponding author
Ethics declarations
Competing interests
The author declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15, Supplementary Tables 1–11 and Supplementary Note (PDF 12442 kb)
Rights and permissions
About this article
Cite this article
the St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45, 602–612 (2013). https://doi.org/10.1038/ng.2611
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2611