Abstract
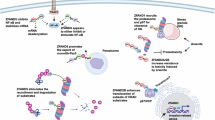
The view of the lysosome as the terminal end of cellular catabolic pathways has been challenged by recent studies showing a central role of this organelle in the control of cell function. Here we show that a lysosomal Ca2+ signalling mechanism controls the activities of the phosphatase calcineurin and of its substrate TFEB, a master transcriptional regulator of lysosomal biogenesis and autophagy. Lysosomal Ca2+ release through mucolipin 1 (MCOLN1) activates calcineurin, which binds and dephosphorylates TFEB, thus promoting its nuclear translocation. Genetic and pharmacological inhibition of calcineurin suppressed TFEB activity during starvation and physical exercise, while calcineurin overexpression and constitutive activation had the opposite effect. Induction of autophagy and lysosomal biogenesis through TFEB required MCOLN1-mediated calcineurin activation. These data link lysosomal calcium signalling to both calcineurin regulation and autophagy induction and identify the lysosome as a hub for the signalling pathways that regulate cellular homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Luzio, J. P., Pryor, P. R. & Bright, N. A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 (2007).
Saftig, P. & Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10, 623–635 (2009).
De Duve, C. The lysosome turns fifty. Nat. Cell Biol. 7, 847–849 (2005).
Luzio, J. P., Parkinson, M. D., Gray, S. R. & Bright, N. A. The delivery of endocytosed cargo to lysosomes. Biochem. Soc. Trans. 37, 1019–1021 (2009).
He, C. & Klionsky, D. J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 (2009).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Roczniak-Ferguson, A. et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 (2012).
Martina, J. A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Martina, J. A. & Puertollano, R. RRAG GTPases link nutrient availability to gene expression, autophagy and lysosomal biogenesis. Autophagy 9, 928–930 (2013).
Ferron, M. et al. A RANKL-PKCbeta-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts. Genes Dev. 27, 955–969 (2013).
Sancak, Y. et al. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Zoncu, R. et al. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683 (2011).
Settembre, C., Fraldi, A., Medina, D. L. & Ballabio, A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296 (2013).
Füllgrabe, J., Klionsky, D. J. & Joseph, B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat. Rev. Mol. Cell Biol. 15, 65–74 (2014).
Morgan, A. J., Platt, F. M., Lloyd-Evans, E. & Galione, A. Molecular mechanisms of endolysosomal Ca2+ signaling in health and disease. Biochem. J. 439, 349–374 (2011).
Rizzuto, R. & Pozzan, T. Microdomains of intracelular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006).
Stewart, A. A., Ingebritsen, T. S., Manalan, A., Klee, C. B. & Cohen, P. Discovery of a Ca2 + - and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 11, 80–84 (1982).
Rao, A., Luo, C. & Hogan, P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Hogan, P. G. & Li, H. Calcineurin. Curr. Biol. 15, R442–443 (2005).
Wu, H. et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20, 6414–6423 (2001).
Mammucari, C. et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6, 458–471 (2007).
Lai, M. M., Burnett, P. E., Wolosker, H., Blackshaw, S. & Snyder, S. H. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 273, 18325–18331 (1998).
Loh, C. et al. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J. Biol. Chem. 271, 10884–10891 (1996).
Soderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006).
Liu, Q. et al. Kinome-wide selectivity profiling of ATP-competitive mammalian target of rapamycin (mTOR) inhibitors and characterization of their binding kinetics. J. Biol. Chem. 287, 9742–9752 (2012).
Klee, C. B., Crouch, T. H. & Krinks, M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl Acad. Sci. USA 76, 6270–6273 (1979).
Liu, Q., Wilkins, B. J., Lee, Y. J., Ichijo, H. & Molkentin, J. D. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol. Cell. Biol. 26, 3785–3797 (2006).
Hoyer-Hansen, M. et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 25, 193–205 (2007).
Morgan, A. J. et al. Bidirectional Ca(2)(+) signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 200, 789–805 (2013).
Calcraft, P. J. et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 (2009).
Patron, M. et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell 53, 726–737 (2014).
Dong, X. P. et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 1, 38 (2010).
Shen, D. et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 3, 731 (2012).
Samie, M. et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell 26, 511–524 (2013).
Medina, D. L. et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430 (2011).
Bassi, M. T. et al. Cloning of the gene encoding a novel integral membrane protein, mucolipidin- and identification of the two major founder mutations causing mucolipidosis type IV. Am. J. Hum. Genet. 67, 1110–1120 (2000).
Bargal, R. et al. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 26, 118–123 (2000).
Palmieri, M. et al. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 20, 3852–3866 (2011).
Axe, E. L. et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701 (2008).
Yang, Z. & Klionsky, D. J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 (2010).
He, C. et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481, 511–515 (2012).
Grumati, P. et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 7, 1415–1423 (2011).
Jamart, C., Naslain, D., Gilson, H. & Francaux, M. Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am. J. Physiol. Endocrinol. Metab. 305, E964–E974 (2013).
Macian, F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5, 472–484 (2005).
Rusnak, F. & Mertz, P. Calcineurin: form and function. Physiol. Rev. 80, 1483–1521 (2000).
Kasahara, A., Cipolat, S., Chen, Y., Dorn, G. W. II & Scorrano, L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science 342, 734–737 (2013).
Mellstrom, B. & Naranjo, J. R. Ca2+-dependent transcriptional repression and derepression: DREAM, a direct effector. Semin. Cell Dev. Biol. 12, 59–63 (2001).
Heineke, J. & Ritter, O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolema to the nucleus and beyond. J. Mol. Cell Cardiol. 52, 62–73 (2012).
Plyte, S. et al. Identification and characterization of a novel nuclear factor of activated T-cells-1 isoform expressed in mouse brain. J. Biol. Chem. 276, 14350–14358 (2001).
Daniele, T., Di Tullio, G., Santoro, M., Turacchio, G. & De Matteis, M. A. ARAP1 regulates EGF receptor trafficking and signalling. Traffic 9, 2221–2235 (2008).
Sittampalam, G. S. et al. (eds) in Assay Guidance Manual [Internet] (Eli Lilly Company and the National Center for Advancing Translational Sciences, 2004)
Pattni, K., Jepson, M., Stenmark, H. & Banting, G. A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr. Biol. 11, 1636–1642 (2001).
Granatiero, V., Patron, M., Tosatto, A., Merli, G. & Rizzuto, R. The use of aequorin and its variants for Ca2+ measurements. Cold Spring Harb. Protoc. 2014, 9–16 (2014).
Sandri, M. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).
Schroder, B. A., Wrocklage, C., Hasilik, A. & Saftig, P. The proteome of lysosomes. Proteomics 10, 4053–4076 (2010).
Amberger, J. et al. McKusick’s Online Mendelian Inheritance in Man (OMIM). Nucleic Acids Res. 37, D793–D796 (2009) (Database issue)
McKusick, V. A. Mendelian Inheritance in Man and its online version, OMIM. Am. J. Hum. Genet. 80, 588–604 (2007).
Carbon, S. et al. AmiGO: online access to ontology and annotation data. Bioinformatics 25, 288–289 (2009).
Belcastro, V. et al. Transcriptional gene network inference from a massive dataset elucidates transcriptome organization and gene function. Nucleic Acids Res. 39, 8677–8688 (2011).
Magrane, M. & U. Consortium, UniProt Knowledgebase: a hub of integrated protein data. Database: J. Biol. Databases Curation 2011, bar009 (2011).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
We thank J. Meldolesi, T. Pozzan, D. Rubinsztein and R. Polishchuk for helpful suggestions and critical review of the manuscript. We thank G. Diez-Roux and A. Burton for their support in manuscript preparation. We are also grateful to R. De Cegli and D. Carrella for their support in the statistical analysis of the results. The TIGEM Bioinformatic and High Content Screening Facilities are gratefully acknowledged for their technological contributions to the project. We also thank J. D. Molkentin for the CanB KO MEFs, B. A. Rothermel for the HA–ΔCnA, and P. Aza-Blanc for suggestions on the reverse transfection protocol. We acknowledge the support of the Italian Telethon Foundation grant numbers TGM11CB6 (A.B.); the Beyond Batten Disease Foundation (A.B.); European Research Council Advanced Investigator grant no. 250154 (CLEAR) (A.B.); US National Institutes of Health (R01-NS078072) (A.B.); Telethon-Italy (TCP04009) (M.S.), European Research Council Consolidator grant no. 282310-(MyoPHAGY) (M.S.).
Author information
Authors and Affiliations
Contributions
D.L.M., S.D.P., I.P., S.M., A.S-R., C.P., R.V., A.F. and C.S. performed all experiments in cultured cells. A.A. and M.S. performed in vivo experiments in the murine muscle. W.W., Q.G., D.D.S., H.X. and R.R. performed electrophysiology experiments. D.L.M., M.A.D.M. and A.B. designed the overall study. D.L.M. and A.B. supervised the work. All authors discussed the results and made substantial contributions to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Starvation and calcineurin overexpression induce NFAT nuclear translocation and de-phosphorylation.
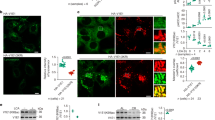
HeLa cells were transiently transfected with a vector carrying NFAT fused to the GPF. (a) The plot shows the Nuc/Cyt ratio of NFAT-GFP. 1, 3, and 6 hours of starvation increased the nuclear translocation of NFAT. Thapsigargin (300 nM, 1 hour) was used as positive control. Bar graphs show mean ± s.d. for n = 3 independent experiments. (b) Starvation (1 and 3 hours, respectively) induces NFAT de-phosphorylation as measured by the mobility of NFAT detected by using anti-GFP antibodies. ΔCaN overexpression in fed conditions also induced NFAT-GFP mobility down-shift (right blot) (n = 3 independent experiments). (c) The overexpression of HA-tagged dominant positive calcineurin (ΔCaN) in HeLa cells transfected with NFAT-GFP induces NFAT nuclear translocation. ΔCaN positive cells were identified using anti-HA antibodies. The graph represents the nucleus/cytosol ratio of NFAT-GFP in both negative and positive calcineurin populations. Bar graphs show mean ± s.d. for n = 3 independent experiments. Scale bar 10 μm. Source data are provided in Supplementary Table 4.
Supplementary Figure 2 PPP3CB binds TFEB.
(a–c) Co-immunoprecipitation experiments performed both in HeLaTFEB–GFP (a,c) and in HEK-293TFEB–GFP cells (b) transfected with PPP3CB-FLAG and treated as indicated (fed or STV during 3 hr). 2 mg of lysates were immunoprecipitated with anti-GFP antibodies or rabbit IgG as a control and immunoblotted with the indicated antibodies. 50 μg of lysates were used to the input. n = 5 independent experiments were performed. (d) Histidine-TFEB pull down assay performed as described in Methods section confirms the interaction between TFEB and calcineurin. The input proportions of each protein are indicated. n = 3 independent experiments.
Supplementary Figure 3 Calcineurin interacts with TFEB and regulates its localization.
(a) Proximity Ligation Assay (PLA) to study TFEB interactions. HeLa cells were pre-treated 10 minutes before fixation with 1 μM ER-tracker (green) as marker for the cytoplasm or with Hoechst 33258 (blue) for nuclei. After fixation, cells were processed for investigating TFEB proximity interactions. Golgin 97 was used as negative control, and mTOR as a positive one. PLA detection red dots indicate positive interactions. White squares contain higher magnification images (n = 3 independent experiments). (b) Validation of the TFEB antibody. HeLa cells were control treated (Mock), or treated with TFEB siRNAs for 72 hours, processed for IF and stained with anti-TFEB (SantaCruz) (green, as a marker of silencing efficiency) or with Hoechst 33258 (blue, as nuclear staining). Mean values ± s.d. of TFEB fluorescence intensity under Mock or TFEB-KD conditions. n = 100 cells pooled from 2 independent experiments, scale bars, 10 μm. (c) Cell lysates (50 μg/sample) were analyzed by SDS-PAGE and immunobloting with anti-TFEB (Cell Signaling). β-actin was used as a loading control. n = 5 independent experiments (d) Cellular localization at the steady state of the endogenous proteins used in the PLA experiment: TFEB (green) (PPP3CB, Golgin97, mTOR, red) in HeLa cells. Hoechst 33258 (blue), was used as nuclear staining. n = 60 cells pooled from 2 independent experiments. Scale bars, 10 μm. (e), Nuclear translocation of endogenous TFEB is reduced in PPP3CB/R1 knock-down cells. Representative images from HC assay of HeLa cells transfected with SCRMBL or a pool of PPP3CB/R1 siRNAs, and then subjected to the indicated conditions. A similar experiment was performed in MCF-7 cells, providing similar results. The plot shows the percentage of nuclear TFEB translocation of PPP3CB/R1 silenced HeLa and MCF-7, compared with their corresponding control-treated cells transfected with scramble siRNA oligonucleotides (n = 3 independent experiments were performed). Scale bar 10 μm. (f) Nuclear fractions from cells treated as indicated for 3 h, WT MEFs (PPP3R1+/+) or PPP3R1−/− were immunobloted, and endogenous TFEB protein was detected using anti-TFEB antibodies. n = 2 independent experiments. (g) Normal mTOR phosphorylation activity in WT MEFs (PPP3R1+/+) or PPP3R1−/−, treated as indicated for 3 h, was assessed by immunoblotting analysis of mTOR substrate P70S6K. n = 2 independent experiments. Source data are provided in Supplementary Table 4.
Supplementary Figure 4 TPCN2 and MICU1 calcium channels do not regulate TFEB localization.
(a) Depletion of the lysosomal two-pore channel TPCN2 does not affect TFEB nuclear translocation during starvation (1 hour) (left graph). Quantification of the efficiency of TPCN2 gene silencing by qPCR analysis (right graph). The plot shows the mean ± s.d. for n = 3 independent experiments. (b) Overexpression of the essential mitochondrial Ca2+ regulator MICU1 does not impact on the subcellular localization of TFEB in fed and starvation conditions (1 hr), as compared to untransfected cells. High content imaging analysis was used to quantify TFEB localization using HeLa-TFEB-GFP cells. The plot shows the mean ± s.d. for n = 3 independent experiments. Source data are provided in Supplementary Table 4.
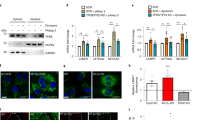
Supplementary Figure 5 MCOLN1 regulates TFEB phosphorylation and localization in a calcineurin-dependent fashion.
(a) Depletion of the lysosomal calcium channel MCOLN1 reduces starvation mediated TFEB nuclear translocation. WT HeLa and HeLa-shMCOLN1cells were starved at different time-points. Following starvation, 50 μg of protein extracts were prepared and probed for endogenous TFEB using anti-TFEB antibodies. TFEB mobility downshift after starvation is reduced in MCOLN1 depleted cells during starvation (black arrows point-out the phosphorylated forms, while red arrows show the downshift corresponding to de-phosphorylated TFEB forms). n = 3 independent experiments. (b) MCOLN1-mediated induction of TFEB nuclear localization is calcineurin-dependent. HeLaTFEB–GFP cells were transfected with FLAG-tagged MCOLN1 gene in combination with siRNAs against PPP3CB and PPP3R1 (siCaN) or scramble siRNA oligonucleotides (SCRMBL). TFEB subcellular localization was assessed in FLAG-positive population using HC imaging. The graph shows the percentage of nuclear TFEB in siCaN knock-down cells compared with control SCRMBL-transfected cells. The plot shows the mean ± s.d. for n = 5 independent experiments. (c) The MCOLN1 agonist SF-51 induces TFEB nuclear translocation. We quantified the effect of SF-51 treatment on HeLaTFEB–GFP cells using an HC assay at the time points indicated in the plot. The graph shows the percentage of treated cells with nuclear TFEB compared to DMSO control cells. The plot shows the mean ± s.d. for n = 3 independent experiments. Source data are provided in Supplementary Table 4.
Supplementary Figure 6 Calcineurin regulates the transcriptional response to starvation.
(a) The figure shows genes that were significantly regulated by starvation ordered by gene ID. A value of “1” was assigned to expression levels under fed conditions. n = 3 independent experiments. (b) The TFEB-mediated transcriptional response to starvation is inhibited in PPP3R1−/− MEFs. PPP3R1−/− and wild type MEFs were starved for 6 hrs or left in fed conditions as indicated, and the mRNA levels of TFEB target genes analyzed by quantitative PCR analysis (qPCR). mRNA expression levels of PPP3R1 were determined to confirm downregulation of PPP3R1 in PPP3R1−/− MEFs. n = 3 independent experiments. (c) HeLa cells were transfected with control SCRMBL siRNA oligonucleotides or depleted of PPP3CB and PPP3R1. 72 hours after silencing, cells were left untreated of starved. Expression levels of TFEB target genes were investigated by qPCR (n = 3 independent experiments). ∗p < 0.05, source data are provided in Supplementary Table 4.
Supplementary Figure 7 Calcineurin and MCOLN1 regulate starvation-mediated increase of autophagosome number.
(a) HeLa cells were left untreated, starved or starved in presence of FK506 (25 μM) or BAPTA-AM (25 μM). Subsequently, cells were incubated with anti-LC3 antibody to detect endogenous LC3 by immunofluorescence. HC imaging analysis was used to quantify the number of LC3-positive vesicles. As shown in the graph, the administration of the calcineurin inhibitor FK506 and calcium chelator BAPTA-AM caused a decrease of LC3-positive puncta during starvation. (n = 2 independent experiments). Scale bar 10 μm. ∗, p < 0.05. (b) Silencing of MCOLN1 reduces the earlier marker of autophagy WIPI. HeLa cells were silenced against MCOLN1 for 72 h and left untreated or starved for 4 h. IF against WIPI endogenous protein was performed. Confocal images of 30 cells per condition were analyzed. The plot shows the mean ± s.em. of WIPI-positive puncta per cell (n = 30 cells pooled from three independent experiments). Source data are provided in Supplementary Table S4.
Supplementary information
Supplementary Information
Supplementary Information (PDF 965 kb)
Supplementary Table 1
Supplementary Information (XLSX 12 kb)
Supplementary Table 2
Supplementary Information (XLSX 47 kb)
Supplementary Table 3
Supplementary Information (XLSX 46 kb)
Supplementary Table 4
Supplementary Information (XLSX 147 kb)
Agonist-dependent activation of MCOLN1 induces TFEB nuclear translocation.
Time-Lapse of mock control HeLa cells showing the induction of TFEB nuclear translocation during the tretament with the agonist of MCOLN1 SF-51. (MOV 1563 kb)
The silencing of calcineurin reduces MCOLN1-dependent TFEB nuclear localization during SF-51 treatment.
Time-Lapse of HeLa cells knock-down for calcineurin (siCaN) showing a reduction of TFEB nuclear translocation during the tretment with the agonist of MCOLN1 SF-51. (MOV 1516 kb)
The silencing of MCOLN1 reduces TFEB nuclear localization during SF-51 treatment.
Time-Lapse of HeLa cells knock-down for MCOLN1 (siMCOLN1) showing a reduction of TFEB nuclear translocation during the treatment with its agonist SF-51. (MOV 1298 kb)
Rights and permissions
About this article
Cite this article
Medina, D., Di Paola, S., Peluso, I. et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299 (2015). https://doi.org/10.1038/ncb3114
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3114
This article is cited by
-
Regulation of autophagy by perilysosomal calcium: a new player in β-cell lipotoxicity
Experimental & Molecular Medicine (2024)
-
Transcriptional dysregulation of autophagy in the muscle of a mouse model of Duchenne muscular dystrophy
Scientific Reports (2024)
-
Activation of Ca2+ phosphatase Calcineurin regulates Parkin translocation to mitochondria and mitophagy in flies
Cell Death & Differentiation (2024)
-
Key genes and convergent pathogenic mechanisms in Parkinson disease
Nature Reviews Neuroscience (2024)
-
Cystinosis — a review of disease pathogenesis, management, and future treatment options
Journal of Rare Diseases (2024)