Abstract
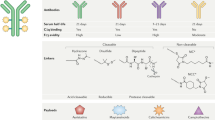
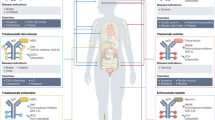
Progress has been made recently in developing antibody-drug conjugates (ADCs) that can selectively deliver cancer drugs to tumor cells. In principle, the idea is simple: by attaching drugs to tumor-seeking antibodies, target cells will be killed and nontarget cells will be spared. In practice, many parameters needed to be addressed to develop safe and effective ADCs, including the expression profiles of tumor versus normal tissues, the potency of the drug, the linker attaching the drug and placement of the drug on the antibody, and the pharmacokinetic and stability profiles of the resulting ADC. All these issues had been taken into account in developing brentuximab vedotin (Adcetris), an ADC that recently received accelerated approval by the US Food and Drug Administration for the treatment of relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma (ALCL). Research is under way to extend the applications of brentuximab vedotin and to advance the field by developing other ADCs with new linker and conjugation strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Katie Vicari


Katie Vicari

Katie Vicari

Similar content being viewed by others
References
Schwartz, R.S. Paul Ehrlich's magic bullets. N. Engl. J. Med. 350, 1079–1080 (2004).
Senter, P.D. Potent antibody drug conjugates for cancer therapy. Curr. Opin. Chem. Biol. 13, 235–244 (2009).
Carter, P.J. & Senter, P.D. Antibody-drug conjugates for cancer therapy. Cancer J. 14, 154–169 (2008).
Wu, A.M. & Senter, P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 23, 1137–1146 (2005).
Bernardes, G.J. et al. A traceless vascular-targeting antibody-drug conjugate for cancer therapy. Angew. Chem. Int. Edn Engl. 51, 941–944 (2012).
Arnon, R. & Sela, M. In vitro and in vivo efficacy of conjugates of daunomycin with anti-tumor antibodies. Immunol. Rev. 62, 5–27 (1982).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Petersen, B.H., DeHerdt, S.V., Schneck, D.W. & Bumol, T.F. The human immune response to KS1/4-desacetylvinblastine (LY256787) and KS1/4-desacetylvinblastine hydrazide (LY203728) in single and multiple dose clinical studies. Cancer Res. 51, 2286–2290 (1991).
Tolcher, A.W. et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J. Clin. Oncol. 17, 478–484 (1999).
Jain, R.K. Tumor physiology and antibody delivery. Front. Radiat. Ther. Oncol. 24, 32–46 (1990).
Sievers, E.L. & Linenberger, M. Mylotarg: antibody-targeted chemotherapy comes of age. Curr. Opin. Oncol. 13, 522–527 (2001).
Petersdorf, S. et al. Preliminary results of Southwest Oncology Group Study S0106: an international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood 114, abstract 790 (2009). Paper presented at the American Society of Hematology National Meeting, New Orleans, December 5–9, 2009.
Ravandi, F. Gemtuzumab ozogamicin: one size does not fit all—the case for personalized therapy. J. Clin. Oncol. 29, 349–351 (2011).
Burnett, A.K. et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J. Clin. Oncol. 29, 369–377 (2011).
Castaigne, S. et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 379, 1508–1516 (2012).
Walter, R.B., Appelbaum, F.R., Estey, E.H. & Bernstein, I.D. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood advance online publication, doi:10.1182/blood-2011-11-325050 (27 January 2012).
Bross, P.F. et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 7, 1490–1496 (2001).
Hollander, I., Kunz, A. & Hamann, P.R. Selection of reaction additives used in the preparation of monomeric antibody-calicheamicin conjugates. Bioconjug. Chem. 19, 358–361 (2008).
Boghaert, E.R. et al. Determination of pharmacokinetic values of calicheamicin-antibody conjugates in mice by plasmon resonance analysis of small (5 microl) blood samples. Cancer Chemother. Pharmacol. 61, 1027–1035 (2008).
Pettit, G.R. et al. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 109, 6883–6885 (1987).
Pettit, G.R. et al. Antineoplastic agents 365. Dolastatin 10 SAR probes. Anticancer Drug Des. 13, 243–277 (1998).
Perez, E.A. et al. Phase II trial of dolastatin-10 in patients with advanced breast cancer. Invest. New Drugs 23, 257–261 (2005).
Doronina, S.O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 (2003).
Chari, R.V. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc. Chem. Res. 41, 98–107 (2008).
Dubowchik, G.M., Mosure, K., Knipe, J.O. & Firestone, R.A. Cathepsin B-sensitive dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (Taxol), mitomycin C and doxorubicin. Bioorg. Med. Chem. Lett. 8, 3347–3352 (1998).
Alley, S.C. et al. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug. Chem. 19, 759–765 (2008).
Hamblett, K.J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Shen, B.Q. et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 30, 184–189 (2012).
LoRusso, P.M., Weiss, D., Guardino, E., Girish, S. & Sliwkowski, M.X. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin. Cancer Res. 17, 6437–6447 (2011).
McDonagh, C.F. et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng. Des. Sel. 19, 299–307 (2006).
Junutula, J.R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932 (2008).
Andersen, D.C. & Reilly, D.E. Production technologies for monoclonal antibodies and their fragments. Curr. Opin. Biotechnol. 15, 456–462 (2004).
Sun, M.M. et al. Reduction-alkylation strategies for the modification of specific monoclonal antibody disulfides. Bioconjug. Chem. 16, 1282–1290 (2005).
Lyon, R.P., Meyer, D.L., Setter, J.R. & Senter, P.D. Conjugation of anticancer drugs through endogenous monoclonal antibody cysteine residues. Methods Enzymol. 502, 123–138 (2012).
Tijink, B.M. et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin. Cancer Res. 12, 6064–6072 (2006).
Deutsch, Y.E., Tadmor, T., Podack, E.R. & Rosenblatt, J.D. CD30: an important new target in hematologic malignancies. Leuk. Lymphoma 52, 1641–1654 (2011).
Wahl, A.F. et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin's disease. Cancer Res. 62, 3736–3742 (2002).
Duckett, C.S. & Thompson, C.B. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 11, 2810–2821 (1997).
Connors, J.M. State-of-the-art therapeutics: Hodgkin's lymphoma. J. Clin. Oncol. 23, 6400–6408 (2005).
Pro, B. et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma: results of a phase 2 study. J. Clin. Oncol. advance online publication doi:10.1200/JCO.2011.38.0402 (21 May 2012).
Ansell, S.M. et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J. Clin. Oncol. 25, 2764–2769 (2007).
Forero-Torres, A. et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 146, 171–179 (2009).
Schnell, R. et al. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin's and non-Hodgkin's lymphoma. Clin. Cancer Res. 8, 1779–1786 (2002).
Kim, K.M. et al. Anti-CD30 diabody-drug conjugates with potent antitumor activity. Mol. Cancer Ther. 7, 2486–2497 (2008).
Oflazoglu, E., Kissler, K.M., Sievers, E.L., Grewal, I.S. & Gerber, H.P. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br. J. Haematol. 142, 69–73 (2008).
Younes, A. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363, 1812–1821 (2010).
Fanale, M.A. et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin. Cancer Res. 18, 248–255 (2012).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J. Clin. Oncol. advance online publication, doi:10.1200/JCO.2011.38.0410 (29 May 2012).
Keir, C.H. & Vahdat, L.T. The use of an antibody drug conjugate, glembatumumab vedotin (CDX-011), for the treatment of breast cancer. Expert Opin. Biol. Ther. 12, 259–263 (2012).
Baselga, J. et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J. Clin. Oncol. 23, 2162–2171 (2005).
Cobleigh, M.A. et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 17, 2639–2648 (1999).
Vogel, C.L. et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20, 719–726 (2002).
Burris, H.A. III et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 (2011).
Hurvitz, S.A., Hu, Y., O'Brien, N. & Finn, R.S. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat. Rev. advance online publication, doi:10.1016/j.ctrv.2012.04.008 (4 June 2012).
Krop, I.E. et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J. Clin. Oncol. 28, 2698–2704 (2010).
Krop, I.E. et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J. Clin. Oncol. advance online publication, doi: 10.1200/JCO.2011.40.5902 (29 May 2012).
Kantarjian, H. et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 13, 403–411 (2012).
Leonard, J.P. et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J. Clin. Oncol. 21, 3051–3059 (2003).
Beck, A., Senter, P. & Chari, R. World Antibody Drug Conjugate Summit Europe: February 21-23 2011, Franfurt, Germany MAbs 3, 331–337 paper presented at the World Antibody Drug Conjugate Summit Europe, Frankfurt, Germany, February 21–23, 2011.
Hartley, J.A. et al. SG2285, a novel C2-aryl-substituted pyrrolobenzodiazepine dimer prodrug that cross-links DNA and exerts highly potent antitumor activity. Cancer Res. 70, 6849–6858 (2010).
Moldenhauer, G. et al. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl. Cancer Inst. 104, 622–634 (2012).
Liu, C.C. & Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Wu, P. et al. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc. Natl. Acad. Sci. USA 106, 3000–3005 (2009).
Friedman, M. & Stahl, S. Engineered affinity proteins for tumour-targeting applications. Biotechnol. Appl. Biochem. 53, 1–29 (2009).
Cheson, B.D. et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 25, 579–586 (2007).
Acknowledgements
We are grateful for meaningful and enduring contributions by our colleagues at Seattle Genetics and various international cancer centers, our corporate partner, Millennium, the Takeda Oncology Company and to patients with life-threatening lymphoma, who collectively participated in clinical trials of brentuximab vedotin —ultimately leading to its approval by the FDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.D.S. and E.L.S. are paid employees and shareholders of Seattle Genetics.
Rights and permissions
About this article
Cite this article
Senter, P., Sievers, E. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol 30, 631–637 (2012). https://doi.org/10.1038/nbt.2289
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2289