Abstract
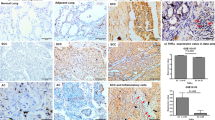
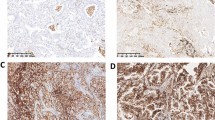
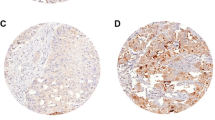
Non-small cell lung cancer (NSCLC) accounts for 80 % of lung cancers, and lung adenocarcinoma (ADC) is one of the main types of NSCLC. Although there are several studies on the relationship between lung ADC immunohistochemical diagnostic markers (thyroid transcription factor 1 (TTF-1) and Napsin A) and survival, some aspects of those studies could be improved. We examined the significance of the commonly used lung ADC diagnostic markers, including TTF-1, Napsin A, and CK7, in the prognosis of early-stage lung ADC. One hundred and nineteen cases of early-stage lung ADC (N0) were selected from the prospective database of lung cancer (Jan 2000 to Dec 2009). The expression levels of TTF-1, Napsin A, and CK7 in inventoried specimens were analyzed using tissue microarray (TMA) and immunohistochemical (IHC) analysis, and the effect of the expression level of each marker on patients’ survival was examined. The diagnostic sensitivity and specificity of each marker for lung ADC were as follows: TTF-1, 87.0 and 90.1 %; Napsin A, 72.2 and 90.4 %; and CK7, 94.6 and 76.0 %, respectively. Patients with high expression levels of TTF-1 and Napsin A, and high co-expression levels of TTF-1/Napsin A had better survival rates than those with low levels of expression (P < 0.05). The expression levels of CK7 were not related to patients’ survival. Multivariate analysis showed that the expression levels of Napsin A and TTF-1/Napsin A are independent prognostic factors for survival. The IHC detection of TTF-1 and Napsin A in specimens should be routinely performed in postoperative early-stage lung ADC patients. Its significance lies not only in the differential diagnosis, but also in determining the prognosis.


Similar content being viewed by others
References
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2011;6:244–85.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–5.
Nobre AR, Albergaria A, Schmitt F. P40: a p63 isoform useful for lung cancer diagnosis—a review of the physiological and pathological role of p63. Acta Cytol. 2013;57:1–8.
Whithaus K, Fukuoka J, Prihoda TJ, Jagirdar J. Evaluation of napsin a, cytokeratin 5/6, p63, and thyroid transcription factor 1 in adenocarcinoma versus squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:155–62.
Berghmans T, Paesmans M, Mascaux C, Martin B, Meert AP, Haller A, et al. Thyroid transcription factor 1—a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol Off J Eur Soc Med Oncol / ESMO. 2006;17:1673–6.
Lee JG, Kim S, Shim HS. Napsin a is an independent prognostic factor in surgically resected adenocarcinoma of the lung. Lung Cancer. 2012;77:156–61.
Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, et al. Nrf2 and keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:3743–53.
Saad RS, Liu YL, Han H, Landreneau RJ, Silverman JF. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol. 2004;35:3–7.
Di Loreto C, Di Lauro V, Puglisi F, Damante G, Fabbro D, Beltrami CA. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50:30–2.
Bohinski RJ, Bejarano PA, Balko G, Warnick RE, Whitsett JA. Determination of lung as the primary site of cerebral metastatic adenocarcinomas using monoclonal antibody to thyroid transcription factor-1. J Neuro-Oncol. 1998;40:227–31.
Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol Off J U S Can Acad Pathol Inc. 2011;24:1348–59.
Ao MH, Zhang H, Sakowski L, Sharma R, Illei PB, Gabrielson E, Askin F, Li QK. The utility of a novel triple marker (combination of TTF1, napsin A, and p40) in the subclassification of non-small cell lung cancer. Hum Pathol. 2014;45:926–34.
Tatnell PJ, Powell DJ, Hill J, Smith TS, Tew DG, Kay J. Napsins: new human aspartic proteinases. Distinction between two closely related genes. FEBS Lett. 1998;441:43–8.
Ordonez NG. Napsin a expression in lung and kidney neoplasia: a review and update. Adv Anat Pathol. 2012;19:66–73.
Brasch F, Ochs M, Kahne T, Guttentag S, Schauer-Vukasinovic V, Derrick M, et al. Involvement of napsin a in the c- and n-terminal processing of surfactant protein b in type-ii pneumocytes of the human lung. J Biol Chem. 2003;278:49006–14.
Ueno T, Linder S, Elmberger G. Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma. Br J Cancer. 2003;88:1229–33.
Debus E, Moll R, Franke WW, Weber K, Osborn M. Immunohistochemical distinction of human carcinomas by cytokeratin typing with monoclonal antibodies. Am J Pathol. 1984;114:121–30.
Sundstrom BE, Stigbrand TI. Cytokeratins and tissue polypeptide antigen. Int J Biol Markers. 1994;9:102–8.
Broers JL, Ramaekers FC, Rot MK, Oostendorp T, Huysmans A, van Muijen GN, et al. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 1988;48:3221–9.
Stieber P, Dienemann H, Hasholzner U, Fabricius PG, Schambeck C, Weinzierl M, et al. Comparison of cyfra 21–1, tpa and tps in lung cancer, urinary bladder cancer and benign diseases. Int J Biol Markers. 1994;9:82–8.
Johansson L. Histopathologic classification of lung cancer: relevance of cytokeratin and ttf-1 immunophenotyping. Ann Diagn Pathol. 2004;8:259–67.
Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum Pathol. 2000;31:980–7.
Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol Off J U S Can Acad Pathol Inc. 2000;13:962–72.
Puglisi F, Barbone F, Damante G, Bruckbauer M, Di Lauro V, Beltrami CA, et al. Prognostic value of thyroid transcription factor-1 in primary, resected, non-small cell lung carcinoma. Mod Pathol Off J U S Can Acad Pathol Inc. 1999;12:318–24.
Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, et al. Suppression of lung adenocarcinoma progression by nkx2-1. Nature. 2011;473:101–4.
Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing ttf-1, napsin a, p63, and ck5/6. Am J Surg Pathol. 2011;35:15–25.
Ueno T, Elmberger G, Weaver TE, Toi M, Linder S. The aspartic protease napsin a suppresses tumor growth independent of its catalytic activity. Lab Invest J Tech Methods Pathol. 2008;88:256–63.
Grant support
This work was supported by the Beijing Academic Leaders Program (Grant 2009-2-17), Beijing Natural Science Foundation (Grant 7102029), Capital Medical Developed Research Found (Grant 2007–1023), New Scholar Star Program of the Ministry of Education, and National Basic Research Program of China (973 programs) (Grant 2011CB504300), Specialized Research Fund for the Doctoral Program of Higher Education (Grant 20130001110108), National Natural Science Foundation (Grant 81301748), and the Education Ministry Innovative Research Team in University (IRT13003).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Yunfan Ma and Mengying Fan contibute equally to this work.
Rights and permissions
About this article
Cite this article
Ma, Y., Fan, M., Dai, L. et al. The expression of TTF-1 and Napsin A in early-stage lung adenocarcinoma correlates with the results of surgical treatment. Tumor Biol. 36, 8085–8092 (2015). https://doi.org/10.1007/s13277-015-3478-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3478-z