Abstract
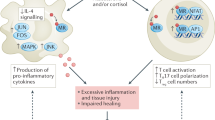
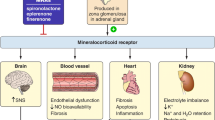
Diabetic kidney disease (DKD) develops in almost half of all patients with diabetes and is the most common cause of chronic kidney disease (CKD) worldwide. Despite the high risk of chronic renal failure in these patients, only few therapeutic strategies are available. The use of renin–angiotensin system blockers to reduce the incidence of kidney failure in patients with DKD was established years ago and remains the hallmark of therapy. The past 2 years have seen a dramatic change in our therapeutic arsenal for CKD. Sodium–glucose co-transporter‑2 inhibitors (SGLT2s) have been successfully introduced for the treatment of CKD. A further addition is a novel compound antagonizing the activation of the mineralocorticoid receptor: finerenone. Finerenone reduces albuminuria and surrogate markers of cardiovascular disease in patients who are already on optimal therapy. In the past, treatment with other mineralocorticoid receptor antagonists was hampered by a significantly increased risk of hyperkalemia. Finerenone had a much smaller effect on hyperkalemia. Together with a reduced effect on blood pressure and no signs of gynecomastia, this therapeutic strategy had a more specific anti-inflammatory effect and a smaller effect on the volume/electrolyte axis. In the FIDELIO-DKD study comparing the actions of the non-steroidal mineralocorticoid receptor antagonist finerenone with placebo, finerenone reduced the progression of DKD and the incidence of cardiovascular events, with a relatively safe adverse event profile. In this article, we summarize the available evidence on the cardioprotective and nephroprotective effects of finerenone and analyze the molecular mechanisms involved. In addition, we discuss the potential future role of mineralocorticoid receptor inhibition in the treatment of patients with diabetic CKD.
Zusammenfassung
Die diabetische Nephropathie ist eine der häufigsten Ursachen der chronischen Nierenerkrankungen und entwickelt sich bei ungefähr der Hälfte aller Patienten mit Diabetes mellitus. Trotz der Häufigkeit der Erkrankung sind die therapeutischen Strategien für die diabetische Nephropathie begrenzt. Lange Zeit war v. a. der Einsatz von Inhibitoren des Renin-Angiotensin-Systems verbunden mit der Blutdrucksenkung die Grundlage der Therapie. In den letzten Jahren sind neue interessante Therapiestrategien eingeführt worden. Zum einen handelt es sich um die sog. SGLT-2-Inhibitoren („sodium–glucose co-transporter‑2 inhibitors“), welche die Progression der chronischen Nierenerkrankung und die Inzidenz der terminalen Niereninsuffizienz nachhaltig beeinflussen. Zum anderen besteht eine Therapiestrategie in neuen Inhibitoren der Mineralokortikoidrezeptoren. Der neue, nichtsteroidale Mineralokortikoidrezeptorantagonist Finerenon reduziert die Albuminurie sowie Surrogatparameter kardiovaskulärer Erkrankungen bei Patienten mit chronischer Niereninsuffizienz und Diabetes, die bereits unter optimaler Therapie stehen. Eine der wichtigen Vorteile dieser Therapiestrategie ist die geringere Auswirkung auf den Kaliumhaushalt als bei anderen Mineralokortikoidrezeptorantagonisten. Dies ist auf eine spezifischere Wirkung dieser Substanz auf Inflammation und Fibrose bei geringerer Wirkung auf den Volumen‑/Elektrolythaushalt und damit auf den Blutdruck zurückzuführen, auch trat keine Gynäkomastie auf. In der FIDELIO-DKD-Studie zum Vergleich der Wirkung von Finerenon mit Placebo verminderte sich unter Finerenon das Fortschreiten der diabetische Nephropathie und die Inzidenz kardiovaskulärer Ereignisse bei einem relativ günstigen Nebenwirkungsprofil. In der vorliegenden Arbeit wird die verfügbare Evidenz zu den kardioprotektiven und nephroprotektiven Wirkungen von Finerenon zusammengefasst und die beteiligten molekularen Mechanismen ausgewertet. Darüber hinaus wird der potentielle zukünftige klinische Stellenwert des Mineralokortikoidrezeptorantagonismus in der Therapie der diabetischen Nephropathie erörtert.



Similar content being viewed by others
References
Jager KJ, Kovesdy C, Langham R et al (2019) A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 34:1803–1805
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 12:2032–2045
Brenner BM, Cooper ME, de Zeeuw D et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345:861–869
Lewis EJ, Hunsicker LG, Clarke WR et al (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345:851–860
Sarafidis P, Ferro CJ, Morales E et al (2019) SGLT‑2 inhibitors and GLP‑1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA‑m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 34:208–230
Perkovic V, Jardine MJ, Neal B et al (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306
Eriksen BO, Ingebretsen OC (2006) The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 69:375–382
Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM et al (2014) Diabetic kidney disease: from physiology to therapeutics. J Physiol 592:3997–4012
Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M (2019) Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143:38–42
Remuzzi G, Perico N, Macia M, Ruggenenti P (2005) The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl 68:S57–S65
Ames MK, Atkins CE, Pitt B (2019) The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med 33:363–382
Hattangady NG, Olala LO, Bollag WB, Rainey WE (2012) Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350:151–162
Alicic RZ, Johnson EJ, Tuttle KR (2018) Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease. Adv Chronic Kidney Dis 25:181–191
Distler JHW, Györfi AH, Ramanujam M et al (2019) Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 15:705–730
Guiteras R, Flaquer M, Cruzado JM (2016) Macrophage in chronic kidney disease. Clin Kidney J 9:765–771
Black LM, Lever JM, Agarwal A (2019) Renal inflammation and fibrosis: a double-edged sword. J Histochem Cytochem 67:663–681
Wada T, Furuichi K, Sakai N et al (2000) Up-regulation of monocyte chemoattractant protein‑1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58:1492–1499
Fuller PJ, Young MJ (2005) Mechanisms of mineralocorticoid action. Hypertension 46:1227–1235
Fuller PJ, Yao Y, Yang J, Young MJ (2012) Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol 213:15–24
Lombes M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet J‑P (1995) Prerequisite for cardiac aldosterone action: mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase in the human heart. Circulation 92(2):175–182
Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M (2007) The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5:e12
Bader M (2010) Tissue renin-angiotensin-aldosterone systems: targets for pharmacological therapy. Annu Rev Pharmacol Toxicol 50:439–465
Queisser N, Schupp N (2012) Aldosterone, oxidative stress, and NF-κB activation in hypertension-related cardiovascular and renal diseases. Free Radic Biol Med 53:314–327
Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM (2006) Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20:1546–1548
Michea L, Villagrán A, Urzúa A, Kuntsmann S, Venegas P, Carrasco L, Gonzalez M, Marusic ET (2008) Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension 52(2):295–300
Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A (2014) eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr Pharm Des 20:3579–3594
Bene NC, Alcaide P, Wortis HH, Jaffe IZ (2014) Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids 91:38–45
Kasal DA, Schiffrin EL (2012) Angiotensin II, aldosterone, and anti-inflammatory lymphocytes: interplay and therapeutic opportunities. Int J Hypertens 2012:829786
Rickard AJ, Morgan J, Chrissobolis S, Miller AA, Sobey CG, Young MJ (2014) Endothelial cell mineralocorticoid receptors regulate DOC/salt-mediated cardiac remodeling and vascular reactivity, but not blood pressure. Hypertension 63:1033–1040
Liu Y, Hirooka K, Nishiyama A, Lei B, Nakamura T, Itano T, Fujita T, Zhang J, Shiraga F (2012) Activation of the aldosterone/mineralocorticoid receptor system and protective effects of mineralocorticoid receptor antagonism in retinal ischemia-reperfusion injury. Exp Eye Res 96:116–123
Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, Lumeng CN, Mortensen RM (2010) Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 120:3350–3364
Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL (2012) T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59:324–330
Herrada AA, Contreras FJ, Marini NP, Amador CA, González PA, Cortés CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF et al (2010) Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol 184:191–202
Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK (2008) Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 117:2253–2261
Anders HJ, Schaefer L (2014) Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25:1387–1400
Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ (2008) Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 51:199–211
Chrysostomou A, Becker G (2001) Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N Engl J Med 345:925–926
Sato A, Hayashi K, Naruse M, Saruta T (2003) Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 41:64–68
Epstein M, Williams GH, Weinberger M et al (2006) Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1:940–951
Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF (2014) Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 4:CD7004
Schwenk MH, Hirsch JS, Bomback AS (2015) Aldosterone blockade in CKD: emphasis on pharmacology. Adv Chronic Kidney Dis 22:123–132
Kolkhof P, Borden SA (2012) Molecular pharmacology of the mineralocorticoid receptor: Prospects for novel therapeutics. Mol Cell Endocrinol 350:310–317
Dooley R, Harvey BJ, Thomas W (2012) Non-genomic actions of aldosterone: from receptors and signals to membrane targets. Mol Cell Endocrinol 350:223–234
Iraqi W, Rossignol P, Angioi M, Fay R, Nuée J, Ketelslegers JM, Vincent J, Pitt B, Zannad F (2009) Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation 119:2471–2479
Yang P, Huang T, Xu G (2016) The novel mineralocorticoid receptor antagonist finerenone in diabetic kidney disease: progress and challenges. Metabolism 65(9):1342–1349
Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, Reyna J, Uribe N, Gamba G, Bobadilla NA (2013) Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int 83:93–103
Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF (2009) Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007004
Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin-Oblin ME, Fagart J (2015) Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor Coactivator‑1. J Biol Chem 290(36):21876–21889
Ruilope LM, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C (2014) Rationale, design, and baseline characteristics of ARTS-DN: a randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. Am J Nephrol 40:572–581
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group (2015) Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314:884–894
Bakris GL, Agarwal R, Anker SD et al (2020) Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383:2219–2229
Pitt B, Filippatos G, Agarwal R et al (2021) Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 385:2252–2263
Ruilope LM, Agarwal R, Anker SD et al (2019) Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol 50:345–356
Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL, FIDELIO-DKD and FIGARO-DKD investigators (2022) Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 43(6):474–484
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Haller has received lecture fees from AstraZeneca, Bayer, Vifor, Otsuka, Glaxo and honoraria for consulting from AstraZeneca, Bayer, Vifor, Travere.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Haller, H. Cardiorenal benefits of mineralocorticoid antagonists in CKD and type 2 diabetes. Herz 47, 401–409 (2022). https://doi.org/10.1007/s00059-022-05138-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-022-05138-2